Abstract
Amidase from Bacillus sp. APB-6 with very good acyltransferase activity was purified to homogeneity with a purification fold of 3.68 and 53.20% enzyme yield. The purified protein's subunit molecular mass was determined approximately 42 kDa. Hyperactivity of the enzyme was observed at pH 7.5 (150 mM, potassium-phosphate buffer) and 50 °C of incubation. An enhancement in activity up to 42% was recorded with ethylenediaminetetraacetic acid and dithiothreitol. The kinetic parameter Km values for substrates: acetamide and hydroxylamine-hydrochloride were 73.0 and 153 mM, respectively. Further, the Vmax for acyltransferase activity was 1667 U/mg of protein and the Ki for acetamide was calculated as 37.0 mM. The enzyme showed tolerance to various organic solvents (10%, v/v) and worked well in the biphasic reaction medium. The acyltransferase activity in presence of solvents i.e. biphasic medium may prove highly favorable for the transformation of hydrophobic amides, which otherwise is not possible in simple aqueous phase.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00836-8) contains supplementary material, which is available to authorized users.
Keywords: Bacillus sp. APB-6, Amidase, Acyltransferase, Biphasic system, Hydrophobic amides
Introduction
Amidases belong to nitrilase-superfamily of enzymes that facilitate hydrolysis of amides to ammonia and carboxylic acid [1]. Based on their amino acid sequences, amidases have been grouped into two types: aliphatic and signature amidases. Aliphatic amidases contain a conserved nucleophilic cysteine at their active site like the nitrilases and other sulphydryl enzymes, forming either homo-tetrameric/hexameric structure [2, 3]. The signature amidases contain conserved catalytic motif (GGSS). In addition, some amino acids (Gly, Asp and Ser) are located downstream from the GGSS signature at positions 17, 19 and 23, respectively [4].
Value-added organic acids such as acrylic acid, p-aminobenzoic acid, and nicotinic acid have been produced by nitriles utilizing amidases in conjunction with nitrile hydratases [3, 5]. Amidases are also used as catalysts in the treatment of industrial effluents and wastewater management [6–8]. Wide spectrum amidases exhibit acyltransferase activity in the presence of hydroxylamine (acyl group acceptor) and amide (acyl group donor) for producing hydroxamic acids that are reported to be tumor inhibitors and anti-cancerous agents [5, 9]. Some hydroxamic acids such as α-amino hydroxamate and acetohydroxamic acid are recommended for treatment of urea plasma infections and are also being investigated for anti-human immunodeficiency viral and anti-malarial activity [5, 10, 11].
Biotransformation of readily soluble amides is easily possible (aqueous conditions). Whereas, low solubility is associated for higher amides under normal conditions. Therefore, organic solvents were used to improve their solubility [12, 13]. Novel compounds in addition to biologically active enantiomers which were difficult to synthesize conventionally, are now possible to synthesize by using enzymes in a solvent medium [13–15]. The stability of enzymes as biocatalysts in the presence of solvents is also an important factor for efficient biotransformation and biotechnological applications [16–19].
Bacillus is generally regarded as safe and widely used in the biotechnological applications such as antimicrobials, biofuels, biopolymers and enzyme production [20–25]. The synthesis of hydroxamic acids is known through chemical and biological methods. Owing to high selectivity, biological methods are more promising over chemical synthesis [1, 5, 10]. Recently, the synthesis of useful products using enzyme catalysis in low-water (non-aqueous) media is gaining more importance. Keeping in view the importance of enzyme reactions in biphasic (water-restricted) medium and the potential applications of hydroxamic acids, in present work, purification and characterization of amidase (Bacillus sp. APB-6) with acyltransferase activity was described. Further, we also report the acyltransferase reaction in the biphasic system i.e. in presence of both aqueous as well as solvents (organic).
Materials and Methods
Reagents, Microbial Strains and Culture Conditions
All the reagents and chemicals were of high purity and molecular biology grade. Solvents were used of high-performance liquid chromatography (HPLC) grade (Merck, India). Culture media components and inorganic salts were procured from HiMedia (Mumbai, India). Bacillus sp. APB-6 was used as a nitrile metabolizing culture [10]. Briefly, N-methyl acetamide (70 mM) was added at 0, 24 and 30 h of intervals to the production medium for induction of acyltransferase activity. After 36 h of incubation, fully-grown culture was recovered by centrifugation and washed twice with potassium-phosphate (0.2 M, pH 7.5) [10].
Acyltransferase Assay
Acyltransferase assay was performed spectrophotometrically (500 nm) as described earlier [10]. Briefly, total 2 mL of reaction with substrates, acetamide (300 mM) and hydroxylamine-hydrochloride [HCl (800 mM)] and resting cells was incubated at 45 °C for 5 min in the glycine–NaOH buffer (0.1 M, pH 7.5). Thereafter, reaction was stopped through addition of FeCl3 reagent [4.0 mL].
