Abstract
Commensal bacterial population is believed to be a reservoir for antibiotic resistance genes (ARGs). The infant gut microbiota has relatively higher abundance of ARGs than the adults. These genes can get transferred from commensals to pathogens by horizontal gene transfer, which magnifies the spectrum of antibiotic resistance in the environment. The presence of ARGs in neo-nates and infants, with no prior antibiotic exposure, questions their origin in the naïve commensal population. Breast milk microbiota that is responsible for the initial seeding of infant gut microbiota has also been found to harbour a vast array of ARGs. This review discusses the recent findings that indicate the potential of breast milk microbiota to act as a vehicle for transmission of ARGs to infants.
Keywords: Antibiotic resistance, Resistome, Breast milk microbiota, Infant gut microbiota, Antibiotic resistance genes
Introduction
Breast milk is a superfood for infants. It is a bioactive fluid which provides hundreds to thousands of macronutrients, micronutrients, growth factors and microorganisms that are important for nutrition and long-term health of infant [1]. The breast milk microbiota and bioactive compounds help in the initial seeding of infant gut commensal population and aids in the maturation of mucosal immune system [2–5]. Depriving the infants of this dynamic fluid may make them susceptible to several gastrointestinal and respiratory infections, chronic malnutrition, neurological disorders and inflammatory diseases [6–8].
Human milk consists of a vast plethora of commensal microorganisms. With the introduction of next generation sequencing technique, the bacterial diversity of low biomass samples like human milk, colostrum and precolostrum (a biological fluid secreted by mammary glands at late stages of pregnancy) are being defined elaborately [4, 9–11]. Human milk is comprised of bacterial genera such as Bifidobacterium, Clostridium, Bacteroides, Faecalibacterium etc. that are strict anaerobes and are usually associated with human gut [4, 10]. This indicates the possible existence of an entero-mammary pathway where the commensal bacteria in the mother’s gut may be engulfed by dendritic cells and macrophages which might, in the due course, carry them to the lactating mammary gland [12].
In addition, human milk microbiota is also comprised of bacterial genera like Streptococcus, Veilonella, Prevotella etc. that are usually inhabitants of human oral cavity. This observation fuels the hypothesis that the milk microbiome is partially derived from infant oral microbiota by retrograde flow while suckling [13]. However, a recent report revealed the presence of typical oral bacteria in the precolostrum of pregnant woman suggesting that this bacterial population will be present in the colostrum as well and at least some oral microbiota of infant will be derived from the mother’s milk [10, 11]. It is a subject of intense research as to how typical oral bacterial population reach the mammary gland even before there is any contact with the infant mouth.
Despite the fact that breast milk is a complete food for infant, there are many factors that influence its nutritional and microbial composition. Such changes can alter the composition of infant gut microbiota in terms of diversity and number which might have an adverse effect on the long-term health of infants. The mode of delivery (caesarean or vaginal), maternal nutrition, maternal age, health status, geographical location and antibiotic exposure have been known to affect breast milk microflora and the infant gut microflora as well [14–17]. Intrapartum antibiotic exposure of the mother enriches the bacterial diversity in milk but reduces the number of initial infant gut colonizer, Bifidobacterium in mother’s milk [18]. It is also associated with emergence of antibiotic resistant phenotype in the infant gut microflora.
Antimicrobial resistance is an alarming phenomenon which severely impacts treatment outcome and increases disease related mortality. Antibiotic resistance genes (ARGs) are not only found in pathogenic bacteria but are present in much higher numbers in commensal and environmental bacteria [19]. The collection of ARGs within a bacterial population is known as the ‘resistome’ [20].
Some clinically important ARGs are transferred from commensal and environmental bacteria to pathogenic ones via horizontal gene transfer. Horizontal transfer of this bacterial resistance occurs in all possible environmental niches like soil, wastewater, marine and in human and animal body as well [9, 21]. The human gut is a favourable niche for transfer of bacterial resistome. It harbours a vast array of commensal bacteria and phages in association with host cells. The dynamic nature of the human gut with continuous supply of food and nutrition, stable temperature and close contact between commensal population make horizontal gene transfer easier [22, 23].
