Abstract
Introduction
The main pathophysiology of sepsis is considered to be circulation crisis with an imbalance of vasodilation and vasoconstriction mechanisms, which contributes to multiple organ failure. However, sepsis-induced hemodynamic changes have not been fully validated by novel arterial stiffness parameter. The aim of this study was to clarify the acute vascular alteration and hemodynamic change in sepsis using cardio-ankle-vascular index (CAVI).
Methods
Twenty-one Japanese patients (14 males and 7 females, age 62.8 ± 19.0 years) with sepsis were recruited. CAVI was measured before and 1-week after sepsis treatment.
Results
The leading underlying cause of sepsis was pyelonephritis, followed by pneumonia, lung abscess, hepatic abscess and cholecystitis. All subjects recovered from sepsis. Analysis of all subjects showed a significant increase in CAVI after 1-week treatment (7.9 ± 2.4 to 9.6 ± 1.8, P < 0.001), but no significant change in blood pressure (BP) was observed. Significant correlations were observed for all combinations among the change in CAVI, systolic BP and ln[procalcitonin (PCT)], respectively. Additionally, in subjects with PCT at presentation ≥2.0 ng/mL, the increase in CAVI after treatment was significantly greater compared to those with PCT < 2.0 ng/mL (2.4 ± 1.6 vs 1.1 ± 0.9, P = 0.037).
Discussion
CAVI may reflect sepsis-induced vascular alteration which is not indicated by BP change, and is associated with sepsis severity. These findings suggest the usefulness of CAVI in the management of circulatory failure in sepsis patients.
Keywords: SIRS, circulation crisis, procalcitonin, arterial stiffness
Introduction
Critically ill septic patients are prone to develop multiple organ failure with high mortality. Sepsis develops as a result of systemic inflammatory response syndrome (SIRS) to infection. Furthermore, the pathophysiologic features of sepsis result from the comprehensive effects of microbes and their products.1 These products can activate cytokines, complement, the coagulation system, plasmakinin, endorphins and the sympathetic nervous system. Recently, procalcitonin (PCT), a 116-amino acid-long precursor of calcitonin, has been proposed as a marker of infection, and serum PCT level positively correlates with the severity of infection. The major benefit of PCT-guided therapy is a shorter duration of antibiotic treatment compared to standard care.2
Disseminated intravascular coagulation (DIC) is a principal complication of sepsis and characterized by massive thrombin formation and widespread microvascular thrombosis involved in multiple organ ischemia.3 Indeed, the pathophysiology of sepsis-induced circulatory failure includes not only coagulation disorders but also decreased blood flow. This hemodynamic change involves some degree of hypovolemia and a decrease in vascular tone.4 Vasodilation and vascular permeability induced by pro- and anti-inflammatory cytokines released in sepsis dramatically induce peripheral circulatory failure. However, blood pressure (BP) does not necessarily reflect the pathophysiology, due to compensated increase in cardiac output. In other words, even when BP is within the normal range, residual risk of circulatory failure induced by sepsis cannot be completely ruled out. It is therefore questionable whether BP is the most suitable non-invasive indicator to detect hemodynamic changes in sepsis.
Arterial stiffness is recognized as a clinical prognostic index and a potential therapeutic target in patients with atherosclerotic risk factors. Several studies have shown that increased arterial stiffness is associated with increased morbidity and mortality of cardiovascular disease (CVD).5 The cardio-ankle vascular index (CAVI) has been developed as an arterial stiffness index, which is derived from the β theory with application of the Bramwell–Hill equation.6 The conspicuous feature of CAVI is independency from BP at the time of measurement. Furthermore, CAVI reflects the stiffness of the arterial tree from the origin of the aorta to the ankle,7 and has adequate reproducibility for clinical use.8,9 Furthermore, the increase in CAVI has also been reported to be associated with increased number of risk factors and severity of CVD10–12 and to be an independent predictor of major adverse cardiovascular events.13 These reports reveal that analyzing arterial stiffness based on CAVI may be helpful for identifying subjects with higher CVD risks.
