Abstract
Phosphatidylinositol 3-kinase-related kinases (PIKKs), are structurally related to phosphatidylinositol 3-kinase (lipid kinase) but possess protein kinase activities. PIKKs include ATM, ATR, DNA-PK, mTOR and SMG1, key regulators of cell proliferation and genome maintenance. TRRAP, which is devoid of protein kinase activity, is the sixth member of the PIKK family. PIKK family members are gigantic proteins in the range of 300–500 kDa. It has become apparent in the last decade that the stability or maturation of the PIKK family members depends on a molecular chaperone called the Tel2-Tti1-Tti2 (TTT) complex. Several lines of evidence have established a model in which TTT connects to the Hsp90 chaperone through the Rvb1-Rvb2-Tah1-Pih1 (R2TP) complex in mammalian and yeast cells. However, recent studies of yeast cells indicate that TTT is able to form different complexes. These observations raise a possibility that several different mechanisms regulate TTT-mediated protein stability of PIKKs.
Keywords: Protein folding, Mec1, Tel1, casein kinase, Asa1, Cdc37
Introduction
The life cycle of a protein kinase is tightly controlled by mechanisms of protein folding for maturation and repair. Failure of these controls leads to rapid degradation; that is, a protein kinase loses stability. Protein folding for canonical protein kinases requires the Hsp90 chaperone and the co-chaperone called Cdc37 [1–3](Fig. 1). Cdc37 forms a complex with Hsp90 and plays a key role in bringing kinase clients to Hsp90. Hsp90/Cdc37 is required for initial folding, but many kinases rely on Hsp90/Cdc37 for repair throughout their lifetimes. Phosphatidylinositol 3-kinase-related kinases (PIKKs) are a family of Ser/Thr-protein kinases with sequence similarity to phosphatidylinositol-3 kinases (PI3Ks) [4, 5]. Several lines of evidence have established that the Tel2-Tti1-Tti2 (TTT) complex collaborates with Hsp90 to promote protein stability of PIKKs [6–11] (Fig. 2). Thus, protein stability of PIKKs is regulated differently from that of canonical protein kinases. This short review summarizes the discovery and complexity of the TTT-dependent PIKK stabilization.
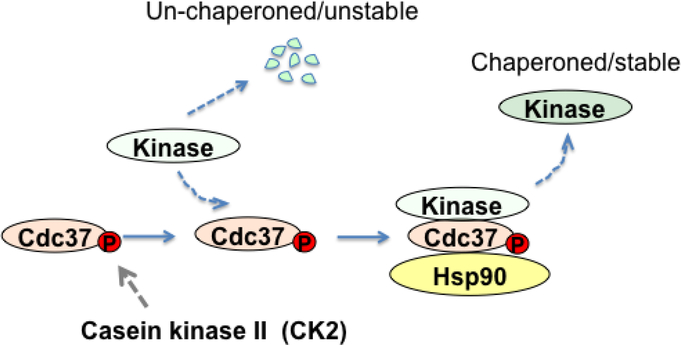
Fig. 1. HSP90-Cdc37-dependent protein kinase stabilization.
Protein kinase folding requires Hsp90 chaperone and the co-chaperone Cdc37. Casein kinase II (CK2) phosphorylates Cdc37 and stimulates Cdc37-client kinase interaction. In turn Hsp90 recognizes the Cdc37-client kinase complex and promotes the maturation of the client kinase.
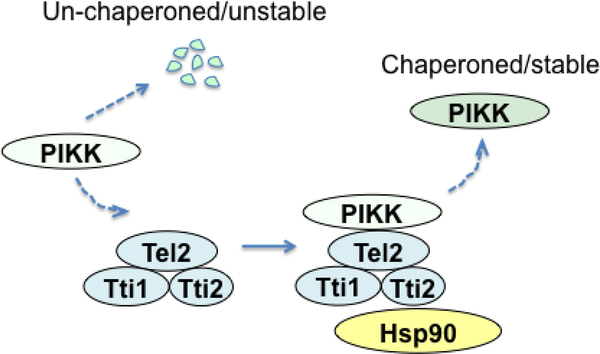
Fig. 2. Requirement of the co-chaperone Tel2-Tti1-Tti2 (TTT) complex for PIKK stabilization.
The TTT complex interacts with newly synthesized PIKKs and plays a key role in protein kinase maturation. TTT also collaborates with Hsp90.
