Abstract
Purpose:
To evaluate the effects of device-guided slow-paced respiration on urgency-associated urinary symptoms, perceived stress and anxiety, and autonomic function in women with overactive bladder syndrome.
Materials and Methods:
We conducted a randomized, parallel-group trial of slow-paced respiration to improve perceived stress and autonomic dysfunction as potential contributors to overactive bladder. Ambulatory women reporting at least 3 voiding or incontinence episodes per day associated with moderate-to-severe urgency were randomized to use a portable biofeedback device to practice daily slow-guided breathing exercises or use an identical-appearing control device reprogrammed to play music without guiding breathing. Over 12 weeks, changes in urinary symptoms were evaluated by voiding diaries, perceived stress and anxiety were assessed by validated questionnaires, and autonomic function was examined using heart rate variability and impedance cardiography.
Results:
Among the 161 participants randomized (82 to paced respiration, 79 to control), the average baseline frequency of voiding or incontinence associated with moderate-to-severe urgency was 6.9 (±3.4) episodes/day. Compared to controls, participants randomized to paced respiration demonstrated greater improvements in perceived stress (average decrease in Perceived Stress Scale score of 2.8 versus 1.1, P=.03), but not autonomic function markers. Over 12 weeks, the average frequency of voiding or incontinence associated with moderate-to-severe urgency (primary outcome) decreased by 0.9 (±3.2) episodes/day, but no significant between-group differences were detected.
Conclusions:
Among women with overactive bladder, slow-paced respiration was associated with modest improvements in perceived stress over 12 weeks, but was not superior to a music-listening control in reducing urinary symptoms or changing autonomic function.
MeSH keywords: Urinary bladder, overactive; Urinary incontinence, urge; Breathing exercises/methods; Relaxation therapy/methods; Stress, psychological; Anxiety; Autonomic nervous system
Introduction
Overactive bladder (OAB), a syndrome characterized by recurrent, strong urges to urinate (i.e., urgency), increased frequency of daytime and nighttime urination, and in some cases urgency incontinence, affects up to one in five women and can have a major impact on functioning and quality of life.1,2 Currently, the most widely used treatments for OAB are anti-muscarinic mediations, which are modestly effective in decreasing urgency-associated voiding but associated with side effects3 and high rates of discontinuation.4 While behavioral management techniques such as bladder re-training are better tolerated, many patients have difficulty practicing them effectively without intensive training from specialized practitioners.5 As a result, alternate treatment strategies are needed that are safer and more accessible.
Epidemiologic studies have documented strong associations between perceived stress, anxiety, and urinary urgency in women with OAB, including an increased risk of new-onset OAB among those with high baseline levels of stress or anxiety.6,7 Clinical studies have also indicated that patients with OAB tend to have abnormalities in peripheral autonomic function that are in turn associated with clinical anxiety disorders.8–10 These findings have generated interest in identifying alternate therapeutic approaches directed at improving perceived stress, anxiety, and associated autonomic dysfunction as potential contributors to OAB.11,12
One such potential therapy is slow-paced respiration, a behavioral technique involving slowing the respiratory rate below 10 breaths per minute with the goal of improving perceived stress, anxiety, and autonomic balance. Slow-breathing exercises are already used to a limited extent in “urge suppression” techniques for OAB, in that patients are told to take slow, deep breaths upon experiencing an urge to urinate to distract themselves from their bladder sensation. However, prior research has not examined whether slow breathing may be useful not just as an ad hoc response to isolated episodes of urgency, but a regular program of breathing exercises to alter underlying susceptibility to OAB.
Materials and Methods
The Controlling Urgency through Relaxation Exercises (CURE) study is a randomized, parallel-group trial of device-guided slow-paced respiration in women with OAB. Participants were ambulatory women recruited from 2014–2017 from two study clinics in San Francisco and Oakland, California affiliated with the University of California San Francisco (UCSF). Women were eligible if they documented an average of at least 3 voiding or incontinence episodes/day associated with at least a moderate sensation of urgency in a voiding diary13 and agreed to temporarily forgo other clinical OAB treatments.
Women were excluded if they reported a history of pelvic cancer or irradiation, prior bladder surgery or other pelvic surgery in the past 3 months, interstitial cystitis, bladder or rectal fistulas, congenital urinary tract defect, symptomatic pelvic organ prolapse, recurrent urinary tract infections (≥3/year), or major neurologic conditions such as stroke or multiple sclerosis. Additionally, women could not have evidence of hematuria or infection on a screening urinalysis test, be pregnant or planning pregnancy, or have a chronic pulmonary condition that would interfere with slow-breathing exercises. Women taking anxiolytics or antidepressants were required to be on steady doses for a month. No exclusions were made for use of beta or alpha blocker or agonist medications. Although women engaged in practitioner-administered behavioral relaxation therapies were excluded, participants were not precluded from using other informal self-administered relaxation practices. All participants provided written informed consent, and the study was approved by UCSF’s institutional review board (#14–13319) and registered on clinicaltrials.gov (#NCT02202031).