Meanwhile, CAVI is useful not only for long-term management of atherosclerotic diseases, but also for evaluation of short-term hemodynamic changes. As for the relationship between CAVI and BP measurement, the independence of CAVI on measured BP has been confirmed experimentally in human subjects in vivo. Shirai et al14 demonstrated that administration of metoprolol, a selective β1-blocker, did not change CAVI while the drug decreased BP, whereas administration of doxazosin, a selective α1-blocker, induced decreases in both BP and CAVI. These data indicate that CAVI is mostly not affected by acute fluctuation in BP alone, but is affected by short-term arterial smooth muscle contraction. In other words, CAVI indicates not only organic stiffness but also functional stiffness. Additionally, Masugata et al15 reported that the increase in CAVI preceded the appearance of hypertension induced by sunitinib, a multi-targeted tyrosine kinase inhibitor. This report suggests that CAVI may detect hemodynamic instability that precedes change in BP.
In this study, using CAVI, we examined the change in arterial stiffness before and after sepsis treatment. Furthermore, we analyzed whether serum PCT level as a sepsis severity marker contributes to hemodynamic change in sepsis.
Subjects And Methods
Design
The study protocol was prepared in accordance with the Declaration of Helsinki, and was approved by the institutional review board of Sakura Hospital, Toho University Medical Center (approval number: 2013–001). Before starting the study, we explained the purpose of the study to each subject and obtained written informed consent for participation in this study.
Patient Recruitment
Inclusion criteria of this study were American College of Chest Physicians/Society of Critical Care Medicine consensus definitions as the criteria for diagnosing SIRS and sepsis.16 Subjects with at least two of the following four clinical findings were considered as having sepsis in this study. Resultantly, 21 Japanese subjects with sepsis (14 males, 7 females, age 66.8 ± 19.0 years) were recruited in this study.
1) Body temperature higher than 38ºC or lower than 36ºC,
2) Heart rate (HR) higher than 90/min,
3) Respiratory rate higher than 20/min or arterial carbon dioxide tension (PaCO2) lower than 32 mmHg,
4) White blood cell (WBC) count higher than 12,000/μL or lower than 4,000/μL, or with 10% immature (band) forms.
In addition, a diagnosis of infectious disease was required by culturing a sample from the infected site or blood, according to the management guidelines for the diagnosis of infection in sepsis developed in 2003.17
Exclusion criteria were a previous history of CVD, other vascular diseases, definitive renal disease, or severe liver dysfunction. Subjects with ankle-brachial indices lower than 0.90 were also excluded because CAVI was apparently low in patients with severe arterial occlusive diseases.7
Resuscitation protocols related to hemodynamic management and antibiotics administration were performed in accordance with Surviving Sepsis Campaign Guidelines 2012 (SSCG 2012).18 However, Japanese guideline was applied only for the use of immunoglobulin, recombinant thrombomodulin or antithrombin III.19
Data Collection And Laboratory Assay Methods
Height and body weight were measured, and body mass index (BMI) was calculated (weight in kilogram divided by square of height in meter). Venous blood samples were obtained in the emergency room at presentation and after 1-week of sepsis treatment, and complete blood count (CBC), C-reactive protein (CRP) and PCT were measured. CBC was performed by the electrical impedance method. C-reactive protein was quantified by latex-enhanced nephelometry. Serum PCT level, measured using the Elecsys BRAHMS PCT assay (Roche Diagnostics K.K, Swiss) based on the ELISA method,20 was also converted to logarithmic scale as ln(PCT) to curb departure from normality.
Measurement Of CAVI And Blood Pressure
CAVI was measured using the VaSera VS-1500 system (Fukuda Denshi Co Ltd, Tokyo, Japan). CAVI was obtained by measuring BP and pulse wave velocity (PWV) according to the following formula: CAVI = a{(2ρ/ΔP)×ln(Ps/Pd)PWV2}+b, where Ps is systolic blood pressure; Pd is diastolic blood pressure; ΔP is Ps – Pd; ρ is blood density, and a and b are constants.
