Abstract
Background
It is well established that inflammation and apoptosis of renal tubular epithelial cells caused by hyperglycemia contribute to the development of diabetic nephropathy (DN). Although microRNAs (miRNAs) are known to have roles in inflammation-related disorders, the exact role of miR-34b in DN has not been defined, and the regulatory mechanism has been unclear. This study aimed to clarify the role of miR-34b in DN pathogenesis.
Material/Methods
Expression of miR-34b, IL-6R, and other key factors of inflammation, apoptosis (TNF-α, IL-1β, IL-6, caspase-3) in high glucose (HG)-induced HK-2 cells were measured by real-time PCR, Western blot, and flow cytometric cell apoptosis assays. We used luciferase reporter assay to detect the target of miR-34b. Moreover, the targeting gene of miR-34b and its downstream JAK2/STAT3 signaling pathway were explored.
Results
It was demonstrated that miR-34b overexpression inhibited apoptosis and expression levels of TNF-α, IL-1β, IL-6, and caspase-3 in HG-treated HK-2 cells. We also found that IL-6R is a direct target of miR-34b, which could rescue inflammation and apoptosis in HG-treated HK-2 cells transfected with miR-34b mimic. Furthermore, we showed that overexpression of miR-34b inhibited the IL-6R/JAK2/STAT3 signaling pathway in HG-treated HK-2 cells.
Conclusions
Our data suggest that overexpression of miR-34b improves inflammation and ameliorates apoptosis in HG-induced HK-2 cells via the IL-6R/JAK2/STAT3 pathway, indicating that miR-34b could be a promising therapeutic target in DN.
MeSH Keywords: Apoptosis, Diabetic Nephropathies, MicroRNAs
Background
Diabetic nephropathy is a leading cause of many high-mortality diseases such as end-stage renal disease (ESRD) and results in nephrotic syndrome [1]. The impaired renal function caused by diabetes can also accelerate microvascular complications such as cardiovascular disease and stroke [1,2]. Although several methods are widely used for treating DN, most of these patients continue and to progress to renal injury [3,4]. Thus, it is important to establish a novel therapeutic method to block DN progression in these patients.
Growing evidence indicate that tubular cells are a primary target of DN, which has an important role in the pathogenesis and progression of this disease [5]. Tubular atrophy results in DN-induced tubular injury, in which there is increased apoptosis in the distal and proximal tubular epithelial cells. Recently, several studies reported that imflamation and apoptosis of renal tubular epithelial cells can be found in both DM patients and DM animal models [6,7]. A preclinical study by Shi et al. showed that high glucose (HG)-induced tubular cell damage suppresses the ability of these cells to maintain homeostasis and ultimately leads to renal insufficiency [8]. Despite these studies of renal tubular cell apoptosis [9], details of the mechanism by which HG induces apoptosis in renal tubular epithelial cells has been unclear.
MicroRNAs (miRNAs) are small non-coding RNAs that perform post-transcriptional mediation of gene expression [10–13] and are involved in various cellular processes such as angiogenesis [14], differentiation [15], proliferation [16], and apoptosis [17]. Furthermore, recent studies have demonstrated that miRNAs targeted against genes involved in renal tubular epithelial cells might be potential candidates for use in anti-apoptotic therapies for DN. For example, Zhu et al. suggested that miR-181a can induce tubular epithelial cell apoptosis by suppressing expression of the Bcl-2 family, and showed that this process was inhibited by cisplatin [18]. A study by Huang et al. reported that miR-125b mediates HG-induced apoptosis and ROS production in HK-2 cells by targeting ACE2 [19]. At present, miRNAs are thought to be involved in regulating the DN process, and investigations on the influence miRNA and their target have shown promising results. Recent studies found that miR-34b levels are greatly increased in inflammation-related diseases such as acute graft-versus-host disease and intracranial aneurysms [20,21], suggesting miR-34b is involved with inflammation-related signaling pathways. However, the mechanism of miR-34b in DN is still unclear.
Therefore, the purpose of this study was to elucidate the possible mechanism by which miR-34b is involved in HG-induced HK-2 cells. In the present study, we assessed miR-34b expression and the effects of miR-34b on accumulation of inflammatory cytokines and apoptosis in HG-treated HK-2 cells, focusing on the target of miR-34b and its downstream JAK2/STAT3 pathway, which mainly involved in inflammation and apoptosis.
Material and Methods
Cell culture
The HK-2 cells, which are considered as to be a proximal tubular cell line [22], were obtained from ScienCell™ Corporation (Carlsbad, CA, USA) and were cultured in DMEM-F12 culture medium (GIBCO, Invitrogen, Grand Island, NY, USA). The culture medium were supplemented with 10% fetal bovine serum (FBS) and mixed with 100 U/ml penicillin and 100 mg/ml streptomycin and incubated in 5% CO2 and 37°C. To assess the effects of HG, HK-2 cells were added and recultured at a density of 5×105 cells in a 12-well plate. After that, cells were cultured in serum-free cell culture medium containing 20 mM glucose (HG) or 5 mM glucose (normal glucose, NG) for 12 h, 24 h, 48 h, and 72 h. The treated cells were incubated and collected for the following experiments. The HEK293 cells were cultured according to the manufacturer’s specifications and as detailed in a previous study [23].