Indiscriminate use of antibiotics for treatment and their prolonged exposure in the environment helps in emergence of antibiotic resistance phenotype and enrichment of ARGs in microbial populations [24, 25]. The neo-natal and infant gut microbiota also harbour ARGs which can be a result of antibiotic exposure in early life, but the presence of such genes in the neo-nates or infants who have never been exposed to antibiotics is surprising and a matter of concern [26]. It is known that infants derive their first microbiota from the mother and hence it is very likely that the antibiotic resistome of the mother gets transferred to her baby during birth and breastfeeding. In this review we try to put forward, (1) the diversity of antibiotic resistome in the infant gut commensal microbial population in absence of any antibiotic treatment to the infant and, (2) the role of breast milk commensals as reservoir of ARGs.
The Antibiotic Resistome of Infant Gut in Absence of Prior Antibiotic Exposure
Some studies have reported that the commensals residing in the gut of infants who have no prior antibiotic exposure have high prevalence of antibiotic resistance genes. These genes encode for proteins that confer beta-lactam, tetracycline, macrolide, aminoglycoside and quinolone resistance. The prevalence rates of ARGs increase significantly from 5 to 31 weeks of age [21]. Functional analysis of 6-months old infant gut microbiota revealed that the earliest colonizers, Escherichia coli, Enterococcus faecalis, Staphylococcus epidermidis and Clostridium difficile carried genes responsible for adenylation, acetylation and phosphorylation of aminoglycosides that render the antibiotic inactive [27].
The infant gut commensals also harbour genes for resistance to folate-synthesis inhibitors in addition to genes encoding for chloramphenicol acetyltransferases and multidrug-efflux pumps [20]. Even the most abundant commensal of infant gut i.e. Bifidobacterium has been reported to carry ARGs during the early weeks of life [28]. This indicates that the resistome of infant gut is very diverse even in the absence of selective antibiotic pressure.
Mobile genetic elements like plasmids and transposons are instrumental in transferring ARGs from commensals to pathogenic species. The IncF plasmid carries beta-lactam, quinolone and aminoglycoside resistance genes along with genes encoding cytotoxins and adhesion factors. The Enterobacteriaceae family residing in the infant gut harbours the IncF plasmid-associated resistance genes [29]. The commensal Enterobacteriaceae population isolated from faecal samples of neo-nates exhibit high level of fluoroquinolone resistance due to mutation in the ‘Quinolone resistance determining region’ of parC and gyrA genes and also because of the presence of ‘Plasmid mediated quinolone resistance genes’ (PMQR) in them [30]. Genetic elements like integrons are also carriers of ARGs. Integrons are non-mobile genetic elements that are associated with mobile genetic elements like transposons and plasmids. Class I integron is present in both commensal and pathogenic bacteria and carry different antibiotic resistance gene cassettes that are responsible for conferring resistance to commonly used antibiotics. Infants of 3 days to 4 months of age, having no prior antibiotic exposure, show highest persistent pattern of class I integrons carrying trimethoprim, streptomycin and spectinomycin resistance genes [31].
Therefore, we can say that the commensal microbiota of infant gut can act as one of the reservoirs of ARGs and help in the dissemination of multidrug resistance to pathogenic bacteria. Also, the fact that a diverse antibiotic resistome is present in the gut of infants who never had any antibiotic encounter points towards the fact that the early infant gut resistome is obtained from the mother during delivery and breastfeeding.
In the next section we discuss about the presence of ARGs in breast milk microbiota and their possible role in transferring these genes to the infant gut commensal population.
The Antibiotic Resistome of Breast Milk
The role of breast milk during community assembly has recently gained a lot of attention. Initially considered to be sterile, breast milk has been found to contain a diverse microbial community, consisting of bacteria also present in the early infant gut [32]. It has been suggested that breast milk presents an important source of bacteria for seeding the gut microbiota, in particular at the very beginning of the colonization process. Immense interest in understanding the role of milk microbiota in infant health has unravelled the presence of ARGs in milk commensal bacteria.
Resistance to some widely administered antibiotics like tetracycline, chloramphenicol, streptomycin, gentamicin and quinupristin has been reported in bacteria isolated from milk of healthy mothers [33]. Vancomycin resistance is seen in Staphylococcus aureus, S. lugdunensis, E. faecalis, Streptococcus pneumoniae and S. parasanguinis isolated from breast milk. The multidrug resistant profile present in the infant gut commensals is also reflected in the milk microbial species where Acinetobacter, Rothia and Corynebacterium species in addition to the most common Staphylococcus and Streptococcus species exhibit resistance to more than one antibiotic which include ampicillin, amoxicillin, oxacillin, cephalothin, erythromycin, clindamycin and oxytetracycline and ciprofloxacin [34].