Measurements of CAVI were performed at the following two time points: presentation at the emergency room and after 1-week of sepsis treatment. Cuffs were applied to bilateral upper arms and ankles, with the subject lying supine and the head held in midline position. After resting for 10 min, the examinations were performed. To detect the brachial and ankle pulse waves with cuffs, a low cuff pressure from 30 to 50 mmHg was used to minimize the effect of cuff pressure on hemodynamics. Thereafter, BP was measured from the cuff of the upper arm. PWV was obtained by dividing the vascular length by the time for which the pulse wave propagated from the aortic valve to the ankle, and was measured using cuffs at the upper arms and ankles. All the measurements and calculations were performed automatically. The details of CAVI and the measurement have been described previously.7
Statistical Analysis
All data are expressed as mean ± standard deviation (SD). The SPSS 15.0 software (SPSS Inc., Chicago, Ill, USA) was used for statistical analyses. Paired t-test was performed to determine whether intragroup differences between presentation and after 1-week of treatment were statistically significant. Student’s t-test or Fischer exact test was performed to determine whether subgroup differences were statistically significant. Pearson’s rank correlation was used to verify the correlation between hemodynamic factors. In all comparisons, P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics Before And After 1-Week Of Sepsis Treatment
We analyzed a total of 21 septic patients (14 males and 7 females, age 62.8 ± 19.0 years), and compared patient characteristics before and after 1-week of sepsis treatment, as shown in Table 1. Among 21 subjects, 33.3% had hypertension and 28.6% had diabetes. The average number of SIRS criteria met at presentation was 2.43 ± 1.12. All subjects received antibiotics including meropenem (33.3%), ceftriaxone (42.9%), sulbactam/ampicillin (14.3%) and tazobactam/piperacillin (9.5%). In some patients, intravenous immunoglobulin (33.3%), recombinant thrombomodulin (23.8%) or antithrombin III (14.3%) was administered. Ultimately, all subjects recovered from sepsis.
Table 1.
Patient Characteristics Before And After 1 Week Of Sepsis Treatment
| At Presentation | After 1 Week | P | |
|---|---|---|---|
| N (male/female) | 21 (14/7) | – | – |
| Age (years) | 62.8 ± 19.0 | – | – |
| Height (cm) | 163.0 ± 9.6 | – | – |
| Body weight (kg) | 64.2 ± 17.9 | – | – |
| BMI (kg/m2) | 24.0 ± 5.7 | – | – |
| CAVI | 7.9 ± 2.4 | 9.6 ± 1.8 | <0.001 |
| SBP (mmHg) | 121 ± 30 | 125 ± 14 | 0.523 |
| DBP (mmHg) | 70 ± 15 | 70 ± 12 | 0.939 |
| HR (/min) | 101 ± 16 | 76 ± 13 | <0.001 |
| WBC (/μL) | 17,104 ± 7,579 | 8,874 ± 4,090 | <0.001 |
| Plt (/μL) | 22.7 ± 11.5 | 31.7 ± 10.7 | 0.001 |
| CRP (mg/dL) | 20.9 ± 8.8 | 4.6 ± 3.9 | <0.001 |
| PCT (ng/mL) | 44.2 ± 102.9 | 0.7 ± 1.3 | 0.064 |
| ln(PCT) | 1.90 ± 1.94 | 0.39 ± 0.46 | <0.001 |
| Hypertension (%) | 7 (33.3) | – | – |
| Diabetes mellitus (%) | 6 (28.6) | – | – |
Notes: Data are presented as mean ± SD. Paired t-test was used to compare before and after 1 week of treatment.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HR, heart rate; WBC, white blood cell; Plt, platelet; CRP, C-reactive protein; PCT; procalcitonin.
After 1-week sepsis treatment, significant increases in CAVI (7.9 ± 2.4 to 9.6 ± 1.8, P < 0.001) and platelet (Plt) count were observed, while decreases in HR, WBC, CRP and ln(PCT) were also found. Significant changes in systolic and diastolic BP were not observed.
Types And Proportions Of Infectious Diseases Diagnosed Among Participant
Most of the patients (42.9%) were diagnosed as having sepsis caused by pyelonephritis, and the other major underlying causes were pneumonia, lung abscess, hepatic abscess and cholecystitis. Iliopsoas abscess, spondylitis, vasculitis and cellulitis were less frequent causes (Table 2).
Table 2.
Types And Proportions Of Infectious Diseases Diagnosed Among Participant
| Infectious Disease | Frequency (%) |
|---|---|
| Pyelonephritis | 9 (42.9) |
| Pneumoniae, lung abscess | 4 (19.0) |
| Hepatic abscess, cholecystitis | 4 (19.0) |
| Iliopsoas abscess | 1 (4.8) |
| Spondylitis | 1 (4.8) |
| Vasculitis | 1 (4.8) |
| Cellulitis | 1 (4.8) |
Correlations Among Changes In Clinical Variables
Correlations between hemodynamic variables or sepsis severity markers were verified in Table 3. The change in CAVI was correlated positively with that in systolic BP (SBP) (R = 0.585, P = 0.005), and negatively with that in ln(PCT) (R = −0.546, P = 0.010). In addition, there were also significant correlation between the change in SBP and that in ln(PCT) (R = −0.611, P = 0.003).