ELISA assays
To detemine the inflammatory factors produced by cells, ELISA was performed according to the manufacturer’s protocols. Different groups of HK-2 cells (4×105/per well) were seeded in a 12-well plate and cultured for 24 h. Then, the cells were collected and lysed, and a BCA protein assay kit was used to detect concentrations of proteins. After that, the specific IL-6 ELISA kit was used to determine the amounts of inflammatory factors present in the supernatants. Enzyme activity was measured by detecting fluorescence using a spectrofluorophotometer. All assays were performed in duplicate and repeated 3 times.
Cell transfections
Before transfection, the HK-2 cells were incubated in a 6-well plate overnight. Next, all cells were transfected with miR-34b mimic, miR-34b inhibitor, and negative controls using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific). The IL-6R sequence (Gene-bank: NM_000565.3) was synthesized according to the full-length IL-6R sequence (based on the IR-6R sequence) and then cloned into a pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific) and empty vector was defined and considered as a control. After 48 h of transfection, the cells were used for further experiments.
qRT-PCR
To detect gene expression, quantitative real-time polymerase chain reaction (qRT-PCR) was performed. A total RNA isolation kit was used to isolate total RNA according to the manufacturer’s instructions. Briefly, total RNA was extracted from podocytes using TRIZOl reagent (Sigma, CA, USA). Then, 1 μg RNA per sample was used to generated cDNA using the Verso cDNA synthesis kit (Thermo Scientific). The RT-qPCR mixture system comprised cDNA templates, primers, and and SYBR Green qPCR Master Mix (Thermo Scientific). Conditions were 95°C for 30 s and 40 cycles of amplification. The results are expressed as fold change using the ΔΔCt method compared with control. GAPDH or U6 was used as an internal control to normalize gene expression for mRNA and miRNA, respectively. The primer information is shown in Table 1.
Table 1.
List of primers used for real-time polymerase chain reaction.
| Name of genes | Sequence (5′-3′) |
|---|---|
| Caspase-3 | Fwd: ACTTCTCCAACATTCACTGG |
| Rev: ATTCTTCTGGAGGAGAGGAG | |
| IL-6 | Fwd: GTCTTCCTCACCGATTCCT |
| Rev: ACCACCCGAGCTCTGTCTTACTC | |
| IL-1β | Fwd: CCTCGTGCTGTCGGACCCATA |
| Rev: CAGGCTTGTGCTCTGCTTGTGA | |
| TNF-α | Fwd: TACGCTCTTCTGCCTGCT |
| Rev: GCTTGT2CACTCGGGGTTC | |
| miR-34b | Fwd: TCTATTTGCCATCGTCTA |
| Rev: CAGGCAGCTCATTTGGAC | |
| GAPDH | Fwd: CACTCACGGCAAATTCAACGGCA |
| Rev: GACTCCACGACATACTCAGCAC | |
| U6 | Fwd: 5′-CTCGCTTCGGCAGCACA-3′ |
| Rev: 5′-AACGCTTCACGAATTTGCGT-3′ |
Western blots
Western blot analysis was used for protein detection, and the process was conducted as previously described [24]. Total proteins were extracted from HK-2 cells. As described previously, BCA protein assay was used to measure protein concentrations. Then, proteins were transferred to polyvinylidene difluoride (PVDF) membranes and the membranes were blocked by 5% nonfat milk for 2 h at 37°C. After rinsing, the membranes were incubated at 4°C overnight with primary antibodies. The primary polyclonal antibodies were: caspase-3; TNF-α, (1: 200, rabbit; Abcam); phosphorylated-STAT3 (p-STAT3), phosphorylated-JAK2 (p-JAK2), total STAT3 (t-STAT3), total JAK2 (t-JAK2) (1: 500, rabbit; Abcam); and IL-1β and IL-6 (1: 3000, rabbit; CA, USA). After adding secondary antibody, the binding was detected by Western Lightning Plus ECL reagent (Waltham, MA, USA). Densitometric analysis was quantified with Image-J software.
