Abstract
Objective
To determine if fetal overnutrition resulting from maternal obesity or gestational diabetes mellitus (GDM) is associated with increased liver fat during adolescence, adjusting for past and current metabolic risk factors.
Study design
Data come from a historical prospective cohort study (Exploring Perinatal Outcomes in Children) of 254 mother-child pairs in Colorado who participated in 2 research visits at T1 (mean age 10.4, SD = 1.5 years) and at T2 (mean age 16.4, SD = 1.5 years), and had complete exposure and outcome data. Multiple linear regression was used to evaluate the effects of pre-pregnancy body mass index (BMI) and GDM on hepatic fat fraction (HFF) by magnetic resonance imaging at T2.
Results
Maternal pre-pregnancy obesity (BMI 30+) was significantly associated (β = 1.59, CI = 0.66, 2.52) with increased HFF relative to mothers with normal pre-pregnancy weight (BMI <25) independent of maternal GDM and sociodemographic factors. Moreover, this association was independent of T2 and T1 metabolic risk factors (acanthosis nigricans, BMI, fasting glucose) (β = 1.03, CI = 0.10, 1.97). Prenatal GDM exposure was not associated with HFF in either unadjusted or adjusted models.
Conclusions
Maternal pre-pregnancy obesity was associated with increased HFF in offspring independent of childhood and adolescent adiposity. Intervention studies are needed to test the hypothesis that maternal obesity is a modifiable risk factor for childhood fatty liver disease.
The intrauterine environment is critical for the growth and development of the fetus. Prior research has also demonstrated clear associations between the intrauterine environment and later metabolic risk in offspring as it relates to fetal overnutrition. Specifically, maternal pre-pregnancy overweight and obesity and gestational diabetes mellitus (GDM) during pregnancy have each been associated with increased risk of obesity, metabolic syndrome, and type 2 diabetes later in life.1-5
Despite interest and research in metabolic risks associated with these intrauterine exposures, there are limited data on potential associations with either adolescent liver fat accumulation or nonalcoholic fatty liver disease (NAFLD), an increasingly prevalent condition in the pediatric population.6 Elevated visceral or organ fat, such as elevated liver fat, is associated with elevated risk of atherosclerosis,7 type 2 diabetes mellitus,8 and long-term liver damage.9 NAFLD includes a spectrum of disorders, including simple steatosis, non-alcoholic steatohepatitis, inflammation and fibrosis, cirrhosis, and hepatocellular carcinoma.10,11 NAFLD has become the leading chronic liver disease in the world, present in ~30% of adults who are usually obese in association with high-fat diets and physical inactivity.10
One large epidemiologic study of these exposures and NAFLD in children and adolescents,12 the Avon Longitudinal Study of Parents and Children (ALSPAC) in the United Kingdom, reported that maternal overweight/obesity and GDM were each associated with higher levels of ultrasound assessed hepatic fat; however, when adjusted for offspring obesity, the association with maternal overweight/obesity disappeared, whereas the association of maternal GDM did not. There were also 2 similar smaller studies conducted in the US.13,14 Using a contemporary US cohort in Colorado, we aimed to study whether maternal pre-pregnancy obesity and prenatal GDM exposure are associated with increased adolescent hepatic fat fraction (HFF) as measured by magnetic resonance imaging (MRI), a marker for fatty liver. In addition, we examined whether such associations were mediated by childhood and adolescent obesity, a marker associated with insulin-resistance and elevated glucose levels.