Table 3.
Correlations Among Changes In Clinical Variables
| ΔCAVI | ΔSBP | ΔDBP | ΔHR | ΔWBC | ΔPlt | ΔCRP | ΔPCT | Δln(PCT) | |
|---|---|---|---|---|---|---|---|---|---|
| ΔCAVI | 0.585 | 0.182 | 0.301 | 0.300 | 0.233 | 0.023 | −0.369 | −0.546 | |
| ΔSBP | 0.005 | 0.522 | −0.201 | 0.013 | 0.096 | 0.061 | −0.278 | −0.611 | |
| ΔDBP | 0.430 | 0.015 | −0.520 | 0.112 | −0.123 | 0.227 | −0.101 | −0.276 | |
| ΔHR | 0.185 | 0.383 | 0.016 | 0.305 | 0.311 | −0.242 | −0.280 | −0.316 | |
| ΔWBC | 0.187 | 0.956 | 0.628 | 0.179 | 0.104 | 0.149 | 0.042 | 0.139 | |
| ΔPlt | 0.310 | 0.680 | 0.595 | 0.170 | 0.654 | −0.335 | −0.141 | −0.387 | |
| ΔCRP | 0.922 | 0.791 | 0.323 | 0.291 | 0.519 | 0.138 | 0.259 | 0.210 | |
| ΔPCT | 0.100 | 0.222 | 0.664 | 0.219 | 0.856 | 0.541 | 0.256 | 0.690 | |
| Δln(PCT) | 0.010 | 0.003 | 0.227 | 0.163 | 0.549 | 0.083 | 0.361 | 0.001 |
Notes: Pearson’s rank correlation matrix showing statistical relationships. Right side (above diagonal) shows correlations of the raw values; left side (below diagonal) shows P values.
Abbreviations: CAVI, cardio-ankle vascular index; HR, heart rate; WBC, white blood cell; Plt, platelet; CRP; C-reactive protein; PCT; procalcitonin.
Comparison Of Characteristics At Presentation And Changes In Parameters In Subjects With Higher Or Lower Pct Level
Additionally, we compared patients stratified by the severity of infectious disease. The subjects were divided into two groups by the cut-off PCT level for severe sepsis:21 PCT level at presentation ≥2.0 ng/mL and <2.0 ng/mL.
Subjects with PCT at presentation ≥2.0 ng/mL had significantly higher ΔCAVI, ΔPlt count, PCT at presentation and lower ΔPCT compared with <2.0 ng/mL. Furthermore, a notable but not significant increase in SBP was observed in subjects with PCT at presentation ≥2.0 ng/mL. These results indicated that higher serum PCT level was associated with relatively larger amplitude of hemodynamic changes after treatment (Table 4).
Table 4.
Comparison Of Characteristics At Presentation And Changes In Parameters In Subjects With Higher Or Lower PCT Level
| Subjects With PCT ≥ 2.0 ng/mL | Subjects With PCT < 2.0 ng/mL | P | |
|---|---|---|---|
| N (male/female) | 10 (7/3) | 11 (7/4) | 1.000* |
| Age (years) | 62.0 ± 19.8 | 63.5 ± 19.3 | 0.858 |
| BMI (kg/m2) | 24.6 ± 4.2 | 23.5 ± 6.9 | 0.667 |
| CAVI | 7.0 ± 2.5 | 8.7 ± 2.1 | 0.098 |
| ΔCAVI | 2.4 ± 1.6 | 1.1 ± 0.9 | 0.037 |
| SBP (mmHg) | 110 ± 30 | 131 ± 27 | 0.103 |
| ΔSBP (mmHg) | 17 ± 35 | −8 ± 17 | 0.051 |
| DBP (mmHg) | 66 ± 20 | 73 ± 7 | 0.232 |
| ΔDBP (mmHg) | 4 ± 24 | −3 ± 6 | 0.378 |
| HR (/min) | 97 ± 15 | 105 ± 16 | 0.218 |
| ΔHR (/min) | −19 ± 13 | −31 ± 12 | 0.048 |
| WBC (/μL) | 15,929 ± 5,604 | 18,172 ± 9,164 | 0.512 |
| ΔWBC (/μL) | −8,699 ± 5,901 | −7,714 ± 5,505 | 0.698 |
| Plt (/μL) | 18.8 ± 9.6 | 26.4 ± 12.3 | 0.133 |
| ΔPlt (/μL) | 14.3 ± 12.0 | 4.2 ± 8.1 | 0.035 |
| CRP (mg/dL) | 22.8 ± 8.6 | 19.2 ± 9.0 | 0.356 |
| ΔCRP (mg/dL) | −17.4 ± 7.3 | −15.4 ± 7.9 | 0.558 |
| PCT (ng/mL) | 92.2 ± 136.5 | 0.5 ± 0.5 | 0.038 |
| ln(PCT) | 3.56 ± 1.55 | 0.39 ± 0.30 | <0.001 |
| ΔPCT (ng/mL) | −90.9 ± 134.8 | −0.3 ± 0.7 | 0.038 |
| Δln(PCT) | −2.92 ± 1.18 | −0.23 ± 0.40 | <0.001 |
Notes: Data are presented as mean ± SD. Student’s t-test and *Fischer exact test are used to compare subjects with or without PCT ≥ 2.0 ng/mL.