HPLC Analysis and Purification of Amidase (Acyltransferase) Enzyme
HPLC analysis was performed as described earlier for the estimation of product formed and the unchanged substrate in the assay mixture [10]. Amidase showing very good activity of acyltransferase (henceforth referred to acyltransferase) was purified using 1.0 mM of each [dithiothreitol (DTT) and ethylene diamine tetra acetic acid (EDTA)] in the buffer (0.2 M, pH 7.5) at 4 °C to study its biochemical and molecular characteristics.
Preparation of Membrane-Free Crude Enzyme Extract
The cell slurry [25 mg of dry cell weight/ml] was disrupted using BeadBeater™ (BioSpec Products, Inc. Bartlesville, USA). Cell suspension (30 ml) was added to the bead beater chamber (50 ml capacity) half filled with 0.1 mm zirconium beads. Cells were disrupted in 5 cycles (each of 1 min). The resulting suspension was centrifuged (30min, 4 ºC) to remove the debris. The clear supernatant was filtered through 0.45 μm polyvinylidene difluoride membrane (using a vacuum pump) and collected as extract without cell.
Ammonium Sulphate Fractionation
The extract was treated with different saturation/concentrations (10–100%) of ammonium sulphate. Protein estimation (by Bradford method) and acyltransferase assay were performed for each of the samples [10, 26]. Finally, the protein of interest was precipitated and subjected to anion exchange chromatography.
Anion Exchange Chromatography (DEAE)
Pre-packed DEAE-Sepharose column (HiPrep™ 16/10 DEAE FF, GE Healthcare, USA) connected to liquid chromatography system (AKTA prime™ V2.00, GE Healthcare, USA) was used for anion exchange chromatography. Briefly, protein fraction (7 ml) obtained after ammonium sulphate precipitation was added to column, pre-equilibrated by potassium-phosphate buffer (0.1 M, pH 7.5) having NaCl (0.1 M). Loosely attached proteins were removed using 100 ml of buffer, whereas NaCl gradient (0.1–1.0 M) was used to elute the bound proteins from the column. The length of the gradient was 200 ml with the flow rate and fraction size of 1.0 ml/min and 2.5 ml, respectively. Eluted proteins were monitored by absorbance at 280 nm using UV–Vis spectrophotometer (Lambda 12, Perkin Elmer, Massachusetts, USA).
Polyacrylamide Gel Electrophoresis (PAGE)
Protein fractions obtained after anion exchange chromatography were analysed by sodium-dodecyl sulfate (SDS)–PAGE to assess the purification status and subunit mass of the enzyme [27]. The protein markers (medium range) used for molecular mass analysis of acyltransferase of Bacillus sp. APB-6 were from Bangalore Genei Pvt. Ltd (India). The Amersham high molecular weight-native protein markers (GE Healthcare, UK) were used for the native-PAGE of the protein.
Zymogram Analysis
Zymogram staining was performed to examine the activity of enzyme in the gel. Briefly, after running the native-PAGE, gel was sliced into two pieces. One piece was stained with coomassie and then destained as usual. The second gel piece was cleaned with distilled-water (twice) and incubated for 15 min (60 °C) in the buffer (0.2 M, pH 7.5) with acetamide (0.3 M) and hydroxylamine-HCl (0.8 M). After incubation, the gel was transferred to a fresh container having FeCl3 reagent. The appearance of reddish-brown band confirmed the presence of acyltransferase activity.
Characterization of Purified Enzyme
Determination of Molecular Mass
Purified protein's molecular weight was measured through SDS–PAGE gel imaging system (Gel documentation system, Alpha Innotech Corporation, USA).
Optimization of pH and Buffer Concentration
The activity of purified enzyme was assayed in 2.0 ml reaction mixture of different pH (4.0–10.5) values buffer (100 mM). Further, influence of buffer concentration (50–400 mM) on the enzyme activity was evaluated at the optimum pH.
Optimization of Temperature and Thermostability
The activity of acyltransferase was assayed at varying incubation temperatures from 35 to 65 °C. Further, the thermostability of the purified protein was investigated by incubating 50 μg of purified enzyme in potassium-phosphate buffer (150 mM, pH 7.5) at various temperatures: 45, 50, 55 and 60 °C to measure the residual activity after every 30 min intervals. Initial activity was considered as 100%.
Effects of Metal Ions, Inhibitors and Organic Solvents
Influence of metals ions and enzyme inhibitors (1 mM, each), including FeCl3, MgCl2·6H2O, ZnSO4·7H2O, CoCl2, CuSO4·5H2O, NaCl, AgNO3, BaCl2·2H2O, HgCl2, NaN3, CaCl2·2H2O, CdCl2·H2O, Pb(NO3)2, MnCl2·2H2O, urea, phenyl methyl sulphonyl fluoride (PMSF), DTT, EDTA, and polyethylene glycol on activity of enzyme was investigated for incubation of 20 min (30 °C). Similarly, solvents (10%, v/v), including 1,4-dioxan, acetone, benzene, butanol, carbon tetrachloride, ethylene glycol, isoamyl alcohol, propane-2-ol, methanol, and toluene were studied. The residual acyltransferase activity was assayed at 50 °C.