Participants were randomly assigned in equal ratios to the paced respiration or control intervention by computer algorithm using randomly permuted block sizes of 2, 4, and 6. Women assigned to paced respiration were instructed to use a commercially available, portable biofeedback device, RESPeRATE (Intercure, Ltd), currently approved by the Food and Drug Administration for adjunctive treatment of hypertension based on evidence that regular use decreases high blood pressure attributable to excess sympathetic tone.14–16 The device senses the user’s respiratory rate using an elastic belt placed around the chest and plays musical tones synchronized to inspiration and expiration. Gradually, the device gradually increases the interval between its musical tones to guide the user in slowing respiration and expiration. Consistent with use for hypertension, CURE participants randomized to paced respiration were instructed to practice slowing their respiration for a minimum of 15 minutes/day for 12 weeks.
To enable rigorous evaluation of efficacy, participants randomized to the control group were given an identical-appearing RESPeRATE device re-programmed to play quiet non-rhythmic music while monitoring spontaneous breathing, and were also instructed to use it for a minimum of 15 minutes/day for 12 weeks. Both participants and study staff responsible for monitoring adherence were aware of intervention assignment. Adherence to device use was recorded automatically by devices in both groups. All participants also received basic written information about behavioral self-management strategies for OAB (timed urination, urge suppression), consistent with usual first-line care.
Overactive bladder symptoms were assessed at baseline and 12 weeks using a validated 3-day voiding diary administered in previous OAB trials.13 Participants recorded each time they experienced an urge to urinate, voided in the toilet, or leaked urine, and rated the severity of urgency associated with each episode using a standardized scale (none, mild, moderate, severe).17 Diary data were abstracted by research staff blinded to intervention assignment. An OAB composite score was also calculated that assigned points to voiding and incontinence based on associated urgency.17
Participants also completed structured-item validated questionnaires of the severity or impact of urinary symptoms at baseline and 12 weeks, including the Overactive Bladder Questionnaire (OAB-Q),18 Urgency Severity and Impact Questionnaire (USIQ),19 and Patient Perception of Bladder Condition (PPBC).20 Additional questionnaires assessed perceived stress and associated anxiety symptoms at baseline at 12 weeks, including the Cohen Perceived Stress Scale (PSS) to assess subjective feelings and thoughts related to perceived stress,21 Spielberger State Trait Anxiety Inventory (STAI)-trait component to assess somatic anxiety,22 and anxiety subscale of the Hospital Anxiety and Depression Scale (HADS) to assess cognitive anxiety23 (score range and directionality provided in Table 1 footnotes).
Table 1:
Baseline Demographic and Clinical Characteristics of Participants, by Intervention Assignment
| Paced Respiration (N=79) |
Music Control (N=82) |
P-Valuea | |
|---|---|---|---|
| Age in years | 60.4 (±11.4) | 61.7 (±10.9) | .42 |
| Race/ethnicity | .53 | ||
| Non-Latina white | 40 (50.6%) | 49 (59.8%) | |
| Latina white | 12 (15.2%) | 6 (7.3%) | |
| Asian/Asian-American | 8 (10.1%) | 6 (7.3%) | |
| African-American | 12 (15.2%) | 12 (14.6%) | |
| Mixed race | 5 (6.3%) | 8 (9.8%) | |
| Unknown | 2 (2.5%) | 1 (1.2%) | |
| Self-Reported General Health | .29 | ||
| Excellent | 19 (24.1%) | 28 (34.1 %) | |
| Very good | 36 (45.6%) | 31 (37.8 %) | |
| Good | 22 (27.8%) | 18 (22.0%) | |
| Fair/poor | 2 (2.5%) | 5 (6.1 %) | |
| Selected Medications | |||
| Diuretic (thiazide or other non-loop) | 8 (16.7%) | 5 (8.3%) | .19 |
| Sedative/hypnotics | 1 (2.1%) | 2 (3.3%) | .69 |
| Tricyclic antidepressants | 4 (8.3%) | 3 (5.0%) | .48 |
| Selective serotonin/norepinephrine reuptake inhibitors | 9 (18.8%) | 11 (18.3%) | .96 |
| Other antidepressants | 2 (4.2% ) | 1 (1.7%) | .43 |
| Beta blockers | 5 (6.3%) | 13 (15.9%) | .06 |
| Beta agonists | 0 (0.0%) | 1 (1.2%) | .33 |
| Alpha blockers | 2 (2.5%) | 1 (1.2%) | .54 |
| Sympathomimetics | 2 (2.5%) | 1 (1.2%) | .54 |
| Parity | .30 | ||
| 0 | 19 (24.1%) | 19 (23.2%) | |
| 1–2 | 35 (44.3%) | 28 (34.1%) | |
| 3 or more | 25 (31.6%) | 35 (42.7%) | |
| Gynecologic History | |||
| Postmenopausal | 62 (78.5%) | 68 (82.9%) | .47 |