Methods
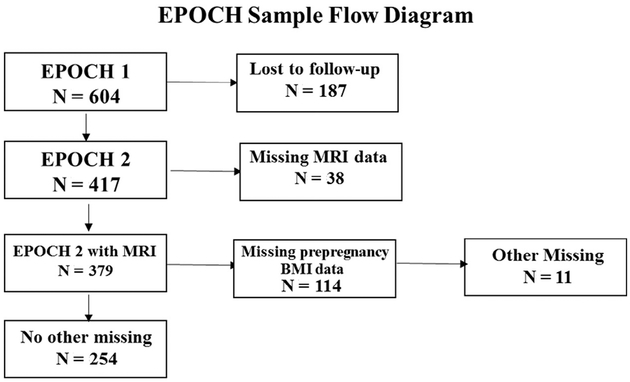
Data come from the Exploring Perinatal Outcomes in Children (EPOCH) study, a historical prospective cohort study of 604 mother-child pairs who were members of Kaiser Permanente of Colorado at the time of the child’s birth.15 The EPOCH study was designed to explore the effects of fetal overnutrition on childhood adiposity-related outcomes, with a special focus on exposure to maternal GDM and obesity. Thus, by design, the study oversampled on maternal obesity and GDM exposure. Approximately one-quarter (24.0%) of the sample youth were exposed to GDM and one-half (50.6%) of the mothers in the sample had pre-pregnancy BMI levels that were either overweight or obese prior to the start of the index pregnancy. A first EPOCH visit (T1) was conducted when youth were, on average, 10.4 years of age (SD = 1.5) during 2005-2010 (n = 604). A second visit (T2) took place during 2011-2015 when youths were on average 16.4 years (SD = 1.5) (n = 417). The Colorado Multiple Institutional Review Board approved the EPOCH study. Mothers provided written informed consent, and youth provided written assent. This report includes 254 participants who completed both T1 and T2 visits and had nonmissing exposure and outcome data (Figure).
Figure.
EPOCH sample inclusion flow diagram.
The exposures of interest were mother’s pre-pregnancy obesity status and GDM. Maternal weight before the last menstrual cycle preceding pregnancy (from the Kaiser Permanente of Colorado records) and measured maternal height at the first research visit were used to calculate pre-pregnancy body mass index (BMI) as weight (kg)/height (m2). Mothers were categorized as underweight or normal (BMI ≤24.9), overweight (25.0-29.9), and obese (30.0+). All pregnant women were routinely screened for GDM at 24-28 weeks using the 2-step standard protocol.16 We elected to combine maternal prepregnant underweight (n = 8) and normal weight (n = 120) because of too few cases of maternal pre-pregnancy underweight for separate analyses. GDM was diagnosed if glucose values exceeded 2 SD thresholds set by the National Diabetes Data Group on the 3-hour, 100 g oral glucose tolerance test.17
The outcome variable in this study was the HFF obtained by MRI at T2. Hepatic imaging was performed using a magnitude based, 6-echo, spoiled gradient-recalled echo sequence,18 which allowed correction for T2* effects. HFF was calculated from the mean pixel signal intensity data, for each echo acquisition using an open source Osirix algorithm.19 This fraction was then multiplied by 100 such that a value of 1 is equivalent to 1% HFF. An HFF of 5.56% or greater was indicative of fatty liver in adults.20
Both T1 and T2 included extensive study questionnaires about the adolescent’s health and family contexts and the mother’s health during and prior to the index pregnancy. Sociodemographic covariates included child age in years at the time of the T2 visit, child sex, child race/ethnicity with options for non-Hispanic black, Hispanic, non-Hispanic other race (non-Hispanic white reference), an indicator variable of low parent education (highest parent education high school or less = 1, more than high school = 0), and an indicator for household income in the bottom 35% of the sample ($74 999 or less = 1, $75 000+ = 0). Specific metabolic risk mediators measured at both visits included child age and sex standardized BMI scores standardized to US youth (BMIz scores), calculated using Centers for Disease Control and Prevention reference standards21,22; elevated fasting glucose level (≥100 vs ≤99 mg/dL) during the research visit; and presence of acanthosis nigricans, a pattern of skin texture and coloring associated with insulin resistance determined by a trained examiner.23
Statistical Analyses
We employed listwise deletion for complete case analyses. Of the 417 respondents who completed both visits, 254 had nonmissing covariate, exposure, and outcome data. A schematic detailing the exclusion criteria and the effects on the sample size are included in the Figure.