Determination of Kinetic Parameters
Purified enzyme parameters (Km and Vmax) were measured by varying the concentrations of acetamide (100–500 mM) at various fixed concentrations of hydroxylamine-HCl (200–1000 mM). The assay was performed in potassium-phosphate buffer (150 mM; pH 7.5) for 5 min (50 °C) and acetohydroxamic acid was quantified by HPLC.
Acyltransferase catalyzes a bi-substrate reaction. The reaction occurs in the manner that substrate A combines with enzyme obligatorily before substrate B, followed by the departure of products in the order P followed by Q (Eq. 1).
| 1 |
where E = enzyme, A = acetamide, B = hydroxylamine-HCl, P = ammonia, and Q = acetohydroxamic acid.
If the concentration of B is held constant in the absence of P and Q, and the velocities are measured at different concentrations of A, Michaelis–Menten kinetics is often followed so that ‘apparent’ values of KmA and Vmax can be determined by the double reciprocal plot (plot 1/v vs 1/[A]). Repetition of the measurements at a different fixed concentration of B yields different ‘apparent’ KmA and Vmax values and a pattern of reciprocal plots is obtained.
If values (1/Vappmax) of intercept (first double reciprocal plot) are plotted with 1/[B], the true values for KmB and Vmax can be deduced (the intercept and slope of replot). A second linear replot of slope values (Kappm/Vappmax) from the first double reciprocal plot against 1/[B] yields KmA and KiA from its intercept and slope, given the values of KmB and Vmax from the first replot.
Results and Discussion
Purification of Acyltransferase
The high specific activity (402 U/mg of protein) was obtained after five beating cycles in the bead-beater. 26% of the residual acyltransferase activity was recorded at the end of the beating cycles from the disrupted cells. Cell-free extract reported 74% of the total acyltransferase activity and was subjected to different ammonium sulphate saturation (10 to 100%). It was observed that although proteins started precipitating from 10% saturation, no acyltransferase activity was recorded till 30% saturation. Protein of interest started precipitating after 40% saturation and maximum specific activity of acyltransferase (689 U/mg of protein) was obtained with 80% saturation. Contaminating proteins were precipitated out by an initial cut of 30%, whereas the remaining supernatant was subjected to a final cut of 80% to precipitate the protein of interest. Finally, the precipitated protein was dissolved in 100 mM of potassium-phosphate buffer (pH 7.5). Previously, ammonium sulphate has also demonstrated for precipitating amidases of Cupriavidus oxalaticus ICTDB921 and Paracoccus sp. SKG [28, 29].
After dialysis, the ammonium sulphate precipitated protein was loaded on to the DEAE Sepharose column for anion exchange chromatography. The eluted fractions (number 35–39) showed very high specific activity with maximum (1553 U/mg of protein) being in the 37th fraction. These fractions gave single band in SDS–polyacrylamide gel electrophoresis which represented the protein of interest which later were pooled and concentrated by lyophilization (Flexi-Dry MP™, FTS, USA) for further characterization of the purified enzyme. Native polyacrylamide gel electrophoresis was also performed along with zymogram analysis (Fig. S1). Purification summary is shown in Table 1. The purification strategies employed resulted in the complete purification of the enzyme in two steps. A similar purification approach for amidase from Paracoccus sp. SKG has also adapted in two steps [29].
Table 1.
Acyltransferase purification of Bacillus sp. APB-6
| Purification stagea | Volume (ml) | Protein (mg/ml) | Total Protein (mg) | Specific activity (U/mg) | Total activity (U) | % Yield | Fold purification |
|---|---|---|---|---|---|---|---|
| CFE | 30 | 2.4 | 72 | 401 | 28,872 | 100 | 1 |
| ASF | 7 | 3.6 | 25.2 | 702 | 17,711 | 61.3 | 1.75 |
| AEC | 12.5 | 0.83 | 10.4 | 1478 | 15,371 | 53.2 | 3.68 |
aCFE, Cell free extract; ASF, Ammonium sulphate fractionation; AEC, Anion exchange chromatography (DEAE)
Characterization of Purified Acyltransferase
Determination of Molecular Mass
In SDS–PAGE the enzyme moved as one band of 42 kDa (Fig. 1), comparing it with the band obtained in native PAGE, it appears that the protein could be a homotrimer. Amidases have molecular masses that range from 38 kDa as in case of Stenotrophomonas maltophila [30] to 480 kDa as observed in case of R. erythropolis MP 50 [31]. Majority of the amidases are dimers and their sub-units varied from 1 to 8 [32]. Amidases are usually homomultimers but amidase (Providencia rettgeri) exhibited heterodimer with α-subunit (23 kDa) and β-subunit of 65 kDa (92 kDa) [33].
Fig. 1.