| Oophorectomy | 13 (16.5%) | 15 (18.3%) | .76 |
| Hysterectomy | 14 (17.7%) | 11 (13.4 %) | .45 |
| Health-Related Habits | |||
| Current cigarette smoking | 0 (0.0%) | 3 (3.7%) | .08 |
| Weekly alcohol consumption | 34 (43.0%) | 44 (53.7%) | .18 |
| Physical Exam Measures | |||
| Body mass index (kg/m2) | 27.8 (±5.8) | 28.4 (±7.7) | .97 |
| Systolic blood pressure (mmHg) | 122.1 (±17.4) | 119.8 (±15.3 ) | .42 |
| Diastolic blood pressure (mmHg) | 75.4 (±10.3) | 74.4 (± 9.1) | .49 |
| Urinary Symptom Frequency | |||
| Incontinence or voiding episodes per day with at least moderate urgency Median (IQR) |
6.7 (4.7, 9.3) | 6.0 (4.0, 8.0) | .16 |
| Incontinence or voiding episodes per day with at least severe urgency Median (IQR) |
2.3 (1.3, 4.0) | 2.0 (0.7, 3.0) | .09 |
| Urgency-type incontinence episodes per day Median (IQR) |
0.7 (0.0, 2.0) | 0.7 (0.0, 1.7) | .89 |
| Daytime voiding episodes per day (regardless of urgency) Median (IQR) |
9.3 (8.0, 11.7) | 8.7 (7.3, 11.0) | .29 |
| Nighttime voiding episodes per night (regardless of urgency) Median (IQR) |
1.0 (0.3, 2.0) | 1.3 (0.7, 2.0) | .23 |
| Overactive Bladder Symptom Composite scoreb Median (IQR) |
28.0 (21.3, 34.7) | 24.3 (20.0, 31.0) | .16 |
| Urinary Symptom Questionnaire Scores | |||
| Overactive Bladder Questionnaire (OAB-Q)c Median (IQR) |
29.7 (21.2, 41.2) | 30.0 (20.6, 38.8) | .83 |
| Urgency Severity and Impact Questionnaire (USIQ)—Severity Subscaled Mean (±SD) |
58.5 (±14.5) | 58.5 (+11.7) | .79 |
| Urgency Severity and Impact Questionnaire (USIQ)—Quality of Life Subscaled Mean (±SD) |
24.6 (±19.4) | 21.9 (+15.4) | .69 |
| Urogenital Distress Inventory Short Form (UDI-6)e Median (IQR) |
44.4 (33.3, 61.1) | 44.4 (33.3, 55.6) | .84 |
| Patient Perception of Bladder Condition (PPBC)f Median (IQR) |
3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | .87 |
| Stress and Anxiety Questionnaire Scores | |||
| Perceived Stress Scale (PSS) Median (IQR)g |
14.0 (8.0, 20.0) | 13.0 (9.0, 18.0) | .41 |
| Spielberger State Trait Anxiety Inventory (STAI) --Trait Component Median (IQR)h |
37.0 (31.0, 45.0) | 35.5 (28.0, 44.0) | .33 |
| Hospital Anxiety and Depression Scale (HADS) -- Anxiety Subscale Median (IQR)i |
7.0 (4.0, 10.0) | 6.0 (3.0, 9.0) | .37 |
| Autonomic Function Parametersj | |||
| Pre-ejection period, msec Mean (±SD) |
117.1 (14.8) | 123.1 (11.5) | .16 |
| Minimum and maximum | 78.0, 144.6 | 102.4, 149.6 | |
| Respiratory sinus arrhythmia, msec2 Mean (±SD) |
5.7 (1.6) | 4.8 ( 1.2) | .052 |
| Minimum and maximum | 2.3, 9.4 | 1.7, 6.4 |
Data are presented as number (percentage), mean (±standard deviation) for normally distributed variables, or median (interquartile range) for non-normally distributed variables.
P-values were calculated using chi-square or Kruskal–Wallis tests, as appropriate.
Calculated by assigning points for mild urgency-associated voiding (1 point), moderate urgency-associated voiding (2 points), severe urgency associated voiding (3 points), and urgency incontinence (5 points each) per day, then taking the average score across all diary days. Voiding episodes that occur without any urgency are assigned 0 points.
The OAB-Q is a 33-item measure of the bothersomness and impact of multiple OAB symptoms along a 100-point scale, with higher scores indicating greater bother and impact.
The USIQ is a 13-item measure of the severity and impact of urinary urgency; scores range from 0 to 100, with higher scores indicating greater severity and impact.
The UDI-6 is a 6-item measure of the bothersomeness of multiple types of urogenital symptoms; scores range from 0 to 75, with higher scores indicating greater bother.
The PPBC is a single-item measure assessing patient’s overall perception of their bladder problems using a 6-point Likert scale, with higher scores indicating greater problems.
The PSS is a 10-item self-administered questionnaire assessing subjective feelings and thoughts related to perceived stress; scores range from 0 to 40, with higher scores indicating greater perceived stress.
The STAI is a 20-item self-administered measure of anxiety, including a trait component designed to measure the affective component of anxiety believed to be related to autonomic physiological arousal response; scores range from 20 to 80, with higher scores indicating greater somatic anxiety.
The 7-item Anxiety Subscale of the HADS measures cognitive anxiety, the mental component of anxiety associated with fear of adverse events; scores range from 0 to 21, with higher scores indicating greater cognitive anxiety.