We first explored bivariate relationships between exposures and all other variables. We also examined correlations between HFF and the hypothesized mediators at T1 and T2 (Table I). We then used multiple linear regression to examine the change in predicted HFF because of the presence, absence, or change in the hypothesized mediators at each visit. Beta coefficients for the predictor variables reflect HHF predicted change in one percent increments (ie, the unit of analysis is one percent of hepatic fat).
Table I.
Correlations between HFF percentage and metabolic risk factors in youth by visit
| Correlation with liver fat % | P value | |
|---|---|---|
| Risk factors at T1 | ||
| Presence of acanthosis nigricans | 0.12 | .05 |
| Fasting glucose | −0.05 | .45 |
| BMIz score | 0.09 | .13 |
| Risk factors at T2 | ||
| Presence of acanthosis nigricans | 0.39 | <.00 |
| Fasting glucose | 0.31 | <.00 |
| BMIz score | 0.27 | <.00 |
We included 5 main models in our analyses. Models 1 and 2 only included maternal pre-pregnancy BMI category (model 1) or GDM exposure (model 2) and potential confounders: participant age, sex, race/ethnicity, family income, and parental education. Model 3 included both exposure variables and potential confounders. Model 4 included model 3 variables and T1 potential metabolic mediators. Model 5 included model 3 variables and T2 potential metabolic mediators. This model structure explores whether an initial relationship exists between exposure variables and adolescent HFF, then tests whether the presence of metabolic risk factors at T1 or T2 mediate this relationship. Analyses were conducted using Stata v 14.2 (StataCorp, College Station, TX).24
Results
This report included 254 participants who had both T1 and T2 visits, maternal pre-pregnancy BMI and GDM information, and outcome data. The analytic sample (254 participants) did not substantially differ from the larger cohort in terms of maternal exposures or key offspring characteristics by race/ethnicity, age, sex, or family socioeconomic indicators (Table II; available at www.jpeds.com).
Table II.
Comparing demographics across the analytic and T2 samples
| Full sample at T2 (n = 417) |
Reported in paper (n = 254) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total N (% of sample) |
Pre-pregnant normal |
Overweight | Obese | Missing Pre-pregnancy BMI* |
Relevant N | Total N (% of sample) |
Pre-pregnant Normal |
Overweight | Obese | ||
| n (%) | 417 | 150 (50.8%) | 83 (28.1%) | 62 (21.0%) | 122 | 295 | n (%) | 254 | 128 (50.4%) | 71 (28.0%) | 55 (21.7%) |
| Exposed to GDM, n, (%) | 74 (17.7%) | 24 (38.1%) | 23 (36.5%) | 16 (25.4%) | 11 | 63 | Exposed to GDM, n, (%) | 61 (24.0%) | 24 (39.3%) | 21 (34.4%) | 16 (26.2%) |
| Current age (mean, SD) | 16.47 (1.50) | 16.52 (1.51) | 16.40 (1.65) | 16.40 (1.55) | 115 | 302 | Current age (mean, SD) | 16.42 (1.58) | 16.47 (1.57) | 16.43 (1.68) | 16.31 (1.52) |
| Sex n (%) | |||||||||||
| Male | 209 (50.1%) | 76 (48.1%) | 44 (27.8%) | 38 (24.1%) | 55 | 158 | Sex: Male n (%) | 139 (54.7%) | 67 (48.2%) | 39 (28.1%) | 33 (23.7%) |
| Female | 205 (49.2%) | 74 (54.0%) | 39 (28.5%) | 24 (17.5%) | 71 | 137 | Female n (%) | 115 (45.3%) | 61 (53.4%) | 32 (28.5%) | 22 (19.1%) |
| Race n (%) | Race n (%) | ||||||||||
| Non-Hispanic white | 210 (50.4%) | 82 (60.3%) | 34 (25.0%) | 20 (14.7%) | 74 | 136 | Non-Hispanic white | 116 (45.7%) | 67 (57.8%) | 31 (26.7%) | 18 (15.5%) |