Luciferase assays
TargetScanHuman version 7.1 (http://www.targetscan.org) was used to predict the target sequences of miR-34b in IL-6R. We constructed the wild-type (WT) luciferase reporter vectors and the IL-6R 3′-untranslated region (3′-UTR), which included the miR-34b seed-binding sites. A pGL3 expression system (Promega, USA) was used to construct the recombinant IL-6R sequence (Gene-bank: NM_000565.3), which was synthesized according to the full-length IL-6R sequence. To build the pGL3-wt- IL-6R′-UTR and pGL3-mut- IL-6R-3′-UTR plasmids, the wild-type or mutant binding sites of IL-6R were trasfacted into the pGL3 vector. For the luciferase reporter assay, HEK-293T cells (1×104) were plated onto a 96-well plate and co-transfected with reporter constructs (0.2 μg), miR-34b mimic, or control miRNA (50 nM), and pRL-TK vector (0.03 μg, Promega). Luciferase activities were measured 48 h after transfection using the Dual-Luciferase Reporter Assay System (Promega).
Flow cytometric cell apoptosis assays
Cell apoptosis was assessed by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit (Sigma, USA) according to the manufacturer’s instructions. The tranfected cells (1×105/well) were collected by trypsinization and resuspended in binding buffer, and then stained by FITC-Annexin V and propidium iodide (PI) in the dark for 15 min. Finally, the stained cells were detected using flow cytometry (BD Biosciences, USA).
Data analysis
All data analyses were performed using SPSS 21.0 software (SPSS, Inc., Chicago, IL, USA), and all data are presented as mean ±SD. The figures were made by using GraphPad Prism 7.0. For two-group analysis, we used Student’s t test, and for multiple groups analysis, we used one-way ANOVA. P-value ≤0.05 was considered as statistically significant.
Results
The expression of miR-34b is downregulated in HG-treated HK-2 cells
In the first experiment we used the DN cell model induced by HG in HK-2 cells. To assess the role of miR-34b in HG-treated HK-2 cells, we first established the HG damaged model as previously described [19]. The expression of miR-34b was detected and analyzed at different time points (25 mM for 12, 24, 48, and 72 h) by using RT-PCR. As shown in Figure 1, the miR-34b expression was significantly downregulated in HG-treated HK-2 cells in a time-dependent manner, suggesting a role in pathological progression of DN.
Figure 1.
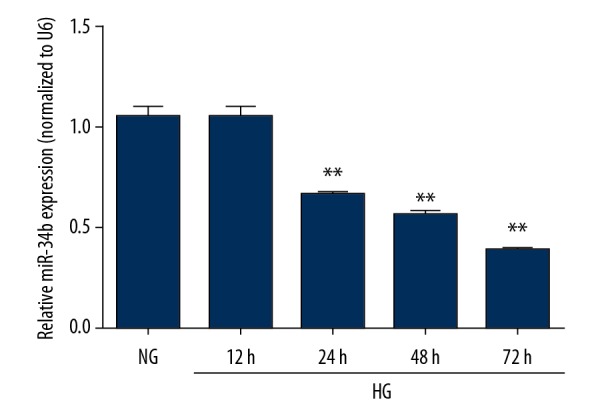
miR-34b was downregulated in HG-treated HK-2 cells. The HK-2 cells were incubated with 5 mM (NG group) or 25 mM (HG group) at different time points (12 h, 24 h, 48 h, 72 h). The expression of miR-34b was measured by qRT-PCR. Data are presented as mean ±SD and shown as fold change relative to the control group. Data were assessed using one-way ANOVA. * p<0.05 and ** p<0.01. HG – high glucose; NG – normal glucose.
miR-34b attenuated inflammation in HG-treated HK-2 cells
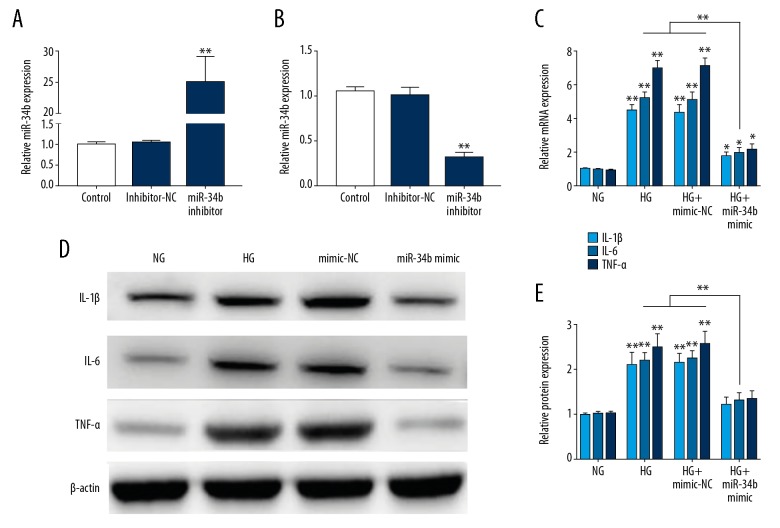
To assess the role of miR-34b in inflammatory response in DN, we detected the inflammatory factor in HG-treated HK-2 cells transfected with miR-34b mimic. The transfection efficiency of miR-34b mimic and miR-34b inhibitor in HK-2 cells was verified by qRT-PCR (Figure 2A). Then, the inflammatiory factors such as TNF-α, IL-1β, and IL-6, which play major roles in DN progression, were measured in each group by RT-PCR and Western bolt. As shown in Figure 2B, mRNA expressions of the TNF-α, IL-1β, and IL-6 were significanlty decreased in the miR-34b overexpression group compared to the control groups. We also found that the protein levels of TNF-α, IL-1β, and IL-6 were remarkably decreased in the miR-34b mimic group (Figure 2C–2E). Taken together, these findings indicate that miR-34b attenuates inflammation in HG-treated HK-2 cells.