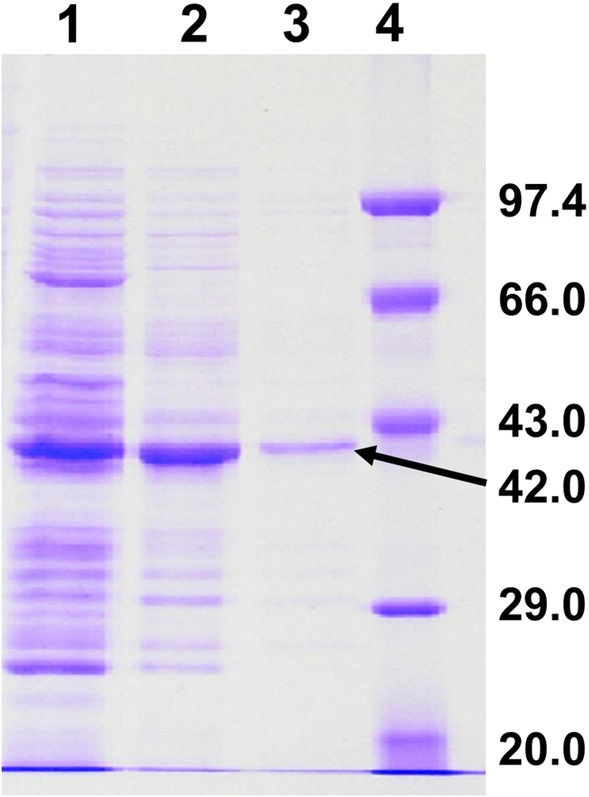
SDS–Polyacrylamide Gel Electrophoresis of the purified acyltransferase of Bacillus sp. APB-6 on 12% gel. Lane 1: Cell free extract; Lane 2: ammonium sulphate fraction; Lane 3: pooled fraction of DEAE and Lane 4: molecular weight standards—phosphorylase B (97.4 kDa), bovine serum albumin (66.0 kDa) ovalbumin (43.0 kDa), carbonic anhydrase (29.0 kDa) and soyabean trypsin inhibitor (20.0 kDa)
pH and Buffer Concentration
The purified acyltransferase exhibited broad pH (4.0–10.5) activity with maximum (1362 U/mg of protein) at pH 7.5 (Fig. 2a). The influence of buffer molarity on enzyme activity is shown in Fig. 2b. The maximum enzyme activity of 1393 U/mg of protein recorded in the 150 mM of buffer (potassium-phosphate, pH 7.5). Overall, purified acyltransferase retained high residual activities of 1250 and 840 U/mg of protein at pH 10.5 and buffer concentration of 400 mM, respectively (Fig. 2). Pseudonocardia thermophila amidase was reported active over at broad pH range (4.0 to 9.0) with an optimum pH of 7.0 [34]. Similarly, acyltransferase of Bacillus sp. APB-6 is active at a much broader pH range. Also, production enhancement of amidotransferase (Bacillus sp. ABP-6) was recorded with optimum pH of 8.0 by using statistical experimental design [35].
Fig. 2.
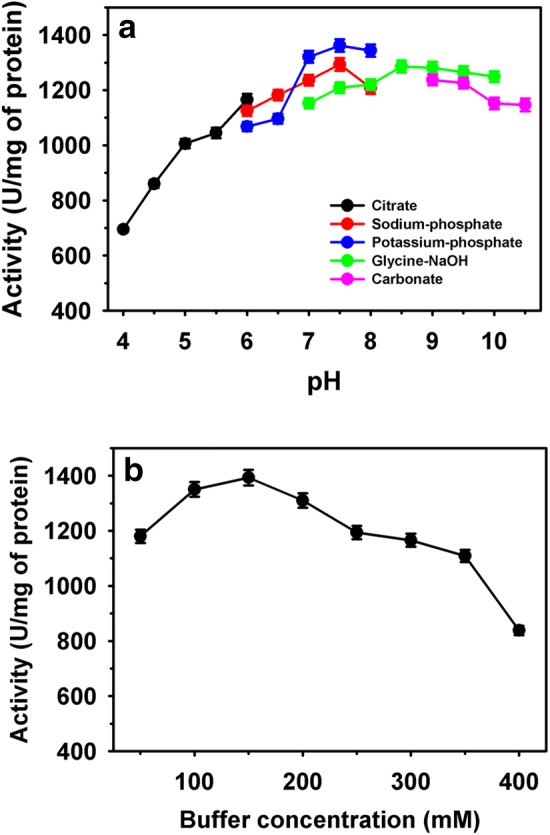
Activity of purified acyltransferase at different: pH (a) and buffer concentrations (b)
Temperature and Thermal Stability
A gradual increase in activity of enzyme was noted from 35 to 50 °C. Thereafter, activity declined significantly at higher incubation temperatures. The optimum temperature was observed 50 °C for purified enzyme activity (Fig. 3a). The thermal stability study revealed the stability of enzyme for 3 h at 45 °C with residual activity of 76%. Whereas, activity decreased to 45% at incubation of 50 °C. At 55 and 60 °C of incubation temperatures, the acyltransferase activity declined at a faster rate with a t1/2 less than 1 h and 30 min, respectively (Fig. 3b). Schar et al. [36] studied the effect of a wide range of temperature (20–80 °C) on the stability of N,N-dimethyl formamidase from Pseudomonas DMF 3/3 and reported rapid inactivation of activity above 40 °C. Whereas, no activity was observed at 60 °C. In contrast, Pseudonocardia thermophile showed amidase activity at 60 °C [24]. Thermostability and reusability in the biotransformation processes can be explored in future by immobilizing the biocatalyst, including purified enzymes through different approaches [37–42]. Also, the properties of enzyme can be improved through protein engineering approaches [27, 43, 44].