| Hispanic | 153 (36.7%) | 54 (46.2%) | 34 (29.1%) | 29 (24.8%) | 36 | 117 | Hispanic | 106 (41.7%) | 50 (47.2%) | 29 (27.4%) | 27 (25.5%) |
| Non-Hispanic black | 31 (7.4%) | 7 (26.9%) | 8 (30.8%) | 11 (42.3%) | 5 | 26 | Non-Hispanic black | 19 (7.5%) | 6 (31.6%) | 4 (21.1%) | 9 (47.4%) |
| Other | 23 (5.5%) | 7 (43.8%) | 7 (43.8%) | 2 (12.5%) | 7 | 16 | Other | 13 (5.1%) | 5 (38.5%) | 7 (53.9%) | 1 (7.7%) |
| Parental education (high school or less) (%) | 107 (25.7%) | 33 (45.8%) | 21 (29.2%) | 18 (25.0%) | 35 | 72 | Parental education (high school or less) (%) | 64 (25.2%) | 31 (48.4%) | 17 (26.6%) | 16 (25.0%) |
| Household Income (bottom 35%) (%) | 147 (35.3%) | 43 (39.8%) | 32 (29.6%) | 33 (30.6%) | 39 | 108 | Household Income (bottom 35%) (%) | 96 (37.8%) | 37 (38.5%) | 29 (30.2%) | 30 (31.3%) |
Those missing pre-pregnancy BMI are excluded from the percentages in the full sample panel, but the N affected are listed to more easily compare percentages from those included in the sample to those in the full sample.
Table III shows descriptive variables and statistics for the participants and includes P values testing for significant differences across maternal pre-pregnancy BMI categories using either χ2 or ANOVA with F-tests depending on the measures compared; 128 (50.4%) of the mothers had normal or underweight BMI levels, 28.0% were overweight and 21.7% were obese before the onset of the index pregnancy. No significant differences were observed for child age, sex, or parental education level by maternal pre-pregnancy BMI status. However, significant differences were observed for race/ethnicity (P = .01) and income (P = .001). Further, among the youth metabolic risk variables, BMIz scores, and clinical obesity levels significantly differed at both T1 and T2 by mother’s pre-pregnancy BMI category. The percent with elevated fasting glucose did not differ at either time. Presence of acanthosis nigricans was generally higher with increasing pre-pregnancy BMI but was not statistically significant at either visit. Both the HFF (P = .001) and the percent with HFF ≥5.56% (P = .001) were higher in offspring of obese mothers. Overall, 15 youth (5.9%) met the criteria for fatty liver.
Table III.
Descriptive characteristics of study participants by maternal pre-pregnancy BMI status
| Total N (% of sample)* | Pre-pregnant normal† | Overweight† | Obese† | P for trend | |
|---|---|---|---|---|---|
| n (%) | 254 | 128 (50.4%) | 71 (28.0%) | 55 (21.7%) | |
| Exposed to GDM, n, (%) | 61 (24.0%) | 24 (39.3%) | 21 (34.4%) | 16 (26.2%) | .20 |
| Current age (mean, SD) | 16.42 (1.58) | 16.47 (1.57) | 16.43 (1.68) | 16.31 (1.52) | .76 |
| Sex n (%) | |||||
| Male | 139 (54.7%) | 67 (48.2%) | 39 (27.8%) | 33 (23.7%) | .63 |
| Female | 115 (45.3%) | 61 (53.4%) | 32 (27.8%) | 22 (19.1%) | |
| Race n (%) | .01 | ||||
| Non-Hispanic white | 116 (45.7%) | 67 (57.8%) | 31 (26.7%) | 18 (15.5%) | |
| Hispanic | 106 (41.7%) | 50 (47.2%) | 29 (27.4%) | 27 (25.5%) | |
| Non-Hispanic black | 19 (7.5%) | 6 (31.6%) | 4 (21.1%) | 9 (47.4%) | |
| Other | 13 (5.1%) | 5 (38.5%) | 7 (53.9%) | 1 (7.7%) | |
| Parental education (high school or less) (%) | 64 (25.2%) | 31 (48.4%) | 17 (26.6%) | 16 (25.0%) | .77 |
| Household income (bottom 35%) (%) | 96 (37.8%) | 37 (38.5%) | 29 (30.21%) | 30 (31.25%) | <.00 |