Antibiotic therapy during pregnancy, childbirth and lactation is one of the prime causes for emergence of antibiotic resistance. Lactational mastitis is one such disease which requires antibiotic treatment. Lactational mastitis in humans is a very common inflammatory condition occurring due to dysbiosis of normal milk microbiota resulting in rapid increase in the number of opportunistic pathogens like S. aureus, S. epidermidis and Corynebacterium [35]. A recent study has shown that Staphylococcus and Streptococcus isolates from milk of mastitis patients were resistant to benzylpenicillin, erythromycin, ampicillin and tetracycline [36]. More than 90% of Staphylococcus and Streptococcus species were found to be resistant to at least one of these antibiotics. In addition to this, a significant number of Streptococcal species exhibited multidrug resistant phenotype. HIV infection is another example where administration of the antiviral drug to mother results in emergence of drug resistant viral population in breast milk. Nevirapine is administered during childbirth and prevents the transmission of virus from mother to infant however, a study based in Uganda reported that breast milk samples of 40% subjects contained Nevirapine resistant strains of HIV subtypes A and D which developed within 4 weeks of drug administration [37].
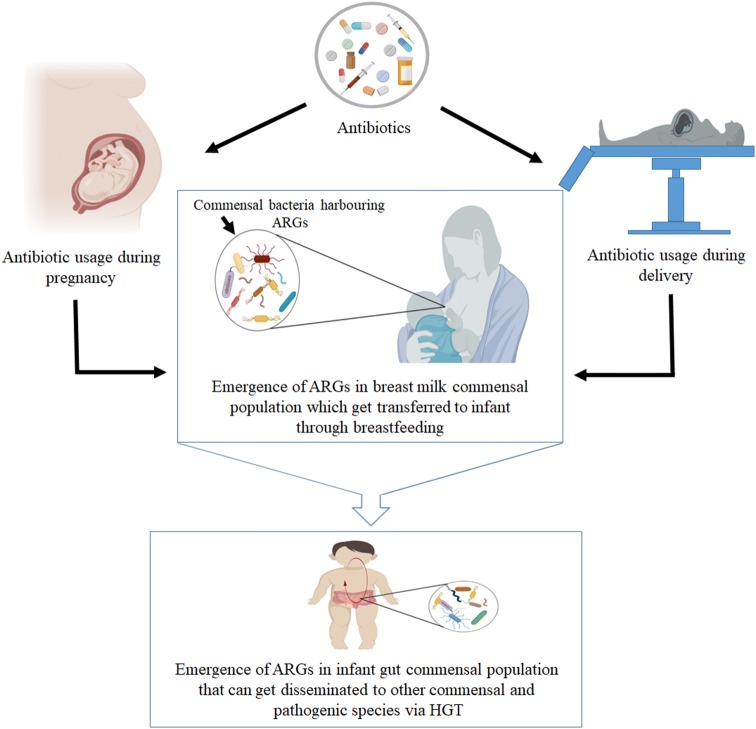
The ARGs in the gut microbiota of infants can be traced back to the resistome present in breast milk microbiota. To get a better understanding of this phenomenon it is important to analyse the bacterial resistome from infant-mother pair, where the ARG signatures in infant gut can be compared with those present in mother’s milk microbiota. Bifidobacterium longum subsp. infantis, Lactobacillus fermentum, L. gasseri and E. faecalis isolates from infant gut bear > 97% similarity with those present in mother’s milk and the antibiotic resistance profiles of these isolates from breast milk are shared with the infant gut [38]. In fact, the relative abundances of ARGs associated with mobile genetic elements are similar in breast milk and infant faecal samples. Interestingly, an antibiotic resistance profile is exclusive to a mother-infant pair as a recent study has reported dissimilarity in ARGs and mobile genetic element profiles with unrelated mother [39]. Although, there are very few studies which have explored the antibiotic resistome of breast milk and studied its acquisition by the infant, the fact that mothers milk microbiota is a vehicle for ARGs is quite evident (Fig. 1).
Fig. 1.