Common features of PIKKs that exhibit diversified functions
PIKKs have Ser/Thr protein kinase activity and no lipid kinase activity although PIKKs have a clear homology with PI3Ks (i.e. lipid kinase) [4, 5]. PIKKs have been discovered as an unconventional family of protein kinases in all eukaryotic cells [4, 5].
PIKKs exhibit diversified functions, serving in both positive and negative cell proliferation pathways [4, 5]. Mammalian cells express six PIKK family proteins, only five of which have been identified as active kinases: ATM, ATR, DNA-PK, mTOR and SMG-1. ATM and ATR control the DNA damage checkpoint pathway [12]. DNA-PK acts in DNA double-strand beak (DSB) repair [12, 13]. mTOR regulates cellular metabolism and proliferation in response to hormones, growth factors, nutrients and stress signals [14]. SMG-1 is involved in nonsense-mediated mRNA decay [15]. The sixth member of the family, TRRAP, retains a catalytic domain but has no associated kinase activity [4, 5]. TRRAP is a common component of histone acetyltransferase (HAT) complexes and is involved in transcription control [16].
In both the budding yeast Saccharomyces cerevisiae and the fission yeast Schizosaccharomyces pombe, orthologs of the PIKK family (ATM, ATR, mTOR and TRRP) have been found (Table 1) (https://www.yeastgenome.org, https://www.pombase.org). Genes corresponding to DNA-PK and SMG-1 have not been identified in budding and fission yeast.
Table 1.
PIKK family member in budding yeast and fission yeast
| Mammals | Budding yeast | Fission yeast |
|---|---|---|
| ATM | Tel1 | Tel1 |
| ATR | Mec1 | Rad3 |
| mTOR | Tor1, Tor2 | Tor1, Tor2 |
| TRRAP | Tra1 | Tra1, Tra2 |
PIKKs share a common domain organization, despite large differences in their sizes. As discussed above, PIKKs show close sequence similarity with PI3Ks in their kinase domains (KDs), but share several motifs found in canonical Ser/Thr-protein kinase [17]. The N-terminal flanking portion of the kinase domain has been referred to as the FAT domain [18] (Fig. 3). The far C-terminus, next to the KD has been named the FATC domain [18]. Structural analyses indicate that both the FAT and FATC domains are positioned close to the KD, thereby forming the conserved core of these enzymes [19, 20]. The N-terminal region consists of more HEAT repeats with little sequence similarity between the kinases and is considered to serve as a protein– protein interaction surface [21].
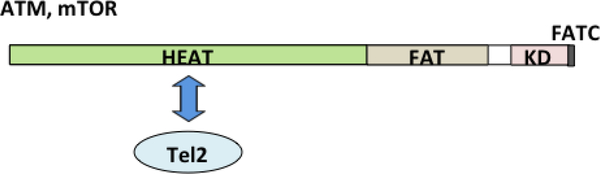
Fig. 3. Interaction of Tel2 with PIKKs through the HEAT repeat domain.
The kinase domains (KDs) of PIKKs are located near C-termini and are flanked by the conserved FRAP-ATM-TRRAP (FAT) and FAT C-terminal (FATC) domains [18]. Nterminal and internal regions of PIKKs contain numerous α-helical Huntington-elongation factor 3-A subunit of protein phosphatase 2A-TOR1 (HEAT) repeats [21]. Human Tel2 has been shown to interact with ATM and mTOR through the HEAT repeat regions.
Requirement of the TTT-R2TP pathway for PIKK expression
The Tel2-Tti1-Tti2 (TTT) complex interacts with and promotes protein stability of PIKKs as a co-chaperone [6–11] (Fig. 2). ATM and ATR family proteins control telomerase length as well as DNA damage response [22]. Note that ATM and ATR correspond to Tel1 and Mec1, respectively, in budding yeast (Table 1). Like Tel1ATM, Tel2 was originally identified as a gene that positively regulates telomere length in budding yeast [23]. Later on, Tel2 was shown to control protein stability of PIKKs [6]. Like Cdc37, TTT collaborates with the Hsp90 chaperone. Unlike Cdc37, however, TTT does not appear to interact directly with Hsp90 proteins. It is not clear whether TTT recognizes the kinase domain of PIKKs. Tel2 has been shown to interact with the N-terminal HEAT repeat of PIKKs [6] (Fig. 3).
