Abstract
Microbial fuel cells (MFCs) are envisioned as an evolving cost-effective process for treating organic wastes to simultaneously generate bioelectricity. Therefore, in present study a single chambered mediator- less air cathode MFC was operated for bioelectricity generation using citrus waste (CW) as a feedstock. The MFC was operated at four organic loading conditions (OLs; 3, 6, 9 and 12 kg/m3). The voltage generation and organic content reduction demonstrated the possibility of utilizing CW as a substrate in MFC. The polarization analysis revealed a high-power generation of 71.1 mW/m2 with low OL of 3 kg/m3. The decrease in pH and high volatile fatty acids (VFAs) generation was noted at high OL. Our current findings suggest better performance of MFC, in terms of energy generation and organic reduction at high OL.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00829-7) contains supplementary material, which is available to authorized users.
Keywords: Microbial fuel cells, Citrus waste, Chemical oxygen demand (COD), Power generation, Bioelectricity
Introduction
The depletion of fossil fuel reserves, increase in atmospheric temperature due to global warming and escalation in discharge of several wastes/wastewaters has directed several researchers in search of alternative technologies to generate energy with a minimal or no excretion of by-products [1–3]. Bioelectricity generation by the use of microbial fuel cell (MFC) has gained the attention of research community, due to their environmental friendly operation, and for efficient transition of waste to energy [4, 5]. In MFCs, the bioelectrogenic anaerobic microbes act as catalysts in direct conversion of organic/chemical energy from waste to power by involving metabolic reactions at ambient conditions [6, 7]. From past decade, several researchers have exploited the use of different waste/wastewaters as a feedstock for energy generation in MFC [8, 9]. However, there are only a limited number of studies, on the use of fruit waste as a substrate for electricity generation.
In urban solid waste (USW) generation, the fruit waste such as citrus fruit peels are generated noticeably. Moreover, the citrus fruits are known to be one of the major fruits, traded around the world [8, 10]. In addition, the citrus fruits contribute about 14% of major fruit crops grown in India and stands at 6th largest producer of worldwide [10]. The use of citrus fruit such as oranges and lime in industries, leaves a considerable number of residues in the form of pulp, seeds and peels which further needs to be processed. The oranges are one of the most commercially important citrus fruits and it is being generated of around 50 million tons around the world. Among, which 34% are used for industrial juice generation and thereby generating around 44% of peel as a by-product [11]. In practical orientation, these citrus peel wastes can be gainfully recycled with eco-friendly, bio conversion strategies; such as by using MFC to produce energy and for simultaneous production of fermentable by-products such as volatile fatty acids (VFAs).
Furthermore, the remains of citrus fruit extract are high in carbohydrates of about 55–60% of the raw fruit [11, 12]. Besides, other reducing sugars and proteins in citrus waste (CW) can be a potential feedstock for renewable energy generation. Previous studies have shown the potential of citrus peel waste to generate biological hydrogen production through dark fermentation [13]. The recovery of energy in terms of bioelectricity from MFC with simultaneous degradation of organics can be an essential alternative in utilization of CW residues. Also, CW is easily available at cheap source for bioelectrogenic microbial feed stock. In addition, the use of CW can skip the disposal cost with simultaneously achieving higher calorific bioenergy with minimal investment. Furthermore, the production of citrus fruit is expanding progressively and there is no clear indication that there will be a shortage of citrus fruit supply in near future [14] Therefore, this can help in skipping of food vs fuel debate. Consequently, the disposal of huge quantities of citrus (Citrus unshiu Marcow) peelings has initiated this study and therefore an effort was made to evaluate the bioelectricity in MFC by using them as a feed stock. The influence of citrus effluent based feedstock in air cathode single chamber MFC was evaluated on basis of organic loading, power generation and chemical oxygen demand (COD) reduction. Amongst the various configurations of MFC accessible, the air cathode single chamber MFC was used in present study to test the influence of CW as a feedstock. These MFCs were chosen due to their uncomplicated model for scale-up and cost minimization; with necessity of just one (anode) compartment and practicability in utilizing O2 in air as a terminal e- acceptor. Furthermore, these can be used in both batch and continuous mode operations.