Abbreviations: BMI, body mass index; CAVI, cardio-ankle vascular index; HR, heart rate; WBC, white blood cell; Plt, platelet; CRP; C-reactive protein; PCT; procalcitonin.
Changes In CAVI, SBP And HR Before And After 1-Week Of Sepsis Treatment
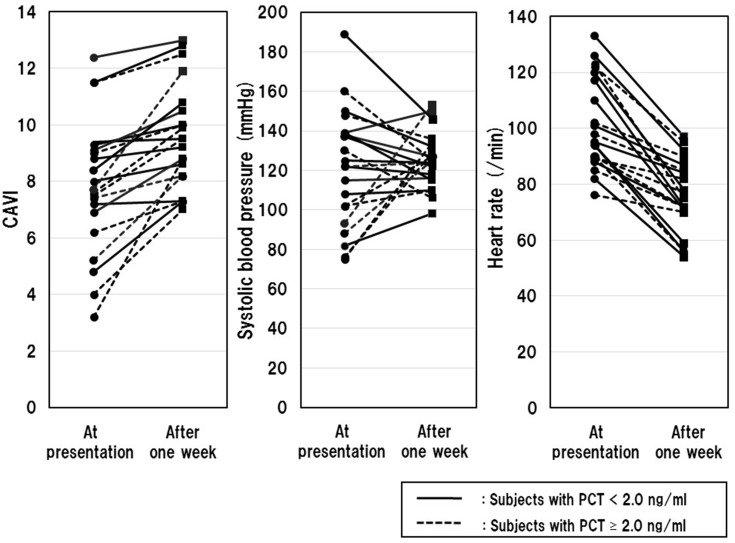
Figure 1 demonstrates the changes in CAVI, SBP and HR from before to after 1-week of sepsis treatment.
Figure 1.
Changes in CAVI, SBP and HR before and after 1 week of sepsis treatment.
Notes: CAVI, SBP or HR for each participant at presentation (circles) and after 1 week of treatment (squares); Continuous lines indicate subjects with serum PCT < 2.0 ng/mL (N=11), and dotted lines indicate those with PCT ≥ 2.0 ng/mL (N=10).
Abbreviations: CAVI, cardio-ankle vascular index; HR, heart rate.
Only 4 of 21 subjects showed SBP below 90 mmHg at presentation, which is the cut-off value of septic shock.22 Almost all (20 of 21) subjects, especially subjects with PCT at presentation ≥2.0 ng/mL (100%), showed an increase in CAVI (ΔCAVI, 1.7 ± 1.4; Δ%CAVI, 20.0 ± 39.2%) after treatment. On the other hand, the change in SBP was not uniform (ΔSBP, 4.1 ± 29.2 mmHg; Δ%SBP, 2.9 ± 22.1%). All subjects showed a decrease in HR after treatment, whereas a greater rate of change was observed in subjects with PCT at presentation <2.0 ng/mL (−28.4 ± 10.2 vs −18.9 ± 11.2%, P=0.046).
There was no association between CAVI and SIRS score, prevalence of hypertension and diabetes, type of infectious disease or treatment.