Autonomic function measures were collected at baseline among the 30 women in the paced respiration and 22 women in the music control group who completed these measures at the San Francisco site. Lower resting PEP corresponds to increased peripheral sympathetic nervous system activity. Higher RSA corresponds to greater peripheral parasympathetic activity
Among participants enrolled in San Francisco, resting cardiac autonomic function was assessed using electrocardiography-derived heart rate variability and impedance cardiography at baseline and 12 weeks. Standardized procedures for these measurements have been described previously.24 Briefly, participants were outfitted with a standard tetrapolar electrode system (two inner electrodes placed at the xiphisternal joint and the base of the neck, outer electrodes place 3 cm distally to the inner electrodes) while sitting quiet, ambient-temperature room. Participants were then asked to view a neutral video for at least 5 minutes while resting measurements were sampled at 1000Hz and stored using a Biopac MP150 data acquisition system (Biopac Systems, Inc.).
For assessment of sympathetic autonomic activity, measurements focused on pre-ejection period (PEP), the time period from the start of cardiac ventricular depolarization to the opening of aortic valve. PEP provides a measure of ventricle contractility that has been shown to provide a relatively pure measure of sympathetic activity, as it occurs during systole when there are no parasympathetic influences on the cardiac cycle. Increases in peripheral sympathetic nervous system activity correspond to shortening PEP.25 For assessment of parasympathetic activity, analyses focused on respiratory sinus arrhythmia (RSA), the variability of the heart rate during the typical respiratory cycle, measured by heart rate variability in the high frequency range, reflecting the amount of influence of the cardiac vagus nerve, with higher RSA corresponding to greater peripheral parasympathetic activity.26 Although there are no validated thresholds for classifying subjects as having abnormal sympathetic or parasympathetic tone based on PEP or RSA, prior studies have reported a mean PEP value of 102 (SE=4.1) and mean RSA value of 6.5 (SE=3.9) in healthy community populations.27,28
Adverse events were assessed by asking participants at follow-up at 1, 6, and 12 weeks if they had experienced any negative changes in health; serious adverse events were defined asdeath, hospitalization, or disability.
The primary outcome was 12-week change in frequency of voiding or incontinence episodes associated with at least moderate urgency. Secondary outcomes included 12-week change in frequency of voiding or incontinence associated with severe urgency, urgency-type incontinence, daytime and nighttime voiding, OAB symptom composite score, and OAB-Q, USIQ, and PPBC scores.
A sample size of 160 (80 per group) was selected to provide 80% power in 2-sided tests with type-I error of 5% to detect between-group difference of more than 20% in the paced respiration versus control group for the primary outcome. This assumed a mean reduction in outcome frequency among controls of 30%, correlation between baseline and follow-up of 0.59, and loss to follow-up of 15%.
Baseline characteristics of participants as well as adherence to interventions at follow-up visits were examined using descriptive statistics. Between-group differences in baseline characteristics were assessed using chi-square for categorical variables and Kruskal–Wallis for continuous variables. Analysis of covariance (ANCOVA) models were developed to estimate least square mean changes in all outcomes within each intervention group over 12 weeks and test for between-group differences, adjusting for baseline values. Change values were 98–99% winsorized if indicated by visual inspection of Q-Q plots. Models examining intervention effects on autonomic outcomes were further adjusted for diabetes mellitus, which was unequally distributed between groups at baseline in the subset of women providing autonomic data, as well as beta or alpha blocker or agonist or sympathomimetic medications.
Only participants who provided 12-week data were included in initial intervention effects models. To examine the implications of missing data, however, 15 multiple-imputed datasets were created using Markov chain Monte Carlo method,29 and summary effect estimates and standard errors were computed using standard methods for imputed data. All analyses were performed using SAS version 9.4.
Results
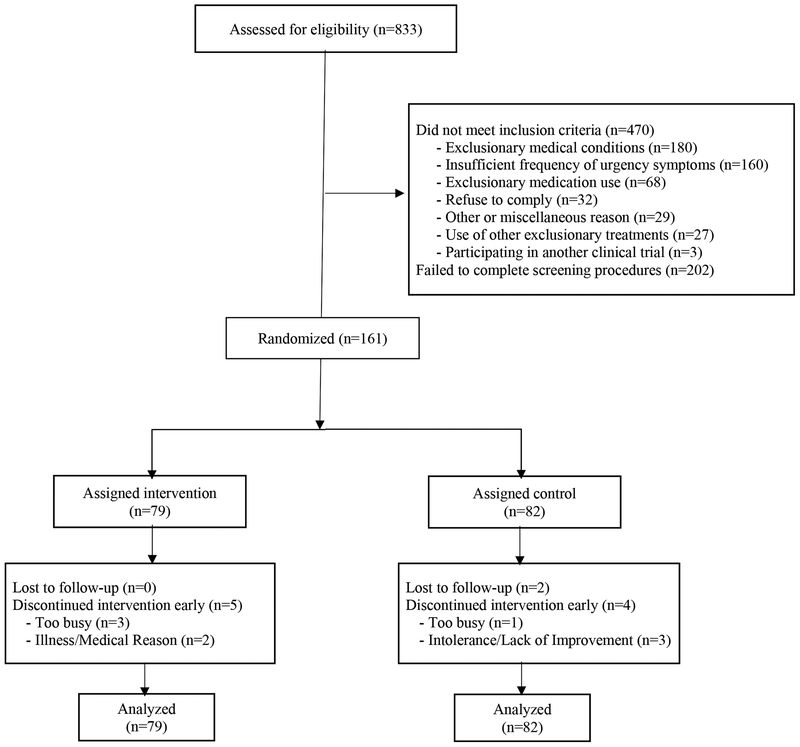
Of the 833 women contacted, 631 completed screening, and 161 were found eligible and randomized (79 to paced respiration, 82 to control) (Figure 1). The most common single reason for ineligibility was insufficient frequency of urgency-associated urinary symptoms. Five assigned to paced respiration (10%) and six to control (7%) discontinued participation early.