Materials and Methods
Extraction of Organics from C. unshiu Marcow Peeling
Citrus peels were procured from the neighbouring fruit market and were sliced into little pieces and submitted to extraction. Typically, 80 g of sliced peels were blended with 350 mL of DI water in a flask and was corked with cotton and autoclaved at 121 °C to extract water soluble organics [13]. The mining of organics from peel was carried out at pH 7 for 40 min. The selection of pH and operational time for organic extraction using autoclave was selected on the basis of other studies [13]. Followed by organic extraction in autoclave, the solution was filtered to remove solid debris and solute was further used as a feed stock for bioelectricity generation. The initial pH, total chemical oxygen demand (TCOD) and soluble oxygen demand (SCOD) of the solute were noted to be 4.6, 53.8 g/l and 42.4 g/l, respectively.
Anodic Bioelectrogenic Mixed Consortium
The anaerobic bioelectrogenic consortia for power generation in the present study were obtained by using anaerobic digestive (AD) effluent as a seed culture. The AD system was operated with food waste leachate as a feed stock. The AD effluent was centrifuged (5000 rpm; 30 °C) and were cleaned thrice with phosphate buffer (PBS; 50 mM) and further enriched with synthetic wastewater (SW). The SW was prepared as described in earlier studies of MFC [15].
MFC Construction and Operation
Air cathode single chamber MFC used in present study to treat CW, was fabricated by using ‘Perspex’ glass (Fig. S1). The anodic chamber of MFC was designed to hold a total volume and working volume of 300 and 200 ml, respectively. Plain graphite plate (Graphite Engineering and technology, Inc., USA) with a surface area of 30 cm2, has served as cathode and anode electrodes, respectively. Both the electrodes were separated by using pre-treated PEM(proton exchange membrane, Nafion-117) [16]. MFC operation was conducted in closed circuit mode with 250 Ω as an external resistor. Batch mode of operation was followed at 8 days as the operating time (hydraulic retention time, HRT) for each feeding event. Prior to operation, the MFC were sparged with nitrogen gas and sealed by using the silica gel to minimize the diffusion of oxygen and to maintain anaerobic conditions.
Prior to the start-up of MFC, anode chamber was inoculated using microflora collected from anaerobic digester (AD) with SW containing acetate (1.2 g/L) as a substrate. After recording a stable voltage generation in repetitive operations with SW, the MFC was further operated with CW at various organic loading conditions. Based on the organic loading conditions adapted in the previous study with vegetable based waste [17]. The present MFC study using CW as substrate, was initially started with a high organic loading condition (OL) of 12 kg COD/m3 and was further decreased to 3 kg COD/m3. Prior to loading of CW to MFC, the influent pH was adjusted to neutral (7.0) by using either hydrochloric acid or sodium hydroxide. The requisite OL with CW was adjusted by mixing with domestic wastewater as mentioned in other studies of bioelectrochemical systems [18]. All the analysis were carried out in repetitive cycles and were averaged. The entire experiments were pursued in one single air cathode microbial fuel cell with 2 to 3 replications at each OL. After 3 cycles of DW operation and stable voltage generation, the CW was used as feedstock for voltage generation. The influent and effluent liquid fractions of MFC collected and analyzed for COD reduction, VFAs and pH by standard APHA methods [19].
Analysis and Calculations
Voltage (V) generation in MFC with CW as a substrate was recorded against the external resistance of 250 Ω using a digital multimeter. Polarization was carried out by recording the voltage with variation of external resistances from 30 KΩ to 50 Ω using a resistance box. The current (mA) and power (mW) were derived from voltage on basis of Ohms law (I = V/R) [20, 21]. The current density (mA/m2) and power density (mW/m2) were calculated as I/A and W/A (A = area of electrode surface area = 30 cm2) respectively.The internal resistance (Rin) of MFC was derived from the slope of I versus V curve of polarization analysis [22]. The columbic efficiency (CE) which relates the number coulombs resulted in electricity generation with respect to total potential of the respective substrate, was calculated on the basis of COD as described in previous studies [23, 24]. In brief the CE was calculated as CE = Cg/CT.C *100%, in which Cg is total coulombs noted by integration of current with time, whereas CT.C is total theoretical coulombs available on the base of COD removal during bioelectrogenic operation of MFC.