Schematic depicting the possible role of breast milk microbiota in transfer of antibiotic resistance genes to infant. Antibiotic exposure during pregnancy and childbirth leads to enrichment of antibiotic resistance genes (ARGs) in mother’s milk microbiota which can be transferred to infant during breastfeeding. These ARGs can get disseminated to other commensal and pathogenic bacteria in infant gut via horizontal gene transfer (HGT)
Conclusion
According to WHO (www.who.int/antimicrobial-resistance/en/) antimicrobial resistance (AMR) is referred to the ability of any microorganism to inhibit the activity of antimicrobials against it. AMR is posing as an increasing threat to mankind as it is making infections persistent and their treatment ineffective. Antimicrobial resistance has co-evolved with human kind and the overuse of antibiotics is accelerating the emergence of multi-drug resistant micro-organisms. Many environmental bacteria are resistant to antibiotics which may be either due to the presence of ARGs or due to their existence as biofilms [40–43]. The most haunting feature of AMR is its ability to spread from environmental sources such as soil, water and air to humans and animals and also within the human population [9, 21]. Antibiotic resistance can arise from either genetic mutation and or by acquisition of resistance genes from other species by horizontal gene transfer or by both the mechanisms [23].
The mobile genetic elements which include transposons, plasmids and integrons help in rapid spread of antimicrobial resistance from a pool of resistance genes [44]. Metagenomic studies have revealed that commensal bacteria harbour a vast plethora of antibiotic resistance genes that get transferred to pathogenic species by HGT. The human gut is one such ecological niche where the enrichment of ARGs occurs within the commensal population and the scope of HGT is also very high [22, 45].
There are many reviews which have extensively elucidated the role of human gut microbiota in the development of early post-natal immune system [46, 47]. Several metabolic diseases and allergies during childhood and adulthood have been linked to imbalances in composition and diversity of gut microbiota. Also, many infectious diseases have been linked to dysbiosis in gut commensal population [47–50]. However, the presence of diverse array of ARGs in infant gut commensal bacteria has drawn the attention of researchers towards understanding the mechanism of ARG acquisition during early life. The existence of ARGs in the gut of neo-nates and infants who have never been exposed to antibiotics (Table 1) is an alarming phenomenon [26]. Acquisition of commensal bacteria by infant starts in utero [51] and the maternal microbiota is also transferred to the infant during childbirth [52] and breastfeeding [2–5].
Table 1.
List of antibiotics for which antibiotic resistance genes have been reported in infant gut (in infants having no prior antibiotic exposure) and human milk commensal bacteria
| Infant gut | Human milk |
|---|---|
| Tetracycline [21] | Tetracycline [33, 36] |
| Macrolides [21] | Oxytetracycline [34] |
| Quinolone [21] | Chloramphenicol [33] |
| Fluoroquinolone [30] | Streptomycin [33] |
| Chloramphenicol [20] | Gentamicin [33] |
| Trimethoprim [31] | Quinupristin [33] |
| Streptomycin [21, 31] | Vancomycin [34] |
| Spectinomycin [21, 31] | Oxacillin [34] |
| Folate-synthesis inhibitors [20] | Ampicillin [36] |
| Cephalothin [34] | |
| Amoxicillin [34] | |
| Ciprofloxacin [34] | |
| Erythromycin [34] | |
| Clindamycin [34] |
Breast milk microbiota helps in the most primitive seeding of infant gut microbiota. Emergence of antibiotic resistant phenotypes and ARGs have been reported in many recent studies [33, 34, 38, 39] (Table 1). Maternal antibiotic usage during pregnancy and delivery (intrapartum) influence the breast milk microbiota and has been associated with the emergence of antimicrobial resistance in infant gut microbiota [18]. Infants acquire the ARGs from their mother through breast milk which results in a shared antibiotic resistome between the two [38, 39]. This acquired resistome can get enriched in the infant gut and get disseminated within the commensal gut bacteria and to pathogenic bacteria in due course of time.
In the present scenario it is nearly impossible to eliminate antibiotic usage. Women often require antibiotic treatment during pregnancy for controlling infections, like urinary tract infections which are very common during pregnancy [53, 54]. Also, intrapartum administration of antibiotics is very much needed especially during C-section in order to prevent post-operative wound infections [55]. However, the indiscriminate and over use of antibiotics can surely be limited. A possible solution can also be the supplementation of breast milk with the right probiotics for infants which can increase the abundance of gut commensals that do not harbour ARGs and help in inhibiting the enrichment of ARGs in the infant gut commensal population.