Figure 1.
CONSORT diagram of participant recruitment, randomization, and follow-up
At baseline, participants reported an average of 6.9 (+3.4) moderate-to-severe urgency-associated voiding or incontinence episodes, 1.3 (±1.0) nocturnal voiding episodes, and 1.3 (±2.0) urgency incontinence episodes per day (Table 1). Mean PSS questionnaire scores were close to the threshold of 14 used to indicate increased perceived stress, but mean scores on other anxiety measures were below standard thresholds for clinically significant anxiety. No significant between-group differences were detected in outcome measures at baseline.
Among 150 participants completing the 12-week visit, women practiced their assigned intervention for an average of 4.8 days/week in the paced respiration and 5.5 days/week in the control group at 12 weeks (Table 2). Average minutes per day of practice on those days was 16.8 in the paced respiration and 16.4 in the control group.
Table 2.
Adherence to Intervention Practice, by Intervention Assignment and Timepoint
| Paced Respiration |
Music Listening |
P-value |
|
|---|---|---|---|
| Average days of practice per week—mean (±SD) | |||
| Week 1 | 5.6 (±1.6) | 5.7 (±1.4) | .71 |
| Week 6 | 5.1 (±1.6) | 5.5 (±1.3) | .18 |
| Week 12 | 4.8 (±2.1) | 5.5 (±2.1) | .048 |
| Average minutes of practice per day on days of practice — mean (±SD) | |||
| Week 1 | 15.2 (±2.7) | 15.5 (±2.7) | .78 |
| Week 6 | 16.7 (±3.4) | 16.2 (±3.1) | .10 |
| Week 12 | 16.8 (±4.0) | 16.4 (±5.3) | .17 |
Adherence to practice was assessed by review of data downloaded from RESPeRATE devices among participants who were confirmed to have received their assigned intervention. In the paced respiration group, adherence is reported for 77 women at week 1, 74 at week 6, and 74 at week 12. In the music-listening group, adherence is reported for 79 women at week 1, 78 at week 6, and 76 at week 12
Participants in both groups reported modest decreases in frequency of OAB symptoms, including voiding or incontinence episodes associated with at least moderate urgency, over 12 weeks (Table 3). No significant between group differences in change in OAB symptoms were detected, however. Participants in both groups also demonstrated modest improvements in multiple urinary symptom questionnaire scores over 12 weeks, but changes did not differ significantly between groups (Table 3). Findings were not significantly affected by analyses using multiple imputation to account for missing data.
Table 3.
Average Change in Urinary Symptom Frequency and Urinary Symptom Questionnaire Scores Over 12 Weeks, by Intervention Assignment
| Paced Respiration | Music Control | Between-Group Difference | ||||
|---|---|---|---|---|---|---|
| Mean (95% CI)a | P-Value | Mean (95% CI)a | P-Value | Mean (95% CI)a | P-Value | |
| Urinary Symptom Frequency (by Diary) | ||||||
| Incontinence or voiding episodes associated with at least moderate urgency per day | −0.8 (−1.5, −0.1) | .02 | −1.1 (−1.7, −0.4) | <.01 | 0.2 (−0.7,1.2) | .62 |
| Incontinence or voiding episodes associated with severe urgency per day | −0.9 (−1.3, −0.6) | <.01 | −0.9 (−1.2, −0.5) | <.01 | −0.1 (−0.6, 0.5) | .77 |
| Urgency incontinence episodes per day | −0.6 (−0.8, −0.3) | <.01 | −0.6 (−0.9, −0.4) | <.01 | 0.1 (−0.2, 0.4) | .65 |
| Daytime voiding episodes per day (regardless of urgency) | −0.7 (−1.1, −0.3) | <.01 | −0.8 (−1.2, −0.3) | <.01 | 0.1 (−0.6, 0.7) | .82 |
| Nighttime voiding episodes per night (regardless of urgency) | −0.4 (−0.6, −0.2) | <.01 | −0.3 (−0.4, −0.1) | <.01 | −0.1 (−0.4, 0.1) | .28 |
| Total voiding episodes per day (regardless of urgency) | −1.1 (−1.5, −0.6) | <.01 | −1.0 (−1.5, −0.6) | <.01 | −0.0 (−0.7, 0.6) | .92 |
| Overactive bladder symptom composite score – modifiedb | −3.2 (−4.9, −1.4) | <.01 | −3.6 (−5.4, −1.8) | <.01 | 0.4 (−2.1, 2.9) | .75 |
| Urinary Symptom Questionnaire Scores | ||||||
| Overactive Bladder Questionnaire (OAB-Q) overall score | −14.3 (−16.9 – −11.7) | <.01 | −15.5 (−18.0, −12.9) | <.01 | 1.2 (−2.5 – 4.8) | .54 |