| T1 | |||||
| Acanthosis nigricans n, (%) | 13 (5.1%) | 3 (23.1%) | 5 (38.4%) | 5 (38.5%) | .13 |
| BMIz (mean, SD) | 0.29 (1.17) | −0.05 (1.11) | 0.49 (1.19) | 0.81 (1.03) | <.00 |
| Obese BMI (%) | 39 (15.3%) | 11 (28.2%) | 13 (33.3%) | 15 (38.5%) | <.00 |
| Fasting glucose (mg/dL) (mean, SD) | 80.32 (16.04) | 79.82 (9.88) | 80.43 (25.98) | 81.31 (8.92) | .84 |
| Fasting glucose ≥100 mg/dL (%) | 4 (1.6%) | 4 (50.0%) | 2 (50.0%) | 0 (0.0%) | .47 |
| T2 | |||||
| Acanthosis nigricans n, (%) | 10 (3.9%) | 4 (40.0%) | 1 (10%) | 5 (50.0%) | .07 |
| BMIz (mean, SD) | −0.01 (0.96) | −0.33 (0.66) | 0.07 (0.96) | 0.61 (1.19) | <.00 |
| Obese BMI (%) | 13 (5.1%) | 1 (7.7%) | 5 (38.4%) | 7 (53.9%) | <.00 |
| Fasting glucose (mg/dL) (mean, SD) | 90.17 (20.55) | 88.13 (6.75) | 90.09 (21.80) | 94.75 (34.3) | .13 |
| Fasting glucose ≥100 mg/dL (%) | 16 (6.3%) | 6 (37.5%) | 3 (18.8%) | 7 (43.8%) | .10 |
| HFF (mean %, SD) | 2.48 (2.99) | 1.98 (1.30) | 2.40 (1.77) | 3.72 (5.60) | <.00 |
| HFF ≥ 5.56% n (%) | 15 (5.9%) | 4 (26.7%) | 2 (13.3%) | 9 (60.0%) | <.00 |
Total column lists N for variable and percentage of sample.
Percentages in maternal pre-pregnancy BMI columns reflect the percentage of each variable in the left column in that maternal pre-pregnancy BMI category by row.
Table I displays the Pearson correlations between risk factors in youth at both T1 and T2 and HFF at the second visit. Significant correlations were observed for 4 of the 6 measures included in Table I. Only fasting glucose at T1 and child BMIz score at T1 were not significantly correlated with HFF (r = −0.05, P = . 45; r = 0.09, P = .13). All 3 measures from T2 had correlations at or above 0.27 with HFF, with presence of acanthosis nigricans having the strongest association (r = 0.39, P = .001) followed by fasting glucose (r = 0.31, P = .001), and child BMIz score (r = 0.27, P = .001). The correlations between the presence of acanthosis nigricans and HFF and those between BMIz score and HFF tripled at T2 compared with T1.
Table IV displays the results for the multiple linear regression models predicting adolescent HFF. There was a strong association between maternal pre-pregnancy obesity and HFF across all models, although no such associations were seen for maternal pre-pregnancy overweight or maternal GDM in any of the models. In model 1, maternal pre-pregnancy obesity was associated with an increase in the HFF (β = 1.59, 95% CI: 0.66-2.52, P < .001), relative to children of normal pre-pregnancy weight mothers, independent of potential confounders (child age, sex, race/ethnicity, family income, and parental education). In model 2, no significant association was observed between GDM exposure and HFF, independent of potential confounders (β = −0.46, 95% CI: −1.37, 0.45). In model 3, both exposures are included and follow the patterns observed in models 1 and 2. Adjusting for T1 hypothesized metabolic mediators in model 4 did little to the predicted relationship between maternal pre-pregnancy obesity and adolescent HFF (1.57; 95% CI: 0.60, 2.55, P < .001). In model 5, adjustment for hypothesized mediators at T2 partially attenuated the association between maternal pre-pregnancy obesity and adolescent HFF, however, the association remained statistically significant (β = 1.03; 95% CI 0.10-1.97, P < .05).