Figure 2.
miR-34b attenuates inflammation in HG-treated HK-2 cells. (A, B) The expression of miR-34b was measured by qRT-PCR. (C) qRT-PCR detection of TNF-α, IL-1β, and IL-6 mRNA expression in HG-treated HK-2 cells in each group. (D, E) Western Blot detection of TNF-α, IL-1β, and IL-6 protein expression in HG-treated HK-2 cells in each group. Data are presented as mean ±SD and shown as fold change relative to the control group. Data were assessed using one-way ANOVA. * p<0.05 and ** p<0.01. HG – high glucose; NG – normal glucose.
miR-34b attenuates apoptosis in HG-treated HK-2 cells
Because inflammation can lead to hyperglycemia-induced apoptosis, we next tested whether miR-34b is involved in apoptosis in HG-treated HK-2 cells. The results showed that, compared to the NG group, the apoptotic cells were significantly increased in HG-induced HK-2 cells. Meanwhile, the number of apoptotic cells was dramatically decreased in the miR-34b mimic group compared with controls (Figure 3A, 3B). As shown in Figure 3C, the caspase-3 mRNA expression was significantly higher in the HG group compared to the NG group, and was remarkably reduced in the miR-34b mimic group, showing that miR-34b can suppress apoptosis in HG-treated HK-2 cells. In addition, our results show that the protien level of cleaved caspase-3 was dramatically upregulated in the HG group compared to the NG group, and was attenuated by transfection of miR-34b mimic (Figure 3D, 3E). Taken together, our results demonstrate that miR-34b can attenuate apoptosis in HG-treated HK-2 cells.
Figure 3.
miR-34b attenuates apoptosis in HG-treated HK-2 cells. (A, B) Percentage appoptosis in HG-treated HK-2 cells transfected with miR-34b mimic or mimic-NC by using flow analysis. (C) qRT-PCR detection of caspase-3 mRNA expression in HG-treated HK-2 cells transfected with miR-34b mimic or mimic-NC. (D, E) Western blot detection of cleaved caspase-3 protein expression in HG-treated HK-2 cells transfected with miR-34b mimic or mimic-NC. Data are presented as mean ±SD and shown as fold change relative to the control group. Data were assessed uding one-way ANOVA. * p<0.05 and ** p<0.01. HG – high glucose; NG – normal glucose.
IL-6R is confirmed to be a direct target of miR-34b
To further explore the molecular mechanisms of miR-34b in the HG-induced cell model, we assessed the potential target IL-6R by using bioinformatics algorithms (Figure 4A). To determine whether IL-6R is a direct target of miR-34b, the dual-luciferase reporter assay was used. We co-transfected wild-type pGL3-IL-6R-WT-3′-UTR or pGL3-IL-6R-Mut-3′-UTR with miR-34b mimic or miR-34b inhibitor in HEK-293T cells. As shown in Figure 4B, miR-34b overexpression clearly reduced the luciferase activity, and miR-34b knockdown showed the opposite effect, in which the relative activity of luciferase was not significantly different from the IL-6R-3′-UTR-Mut groups. To identify whether miR-34b regulated the expression of IL-6R in DN, we further measured the mRNA and protein level of IL-6R in HG-treated HK-2 cells. The results identified that overexpression of miR-34b suppressed the mRNA and protein levels of IL-6R, which was promoted by miR-34b knockdown (Figure 4C–4E). These data sugget that IL-6R is a direct target of miR-34b in HG-treated HK-2 cells.
Figure 4.
IL-6R is a direct target of miR-34b. (A) The predicted binding sequences of the 3′-UTR of IL-6R. (B) Relative luciferase activity was analyzed by luciferase analysis (C–E) The mRNA and protein expression of IL-6R in HG-treated HK-2 cells transfected with miR-34b mimic or miR-34b inhibitors. Data are presented as mean ±SD and shown as fold change relative to the control group. Data were assessed using one-way ANOVA. * p<0.05 and ** p<0.01. NC – negative control.