Discussion
We analyzed 21 septic patients before and after 1-week of treatment. Analysis of all subjects showed an increase in CAVI after sepsis treatment but no significant change in BP. Besides, significant correlations were observed for all combinations among the change in CAVI, SBP and ln(PCT), respectively. Consequently, CAVI may reflect hemodynamic change in sepsis more sensitively than BP. Additionally, the increase in CAVI after treatment in subjects with PCT at presentation ≥ 2.0 ng/mL was greater than that in subjects with PCT <2.0 ng/mL. Serum levels of PCT have also been observed to increase with increasing severity of sepsis and organ dysfunction.23 Therefore, the present study may suggest that arterial stiffness is altered depending on sepsis severity. CAVI might decrease at acute phase of sepsis, and rise to original levels after treatment. However, we were not able to verify sepsis-induced decrease in CAVI at presentation, because most of the data before presentation were missing in this study. However, a 67-year-old man who presented with sepsis and cholecystitis underwent CAVI measurement before developing sepsis. His CAVI was 9.3 before sepsis, 7.6 at presentation and 9.9 after 1-week of sepsis treatment.
Several reports have revealed that hyperproduction of nitric oxide (NO) by the inducible form of NO synthase contributes to vascular collapse and septic shock.24 This pathophysiology is suspected to cause the decreased CAVI observed in septic patients at presentation. Incidentally, which is the type of artery mainly involved in decreased arterial stiffness caused by sepsis? There are three main types of arteries: elastic arteries that receive blood directly from the heart to the aorta and pulmonary artery, muscular arteries that distribute blood from elastic arteries to various parts of the body, and arterioles that deliver blood from muscular arteries to capillaries. For example, calcium channel blockers that reduce resistance in arterioles preferential to elastic and muscular arteries almost do not decrease CAVI.25,26 On the other hand, nitroglycerin (NTG) that liberates NO decreases CAVI probably through functionally reducing arterial stiffness from the aorta to the ankle.26,27 In addition, Yamamoto et al28 have reported that NTG-induced decrease in CAVI depends on relaxation of both elastic and muscular arteries especially in healthy young subjects (age 30.9 ± 3.9 years), whereas elastic arterial stiffness scarcely decreases after NTG administration in elderly subjects with arteriosclerosis (age 72 ± 6.4 years). These findings may indicate that sepsis reduces CAVI through NO-mediated vasodilation, which occurs mainly in muscular arteries, and partially in elastic arteries in young persons.
On the other hand, chronic inflammatory diseases are known to contribute to increased arterial stiffness involved in premature atherosclerosis.29 For example, chronic inflammation based on visceral adiposity such as diabetes, hypertension and metabolic dyslipidemia is associated with high CAVI.30,31 Furthermore, the same is true of chronic inflammation associated with malignant tumors.32 Besides, CAVI can be reduced by proper anti-inflammatory approaches.33,34 These reports therefore appear to contradict our conclusion of the present study. However, sepsis-induced transient decrease in CAVI observed at presentation of our patients should not be expected to contribute to reduction of future vascular events. Transiently decreased CAVI in acute inflammation accompanied by hypercytokinemia may imply pathological vasodilation responses, but not anti-atherosclerotic action to resolve the chronic inflammation of blood vessel wall.
Interestingly, analysis of all subjects in the present study showed no significant BP change during sepsis treatment, whereas CAVI increased significantly. These findings suggest that even when arterial stiffness is reduced by sepsis, BP can be maintained as long as cardiac compensation is effective. Measuring CAVI of a septic patient in the emergency room therefore seems to be helpful to evaluate the risk of circulation crisis. Furthermore, for septic patients with markedly decreased CAVI, starting vasoconstrictor agents may be effective to prevent circulation failure even if they are not yet in a hypotensive state. In addition, an interpretable machine learning model for accurate prediction of sepsis has been reported recently.35 This amazing finding suggests that BP variabilities may be useful for real-time prediction of sepsis onset in applying artificial intelligence. In the future, the algorithm to predict the onset of sepsis may be constructed by combining CAVI and artificial intelligence.
If, instead of CAVI, PWV was used as an arterial stiffness marker, then we would not have obtained definitive results. There are many methods for measuring PWV, such as carotid-femoral PWV,36 heart-to-femoral PWV37 and brachial-ankle PWV.38 However, PWV is known to depend strongly on BP at the time of measurement. Therefore, the validity of PWV in reflecting actual arterial stiffness is controversial, and this parameter is unsuitable for evaluating the vascular alteration in sepsis. Furthermore, carotid-femoral PWV has already been reported to have no association with sepsis severity and prognosis.39
However, our study has several limitations. First, the protocol in the present study was conducted on the assumption of 1 week after sepsis treatment as the recovery phase, whereas that might not always be certain. Furthermore, another problem was the lack of data to link hemodynamic variables such as cardiac output and systemic vascular resistance during the initial resuscitation phase of sepsis. Next, the sample size was relatively small. There were almost no pre-CAVI values for study participants. Finally, although the sepsis criteria were revised after the start of this study, the old criteria had to be used in compliance with the protocol. From these viewpoints, a large-scale prospective multicenter cohort study using the latest criteria is necessary.