Fig. 3.
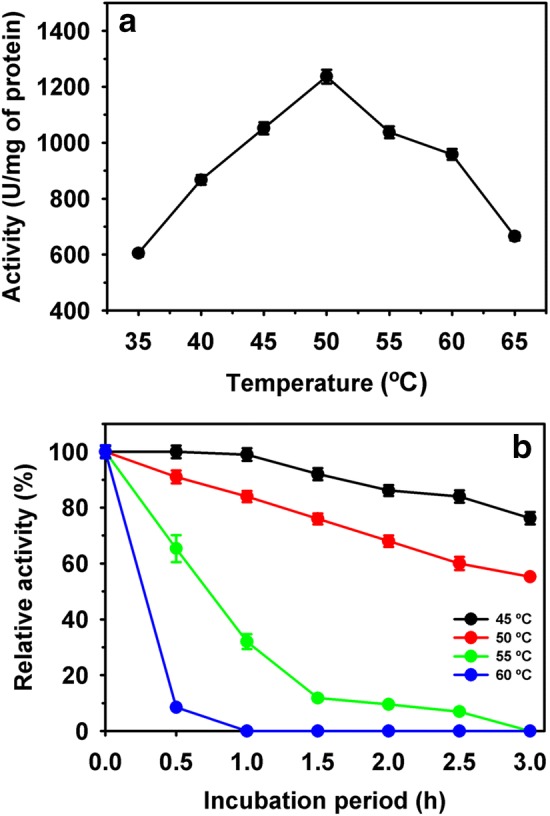
Purified acyltransferase activity at various temperatures (a) and thermostability (b)
Metal Ions and Inhibitors
The residual activity of enzyme was significantly varied in the presence of metal ions and inhibitors (Fig. 4). Metal ions, CuSO4·5H2O substantially, and AgNO3 and HgCl2 strongly inhibited the acyltransferase enzyme activity as compared with control. Whereas, DDT and EDTA, showed an enhancement of 42 and 36%, respectively. Since, the disulphide reductant DTT significantly increased the enzyme activity and free thiol blocking reagents HgCl2 and AgNO3 completely inhibited the enzyme activity, it indicates that free thiols are essential for enzyme activity. This study showed the availability of sulphydryl groups (cysteine residues), at catalytic site as similar to amidases (aliphatic), which are evolutionarily associated with nitrilases [1, 4]. Amidase of R. rhodochrous M8 showed complete inhibition of enzyme activity by Fe2+ and heavy metal ions [45]. They also observed the ~ 1.5-fold enhancement of amidase activity in DTT. Whereas o-phenanthroline, EDTA, and serine protease inhibitor (PMSF) did not affect activity.
Fig. 4.
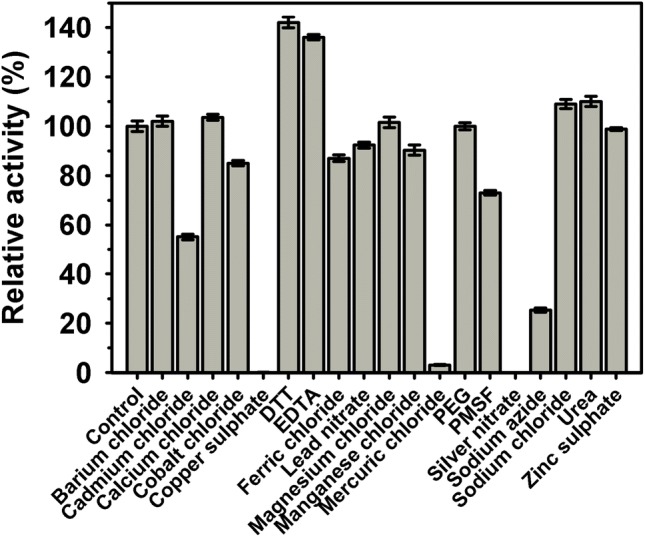
Influence of metal ions and inhibitors on purified acyltransferase activity
Organic Solvents
Acyltransferase activity assay was also performed in water restricted system (Fig. 5). Among the organic solvents [10% (v/v)], the relative activity in carbon tetrachloride, toluene, benzene, and ethylene glycol was 99, 98, 91 and 88%, respectively as compared to control. The high tolerance of this amidase towards carbon-tetrachloride and toluene may prove beneficial for transformation of amides (hydrophobic) to corresponding hydroxamic acids. Solvents like acetone completely inhibited the acyltransferase whereas butanol reported 6.7% relative activity. Doukyu and Ogino [46] have demonstrated the tremendous potential of solvent tolerating enzymes for industrials application. In future, we intend to use this enzyme (in presence of solvents) for the biotransformation of aromatic and hydrophobic amides to corresponding hydroxamic acids, which are commodity chemicals having immense medical applications.