The TTT-Hsp90 interaction depends on another protein complex, R2TP, consisting of AAA-ATPase Rvb1 and Rvb2 as well as Tah1 and Pih1 [11, 24–26] (Fig. 4 and 5). Protein organization of the R2TP complex has been extensively studied [24]. Pih1 interacts with the Rvb1-Rvb2 complex and Tah1, and targets phosphorylated substrates through the N-terminal phospho-peptide binding domain [26, 27]. Tah1 contains the tetratricopeptide repeat (TPR) domain that mediates the interaction with Hsp90 [28]. R2TP-Hsp90 interacts with newly synthesized and phosphorylated substrates to mediate the assembly of large protein complexes such as box C/D snoRNPs and RNA polymerase II [25, 29, 30]. R2TP does not target PIKKs; instead, it recognizes TTT. Casein-kinase-II (CK2) phosphorylates Tel2 and Tti1 [11, 31]. Phosphorylated Tel2 is captured by the N-terminal phospho-peptide binding domain of Pih1 in mammalian and budding yeast cells [26, 27]. Thus, the R2TP-Hsp90 complex appears to promote protein-folding of PIKKs through the interaction with the TTT complex. More comprehensive discussion about R2TP can be found elsewhere [32].
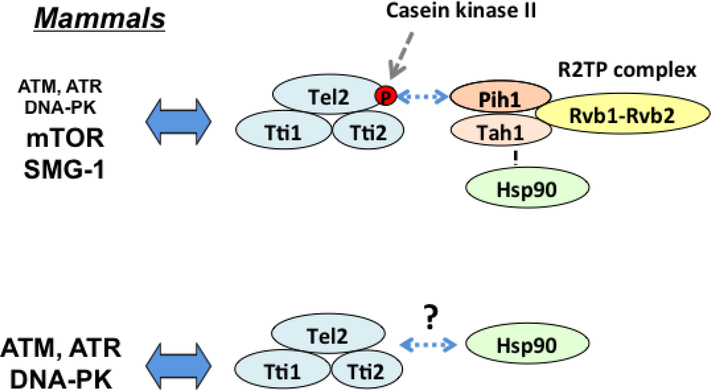
Fig. 4. TTT pathway in mammalian cells.
The TTT-R2TP pathway contributes mainly to protein stabilization of mTOR and SMG1. TTT appears to connect Hsp90 independently of R2TP and regulate protein stabilization of ATM, ATR and DNA-PK.
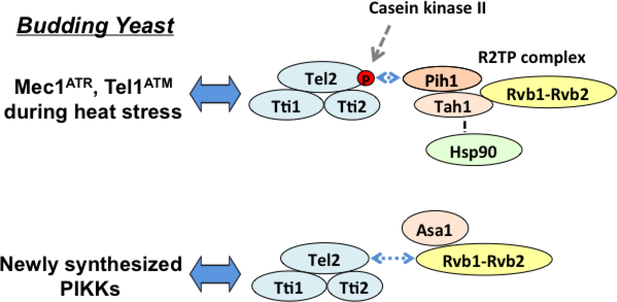
Fig. 5. TTT pathway in budding yeast.
The TTT-Asa1 pathway plays a key role in protein stabilization of newly synthesized PIKKs whereas the TTT-R2TP pathway contributes to protein stabilization of Mec1ATR and Tel1ATM at higher temperatures.
Although Cdc37 does not interact directly with the TTT-R2TP-Hsp90 complex, Cdc37 contributes to the establishment of the TTT-Hsp90 interaction. Like other protein kinases, CK2 depends on Cdc37 activities for protein stability [33–35] (Fig. 1). Thus, Cdc37 controls protein stability of PIKKs by modulating CK2 activity.
Involvement of a separate TTT pathway in PIKK expression
TTT is highly conserved among eukaryotes [6–11]. However, the TTT pathway is not always connected to R2TP; there seem to be different TTT networks present among eukaryotes. It has been suggested that mechanisms other than the TTT-R2TP pathway control TTT-dependent functions in mammalian cells, because defective Tel2-Pih1 interaction decreases the stability of mTOR and SMG-1 but has minor effect on the stability of ATM, ATR and DNA-PK [11]. One possible explanation is that the TTT-R2TP pathway preferentially acts on mTOR and SMG1 but other TTT pathways modulate the protein stability of ATM, ATR and DNA-PK [11] (Fig. 4).