| Overactive Bladder Questionnaire (OAB-Q), Bother Subscale | −19.2 (−22.5 – −15.8) | <.01 | −17.9 (−21.2, −14.6) | <.01 | −1.3 (−6.0 – 3.4) | .60 |
| Overactive Bladder Questionnaire (OAB-Q), Health-Related Quality of Life Subscale | −12.8 (−15.4 – −10.2) | <.01 | −14.6 (−17.1, −12.1) | <.01 | 1.8 (−1.8 – 5.4) | .33 |
| Urgency Severity and Impact Questionnaire (USIQ), Severity Subscale | −13.9 (−17.1 – −10.6) | <.01 | −15.6 (−18.8, −12.3) | <.01 | 1.7 (−2.9 – 6.3) | .47 |
| Urgency Severity and Impact Questionnaire (USIQ), Health-Related Quality of Life Subscale | −10.2 (−13.2 – −7.2) | <.0001 | −12.4 (−15.3, −9.4) | <.0001 | 2.2 (−2.1 – 6.4) | .31 |
| Urogenital Distress Inventory Short Form (UDI-6) | −17.1 (−20.9 – −13.4) | <.0001 | −15.4 (−19.1, −11.6) | <.0001 | −1.8 (−7.1 – 3.5) | .51 |
| Patient Perception of Bladder Condition (PPBC) | −0.9 (−1.1 – −0.7) | <.0001 | −0.7 ( −1.0, −0.5) | <.0001 | −0.1 (−0.4 – 0.2) | .36 |
Least square mean estimates of change and 95% confidence intervals were derived from ANCOVA models, adjusted for baseline values.
Calculated by assigning points for each episode of mild urgency-associated voiding (1 point), moderate urgency-associated voiding (2 points), severe urgency-associated voiding (3 points), and urgency incontinence (5 points each) per day, then taking the average across all diary days. Note that voiding episodes that occur without any urgency are assigned 0 points.
Average scores on perceived stress and anxiety symptom questionnaires decreased modestly in both groups over 12 weeks (Table 4). Participants assigned to slow-paced respiration demonstrated greater improvements in PSS scores than those assigned to music control, but no other significant between-group differences in change in anxiety or symptoms were observed (Table 4).
Table 4.
Average Change in Perceived Stress, Anxiety, and Depression Measures Over 12 Weeks, by Intervention Assignment
| Paced Respiration | Music Control | Between-Group Difference | ||||
|---|---|---|---|---|---|---|
| Mean (95% CI)a | P-Value | Mean (95% CI)a | P-Value | Mean (95% CI)a | P-Value | |
| Perceived Stress Scale (PSS) | −2.8 (−4.0, −1.7) | <.01 | −1.1 (−2.2, −0.0) | 0.05 | −1.7 (−3.3, −0.1) | .03 |
| Spielberger State Trait Anxiety Inventory (STAI) – Trait Component | −2.9 (−4.3, −1.4) | <.01 | −2.4 (−3.8, −1.0) | <0.01 | −0.4 (−2.4, 1.6) | .67 |
| Hospital Anxiety and Depression Scale (HADS) – Anxiety Subscaleb | −1.4 (−1.9, −0.9) | <.01 | −0.7 (−1.2, −0.2) | <0.01 | −0.7 (−1.4, 0.1) | .08 |
Least square mean estimates of change and 95% confidence intervals were derived from ANCOVA models, adjusted for baseline values.
P-values derived from models using Winsorized (98–99th percentile) values due to skewed distributions, estimated mean change values represent raw values
Among the 44 women who underwent autonomic function assessments at baseline and 12 weeks (25 in the paced respiration and 19 in the control group), no significant within-group changes in resting parasympathetic or sympathetic function were observed over 12 weeks (Table 5). Additionally, no significant between-group differences in autonomic function were observed.
Table 5.
Average Change in Resting Sympathetic and Parasympathetic Autonomic Function Markers Over 12 Weeks, by Intervention Assignment
| Paced Respiration | Music Control | Between-Group Difference | ||||
|---|---|---|---|---|---|---|
| Mean (95% CI)a | P-Value | Mean (95% CI)a | P-Value | Mean (95% CI)a | P-Value | |
| Pre-Ejection Period (PEP)b, msec | 0.34 (−20.2, 20.9) | .97 | −3.4 (−21.9, 15.1) | .72 | 3.8 (−7.1, 14.6) | .49 |
| Respiratory Sinus Arrhythmia (RSA)b, msec2 | −0.27 (−1.1, 0.6) | .52 | −0.4 (−1.1, 0.3) | .27 | 0.14 (−0.3, 0.6) | .56 |
Least square mean estimates of change and 95% confidence intervals were derived from ANCOVA models, adjusted for baseline values, diabetes, and selected medications (beta blockers, beta agonists, alpha blockers, and sympathomimetics).
Lower resting PEP corresponds to increased peripheral sympathetic nervous system activity. Higher RSA corresponds to greater peripheral parasympathetic activity.