Despite the fact that breast milk microbiota can be a potential vehicle for transfer of antibiotic resistance to infants, breastfeeding should not be discouraged. It is the ultimate food for infants which provides tailored nutrition at all stages of development and helps in protecting infants from many infections, allergies and metabolic diseases later in life. However, intense research will help to understand the complexity of human milk microbiota in order to unravel its origin and bring forth its yet hidden role in infant health and nutrition.
Acknowledgement
UGC-D. S. Kothari Post-doctoral fellowship to LD and CSIR Junior research fellowship to VS are duly acknowledged.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lahari Das, Email: laharidas04@gmail.com.
Yogendra Singh, Email: ysinghdu@gmail.com.
References
- 1.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am. 2013;60:49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother’s milk: a purposeful contribution to the development of the infant microbiota and immunity. Front Immunol. 2018;9:361. doi: 10.3389/fimmu.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toscano M, De Grandi R, Grossi E, Drago L. Role of the human breast milk-associated microbiota on the newborns’ immune system: a mini review. Front Microbiol. 2017;8:2100. doi: 10.3389/fmicb.2017.02100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy K, Curley D, O’Callaghan TF, O’Shea CA, Dempsey EM, O’Toole PW, Ross RP, Ryan CA, Stanton C. The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep. 2017;7:40597. doi: 10.1038/srep40597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonnerdal B. Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J Pediatr. 2016;173:S4–S9. doi: 10.1016/j.jpeds.2016.02.070. [DOI] [PubMed] [Google Scholar]
- 6.Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351:aad3311. doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz Heijtz R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21:410–417. doi: 10.1016/j.siny.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Oddy WH. Breastfeeding, childhood asthma, and allergic disease. Ann Nutr Metab. 2017;70:26–36. doi: 10.1159/000457920. [DOI] [PubMed] [Google Scholar]
- 9.Penders J, Stobberingh EE, Savelkoul PH, Wolffs PF. The human microbiome as a reservoir of antimicrobial resistance. Front Microbiol. 2013;4:87. doi: 10.3389/fmicb.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz L, Garcia-Carral C, Rodriguez JM. Unfolding the human milk microbiome landscape in the omics era. Front Microbiol. 2019;10:1378. doi: 10.3389/fmicb.2019.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz L, Bacigalupe R, Garcia-Carral C, Boix-Amoros A, Arguello H, Silva CB, de Los Angeles Checa M, Mira A, Rodriguez JM. Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth. Sci Rep. 2019;9:8435. doi: 10.1038/s41598-019-42514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. 2014;5:779–784. doi: 10.3945/an.114.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biagi E, Quercia S, Aceti A, Beghetti I, Rampelli S, Turroni S, Faldella G, Candela M, Brigidi P, Corvaglia L. The bacterial ecosystem of mother’s milk and infant’s mouth and gut. Front Microbiol. 2017;8:1214. doi: 10.3389/fmicb.2017.01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar H, du Toit E, Kulkarni A, Aakko J, Linderborg KM, Zhang Y, Nicol MP, Isolauri E, Yang B, Collado MC, Salminen S. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front Microbiol. 2016;7:1619. doi: 10.3389/fmicb.2016.01619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE. 2011;6:e21313. doi: 10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabrera-Rubio R, Mira-Pascual L, Mira A, Collado MC. Impact of mode of delivery on the milk microbiota composition of healthy women. J Dev Orig Health Dis. 2016;7:54–60. doi: 10.1017/s2040174415001397. [DOI] [PubMed] [Google Scholar]