Recent studies of budding yeast have provided evidence indicating that two different pathways, the TTT-R2TP and the TTT-Asa1 pathway, contribute to the quality control of PIKKs in budding yeast [36] (Fig. 5). Asa1 associates with Rvb1-Rvb2 but forms a different complex from R2TP [37]. It has been shown that Tel2 preferentially recognizes newly synthesized ATM and ATR under non-stress conditions [10]. The TTT-Asa1 pathway appears to act on newly synthesized Mec1ATR and Tel1ATM proteins whereas the TTT-R2TP pathway is primarily required for Mec1ATR and Tel1ATM protein stabilization at high temperatures [36]. Asa1 is largely located in the cytoplasm whereas Pih1 is distributed throughout the cell [36]. These results suggest the model in which the TTT-Asa1 pathway promotes protein folding of nascent Mec1ATR and Tel1ATM in the cytoplasm whereas the TTT-R2TP pathway stimulates protein refolding during heat stress (Fig. 5).
It seems likely that all PIKKs depend on the Tel2-Asa1 pathway for proper folding in budding yeast because the asa1–1 mutation decreases the expression levels of Tor1 protein as well [38]. Tel2 has been shown to interact with Mec1ATR and Tel1ATM in an Asa1-dependent manner [36]. Asa1 contains WD40 repeats; therefore, it may constitute a WD40 domain that organizes multi-protein complex assemblies [39]. Hsp90 has been proposed to interact with various types of proteins [40, 41]. Thus, it is possible that Asa1 mediates Hsp90-chaperone functions in collaboration with the Rvb1-Rvb2 complex. Tel2 has been shown to interact with the N-terminal HEAT repeat region of ATM and mTOR in vitro [6] (Fig. 3). Because the sequence similarity at the N-terminal region of PIKKs is relatively low compared with that at the C-terminal catalytic domain [21], the TTT pathway is expected to process PIKKs with different efficiencies. Since PIKKs do not share significant amino acid sequence similarities in the N-terminal region, TTT could interact with PIKKs with different affinities. The Asa1-Rvb1-Rvb2 complex could make TTT a good fit for newly synthesized PIKK proteins.
The above observations from studies of mammalian and yeast TTT pathways provide a model in which the R2TP and the Asa1-Rvb1-Rvb2 complex determine substrate specificity of TTT. That is, R2TP allows TTT to recognize mTOR and SMG-1 rather than ATM, ATR and DNA-PK in mammalian cells whereas R2TP helps TTT to capture heat-denatured Mec1ATR and Tel1ATM in budding yeast.
Operation of CK2-independent mechanisms in fission yeast
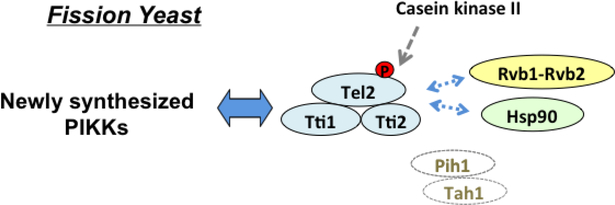
A different TTT pathway may operate in fission yeast. The R2TP complex is found in organisms from budding yeast to humans; however, Pih1 and Tah1 homologs have not been identified in fission yeast [42] (Fig. 6). Curiously, TTT was found to interact with Rvb1-Rvb2 and Hsp90 in fission yeast [42]. It is not clear whether proteins other than Pih1 and Tah1 need to connect TTT to Rvb1-Rvb2 and Hsp90. CK2 phosphorylates Tel2 in fission yeast as well [42]. Not surprisingly, Tel2 phosphorylation is dispensable for TTT-mediated PIKK biogenesis in fission yeast [42] (Fig. 6). Proteins homologous to Asa1 are found in fission yeast and other eukaryotes including humans [37]. Asa1 homologs may act in the TTT pathway; however, their function in the TTT pathway has not been characterized yet.
Fig. 6. TTT pathway in fission yeast.
The TTT pathway plays a key role in protein stabilization of newly synthesized PIKKs. CK2 phosphorylates Tel2, but phosphorylation is dispensable for protein stabilization of PIKKs. No Pih1 or Tah1 counterpart is found in fission yeast.
Conclusion
Protein folding of most canonical protein kinase depends on Cdc37 and Hsp90. PIKKs are large protein kinases with the similarity to lipid kinases. Recent evidence has provided the model in which TTT collaborates with Hsp90 to convert newly synthesized or denatured PIKKs into matured stable forms. As discussed above, however, TTT could form different complexes in different organisms. At the moment, the role of the Rvb1-Rvb2 complex in the TTT pathway is unknown. Curiously enough, the Rvb1-Rvb2 complex is implicated in chaperone for Ino80- and Swr1- chromatin remodeling complexes [43]. That is, TTT could be associated with two different chaperones. People may wonder how intricate the protein maturation processes of PIKKs are.