miR-34b attenuates inflammation and apoptosis by targeting IL-6R in HG-treated HK-2 cells
Previously, we comfirmed that IL-6R was a direct target of miR-34b, so we wanted to further investigate whether miR-34b mediates apoptosis and inflammatory response by IL-6R. Therefore, we investigated the factors related to inflammation and apoptotosis in HG-treated HK-2 cells transfected with pcDNA-IL-6R, empty vector, miR-34b mimic, or mimic-NC. Our results indicated that mRNA expression of caspase-3 and inflammatory factors as IL-1β, IL-6, and TNF-α was lower in HG-treated HK-2 cells transfected with miR-34b mimics, which could be rescued by co-transfection of pcDNA- IL-6R (Figure 5A). As illustrated in Figure 5B, 5C, the IL-6R overexpression also evidently abrogated the effects of miR-34b mimics on the protein expression of TNF-α, IL-1β, and IL-6 and activity of caspase-3 in HG-treated HK-2 cells. These results indicate that miR-34b protects HG-treated HK-2 cells from inflammation and apoptosis by targeting IL-6R.
Figure 5.
miR-34b attenuates inflammation and apoptosis by targeting IL-6R in HG-treated HK-2 cells. (A) The mRNA expression of TNF-α, IL-1β, IL-6, and caspase-3 in HG-induced HK-2 cells co-transfected with pcDNA-IL-6R, empty vector, miR-34b mimic, or mimic-NC. (B, C) The protein expression of TNF-α, IL-1β, IL-6, and cleaved caspase-3 in HG-induced HK-2 cells co-transfected with pcDNA-IL-6R, empty vector, miR-34b mimic, or mimic-NC. Data are presented as mean ±SD and shown as fold change relative to the control group. Data were assessed with one-way ANOVA. * p<0.05 and ** p<0.01.
miR-34b suppresses the activation of the JAK2/STAT3 signaling pathway
In order to explore the deep mechanism of miR-34b in regulating inflammation and apoptosis in HG-treated HK-2 cells, we detected the activation of JAK2/STAT3 pathway, as previous reported. As shown in Figure 6A, 6B, after transfection of miR-34b mimic into HG-induced HK-2 cells, the levels of p-STAT3 and p-JAK2 were significantly reduced compared with controls. Moreover, overexpression IL-6R distinctly suppressed effects of miR-34b mimic on the protein expression of p-STAT3 and p-JAK2 in HG-treated HK-2 cells (Figure 6C, 6D). These data suggest that miR-34b can regulate the JAK2/STAT3 signaling pathway by targeting IL-6R in HG-treated HK-2 cells.
Figure 6.
miR-34b suppresses activation of the JAK2/STAT3 signaling pathway. (A, B) The p-STAT3, t-STAT3, p-JAK2, and t- JAK2 expression in HG-treated HK-2 cells tranfected with miR-34b mimic or controls. (C, D) The p-STAT3, t-STAT3, p-JAK2, and t-JAK2 expression in HG-treated HK-2 cells co-transfected with pcDNA-IL-6R, empty vector, miR-34b mimic, or mimic-NC. Data are presented as mean ±SD and shown as fold change relative to the control group. Data were assessed with one-way ANOVA. * p<0.05, ** p<0.01, and *** p<0.001. p-STAT3 – phosphorylated STAT3; p-JAK2 – phosphorylated JAK2; t-STAT3 – total STAT3; t-JAK2 – total JAK2; HG – high glucose; NG – normal glucose.
Discussion
The correlation between renal tubular epithelial cell death and the generation and progression of DN is explicit. Damage of renal tubular epithelial cells is one of the important pathogeneses associated with the development of DN and ESRD, and increasingly more researchers have suggested that renal tubular epithelial cells death participates in DN [25]. Risk factors leading to renal tubular epithelial cells death in DN are diverse, including nitrous oxide, inflammatory cytokine, fas ligand, and osmotic change [26]. Recently, increasing evidence suggests that inflammatory factors have key roles in damage to tubular epithelial cells [25]. For example, Ma et al. reported that IL-17a takes part in HG-induced renal injury by controlling expression of pro-inflammatory cytokines such as TNF-α, IL-6, CCL2, and CCL10 [27]. Therefore, the use of effective new therapeutic agents that specifically suppress inflammatory injury in DN may be useful strategies for protecting renal function.
miRNAs are a group of 21–23 nucleotide small non-coding RNAs, which have an effect on modulating targeted mRNA expression [10–13]. Notably, there is already sufficient evidence to show that several miRNAs are key factors in regulating the pathological process of DN – some of them, such as miR-15b [28], miR-214 [29], and miR-29a [30] play protective roles, whereas others, such as miR-22 [31] and miR-31 [32], serve as injury elements. The miR-34 family includes 3 miRNAs: miRNA-34a, which is encoded by its own transcript, and miRNA-34b and miRNA-34c [33]. miR-34 family members are found at high levels in colorectal cancer [34–36], gastric cancer [37], ovarian cancer [38], pediatric leukemia [39], and kidney cell cancer [40,41]. These members are mainly induced by the tumor supressor p53 [42] and are involved in apoptosis [43], indicating that miR-34b has important roles in cell apoptotis and progression. Similarily, considerable evidence shows that miR-34b is prominently induced in inflammation-related diseases, suggesting the underlying function of miR-34b in the inflammatory signaling pathway. For example, Xie et al. showed that knockdown of miR-34b-5p regulates inflammation factors and apoptosis in an LPS-induced ALI mouse model [44], and Singh et al. demonstrated that miR-34b is involved in hepatitis B virus-induced chronic inflammation reaction and regulates the innate immune response [45].