Fig. 5.
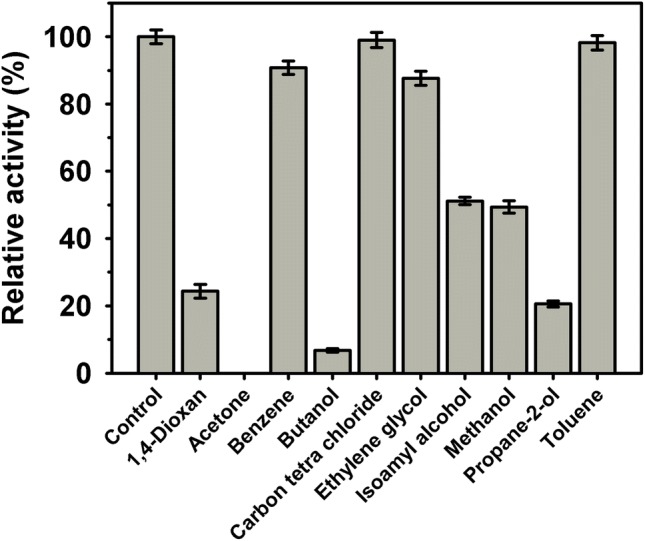
Purified acyltransferase activity in various organic solvents
Kinetic Parameters
Km and Vmax of acyltransferase was determined, by varying the concentration of acetamide (A) at a different fixed concentrations of hydroxylamine-HCl (B). A double reciprocal plot of 1/v versus 1/[acetamide] was plotted and the ‘apparent’ values of KmA and Vmax were obtained (Fig S2a). Further, intercept values (1/Vmax) versus 1/[hydroxylamine HCl] provided the true values of KmB and Vmax as 153 mM and 1667 U/mg of protein, respectively (Fig. S2b). Finally, from slope values (Kappm/Vappmax) against 1/[B], the true values of KmA (73.0 mM) and KiA (37.0 mM) were obtained (Fig. S2c). The Km values obtained for the substrates indicated the higher enzymatic affinity towards acetamide than hydroxylamine-HCl and for successful acyltransfer reaction, the concentration of hydroxylamine-HCl should be almost twice as that of acetamide in the reaction mixture. Rhodococcus sp. R312 amidase (acyltransferase activity) reported a Km of 70 mM for acetamide [9]. Our enzyme shows similar Km and is also solvent tolerant, so it could prove an efficient biocatalyst to synthesize different pharmaceutically vital hydroxamic acids.
Conclusion
This study demonstrated the acyltransferase is active at a broad pH range. DTT enhances activity indicating the availability of free sulphydryl groups (cysteine residue) on catalytic site. It seems the enzyme belongs to class of aliphatic amidases related to nitrilases. Further, the enzyme is tolerant to many organic solvents (10%, v/v) and can be used for the hydroxamic acids synthesis from hydrophobic amides. As per our literature search and knowledge, this is the new report demonstrating the acyltransfer reaction in presence of organic solvents. In future, we would like to immobilize this enzyme for biotransformation applications in organic solvents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Department of Biotechnology, Himachal Pradesh University, Shimla is duly acknowledged for extending their research laboratory facility.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deepak Pandey, Email: deepak4jul@gmail.com.
Duni Chand, Email: profdunichand@gmail.com.
References
- 1.Chhiba-Gonindjee VP, van der Westhuyzen CW, Bode ML, Brady D. Bacterial nitrilases and their regulation. Appl Microbiol Biotechnol. 2019;103:4679–4692. doi: 10.1007/s00253-019-09776-1. [DOI] [PubMed] [Google Scholar]
- 2.Novo C, Farnaud S, Tata R, Clemente A, Brown PR. Support for a three-dimensional structure predicting a Cys-Glu-Lys catalytic triad for Pseudomonas aeruginosa amidase comes from site-directed mutagenesis and mutations altering substrate specificity. Biochem J. 2002;365:731–738. doi: 10.1042/BJ20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma M, Sharma NN, Bhalla TC. Amidases: versatile enzymes in nature. Rev Environ Sci Biotechnol. 2009;8:343. doi: 10.1007/s11157-009-9175-x. [DOI] [Google Scholar]
- 4.Yun H, Liang B, Qiu J, Zhang L, Zhao Y, Jiang J, Wang A. Functional characterization of a novel amidase involved in biotransformation of triclocarban and its dehalogenated congeners in Ochrobactrum sp. TCC-2. Environ Sci Technol. 2017;51:291–300. doi: 10.1021/acs.est.6b04885. [DOI] [PubMed] [Google Scholar]
- 5.Gupta SP. Studies on hydroxamic acids: a fascinating family of chemicals with a wide spectrum of activities. Chem Rev. 2015;115:6427–6490. doi: 10.1021/cr500483r. [DOI] [PubMed] [Google Scholar]
- 6.Chand D, Kumar H, Dutt US, Kumar D, Vitzthum F, Bhalla TC. Treatment of simulated wastewater containing toxic amides by immobilized Rhodococcusrhodochrous NHB-2 using a highly 5-stage plug flow reactor. World J Microbiol Biotechnol. 2004;20:679–686. doi: 10.1007/s11274-004-2158-8. [DOI] [Google Scholar]
- 7.Supreetha K, Rao SN, Srividya D, Anil HS, Kiran S. Advances in cloning, structural and bioremediation aspects of nitrile hydratases. Mol Biol Rep. 2019;46:4661. doi: 10.1007/s11033-019-04811-w. [DOI] [PubMed] [Google Scholar]