Acknowledgements
I thank John Kang, Hisao Masukata and Carol Newlon for helpful comments.
References
- 1.Caplan AJ, Mandal AK, Theodoraki MA. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 2007;17(2):87–92. doi: 10.1016/j.tcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Verba KA, Agard DA. How Hsp90 and Cdc37 Lubricate Kinase Molecular Switches. Trends Biochem Sci. 2017;42(10):799–811. doi: 10.1016/j.tibs.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schopf FH, Biebl MM, Buchner J. The HSP90 chaperone machinery. Nat Rev Mol Cell Biol. 2017;18(6):345–60. doi: 10.1038/nrm.2017.20. [DOI] [PubMed] [Google Scholar]
- 4.Abraham RT. PI 3-kinase related kinases: ‘big’ players in stress-induced signaling pathways. DNA Repair (Amst). 2004;3(8–9):883–7. doi: 10.1016/j.dnarep.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Lempiainen H, Halazonetis TD. Emerging common themes in regulation of PIKKs and PI3Ks. EMBO J. 2009;28(20):3067–73. doi: 10.1038/emboj.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takai H, Wang RC, Takai KK, Yang H, de Lange T. Tel2 regulates the stability of PI3K-related protein kinases. Cell. 2007;131(7):1248–59. doi: 10.1016/j.cell.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, et al. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells. 2007;12(12):1357–70. doi: 10.1111/j.1365-2443.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson CM, Korkin D, Smith DL, Makovets S, Seidel JJ, Sali A, et al. Tel2 mediates activation and localization of ATM/Tel1 kinase to a double-strand break. Genes Dev. 2008;22(7):854–9. doi: 10.1101/gad.1646208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurov KE, Cotta-Ramusino C, Elledge SJ. A genetic screen identifies the Triple T complex required for DNA damage signaling and ATM and ATR stability. Genes Dev. 2010;24(17):1939–50. doi: 10.1101/gad.1934210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takai H, Xie Y, de Lange T, Pavletich NP. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes Dev. 2010;24(18):2019–30. doi: 10.1101/gad.1956410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horejsi Z, Takai H, Adelman CA, Collis SJ, Flynn H, Maslen S, et al. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell. 2010;39(6):839–50. doi: 10.1016/j.molcel.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Blackford AN, Jackson SP. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol Cell. 2017;66(6):801–17. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Gopinathikova K, Chovanec M. Regulation of non-homologous end joining via post-translational modifications of components of the ligation step. Curr Genet. 2017;63(4):591–605. doi: 10.1007/s00294-016-0670-7. [DOI] [PubMed] [Google Scholar]
- 14.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He F, Jacobson A. Nonsense-Mediated mRNA Decay: Degradation of Defective Transcripts Is Only Part of the Story. Annu Rev Genet. 2015;49:339–66. doi: 10.1146/annurev-genet-112414-054639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284–95. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 17.Hunter T. When is a lipid kinase not a lipid kinase? When it is a protein kinase. Cell. 1995;83(1):1–4. [DOI] [PubMed] [Google Scholar]
- 18.Bosotti R, Isacchi A, Sonnhammer EL. FAT: a novel domain in PIK-related kinases. Trends Biochem Sci. 2000;25(5):225–7. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497(7448):217–23. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibanda BL, Chirgadze DY, Ascher DB, Blundell TL. DNA-PKcs structure suggests an allosteric mechanism modulating DNA double-strand break repair. Science. 2017;355(6324):520–4. doi: 10.1126/science.aak9654. [DOI] [PubMed] [Google Scholar]
- 21.Perry J, Kleckner N. The ATRs, ATMs, and TORs are giant HEAT repeat proteins. Cell. 2003;112(2):151–5. [DOI] [PubMed] [Google Scholar]
- 22.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. [DOI] [PubMed] [Google Scholar]
- 23.Greenwell PW, Kronmal SL, Porter SE, Gassenhuber J, Obermaier B, Petes TD. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell. 1995;82:823–9. [DOI] [PubMed] [Google Scholar]