Results and Discussion
Bioelectricity Generation in MFC with CW
At First, the MFC was run with SW containing acetate, at an OL of 1.3 kg COD/m3 for three consecutive operational cycles in a batch mode at neutral pH. Each cycle comprising 8 days as HRT was developed electrochemically active biofilm on anode surface. Acetate is the simple organic substrate that is more amenable for the biofilm formation under anaerobic conditions was supported for quicker biofilm formation [25]. Improvement in MFC performance was noted with repetitive operational cycles. Average maximum voltage of 284 mV with an average current density of 378 mA/m2 were noted during acclimatization phase of operation (Data not shown). The gradual improvement in bioelectricity was evidenced by the adaptation of inoculated microbes to the bioelectrogenic conditions and electrochemically active biofilm formation in the system environment [26]. Subsequently from fourth cycle onwards, the MFC was shifted to operate with CW as a feed stock and with an OL of 12 kg/m3 (Fig. 1). Instantaneously, after change in feed stock from SW to CW, a visible drop in voltage (219 mV) generation and current density (291 mA/m2) were noted. Further the feed was changed to an OL of 9 kg/m3. A considerable enhancement in MFC performance was noted after decreasing of OL (Table 1). The maximum voltage generation of 262 mV with a corresponding current density of 349 mA/m2 was noted at 3 kg/m3. Further, operating the MFC at CW loading condition of 2.0 kg/m3 saw a sudden drop in voltage to 140 mV (0.56 mA/m2) and eventually found unstable (data not shown). This suggested that, the operation of MFC at OL of 3 kg/m3 is optimum for better performance. The similar trend in voltage generation with variation in OL was noted in other studies of MFC operating with vegetable-based waste [17].
Fig. 1.
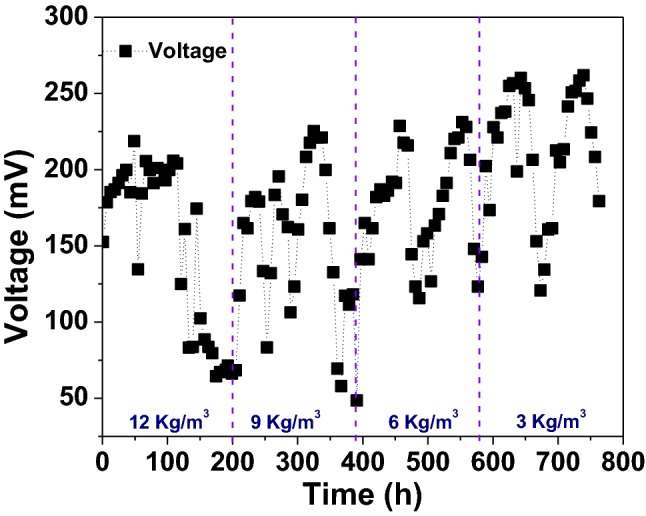
Bioelectricity generation in single chamber air cathode MFC operating with citrus waste as a feedstock
Table 1.
Performance of single chamber air cathode MFC at different organic loadings by using citrus waste as a feed stock
| OLa (kg/m3) | Voltage (mV) | Current density (mA/m2) | Power density (mW/m2) | Anode potential (mV) | Cathode potential (mV) | Open circuit voltage (mV) | CODb removal (%) | CEc (%) |
|---|---|---|---|---|---|---|---|---|
| 3 | 262 | 349 | 71.1 | − 410 | 310 | 740 | 63.8 | 33.2 |
| 6 | 231 | 308 | 63.4 | − 368 | 289 | 672 | 53.7 | 28.6 |
| 9 | 225 | 300 | 56.2 | − 309 | 279 | 605 | 48.1 | 24.4 |
| 12 | 219 | 291 | 48.5 | − 295 | 265 | 598 | 45.8 | 21.3 |
aOrganic loading
bChemical oxygen demand
cColumbic efficiency
Polarization of MFC Using CW as a Feed Stock
Polarization of single chamber air cathode MFC with CW was recorded by differing the external resistance from 30 KΩ to 50 Ω during highest stable voltage generation period. The power density of MFC varied with variation in OL (Fig. 2, Table 1). The MFC operation with low OL of 3 kg/m3, exhibited a power density of 71.1 mW/m2, at a current density of 281 mA/m2. While, the MFC operation with other OLs of 6 kg/m3, 9 kg/m3, and 12 kg/m3 exhibited a power density of 63.4 mW/m2, 56.2 mW/m2, and 48.5 mW/m2, respectively. These power densities were observed at a current densities of 265 mA/m2, 250 mA/m2, and 232 mA/m2. The variation of anode potential, cathode potential and open circuit voltage with respect to change in OL were presented in Table 1. The power densities stated in present study were observed to be higher in comparison to other studies of MFC operating with vegetable waste composite [17]. In their study, a maximum power density of 26.41 mW/m2 was noted at low OL of 0.70 kg COD/m3. These power density values were decreased to 15.42 mW/m2 with increase in OL to 2.08 kg COD/m3. This suggests that, influence of OL is one of the crucial parameter in determining the maximum power densities of MFC. However, these values are lower in contrast to other studies of MFC operating with acetate [27–29] and by using citrus based orange peel waste (358 mW/m2) [30]. These differences in power densities can be attributed to several factors such as variation in biomass (type of citrus peel), pre-treatment, OL, electrode type, reactor configuration and internal resistance [31]. Based on the polarization analysis, the calculated internal resistance from I–V curve of MFC was noted to be 285 Ω, and it was found to be similar at all OL conditions. This was in agreement with external resistance of 300 Ω; the point at which the highest power densities and current densities were noted [20, 32, 33].