- 8.Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:174. doi: 10.1007/s13205-016-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournand D, Bigey F, Arnaud A, Fournand D, Bigey F, Arnaud A. Acyl transfer activity of an amidase from Rhodococcus sp. R312: formation of a wide range of hydroxamic acids. Appl Environ Microbiol. 1998;64:2844–2852. doi: 10.1128/aem.64.8.2844-2852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandey D, Singh R, Chand D. An improved bioprocess for synthesis of acetohydroxamic acid using DTT (dithiothreitol) treated resting cells of Bacillus sp. APB-6. Bioresour Technol. 2011;102:6579–6586. doi: 10.1016/j.biortech.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 11.De Vreese R, D’hooghe M. Synthesis and applications of benzohydroxamic acid-based histone deacetylase inhibitors. Eur J Med Chem. 2017;135:174–195. doi: 10.1016/j.ejmech.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Klibanov AM. Improving enzymes by using them in organic solvents. Nature. 2001;409:241–246. doi: 10.1038/35051719. [DOI] [PubMed] [Google Scholar]
- 13.Stepankova V, Bidmanova S, Koudelakova T, Prokop Z, Chaloupkova R, Damborsky J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013;3:2823–2836. doi: 10.1021/cs400684x. [DOI] [Google Scholar]
- 14.Anwar MZ, Kim DJ, Kumar A, Patel SKS, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK. SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep. 2017;7:15333. doi: 10.1038/s41598-017-15550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Kim I-W, Patel SKS, Lee J-K. Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol. 2018;58:100–104. doi: 10.1007/s12088-017-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel SKS, Choi SH, Kang YC, Lee J-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale. 2016;8:6728–6738. doi: 10.1039/C6NR00346J. [DOI] [PubMed] [Google Scholar]
- 17.Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho B-K, Kalia VC, Kang YC, Lee J-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater. 2018;347:442–450. doi: 10.1016/j.jhazmat.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Park GD, Patel SKS, Kondaveeti S, Otari S, Anwar MZ, Kalia VC, Singh Y, Kim SC, Cho B-K, Sohn J-H, Kim D-R, Kang YC, Lee J-K. SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J. 2019;359:1252–1264. doi: 10.1016/j.cej.2018.11.052. [DOI] [Google Scholar]
- 19.Otari SV, Patel SKS, Kim S-Y, Haw JR, Kalia VC, Kim I-W, Lee J-K. Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol. 2019;59:105–108. doi: 10.1007/s12088-018-0768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit HJ, Sharma R, Patel SKS, Kalia VC. Hydrogen and polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol. 2008;99:5444–5451. doi: 10.1016/j.biortech.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Kumar P, Patel SKS, Lee JK, Kalia VC. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol Adv. 2013;31:1543–1561. doi: 10.1016/j.biotechadv.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Patel SKS, Lee J-K, Kalia VC. Dark-fermentative biological hydrogen production from mixed biowastes using defined mixed cultures. Indian J Microbiol. 2017;57:171–176. doi: 10.1007/s12088-017-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalia VC, Patel SKS, Kang YC, Lee J-K. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotech Adv. 2019;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Patel SKS, Kim J-H, Kalia VC, Lee J-K. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-00764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SKS, Ray S, Prakash J, Wee JH, Kim S-Y, Lee J-K, Kalia VC. Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol. 2019;59:154–160. doi: 10.1007/s12088-018-00777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel SKS, Choi H, Lee J-K. Multimetal-based inorganic–protein hybrid system for enzyme immobilization. ACS Sustainable Chem Eng. 2019;7:13633–13638. doi: 10.1021/acssuschemeng.9b02583. [DOI] [Google Scholar]
- 27.Gao H, Li J, Sivakumar D, Kim T-S, Patel SKS, Kalia VC, Kim I-W, Zhang Y-W, Lee J-K. NADH oxidase from Lactobacillus reuteri: a versatile enzyme for oxidized cofactor regeneration. Int J Biol Macromol. 2019;123:629–636. doi: 10.1016/j.ijbiomac.2018.11.096. [DOI] [PubMed] [Google Scholar]
- 28.Bedade DK, Dev MJ, Singhal RS. Bioreactor studies on acrylamidase produced from Cupriavidus oxalaticus ICTDB921: Production, kinetic modeling, and purification. Biochem Eng J. 2019;149:107245. doi: 10.1016/j.bej.2019.107245. [DOI] [Google Scholar]