- 24.Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120(5):715–27. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhao R, Kakihara Y, Gribun A, Huen J, Yang G, Khanna M, et al. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J Cell Biol. 2008;180(3):563–78. doi: 10.1083/jcb.200709061; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal M, Morgan M, Phelps SE, Roe SM, Parry-Morris S, Downs JA, et al. Structural basis for phosphorylation-dependent recruitment of Tel2 to Hsp90 by Pih1. Structure. 2014;22(6):805–18. doi: 10.1016/j.str.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horejsi Z, Stach L, Flower TG, Joshi D, Flynn H, Skehel JM, et al. Phosphorylation-dependent PIH1D1 interactions define substrate specificity of the R2TP cochaperone complex. Cell Rep. 2014;7(1):19–26. doi: 10.1016/j.celrep.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millson SH, Vaughan CK, Zhai C, Ali MM, Panaretou B, Piper PW, et al. Chaperone ligand-discrimination by the TPR-domain protein Tah1. Biochem J. 2008;413(2):261–8. doi: 10.1042/BJ20080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKeegan KS, Debieux CM, Watkins NJ. Evidence that the AAA+ proteins TIP48 and TIP49 bridge interactions between 15.5K and the related NOP56 and NOP58 proteins during box C/D snoRNP biogenesis. Mol Cell Biol. 2009;29(18):4971–81. doi: 10.1128/MCB.00752-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulon S, Pradet-Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, et al. HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell. 2010;39(6):912–24. doi: 10.1016/j.molcel.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, et al. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Mol Cell. 2014;54(1):119–32. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Morgen P, Horejsi Z, Macurek L. Substrate recognition and function of the R2TP complex in response to cellular stress. Front Genet. 2015;6:69. doi: 10.3389/fgene.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, et al. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11(14):1775–85. [DOI] [PubMed] [Google Scholar]
- 34.Miyata Y, Nishida E. CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol Cell Biol. 2004;24(9):4065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandal AK, Lee P, Chen JA, Nillegoda N, Heller A, DiStasio S, et al. Cdc37 has distinct roles in protein kinase quality control that protect nascent chains from degradation and promote posttranslational maturation. J Cell Biol. 2007;176(3):319–28. doi: 10.1083/jcb.200604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto GH, Ogi H, Biswas H, Ghosh A, Tanaka S, Sugimoto K. Two separate pathways regulate protein stability of ATM/ATR-related protein kinases Mec1 and Tel1 in budding yeast. PLoS Genet. 2017;13(8):e1006873. doi: 10.1371/journal.pgen.1006873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shevchenko A, Roguev A, Schaft D, Buchanan L, Habermann B, Sakalar C, et al. Chromatin Central: towards the comparative proteome by accurate mapping of the yeast proteomic environment. Genome biology. 2008;9(11):R167. doi: 10.1186/gb2008-9-11-r167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stirling PC, Bloom MS, Solanki-Patil T, Smith S, Sipahimalani P, Li Z, et al. The complete spectrum of yeast chromosome instability genes identifies candidate CIN cancer genes and functional roles for ASTRA complex components. PLoS Genet. 2011;7(4):e1002057. doi: 10.1371/journal.pgen.1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stirnimann CU, Petsalaki E, Russell RB, Muller CW. WD40 proteins propel cellular networks. Trends Biochem Sci. 2010;35(10):565–74. doi: 10.1016/j.tibs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Makhnevych T, Houry WA. The role of Hsp90 in protein complex assembly. Biochim Biophys Acta. 2012;1823(3):674–82. doi: 10.1016/j.bbamcr.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Gopinath RK, Leu JY. Hsp90 mediates the crosstalk between galactose metabolism and cell morphology pathways in yeast. Curr Genet. 2017;63(1):23–7. doi: 10.1007/s00294-016-0614-2. [DOI] [PubMed] [Google Scholar]
- 42.Inoue H, Sugimoto S, Takeshita Y, Takeuchi M, Hatanaka M, Nagao K, et al. CK2 phospho-independent assembly of the Tel2-associated stress-signaling complexes in Schizosaccharomyces pombe. Genes Cells. 2017;22(1):59–70. doi: 10.1111/gtc.12454. [DOI] [PubMed] [Google Scholar]
- 43.Zhou CY, Stoddard CI, Johnston JB, Trnka MJ, Echeverria I, Palovcak E, et al. Regulation of Rvb1/Rvb2 by a Domain within the INO80 Chromatin Remodeling Complex Implicates the Yeast Rvbs as Protein Assembly Chaperones. Cell Rep. 2017;19(10):2033–44. doi: 10.1016/j.celrep.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]