There has neen little systematic reasearch reporting the function of miR-34b in DN, and only Greco et al. reported that miR-34b is differentially expressed in ischemic heart failure patients with diabetes mellitus and ischemic simple heart failure patients, indicating miR-34b participates in diabetic injury [46]. Although there is no direct proof that miR-34b regulates HK-2 cell death and inflammatory injury, we hypothesized that miR-34b suppression alleviates functional abnormalities via ameliorating the apoptosis and inflammation response in DN, as suggested by Liu et al. [47]. To determine the role of miR-34b in DN, we initially examined the in vitro expression of miR-34b in different groups, including an HG (25 mM glucose) group and an NG (5 mM glucose) group. We found that the expression of miR-34b was much lower in HG-treated HK-2 cells. Then, we assessed the influence of excessive expression of miR-34b on apoptosis, inflammation, and related functional experiments of HG-treated HK-2 cells. We found that miR-34b weakened the activation and release of caspase-3 and inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in the DN cell model. Furthermore, we found that miR-34b can prevent inflammation and apoptosis in HG-treated HK-2 cells by targeting IL-6R. Our findings appear to contradict previous studies reporting miR-34b promotes apoptotis and inflammation [42,44]. Likewise, Catuogno et al. reported that miR-34c-5p clearly increased resistance to paclitaxel-induced apoptosis by inhibiting p53 in lung cancer [48]. Similar to our results, another study found that miR-34c suppresses HG-induced apoptosis in podocytes via the Notch signaling pathway [47]. These conflicting results are mainly caused by different conditions of stimulation and cell types, indicating miR-34b might have various biological functions in different diseases. However, we believe it is necessary to further explore the mechanisms by which miR-34b induces apoptosis and inflammation in HG conditions.
Recent studies have suggested that the JAK2/STAT3 signaling pathway has a pivotal role in HG-induced injury by regulating inflammatory response, promoting insulin resistance, and modulating glucose intolerance [49,50]. In light of the pathological progression in DN, Kan et al. reported that suppression of the JAK2/STAT3 signaling pathway attenuates glycation end-products-induced renal tubular hypertrophy [49]. Sun et al. also showed that inhibition of the JAK2/STAT3 signaling pathway ameliorates diabetic renal dysfunction and reduces the expression of inflammatory factor such as IL-6, TNF-α, and MCP-1 [50]. The current consensus is that IL-6 and IL-6R interaction modifies the dimerization of glycoprotein 130 (gp130) to activate the STAT3 signaling pathway [51]. Misso et al. previously demonstrated that miR-34a serves as regulator for phosphorylation of JAK2 and STAT3, and the IL-6R signal transducer feedback loop [52]. Rokavec et al. [53] found that the IL-6R/STAT3/miR-34a signaling pathway plays a key role in regulating p53 expression, which is widely believed to be involved in the apoptosis process. Therefore, we hypothesized that overexpression of miR-34b alleviates apoptosis and inflammation response in HG-induced HK-2 via the JAK2/STAT3 signaling pathway. Our results show that upregulation of miR-34b enhances phosphorylation of JAK2 and STAT3 in HG-treated HK-2 cells, which was rescued by IL-6R overexpression, demonstrating that miR-34b represses HG-induced inflammation and apoptosis via the IL-6R/JAK2/STAT3 pathway.