Fig. 2.
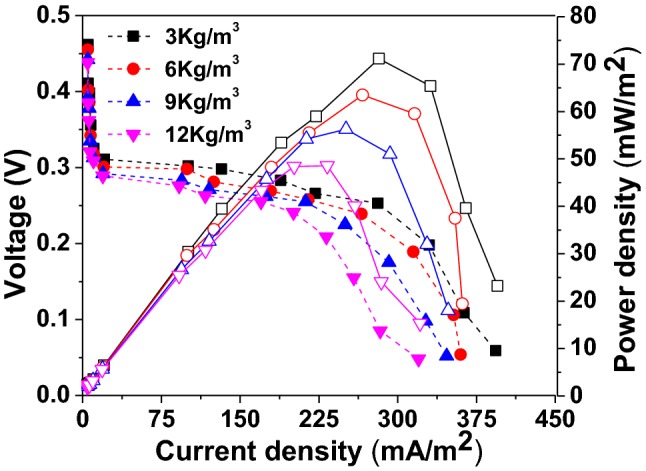
Voltage (closed symbol) and power density (open symbol) generation during polarization analysis of MFC at different organic loadings
Organic Reduction and pH Variation in Anode of MFC
MFC recorded a good reduction of organics present in feed wastewater with simultaneous power generation (Fig. 3). Reduction in organic content in anolyte solution confirms the effective action of electroctive bacteria present in anode surface of MFC. During the acclimatization phase with acetate, MFC exhibited a 85.3% of COD removal (CODr) with a organic reduction (OR) of 1.3 kg COD/m3. The shift in substrate to CW exhibited a drop in organic reduction. Compared to SW with acetate, CW had different types of organics, which are complex in nature. The drop in substrate degradation might be due to the complexity of the substrate. The maximum CODr percentage of 63.8% was noted with OL of 3 kg/m3, this was followed by 6 kg/m3, 9 kg/m3, and 12 kg/m3 with a CODr percentage of 53.7%, 48.1% and 45.8%, respectively. The maximum CODr percentage was recorded at low OL and found to decrease with an increase in OL. On the contrary, the OR increased with increase in OL. On the basis of effluent COD concentration, the maximum OR was registered with 12 kg/m3 (5.4 kg COD/m3), which was followed by 9 kg/m3 (4.3 kg COD/m3), 6 kg/m3 (3.2 kg COD/m3) and 3 kg/m3 (1.9 kg COD/m3). An immediate drop in OR was noted after shifting of MFC from simple organic substrate (acetate) to complex CW. A similar change in substrate degradation effeciency was noted in other studies of MFC by varying the OL and type of feedstock [13]. The maximum specific power yield (SPY) of 0.141 W/kg CODr was noted with 3 kg/m3 OL operation compared to other OL of 6 kg/m3 (0.068 W/kg CODr), 9 kg/m3 (0.048 W/kg CODr), and 12 kg/m3 (0.034 W/kg CODr) operations. On basis of substrate degradation effeciency, the calculated columbic effeciency (CE) of MFC with variation in OL was noted to be 33.2% (3 kg/m3), 28.6% (6 kg/m3), 24.4%(9 kg/m3), and 21.3% (12 kg/m3).