- 17.Lackey KA, Williams JE, Meehan CL, Zachek JA, Benda ED, Price WJ, Foster JA, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, Prentice AM, Debela Gindola K, Kvist LJ, Otoo GE, Garcia-Carral C, Jimenez E, Ruiz L, Rodriguez JM, Pareja RG, Bode L, McGuire MA, McGuire MK. What’s normal? Microbiomes in human milk and infant feces are related to each other but vary geographically: the INSPIRE study. Front Nutr. 2019;6:45. doi: 10.3389/fnut.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermansson H, Kumar H, Collado MC, Salminen S, Isolauri E, Rautava S. Breast milk microbiota is shaped by mode of delivery and intrapartum antibiotic exposure. Front Nutr. 2019;6:4. doi: 10.3389/fnut.2019.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bag S, Ghosh TS, Banerjee S, Mehta O, Verma J, Dayal M, Desigamani A, Kumar P, Saha B, Kedia S, Ahuja V, Ramamurthy T, Das B. Molecular insights into antimicrobial resistance traits of commensal human gut microbiota. Microb Ecol. 2019;77:546–557. doi: 10.1007/s00248-018-1228-7. [DOI] [PubMed] [Google Scholar]
- 20.Moore AM, Patel S, Forsberg KJ, Wang B, Bentley G, Razia Y, Qin X, Tarr PI, Dantas G. Pediatric fecal microbiota harbor diverse and novel antibiotic resistance genes. PLoS ONE. 2013;8:e78822. doi: 10.1371/journal.pone.0078822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Wintersdorff CJ, Penders J, van Niekerk JM, Mills ND, Majumder S, van Alphen LB, Savelkoul PH, Wolffs PF. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front Microbiol. 2016;7:173. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casals-Pascual C, Vergara A, Vila J. Intestinal microbiota and antibiotic resistance: perspectives and solutions. Hum Microbiome J. 2018;9:11–15. doi: 10.1016/j.humic.2018.05.002. [DOI] [Google Scholar]
- 23.Lerner A, Matthias T, Aminov R. Potential effects of horizontal gene exchange in the human gut. Front Immunol. 2017;8:1630. doi: 10.3389/fimmu.2017.01630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canton R, Morosini MI. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol Rev. 2011;35:977–991. doi: 10.1111/j.1574-6976.2011.00295.x. [DOI] [PubMed] [Google Scholar]
- 25.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74:417–433. doi: 10.1128/mmbr.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumpert H, Kubicek-Sutherland JZ, Porse A, Karami N, Munck C, Linkevicius M, Adlerberth I, Wold AE, Andersson DI, Sommer MOA. Transfer and persistence of a multi-drug resistance plasmid in situ of the infant gut microbiota in the absence of antibiotic treatment. Front Microbiol. 2017;8:1852. doi: 10.3389/fmicb.2017.01852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fouhy F, Ogilvie LA, Jones BV, Ross RP, Ryan AC, Dempsey EM, Fitzgerald GF, Stanton C, Cotter PD. Identification of aminoglycoside and beta-lactam resistance genes from within an infant gut functional metagenomic library. PLoS ONE. 2014;9:e108016. doi: 10.1371/journal.pone.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duranti S, Lugli GA, Mancabelli L, Turroni F, Milani C, Mangifesta M, Ferrario C, Anzalone R, Viappiani A, van Sinderen D, Ventura M. Prevalence of antibiotic resistance genes among human gut-derived bifidobacteria. Appl Environ Microbiol. 2017;83:e02894-16. doi: 10.1128/aem.02894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi A, Valdes-Varela L, Gueimonde M, Rudi K. Transmission and persistence of IncF conjugative plasmids in the gut microbiota of full-term infants. FEMS Microbiol Ecol. 2018;94:fix158. doi: 10.1093/femsec/fix158. [DOI] [PubMed] [Google Scholar]
- 30.Saksena R, Gaind R, Sinha A, Kothari C, Chellani H, Deb M. High prevalence of fluoroquinolone resistance amongst commensal flora of antibiotic naive neonates: a study from India. J Med Microbiol. 2018;67:481–488. doi: 10.1099/jmm.0.000686. [DOI] [PubMed] [Google Scholar]
- 31.Ravi A, Avershina E, Foley SL, Ludvigsen J, Storro O, Oien T, Johnsen R, McCartney AL, L’Abee-Lund TM, Rudi K. The commensal infant gut meta-mobilome as a potential reservoir for persistent multidrug resistance integrons. Sci Rep. 2015;5:15317. doi: 10.1038/srep15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger K, Bailey A, Bushman FD, Sleasman JW, Aldrovandi GM. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171:647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang MS, Cheng CC, Tseng SY, Lin YL, Lo HM, Chen PW. Most commensally bacterial strains in human milk of healthy mothers display multiple antibiotic resistance. Microbiologyopen. 2019;8:e00618. doi: 10.1002/mbo3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen PW, Tseng SY, Huang MS. Antibiotic susceptibility of commensal bacteria from human milk. Curr Microbiol. 2016;72:113–119. doi: 10.1007/s00284-015-0925-4. [DOI] [PubMed] [Google Scholar]