Conclusions
Our results suggest that the expression of miR-34b is significantly decreased in HG-induced HK-2 cells. Furthermore, miR-34b overexpression can attenuate HG-induced inflammation and apoptosis in HK-2 cells though the IL-6R/JAK2/STAT3 signaling pathway, indicating that miR-34b is a promising therapeutic traget of DN.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2016;108:2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: An update. Ren Fail. 2017;39:474–83. doi: 10.1080/0886022X.2017.1313164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhury D, Tuncel M, Levi M. Diabetic nephropathy – a multifaceted target of new therapies. Discov Med. 2010;10:406–15. [PubMed] [Google Scholar]
- 4.Seaquist ER, Ibrahim HN. Approach to the patient with type 2 diabetes and progressive kidney disease. J Clin Endocrinol Metab. 2010;95:3103–10. doi: 10.1210/jc.2010-0504. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 2017;56:1627–37. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 6.Brezniceanu ML, Lau CJ, Godin N, et al. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943–54. doi: 10.1681/ASN.2009030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdo S, Lo CS, Chenier I, et al. Heterogeneous nuclear ribonucleoproteins F and K mediate insulin inhibition of renal angiotensinogen gene expression and prevention of hypertension and kidney injury in diabetic mice. Diabetologia. 2013;56:1649–60. doi: 10.1007/s00125-013-2910-4. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Lo CS, Padda R, et al. Angiotensin-(1-7) prevents systemic hypertension, attenuates oxidative stress and tubulointerstitial fibrosis, and normalizes renal angiotensin-converting enzyme 2 and Mas receptor expression in diabetic mice. Clin Sci (Lond) 2015;128:649–63. doi: 10.1042/CS20140329. [DOI] [PubMed] [Google Scholar]
- 9.Sehgel NL, Zhu Y, Sun Z, et al. Increased vascular smooth muscle cell stiffness: A novel mechanism for aortic stiffness in hypertension. Am J Physiol Heart Circ Physiol. 2013;305:H1281–87. doi: 10.1152/ajpheart.00232.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves P, Zeng Y. Biogenesis of mammalian microRNAs: A global view. Genomics Proteomics Bioinformatics. 2012;10:239–45. doi: 10.1016/j.gpb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambros V. The functions of animal microRNAs. Nature. 2014;431:350–54. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 12.Almeida MI, Reis RM, Calin GA. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat Res. 2011;717:1–8. doi: 10.1016/j.mrfmmm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Duarte FV, Palmeira CM, Rolo AP. The Role of microRNAs in mitochondria: Small players acting wide. Genes (Basel) 2014;5:865–86. doi: 10.3390/genes5040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landskroner-Eiger S, Moneke I, Sessa WC. miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect Med. 2013;3:a006643. doi: 10.1101/cshperspect.a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeker LT, Bluestone JA. MicroRNA regulation of T-cell differentiation and function. Immunol Rev. 2013;253:65–81. doi: 10.1111/imr.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao L, Jiang F. MicroRNA (miRNA) profiling. Methods Mol Biol. 2016;1381:151–61. doi: 10.1007/978-1-4939-3204-7_8. [DOI] [PubMed] [Google Scholar]
- 17.Su Z, Yang Z, Xu Y, et al. MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget. 2015;6:8474–90. doi: 10.18632/oncotarget.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu HY, Liu MY, Hong Q, et al. Role of microRNA-181a in the apoptosis of tubular epithelial cell induced by cisplatin. Chin Med J (Engl) 2012;125:523–26. [PubMed] [Google Scholar]
- 19.Huang YF, Zhang Y, Liu CX, et al. microRNA-125b contributes to high glucose-induced reactive oxygen species generation and apoptosis in HK-2 renal tubular epithelial cells by targeting angiotensin-converting enzyme 2. Eur Rev Med Pharmacol Sci. 2016;20:4055–62. [PubMed] [Google Scholar]
- 20.Jalapothu D, Boieri M, Crossland RE, et al. Tissue-specific expression patterns of microRNA during acute graft-versus-host disease in the rat. Front Immunol. 2016;7:361. doi: 10.3389/fimmu.2016.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Z, Li Q, Han Y, et al. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/540794. 540794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan MJ, Johnson G, Kirk J, et al. HK-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 2015;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 23.Shepard BD, Natarajan N, Protzko RJ, et al. A cleavable N-terminal signal peptide promotes widespread olfactory receptor surface expression in HEK293T cells. PLoS One. 2013;8:e68758. doi: 10.1371/journal.pone.0068758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hnasko TS, Hnasko RM. The Western blot. Methods Mol Biol. 2015;1318:87–96. doi: 10.1007/978-1-4939-2742-5_9. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Kong C, Tsun A, Chen C, et al. MiR-125a-5p decreases the sensitivity of Treg cells toward IL-6-mediated conversion by inhibiting IL-6R and STAT3 expression. Sci Rep. 2015;5:14615. doi: 10.1038/srep14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantaluppi V, Quercia AD, Dellepiane S, et al. Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol Dial Transplant. 2014;29:2004–11. doi: 10.1093/ndt/gfu046. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Li YJ, Chen X, et al. Interleukin 17A promotes diabetic kidney injury. Sci Rep. 2019;9:2264. doi: 10.1038/s41598-019-38811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LP, Gao YZ, Song B, et al. MicroRNAs in the progress of diabetic nephropathy: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/3513179. 