Table IV.
Multiple linear regression models predicting HFF
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
|
|---|---|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Maternal exposures | |||||
| Pre-pregnancy overweight | 0.39 (−0.44,1.23) | 0.46 (−0.37,1.30) | 0.39 (−0.46,1.24) | 0.20 (−0.58,0.99) | |
| Pre-pregnancy obesity | 1.59 (0.66,2.52)*** | 1.67 (0.73,2.60)*** | 1.57 (0.60,2.55)*** | 1.03 (0.10,1.97)* | |
| GDM | −0.46 (−1.37,0.45) | −0.63 (−1.54,0.27) | −0.65 (−1.55,0.25) | −0.53 (−1.36,0.31) | |
| Risk factors from T1 | |||||
| Acanthosis nigricans | 1.19 (−0.52,2.90) | ||||
| Fasting glucose | −0.01 (−0.04,0.01) | ||||
| BMIz | 0.09 (−0.24,0.42) | ||||
| Risk factors from T2 | |||||
| Acanthosis nigricans | 4.74 (2.89,6.59)*** | ||||
| Fasting glucose | 0.03 (0.02,0.05)*** | ||||
| BMIz | 0.26 (−0.15,0.66) | ||||
All models adjust for participant age, sex, race/ethnicity, family income, and parent education level; A value of 1 indicates a predicted 1% increase in the amount of liver fat observed on MRI.
P < .05,
P < .01,
P < .001.
Discussion
We found that maternal pre-pregnancy obesity was significantly associated with offspring HFF during adolescence, and this association was not fully mediated by adolescent obesity, dysglycemia, or acanthosis nigricans, suggesting a specific intrauterine effect. However, in our cohort, GDM exposure was not associated with HFF, contrary to our original hypothesis.
Previous research has linked pre-pregnancy obesity to offspring cardiometabolic risk factors,4 higher birthweights,25,26 increased body mass across the life course,27 and elevated risk of glucose dysregulation and type 2 diabetes later in life.2,28 Our findings now extend the impact of maternal pre-pregnancy obesity to elevated HFF in adolescence. This finding is important for several reasons. First, the magnitude of this effect is notable, even in the mediated model, because it represents roughly one-fifth of the threshold for clinical pediatric fatty liver.20 Second, elevated HFF increases the risk of developing NAFLD, which can be detrimental to health in and of itself, but also increases the risk of several associated conditions such as metabolic syndrome,29 cardiovascular disease and atherosclerosis,30 and diabetes.31 Third, because maternal pre-pregnancy obesity has already been associated with several of the related conditions that follow fatty liver disease, it is possible that fatty liver can be regarded as potential mediating pathway to future metabolic risk if a youth is exposed to fetal overnutrition in utero.
Our findings that the association between maternal obesity and adolescent HFF is not completely mediated by youth obesity, the presence of acanthosis nigricans and elevated fasting glucose suggest a specific intrauterine programming effect. Although the mechanism responsible for this association is not clear, there is evidence that the fetus may be vulnerable to steatosis because immature fetal adipose depots are not available to buffer the excess transplacental fuel delivery (glucose and fatty acids) in maternal obesity.32 Animal studies have shown that murine dams that were obese prior to and during pregnancy produced offspring that had a dysmetabolic, insulin resistant, and NAFLD phenotype compared with offspring of lean dams.33 In addition, lactation from obese dams caused lean healthy offspring to develop a similar phenotype. Human studies have demonstrated increased intrahepatocellular lipid in offspring of mothers with diabetes compared with mothers without diabetes at 1-3 weeks of age,13 and in offspring of pre-pregnancy obese mothers at 11.7 average days of age.14 However, these studies were not able to look at GDM independently because of the small sample size,14 or were not able to separate maternal obesity from GDM.13 Neither study reported long-term follow-up data, so it is unclear whether the associations seen in newborns persisted to older ages, though our study suggests they will.