Conclusion
In summary, CAVI may reflect sepsis-induced vascular alteration not indicated by BP change and is associated with sepsis severity. These findings suggest the usefulness of CAVI for managing circulatory failure in septic patients.
Acknowledgment
We would like to thank all the investigators who participated in the study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nyström PO. The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother. 1998;41(Suppl A):1–7. doi: 10.1093/jac/41.suppl_1.1 [DOI] [PubMed] [Google Scholar]
- 2.Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock- a systematic review and meta-analysis. Crit Care. 2013;17(6):R291. doi: 10.1186/cc12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi M, van der Poll T. A short contemporary history of disseminated intravascular coagulation. Semin Thromb Hemost. 2014;40:874–880. doi: 10.1055/s-0034-1395155 [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satoh-Asahara N, Kotani K, Yamakage H, et al. Cardio-ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242(2):461–468. doi: 10.1016/j.atherosclerosis.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech. 1980;13(2):175–184. doi: 10.1016/0021-9290(80)90191-8 [DOI] [PubMed] [Google Scholar]
- 7.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardioankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101–107. doi: 10.5551/jat.13.101 [DOI] [PubMed] [Google Scholar]
- 8.Matsui Y, Kario K, Ishikawa J, Eguchi K, Hoshide S, Shimada K. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res. 2004;27(11):851–857. [DOI] [PubMed] [Google Scholar]
- 9.Kubozono T, Miyata M, Ueyama K, et al. Clinical significance and reproducibility of new arterial distensibility index. Circ J. 2007;71(1):89–94. doi: 10.1253/circj.71.89 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Iizuka T, Takahashi M, et al. Association between cardioankle vascular index and serum cystatin C levels in patients with cardiovascular risk factor. J Atheroscler Thromb. 2009;16(4):371–379. doi: 10.5551/jat.no687 [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi T, Doi M, Hirohata S, et al. Cardio-ankle vascular index is independently associated with the severity of coronary atherosclerosis and left ventricular function in patients with ischemic heart disease. J Atheroscler Thromb. 2010;17(3):249–258. doi: 10.5551/jat.1636 [DOI] [PubMed] [Google Scholar]
- 12.Nagayama D, Yamaguchi T, Saiki A, et al. High serum uric acid is associated with increased cardio-ankle vascular index (CAVI) in healthy Japanese subjects: a cross-sectional study. Atherosclerosis. 2015;239(1):163–168. doi: 10.1016/j.atherosclerosis.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Nagayama D, Saiki A, et al. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23(5):596–605. doi: 10.5551/jat.31385 [DOI] [PubMed] [Google Scholar]
- 14.Shirai K, Hiruta N, Song M, et al. Cardio-ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18:924–938. doi: 10.5551/jat.7716 [DOI] [PubMed] [Google Scholar]
- 15.Masugata H, Senda S, Himoto T, et al. Early detection of hypertension in a patient treated with sunitinib by measuring cardio-ankle vascular index. Tohoku J Exp Med. 2009;218(2):115–119. doi: 10.1620/tjem.218.115 [DOI] [PubMed] [Google Scholar]
- 16.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 17.Cohen J, Brun-Buisson C, Torres A, Jorgensen J. Diagnosis of infection in sepsis: an evidence-based review. Crit Care Med. 2004;32(11 Suppl):S466–S494. doi: 10.1097/01.ccm.0000145917.89975.f5 [DOI] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 19.Oda S, Aibiki M, Ikeda T, et al. Sepsis Registry Committee of the Japanese Society of Intensive Care Medicine. The Japanese Guidelines for the Management of Sepsis. J Intensive Care. 2014;2(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller B, Becker KL. Procalcitonin: how a hormone became a marker and mediator of sepsis. Swiss Med Wkly. 2001;131(41–42):595–602. 2001/41/smw-09751 [DOI] [PubMed] [Google Scholar]