Fig. 3.
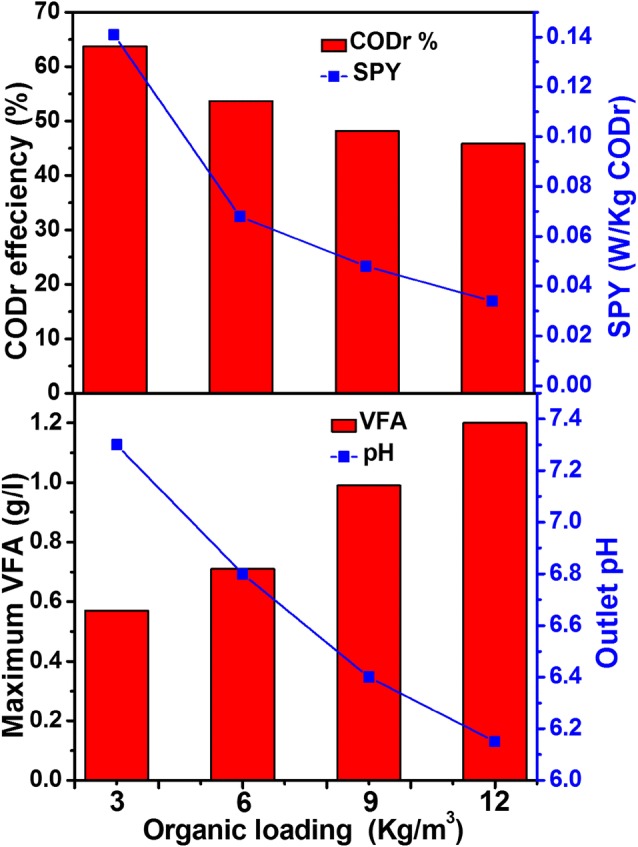
Performance estimation of single chamber air cathode MFC at various organic loadings. a COD removal (CODr) efficiency and specific power yield (SPY), b Effluent VFAs concentration and pH noted in MFC with alteration of organic loadings
Bioelectrogenic anodic pH is one of the crucial parameter, which can influence bacterial metabolism and lead to alteration in substrate degradation effeciency and power generation [34]. The effluent pH of MFC varied between 7.3 and 6.2 during operation with CW and by changing the OL. The low pH of 6.2 was noted with OL of 12 kg/m3, this was increased to 6.45 (9 kg/m3), 6.81 (6 kg/m3), and 7.2 (3 kg/m3) with decrease in OL. The variation in effluent pH might be due to VFA generation. The elevated VFAs generation was noted at high OL and found to decrease along with OL [35]. The maximum VFA generation was observed at 12 kg/m3 (1.2 g/l), in comparison to 9 kg/m3(0.9 g/l), 6 kg/m3 (0.7 g/l), 3 kg/m3 (0.5 g/l), operations.
Conclusion
The viability of single chambered air cathode MFC for bioelectricity generation with simultaneous organic reduction was successfully proved for the treatment of CW. The better functioning of MFC in terms of voltage production and organic reduction was noted at low OL. The execution of MFC, that was categorised on the basis polarization analysis, and SPY are well supported with above observations. The variation in OL influence the performance of MFC. The additional studies are essential to evaluate the economics and tuning of MFC performance for using, CW as a feed stock for practical application.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by Brain Pool Grant (NRF-2019H1D3A2A01060226) by National Research Foundation of Korea to work at Konkuk University (VCK). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013M3A6A8073184). This research was supported by 2018 KU Brain Pool of Konkuk University.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jung-Kul Lee, Email: jkrhee@konkuk.ac.kr.