The most directly comparable study with ours is the report from the ALSPAC investigators who studied 2753 mother-infant dyads using ultrasound assessment of fatty liver (USS-FL) in a subset of 1215 at 17-18 years of age.12 Diabetes during pregnancy was marked only by glycosuria because routine GDM screening was not the practice in the United Kingdom when the cohort was formed. The prevalence of USS-FL was 2.1%, lower than our results of 5.9% (Table III). They found that maternal diabetes (as defined by presence of glycosuria) was associated with a 6.7 (95% CI: 2.5-18.4) fold increase in the odds of USS-FL. In a mediation analysis using birthweight and measures of offspring adiposity, the aOR decreased from 9.1 to 6.7 but remained highly statistically significant (P < .001). We found no association between maternal GDM and HFF, regardless of adjustment. In ALSPAC, maternal pre-pregnancy overweight/obesity (as a combined category) had an aOR of 2.7 (1.2-6.2) for offspring USS-FL.12 In contrast to maternal diabetes/glycosuria, the OR for maternal pre-pregnancy overweight/obesity was greatly reduced and became nonsignificant on adjustment for offspring adiposity, suggesting that offspring adiposity was completely mediating the association. We report here a different finding, that maternal pre-pregnancy obesity (in a category separate from overweight) remained significantly associated with HFF after adjustment for offspring BMIz score as a marker of adiposity, as well as markers of dysglycemia and acanthosis nigricans, and, therefore, the association was not fully mediated by offspring risk factors directly related to HFF.
It is unclear why we did not detect associations between maternal GDM and HFF as found in the ALSPAC study. Differences in methods are the likely explanations, and our definition of GDM from routine screening is more accurate than that used in ALSPAC. Nevertheless, our participants with clinically diagnosed GDM were treated (27.9% with insulin), and it is likely that most had better controlled hyperglycemia than those included in ALSPAC. Although we do not have data on glucose control in our study, it is possible that uncontrolled hyperglycemia during pregnancy is more strongly associated with offspring fatty liver than controlled GDM, a hypothesis that requires further testing. Maternal GDM has been associated with offspring adiposity in several studies,34 including from our cohort15 but our current findings do not support an association with adolescent HFF.
Our study has limitations. No liver biopsies were conducted to determine the histologic diagnosis of youth with elevated HFF, therefore, we do not know the proportion with steatosis or fibrosis. Given the early focal nature of hepatic lesions, the limited interventions available, and the risk of complications to the participants, we did not believe it was appropriate to conduct biopsies in this epidemiologic study.11 We had a small number of youth with elevated HFF, which limits the analysis of mediation and confounding. The ALSPAC study of a larger cohort had only 25 youth with USS-FL (2.1%) compared with 15 (5.9%) in our study, likely because of the lower sensitivity of hepatic ultrasound to identify fatty liver compared with the MRI approach we used.12
Our study has several strengths as well. It was a cohort study with repeated visits to assess mediation by offspring risk factors at 2 visits. We measured liver fat using MRI, the most sensitive noninvasive technique available. Numerous potential confounding factors have been measured in this medium sized cohort, and the analysis explored both confounding and mediation.
In conclusion, our findings suggest that at least part of the variation in adolescent liver fat is associated with maternal pre-pregnancy obesity. This effect is independent of known childhood and adolescent risk factors, demographic and socioeconomic factors, and of exposure to GDM. Further research is needed to determine the mechanisms responsible for this association as well as whether this relationship is part of a biological pathway linking pre-pregnancy obesity to adult cardiometabolic outcomes. Because maternal obesity is a potentially modifiable risk factor, intervention studies that measure offspring liver fat along with other common early life adiposity-related outcomes are needed. ■
Acknowledgments
Supported by the National Institutes of Health (R01DK068001). The authors declare no conflicts of interest.