- 21.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 23.Giamarellos-Bourboulis EJ, Mega A, Grecka P, et al. Procalcitonin: a marker to clearly differentiate systemic inflammatory response syndrome and sepsis in the critically ill patient? Intensive Care Med. 2002;28:1351–1356. doi: 10.1007/s00134-002-1398-z [DOI] [PubMed] [Google Scholar]
- 24.Kirkebøen KA, Strand OA. The role of nitric oxide in sepsis–an overview. Acta Anaesthesiol Scand. 1999;43(3):275–288. doi: 10.1034/j.1399-6576.1999.430307.x [DOI] [PubMed] [Google Scholar]
- 25.Miyashita Y, Saiki A, Endo K, et al. Effects of olmesartan, an angiotensin II receptor blocker, and amlodipine, a calcium channel blocker, on Cardio-Ankle Vascular Index (CAVI) in type 2 diabetic patients with hypertension. J Atheroscler Thromb. 2009;16(5):621–626. doi: 10.5551/jat.497 [DOI] [PubMed] [Google Scholar]
- 26.Chiba T, Yamanaka M, Takagi S, et al. Cardio-ankle vascular index (CAVI) differentiates pharmacological properties of vasodilators nicardipine and nitroglycerin in anesthetized rabbits. J Pharmacol Sci. 2015;128(4):185–192. doi: 10.1016/j.jphs.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 27.Shimizu K, Yamamoto T, Takahashi M, Sato S, Noike H, Shirai K. Effect of nitroglycerin administration on cardio-ankle vascular index. Vasc Health Risk Manag. 2016;12:313–319. doi: 10.2147/VHRM.S106542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto T, Shimizu K, Takahashi M, Tatsuno I, Shirai K. The effect of nitroglycerin on arterial stiffness of the aorta and the femoral-tibial arteries. J Atheroscler Thromb. 2017;24(10):1048–1057. doi: 10.5551/jat.38646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masugata H, Senda S, Himoto T, et al. Detection of increased arterial stiffness in a patient with early stage of large vessel vasculitis by measuring cardio-ankle vascular index. Tohoku J Exp Med. 2009;219(2):101–105. doi: 10.1620/tjem.219.101 [DOI] [PubMed] [Google Scholar]
- 30.Satoh N, Shimatsu A, Kato Y, et al. Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res. 2008;31(10):1921–1930. doi: 10.1291/hypres.31.1921 [DOI] [PubMed] [Google Scholar]
- 31.Nagayama D, Watanabe Y, Saiki S, Shirai K, Tatsuno I. Lipid parameters are independently associated with Cardio–Ankle Vascular Index (CAVI) in healthy Japanese subjects. J Atheroscler Thromb. 2018;25(7):621–633. doi: 10.5551/jat.42291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimizu N, Ban N, Watanabe Y, et al. The elevation of cardio-ankle vascular index in a patient with malignant lymphoma treated with a combination therapy of rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisolone. J Clin Med Res. 2017;9(8):729–732. doi: 10.14740/jocmr3071w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagayama D, Saiki A, Endo K, et al. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract. 2010;64(13):1796–1801. doi: 10.1111/j.1742-1241.2010.02399.x [DOI] [PubMed] [Google Scholar]
- 34.Masugata H, Senda S, Dobashi H, et al. Cardio-ankle vascular index for evaluating immunosuppressive therapy in a patient with aortitis syndrome. Tohoku J Exp Med. 2010;222(1):77–81. doi: 10.1620/tjem.222.77 [DOI] [PubMed] [Google Scholar]
- 35.Nemati S, Holder A, Razmi F, Stanley MD, Clifford GD, Buchman TG. An interpretable machine learning model for accurate prediction of sepsis in the ICU. Crit Care Med. 2018;46(4):547–553. doi: 10.1097/CCM.0000000000002936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6 [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa M. Fundamental research on human aortic pulse wave velocity. Jikei Med J. 1970;85:742–760. [Google Scholar]
- 38.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002;25(3):359–364. [DOI] [PubMed] [Google Scholar]
- 39.Kazune S, Grabovskis A, Cescon C, Strike E, Vanags I. Association between increased arterial stiffness and clinical outcomes in patients with early sepsis: a prospective observational cohort study. Intensive Care Med Exp. 2019;7(1):26. doi: 10.1186/s40635-019-0256-z [DOI] [PMC free article] [PubMed] [Google Scholar]