Vipin C. Kalia, Email: vckaliaku@gmail.com
References
- 1.Kondaveeti S, Abu-Reesh IM, Mohanakrishna G, Pant D, He Z. Utilization of residual organics of Labaneh whey for renewable energy generation through bioelectrochemical processes: strategies for enhanced substrate conversion and energy generation. Bioresour Technol. 2019;286:121409. doi: 10.1016/j.biortech.2019.121409. [DOI] [PubMed] [Google Scholar]
- 2.Kondaveeti S, Mohanakrishna G, Lee J-K, Kalia VC. Methane as a substrate for energy generation using microbial fuel cells. Indian J Microbiol. 2019;59:121–124. doi: 10.1007/s12088-018-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh RK, Singh R, Sivakumar D, Kondaveeti S, Kim T, Li J, Sung BH, Cho B-K, Kim DR, Kim SC, Kalia VC, Zhang Y-HPJ, Zhao H, Kang YC, Lee J-K. Insights into cell-free conversion of CO2 to chemicals by a multienzyme cascade reaction. ACS Catal. 2018;8:11085–11093. doi: 10.1021/acscatal.8b02646. [DOI] [Google Scholar]
- 4.Kondaveeti SK, Seelam JS, Mohanakrishna G. Anodic electron transfer mechanism in bioelectrochemical systems. In: Das D, editor. Microbial fuel cell: a bioelectrochemical system that converts waste to watts. Cham: Springer; 2018. pp. 87–100. [Google Scholar]
- 5.Kondaveeti S, Mohanakrishna G, Pagolu R, Kim I-W, Kalia VC, Lee J-K. Bioelectrogenesis from raw algal biomass through microbial fuel cells: effect of acetate as co-substrate. Indian J Microbiol. 2019;59:22–26. doi: 10.1007/s12088-018-0769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohanakrishna G, Abu-Reesh IM, Kondaveeti S, Al-Raoush RI, He Z. Enhanced treatment of petroleum refinery wastewater by short-term applied voltage in single chamber microbial fuel cell. Bioresour Technol. 2018;253:16–21. doi: 10.1016/j.biortech.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Venkata Mohan S, Mohanakrishna G, Reddy BP, Saravanan R, Sarma PN. Bioelectricity generation from chemical wastewater treatment in mediatorless (anode) microbial fuel cell (MFC) using selectively enriched hydrogen producing mixed culture under acidophilic microenvironment. Biochem Engn J. 2008;39:121–130. doi: 10.1016/j.bej.2007.08.023. [DOI] [Google Scholar]
- 8.Chae K-J, Choi M-J, Lee J-W, Kim K-Y, Kim IS. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol. 2009;100:3518–3525. doi: 10.1016/j.biortech.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 9.Mohanakrishna G, Venkata Mohan S, Sarma PN. Utilizing acid-rich effluents of fermentative hydrogen production process as substrate for harnessing bioelectricity: an integrative approach. Int J Hydrog Energy. 2010;35:3440–3449. doi: 10.1016/j.ijhydene.2010.01.084. [DOI] [Google Scholar]
- 10.Sharma K, Mahato N, Cho MH, Lee YR. Converting citrus wastes into value-added products: economic and environmently friendly approaches. Nutrition. 2017;34:29–46. doi: 10.1016/j.nut.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Marín FR, Soler-Rivas C, Benavente-García O, Castillo J, Pérez-Alvarez JA. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007;100:736–741. doi: 10.1016/j.foodchem.2005.04.040. [DOI] [Google Scholar]
- 12.Crawshaw R. Co-product feeds: animal feeds from the food and drinks industries. R Crawshaw Nottingham University Press, Nottingham, 2001 pp 285. J Sci Food Agr. 2003;83:362–362. doi: 10.1002/jsfa.1326. [DOI] [Google Scholar]
- 13.Venkata Mohan S, Lenin Babu M, Venkateswar Reddy M, Mohanakrishna G, Sarma PN. Harnessing of biohydrogen by acidogenic fermentation of citrus limetta peelings: effect of extraction procedure and pretreatment of biocatalyst. Int J Hydrog Energy. 2009;34:6149–6156. doi: 10.1016/j.ijhydene.2009.05.056. [DOI] [Google Scholar]
- 14.Khan AM, Obaid M. Comparative bioelectricity generation from waste citrus fruit using a galvanic cell, fuel cell and microbial fuel cell. J Energy South Afr. 2015;26:90–99. doi: 10.17159/2413-3051/2015/v26i3a2143. [DOI] [Google Scholar]
- 15.Kakarla R, Min B. Photoautotrophic microalgae Scenedesmus obliquus attached on a cathode as oxygen producers for microbial fuel cell (MFC) operation. Int J Hydrog Energy. 2014;39:10275–10283. doi: 10.1016/j.ijhydene.2014.04.158. [DOI] [Google Scholar]