3513179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Shen E, Wang Y, et al. Cross talk between miR-214 and PTEN attenuates glomerular hypertrophy under diabetic conditions. Sci Rep. 2016;6:31506. doi: 10.1038/srep31506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tung CW, Ho C, Hsu YC, et al. MicroRNA-29a attenuates diabetic glomerular injury through modulating cannabinoid receptor 1 signaling. Molecules. 2019;24 doi: 10.3390/molecules24020264. pii: E264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pofi R, Fiore D, De Gaetano R, et al. Phosphodiesterase-5 inhibition preserves renal hemodynamics and function in mice with diabetic kidney disease by modulating miR-22 and BMP7. Sci Rep. 2017;7:44584. doi: 10.1038/srep44584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rovira-Llopis S, Escribano-Lopez I, Diaz-Morales N, et al. Downregulation of miR-31 in diabetic nephropathy and its relationship with inflammation. Cell Physiol Biochem. 2018;50:1005–14. doi: 10.1159/000494485. [DOI] [PubMed] [Google Scholar]
- 33.Agostini M, Knight RA. miR-34: From bench to bedside. Oncotarget. 2014;5:872–81. doi: 10.18632/oncotarget.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Zhang P, Li Y, et al. The quantitative analysis by stem-loop real-time PCR revealed the microRNA-34a, microRNA-155 and microRNA-200c overexpression in human colorectal cancer. Med Oncol. 2012;29:3113–18. doi: 10.1007/s12032-012-0241-9. [DOI] [PubMed] [Google Scholar]
- 35.Hiyoshi Y, Schetter AJ, Okayama H, et al. Increased microRNA-34b and -34c predominantly expressed in stromal tissues is associated with poor prognosis in human colon cancer. PLoS One. 2015;10:e0124899. doi: 10.1371/journal.pone.0124899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim NH, Cha YH, Kang SE, et al. p53 regulates nuclear GSK-3 levels through miR-34-mediated Axin2 suppression in colorectal cancer cells. Cell Cycle. 2013;12:1578–87. doi: 10.4161/cc.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katada T, Ishiguro H, Kuwabara Y, et al. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–42. [PubMed] [Google Scholar]
- 38.Corney DC, Hwang CI, Matoso A, et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res. 2016;16:1119–28. doi: 10.1158/1078-0432.CCR-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Luo XQ, Zhang P, et al. MicroRNA patterns associated with clinical prognostic parameters and CNS relapse prediction in pediatric acute leukemia. PLoS One. 2009;4:e7826. doi: 10.1371/journal.pone.0007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juan D, Alexe G, Antes T, et al. Identification of a microRNA panel for clear-cell kidney cancer. Urology. 2010;75:835–41. doi: 10.1016/j.urology.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Jung M, Mollenkopf HJ, Grimm C, et al. MicroRNA profiling of clear cell renal cell cancer identifies a robust signature to define renal malignancy. J Cell Mol Med. 2009;13:3918–28. doi: 10.1111/j.1582-4934.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rokavec M, Li H, Jiang L, Hermeking H. The p53/miR-34 axis in development and disease. J Mol Cell Biol. 2014;6:214–30. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 43.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–99. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 44.Xie W, Lu Q, Wang K, et al. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J Cell Physiol. 2017;24:558–63. doi: 10.1002/jcp.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh AK, Rooge SB, Varshney A, et al. Global microRNA expression profiling in the liver biopsies of hepatitis B virus-infected patients suggests specific microRNA signatures for viral persistence and hepatocellular injury. Hepatology. 2018;67:1695–709. doi: 10.1002/hep.29690. [DOI] [PubMed] [Google Scholar]
- 46.Greco S, Fasanaro P, Castelvecchio S, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61:1633–41. doi: 10.2337/db11-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu XD, Zhang LY, Zhu TC, et al. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int J Clin Exp Pathol. 2015;8:4525–34. [PMC free article] [PubMed] [Google Scholar]
- 48.Catuogno S, Cerchia L, Romano G, et al. miR-34c may protect lung cancer cells from paclitaxel-induced apoptosis. Oncogene. 2013;32:341–51. doi: 10.1038/onc.2012.51. [DOI] [PubMed] [Google Scholar]
- 49.Kan WC, Hwang JY, Chuang LY, et al. Effect of osthole on advanced glycation end products-induced renal tubular hypertrophy and role of klotho in its mechanism of action. Phytomedicine. 2019;53:205–12. doi: 10.1016/j.phymed.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Bu W, Li Y, et al. Danzhi Jiangtang Capsule ameliorates kidney injury via inhibition of the JAK-STAT signaling pathway and increased antioxidant capacity in STZ-induced diabetic nephropathy rats. Biosci Trends. 2019;12:595–604. doi: 10.5582/bst.2018.01255. [DOI] [PubMed] [Google Scholar]
- 51.Rokavec M, Oner MG, Li H, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–67. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan SA. Re: Prostatic artery embolization (PAE) for benign prostatic hyperplasia (BPH) with haematuria in the absence of an upper urinary tract pathology. J Urol. 2019;201:834–35. doi: 10.1097/01.JU.0000554113.47614.7d. [DOI] [PubMed] [Google Scholar]
- 53.Rokavec M, Oner MG, Li H, et al. Corrigendum. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2015;125:1362. doi: 10.1172/JCI81340. [DOI] [PMC free article] [PubMed] [Google Scholar]