- 29.Santoshkumar M, Ismailsab M, Nayak AS, Mashetty SB, Karegoudar TB. Purification and characterization of amidase from Paracoccus sp. SKG: Utilization of amidase-inhibited whole cells for bioconversion of acrylonitrile to acrylamide. Biocatal Agric Biotechnol. 2017;10:256–263. doi: 10.1016/j.bcab.2017.04.001. [DOI] [Google Scholar]
- 30.Stelkes-Ritter U, Wyzgol K, Kula MR. Purification and characterization of a newly screened microbial peptide amidase. Appl Microbiol Biotechnol. 1995;44:393–398. doi: 10.1007/bf00169934. [DOI] [PubMed] [Google Scholar]
- 31.Hirrlinger B, Stolz A, Knackmuss HJ. Purification and properties of an amidase from Rhodococcus erythropolis MP 50 which enantio-selectively hydrolyzes 2-arylpropionamides. J Bacteriol. 1996;178:3501–3507. doi: 10.1128/jb.178.12.3501-3507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi M, Komeda H, Nagasawa T, Yamada H, Shimizu S. Occurrence of amidases in the industrial microbe Rhodococcus rhodochrous J1. Biosc Biotechnol Biochem. 1993;57:1949–1950. doi: 10.1271/bbb.57.1949. [DOI] [Google Scholar]
- 33.Sevo M, Degrassi G, Skoko N, Venturi V, Ljubijankic G. Production of glycosylated thermostable Providencia rettgeri penicillin G amidase in Pichia pastoris. FEMS Yeast Res. 2002;1:271–277. doi: 10.1111/j.1567-1364.2002.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 34.Egorova K, Trauthwein H, Verseck S, Antranikian G. Purification and properties of an enantioselective and thermos-active amidase from the thermophilic actinomycetes Pseudonocardia thermophila. Appl Microbiol Biotechnol. 2004;65:38–45. doi: 10.1007/s00253-004-1607-5. [DOI] [PubMed] [Google Scholar]
- 35.Chand D, Kumari P, Devi N. Enhanced production of amidotransferase from Bacillus Sp. ABP-6 by optimization of nutritional parameters using statistical experimental design. Int J Eng Sci Inv. 2017;6:12–22. [Google Scholar]
- 36.Schar HP, Holzmann W, Ramos Tombo GM, Ghisalba O. Purification and characterization of N, N-dimethyl formamidase from Pseudomonas DMF 3/3. Eur J Biochem. 1986;158:469–475. doi: 10.1111/j.1432-1033.1986.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim T-S, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee J-K. A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep. 2016;6:33438. doi: 10.1038/srep33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel SKS, Choi SH, Kang YC, Lee J-K. Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Inter. 2017;9:2213–2222. doi: 10.1021/acsami.6b05165. [DOI] [PubMed] [Google Scholar]
- 39.Patel SKS, Anwar MZ, Kumar A, Otari SV, Pagolu RT, Kim S-Y, Kim I-W, Lee J-K. Fe2O3 yolk-shel particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem Eng J. 2018;132:1–8. doi: 10.1016/j.bej.2017.12.013. [DOI] [Google Scholar]
- 40.Prakash J, Sharma R, Patel SKS, Kim IW, Kalia VC. Bio-hydrogen production by co-digestion of domestic wastewater and biodiesel industry effluent. PLoS ONE. 2018;13:e0199059. doi: 10.1371/journal.pone.0199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel SKS, Gupta RK, Kumar V, Mardina P, Lestari R, Kalia VC, Choi M-S, Lee J-K. Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol. 2019;59:370–374. doi: 10.1007/s12088-019-0796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel SKS, Jeon MS, Gupta RK, Jeon Y, Kalia VC, Kim SC, Cho B-K, Kim DR, Lee J-K. Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces. 2019;11:18968–18977. doi: 10.1021/acsami.9b03420. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran P, Jagtap SS, Patel SKS, Li J, Kang YC, Lee J-K. Role of the non-conserved amino acid asparagine 285 in the glycone-binding pocket of Neosartorya fischeri β-glucosidase. RSC Adv. 2016;6:48137–48144. doi: 10.1039/c5ra28017f. [DOI] [Google Scholar]
- 44.Selvaraj C, Krishnasamy G, Jagtap SS, Patel SKS, Dhiman SS, Kim T-S, Singh SK, Lee J-K. Structural insights into the binding mode of d-sorbitol with sorbitol dehydrogenase using QM-polarized ligand docking and molecular dynamics simulations. Biochem Eng J. 2016;114:244–256. doi: 10.1016/j.bej.2016.07.008. [DOI] [Google Scholar]
- 45.Kotlova EK, Chestukhina GG, Astaurova OB, Leonova TE, Yanenko AS, Debabov VG. Isolation and primary characterization of an amidase from Rhodococcus rhodochrous. Biochemistry (Mosc) 1999;64:384–389. [PubMed] [Google Scholar]
- 46.Doukyu N, Ogino H. Organic solvent-tolerant enzymes. Biochem Eng J. 2010;48:270–282. doi: 10.1016/j.bej.2009.09.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