- 16.Kondaveeti S, Lee J, Kakarla R, Kim HS, Min B. Low-cost separators for enhanced power production and field application of microbial fuel cells (MFCs) Electrochim Acta. 2014;132:434–440. doi: 10.1016/j.electacta.2014.03.046. [DOI] [Google Scholar]
- 17.Venkata Mohan S, Mohanakrishna G, Sarma PN. Composite vegetable waste as renewable resource for bioelectricity generation through non-catalyzed open-air cathode microbial fuel cell. Bioresour Technol. 2010;101:970–976. doi: 10.1016/j.biortech.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Kondaveeti S, Kang E, Liu H, Min B. Continuous autotrophic denitrification process for treating ammonium-rich leachate wastewater in bioelectrochemical denitrification system (BEDS) Bioelectrochemistry. 2019;130:107340. doi: 10.1016/j.bioelechem.2019.107340. [DOI] [PubMed] [Google Scholar]
- 19.APHA . Standard methods for the examinatinon of water and wastewater. Washington, DC: American Public Health Association; 2005. [Google Scholar]
- 20.Logan BE. Microbial fuel cells. Hobokem: Wiley; 2008. [Google Scholar]
- 21.Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 22.Fan Y, Sharbrough E, Liu H. Quantification of the internal resistance distribution of microbial fuel cells. Environ Sci Technol. 2008;42:8101–8107. doi: 10.1021/es801229j. [DOI] [PubMed] [Google Scholar]
- 23.Girguis P, Reimers CE (2011) Methane-powered microbial fuel cells. Google Patents
- 24.He Z, Wagner N, Minteer SD, Angenent LT. An upflow microbial fuel cell with an interior cathode: assessment of the internal resistance by impedance spectroscopy. Environ Sci Technol. 2006;40:5212–5217. doi: 10.1021/es060394f. [DOI] [PubMed] [Google Scholar]
- 25.Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol. 2003;69:1548–1555. doi: 10.1128/aem.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajracharya S, Sharma M, Mohanakrishna G, Dominguez Benneton X, Strik DPBTB, Sarma PM, Pant D. An overview on emerging bioelectrochemical systems (BESs): technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew Energy. 2016;98:153–170. doi: 10.1016/j.renene.2016.03.002. [DOI] [Google Scholar]
- 27.Singh HM, Pathak AK, Chopra K, Tyagi VV, Anand S, Kothari R. Microbial fuel cells: a sustainable solution for bioelectricity generation and wastewater treatment. Biofuels. 2018;10:11–31. doi: 10.1080/17597269.2017.1413860. [DOI] [Google Scholar]
- 28.Wang Z, Mahadevan GD, Wu Y, Zhao F. Progress of air-breathing cathode in microbial fuel cells. J Power Sour. 2017;356:245–255. doi: 10.1016/j.jpowsour.2017.02.004. [DOI] [Google Scholar]
- 29.Santoro C, Arbizzani C, Erable B, Ieropoulos I. Microbial fuel cells: from fundamentals to applications. A review. J Power Sour. 2017;356:225–244. doi: 10.1016/j.jpowsour.2017.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miran W, Nawaz M, Jang J, Lee DS. Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci Total Environ. 2016;547:197–205. doi: 10.1016/j.scitotenv.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Mohanakrishna G, Al-Raoush RI, Abu-Reesh IM. Induced bioelectrochemical metabolism for bioremediation of petroleum refinery wastewater: optimization of applied potential and flow of wastewater. Bioresour Technol. 2018;260:227–232. doi: 10.1016/j.biortech.2018.03.122. [DOI] [PubMed] [Google Scholar]
- 32.Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- 33.Oh S, Logan BE. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005;39:4673–4682. doi: 10.1016/j.watres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Mohanakrishna G, Abu-Reesh IM, Al-Raoush RI, He Z. Cylindrical graphite based microbial fuel cell for the treatment of industrial wastewaters and bioenergy generation. Bioresour Technol. 2018;247:753–758. doi: 10.1016/j.biortech.2017.09.174. [DOI] [PubMed] [Google Scholar]
- 35.Velvizhi G, Babu PS, Mohanakrishna G, Srikanth S, Mohan SV. Evaluation of voltage sag-regain phases to understand the stability of bioelectrochemical system: electro-kinetic analysis. RSC Adv. 2012;2(4):1379–1386. doi: 10.1039/c1ra00674f. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