In safety assessments, 30 women assigned to paced respiration and 29 to control reported one or more adverse events (P=.73), but no serious adverse events, nor any adverse events directly attributed to study interventions.
Discussion
In this randomized trial, women with OAB assigned to practice daily slow-guided breathing exercises reported greater improvements in perceived stress over 12 weeks relative to a music-listening control. However, they did not demonstrate greater improvement in frequency of urgency-associated voiding or incontinence, other OAB questionnaires, or autonomic function. Although paced respiration has been used successfully to treat other conditions associated with high levels of perceived stress and/or peripheral autonomic dysfunction, our findings do not support paced respiration as a uniquely effective treatment for OAB.
Slow breathing has already been incorporated to a limited extent into conservative “urge suppression” distraction and relaxation techniques to manage acute urgency episodes in OAB. When feeling an urge to urinate, patients are often told to sit down or stand still, take deep, slow breaths, and try to distract themselves from their bladder sensation. Combined with other behavioral modification strategies, urge suppression appears moderately effective in reducing OAB symptoms.30 However, the breathing component of urge suppression has not previously been studied apart from other components of OAB self-management.
Slow-paced respiration has also been used to manage other chronic conditions associated with high levels of perceived stress or autonomic dysfunction. In particular, the RESPeRATE guided-breathing device is currently used for adjunctive treatment of hypertension.14–16 However, our study did not detect significant changes in resting autonomic function associated with paced respiration in women with OAB, and a recent trial of RESPeRATE for a different indication (menopause-related vasomotor symptoms) also reported no significant effects on similar autonomic measures in women.24
This research benefits from use of a rigorous time-equivalent control, high rates of retention, and objective confirmation of intervention adherence. Nevertheless, several limitations should be noted. First, women were classified as having OAB based on self-reported history and urinalysis testing, without urodynamic or other clinical evaluation to further characterize pathophysiology. Change in urinary symptoms was assessed by voiding diary, which can be associated with measurement error despite its widespread use as an outcome measure in OAB trials. Third, while the music-listening intervention was selected to provide rigorous control for the time and attention spent on paced respiration, it was also an intervention with potential relaxing effects in its own right, which could play a role in improving participant-perceived OAB symptoms. Consequently, study results should not be used to draw conclusions about the effects of paced respiration versus no intervention at all. Autonomic measurements were available in only the subset of participants seen in the San Francisco clinic, which may have limited our ability to detect changes in these outcomes. Additionally, participants with OAB were not required to demonstrate clinically significant perceived stress or anxiety or abnormal autonomic function at baseline, which may have resulted in ceiling/floor effects for these outcomes.
Conclusions
Findings from this randomized trial indicate that women with OAB who practice daily device-guided paced respiration exercises may experience greater improvements in perceived stress, but are no more likely to report improvement in urgency-associated urinary symptoms than those spending equivalent time listening to music. While behavioral relaxation-based therapies may offer general benefits for OAB, this study does not support unique benefits of paced respiration for urgency-associated urinary symptoms.
Acknowledgements
The CURE trial was funded by National Institute on Aging grant #R01AG047894. Dr. Subak was also supported by grant #5K24DK080775 from the National Institute of Diabetes and Digestive and Kidney Disorders.
The RESPeRATE devices and data cables used in this study were purchased at the wholesale commercial price from Intercure, Ltd. Intercure Ltd. also provided the software used to download device data and re-program devices, but provided no other financial or material support for this research.
Drs. Alison Huang and Leslee Subak have previously received funding from Pfizer Inc. and Astellas Pharma through grants awarded to the University of California San Francisco to conduct research unrelated to this report. No other potential conflicts of interest are reported by the authors.
The authors also gratefully acknowledge the contributions of Ann Chang, Lisa Abinanti, Traci Plaut, Sarah Jorgensen, Sarah Chatfield, and Amy Du in developing the study database, recruiting and following participants, and abstracting and cleaning data.
Funding information: The CURE trial was funded by National Institute on Aging grant #R01AG047894. Dr. Subak was also supported by grant #5K24DK080775 from the National Institute of Diabetes and Digestive and Kidney Disorders.