The authors thank the EPOCH participants, study coordinator, and the study staff for their dedicated contribution to this work.
Glossary
- ALSPAC
Avon Longitudinal Study of Parents and Children
- EPOCH
Exploring Perinatal Outcomes in Children
- GDM
Gestational diabetes mellitus
- HFF
Hepatic fat fraction
- MRI
Magnetic resonance imaging
- NAFLD
Nonalcoholic fatty liver disease
- USS-FL
Ultrasound assessment of fatty liver
References
- 1.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–11. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007;30 Supplement 2:S169–74. [DOI] [PubMed] [Google Scholar]
- 3.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–6. [DOI] [PubMed] [Google Scholar]
- 4.Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, et al. Associations of maternal pre-pregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors the Jerusalem Perinatal Family Follow-up Study. Circulation 2012;125:1381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirkola J, Pouta A, Bloigu A, Hartikainen AL, Laitinen J, Järvelin MR, et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal pre-pregnancy overweight and gestational diabetes mellitus. Diabetes Care 2010;33:1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010;28:155–61. [DOI] [PubMed] [Google Scholar]
- 7.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2006;2:367–73. [DOI] [PubMed] [Google Scholar]
- 8.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–57. [DOI] [PubMed] [Google Scholar]
- 9.Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, et al. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology 2006;147:1140–7. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Marsh S, Hu J, Feng W, Wu C. The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterol Res Pract 2016;2016:2862173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temple JL, Cordero P, Li J, Nguyen V, Oben JA. A guide to non-alcoholic fatty liver disease in childhood and adolescence. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel S, Lawlor DA, Callaway M, Macdonald-Wallis C, Sattar N, Fraser A. Association of maternal diabetes/glycosuria and pre-pregnancy body mass index with offspring indicators of non-alcoholic fatty liver disease. BMC Pediatr 2016;16:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brumbaugh DE, Tearse P, Cree-Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr 2013;162:930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res 2011;70:287–91. [DOI] [PubMed] [Google Scholar]
- 15.Crume T, Ogden L, West NA, Vehik K, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the exploring perinatal outcomes among children (EPOCH) study. Diabetologia 2011;54:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 2016;39(Suppl 1):S13–22. [DOI] [PubMed] [Google Scholar]
- 17.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 18.Tyagi A, Yeganeh O, Levin Y. Intra- and Inter-examination repeatability of magnetic resonance spectroscopy, magnitude-based MRI, and complex-based MRI for estimation of hepatic proton density fat fraction in overweight and obese children and adults. Abdom Imaging 2015;40:3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang AA, Tan J, Sun M. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoo T, Bydder M, Hamilton G, Middleton MS, Gamst AC, Wolfson T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T 1. Radiology 2009;251:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data 2000;1–27. [PubMed] [Google Scholar]
- 22.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr 2000;72:1074–81. [DOI] [PubMed] [Google Scholar]
- 23.Kobaissi HA, Weigensberg MJ, Ball GD, Cruz ML, Shaibi GQ, Goran MI. Relation between acanthosis nigricans and insulin sensitivity in overweight Hispanic children at risk for type 2 diabetes. Diabetes Care 2004;27:1412–6. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. Stata statistical software: release 14. College Station (TX): StataCorp LP; 2015. [Google Scholar]
- 25.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006;195:1100–3. [DOI] [PubMed] [Google Scholar]
- 26.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol 2008;198:416–e1. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS ONE 2013;8:e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norman JE, Reynolds R. The consequences of obesity and excess weight gain in pregnancy. Proc Nutr Soc 2011;70:450–6. [DOI] [PubMed] [Google Scholar]
- 29.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–23. [DOI] [PubMed] [Google Scholar]
- 30.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600–7. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003;285:E906–16. [DOI] [PubMed] [Google Scholar]
- 32.Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res 2013;75:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 2010;52913–20. [DOI] [PubMed] [Google Scholar]
- 34.Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 2011;54:1957–66. [DOI] [PubMed] [Google Scholar]