- 35.Ojo-Okunola A, Nicol M, du Toit E. Human breast milk bacteriome in health and disease. Nutrients. 2018;10:e1643. doi: 10.3390/nu10111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin M, Arroyo R, Espinosa-Martos I, Fernandez L, Rodriguez JM. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front Microbiol. 2017;8:1258. doi: 10.3389/fmicb.2017.01258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudelson SE, McConnell MS, Bagenda D, Piwowar-Manning E, Parsons TL, Nolan ML, Bakaki PM, Thigpen MC, Mubiru M, Fowler MG, Eshleman SH. Emergence and persistence of nevirapine resistance in breast milk after single-dose nevirapine administration. AIDS. 2010;24:557–561. doi: 10.1097/QAD.0b013e3283346e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozak K, Charbonneau D, Sanozky-Dawes R, Klaenhammer T. Characterization of bacterial isolates from the microbiota of mothers’ breast milk and their infants. Gut Microbes. 2015;6:341–351. doi: 10.1080/19490976.2015.1103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pärnänen K, Karkman A, Hultman J, Lyra C, Bengtsson-Palme J, Larsson DGJ, Rautava S, Isolauri E, Salminen S, Kumar H, Satokari R, Virta M. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat Commun. 2018;9:3891. doi: 10.1038/s41467-018-06393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed MN, Porse A, Sommer MOA, Hoiby N, Ciofu O. Evolution of antibiotic resistance in biofilm and planktonic Pseudomonas aeruginosa populations exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 2018;62:e00320-18. doi: 10.1128/aac.00320-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virmani R, Hasija Y, Singh Y. Effect of homocysteine on biofilm formation by mycobacteria. Indian J Microbiol. 2018;58:287–293. doi: 10.1007/s12088-018-0739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das L, Singh Y. Quorum sensing inhibition: a target for treating chronic wounds. In: Kalia VC, editor. Biotechnological applications of quorum sensing inhibitors. Singapore: Springer; 2018. pp. 111–126. [Google Scholar]
- 44.Partridge SR, Kwong SM, Firth N, Jensen SO. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev. 2018;31:e00088-17. doi: 10.1128/cmr.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubey GP, Ben-Yehuda S. Intercellular nanotubes mediate bacterial communication. Cell. 2011;144:590–600. doi: 10.1016/j.cell.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Dzidic M, Boix-Amoros A, Selma-Royo M, Mira A, Collado MC. Gut microbiota and mucosal immunity in the neonate. Med Sci. 2018;6:e56. doi: 10.3390/medsci6030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazar V, Ditu LM, Pircalabioru GG, Gheorghe I, Curutiu C, Holban AM, Picu A, Petcu L, Chifiriuc MC. Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol. 2018;9:1830. doi: 10.3389/fimmu.2018.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libertucci J, Young VB. The role of the microbiota in infectious diseases. Nat Microbiol. 2019;4:35–45. doi: 10.1038/s41564-018-0278-4. [DOI] [PubMed] [Google Scholar]
- 49.Maji A, Misra R, Dhakan DB, Gupta V, Mahato NK, Saxena R, Mittal P, Thukral N, Sharma E, Singh A, Virmani R, Gaur M, Singh H, Hasija Y, Arora G, Agrawal A, Chaudhry A, Khurana JP, Sharma VK, Lal R, Singh Y. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol. 2018;20:402–419. doi: 10.1111/1462-2920.14015. [DOI] [PubMed] [Google Scholar]
- 50.Sood U, Bajaj A, Kumar R, Khurana S, Kalia VC. Infection and microbiome: impact of tuberculosis on human gut microbiome of indian cohort. Indian J Microbiol. 2018;58:123–125. doi: 10.1007/s12088-018-0706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Argenio V. The prenatal microbiome: a new player for human health. High Throughput. 2018;7:e38. doi: 10.3390/ht7040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Matuszkiewicz-Rowinska J, Malyszko J, Wieliczko M. Urinary tract infections in pregnancy: old and new unresolved diagnostic and therapeutic problems. Arch Med Sci. 2015;11:67–77. doi: 10.5114/aoms.2013.39202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bromiker R, Ernest N, Meir MB, Kaplan M, Hammerman C, Schimmel MS, Schlesinger Y. Correlation of bacterial type and antibiotic sensitivity with maternal antibiotic exposure in early-onset neonatal sepsis. Neonatology. 2013;103:48–53. doi: 10.1159/000342215. [DOI] [PubMed] [Google Scholar]
- 55.Liu R, Lin L, Wang D. Antimicrobial prophylaxis in caesarean section delivery. Exp Ther Med. 2016;12:961–964. doi: 10.3892/etm.2016.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]