Abbreviations
- OAB
overactive bladder
- PEP
pre-ejection period
- RSA
respiratory sinus arrhythmia
Footnotes
References
- 1.Sand PK, Appell R. Disruptive effects of overactive bladder and urge urinary incontinence in younger women. Am J Med. 2006;119(3 Suppl 1):16–23. [DOI] [PubMed] [Google Scholar]
- 2.Sexton CC, Coyne KS, Thompson C, Bavendam T, Chen CI, Markland A. Prevalence and effect on health-related quality of life of overactive bladder in older americans: results from the epidemiology of lower urinary tract symptoms study. J Am Geriatr Soc. 2011;59(8):1465–1470. [DOI] [PubMed] [Google Scholar]
- 3.Chapple C, Khullar V, Gabriel Z, Dooley JA. The effects of antimuscarinic treatments in overactive bladder: a systematic review and meta-analysis. Eur Urol. 2005;48(1):5–26. [DOI] [PubMed] [Google Scholar]
- 4.Diokno A, Yuhico M, Jr. Preference, compliance and initial outcome of therapeutic options chosen by female patients with urinary incontinence. J Urol. 1995;154(5):1727–1730; discussion 1731. [PubMed] [Google Scholar]
- 5.Wyman JF, Fantl JA, McClish DK, Bump RC. Comparative efficacy of behavioral interventions in the management of female urinary incontinence. Continence Program for Women Research Group. Am J Obstet Gynecol. 1998;179(4):999–1007. [DOI] [PubMed] [Google Scholar]
- 6.Felde G, Ebbesen MH, Hunskaar S. Anxiety and depression associated with urinary incontinence. A 10-year follow-up study from the Norwegian HUNT study (EPINCONT). Neurourol Urodyn. 2017;36(2):322–328. [DOI] [PubMed] [Google Scholar]
- 7.Lai HH, Rawal A, Shen B, Vetter J. The Relationship Between Anxiety and Overactive Bladder or Urinary Incontinence Symptoms in the Clinical Population. Urology. 2016;98:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JB, Kim YB, Kim BT, Kim YS. Analysis of heart rate variability in female patients with overactive bladder. Urology. 2005;65(6):1109–1112; discussion 1113. [DOI] [PubMed] [Google Scholar]
- 9.Hubeaux K, Deffieux X, Ismael SS, Raibaut P, Amarenco G. Autonomic nervous system activity during bladder filling assessed by heart rate variability analysis in women with idiopathic overactive bladder syndrome or stress urinary incontinence. J Urol. 2007;178(6):2483–2487. [DOI] [PubMed] [Google Scholar]
- 10.Liao WC, Jaw FS. A noninvasive evaluation of autonomic nervous system dysfunction in women with an overactive bladder. Int J Gynaecol Obstet. 2010;110(1):12–17. [DOI] [PubMed] [Google Scholar]
- 11.Baker J, Costa D, Guarino JM, Nygaard I. Comparison of mindfulness-based stress reduction versus yoga on urinary urge incontinence: a randomized pilot study. with 6-month and 1-year follow-up visits. Female Pelvic Med Reconstr Surg. 2014;20(3):141–146. [DOI] [PubMed] [Google Scholar]
- 12.Komesu YM, Rogers RG, Sapien RE, et al. Methodology for a trial of brain-centered versus anticholinergic therapy in women with urgency urinary incontinence. Int Urogynecol J. 2017;28(6):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JS, McNaughton KS, Wyman JF, et al. Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology. 2003;61(4):802–809. [DOI] [PubMed] [Google Scholar]
- 14.Elliot WJ, Izzo JL Jr., White WB, et al. Graded blood pressure reduction in hypertensive outpatients associated with use of a device to assist with slow breathing. J Clin Hypertens. 2004;10:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schein MH, Gavish B, Herz M, et al. Treating hypertension with a device that slows and regularizes breathing: a randomised, double-blind controlled study. J Human Hypertension. 2001;15:271–278. [DOI] [PubMed] [Google Scholar]
- 16.Anderson DE, McNeely JD, Windham BG. Device-guided slow-breathing effects on end-tidal CO(2) and heart-rate variability. Psychol Health Med. 2010;14(6):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinner N, Harnett M, Sabounjian L, Sandage B Jr., Dmochowski R, Staskin D, The overactive bladder-symptom composite score: a composite symptom score of toilet voids, urgency severity and urge urinary incontinence in patients with overactive bladder. J Urol. 2005;173(5):1639–1643. [DOI] [PubMed] [Google Scholar]
- 18.Coyne KS, Matza LS, Thompson CL, Kopp ZS, Khullar V. The responsiveness of the Overactive Bladder Questionnaire (OAB-q). Qual Life Res. 2005;14(3):849–855. [DOI] [PubMed] [Google Scholar]
- 19.Lowenstein L, Rickey L, Kenton K, et al. Reliability and responsiveness of the Urgency Severity and Life Impact Questionnaire (USIQ). Int Urogynecol J. 2012;23(2):193–196. [DOI] [PubMed] [Google Scholar]
- 20.Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol. 2006;49(6):1079–1086. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 22.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 23.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 24.Gibson CJ, Mendes WB, Schembri M, Grady D, Huang AJ. Cardiac autonomic function and hot flashes among perimenopausal and postmenopausal women. Menopause. 2017;24(7):756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. [DOI] [PubMed] [Google Scholar]
- 26.Berntson GG, Bigger JT Jr., Eckberg DL, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. [DOI] [PubMed] [Google Scholar]
- 27.Brownley KA, Hurwitz BE, Schneiderman N. Cardiovascular psychophysiology In: Cacioppo JT, Tassinary LG, Berntson GG, eds. Handbook of psychophysiology. New York, NY: Cambridge University Press; 2000. [Google Scholar]
- 28.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. Journal of the American College of Cardiology. 1998;31(3):593–601. [DOI] [PubMed] [Google Scholar]
- 29.Little RJA, Rubin DB. Statistical analysis with missing data. In: New York: Wiley-Interscience; 1987:209–214. [Google Scholar]
- 30.Fantl JA, Wyman JF, McClish DK, et al. Efficacy of bladder training in older women with urinary incontinence. JAMA. 1991;265(5):609–613. [PubMed] [Google Scholar]