Abstract
Objective
To add to data on adverse birth outcomes accounting for disease activity in women with psoriatic arthritis (PsA) and ankylosing spondylitis (AS).
Methods
Data were analyzed from women enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease Project from 2004–2018. Disease activity was measured by the Health Assessment Questionnaire (HAQ) or Routine Assessment of Patient Index Data 3 (RAPID3). Poisson regression was used to estimate adjusted risk ratios (aRR) with 95% Confidence Intervals (CI) for selected adverse pregnancy outcomes.
Results
Compared to healthy controls (n=717), PsA (n = 117) was associated with increased risk for moderate preterm delivery (32–36 weeks’ gestation) (aRR 1.81, 95% CI 1.01–3.26), oligohydramnios (aRR 3.79, 95% CI 1.34–10.74), and Caesarean delivery (aRR 1.63, 95% CI 1.26–2.12). Women with AS (n = 129) had an increased risk of delivering infants requiring intensive care (aRR 1.67, 95% CI 1.05–2.67). High 32 week HAQ score was associated with preterm delivery in women with PsA (aRR 3.82, 95% CI 1.51–9.67). In women with AS, high RAPID3 score was associated with Caesarian delivery (aRR 5.82, 95% 1.06–31.78), and 2nd trimester corticosteroid use was associated with preterm delivery (aRR 4.41, 95% CI 1.57–12.41).
Conclusion
Women with PsA and AS have increased risk for selected adverse pregnancy outcomes. Active disease and corticosteroid use may increase the risk for some adverse pregnancy outcomes in women with these conditions.
Introduction
Psoriatic arthritis (PsA) and ankylosing spondylitis (AS) are chronic inflammatory conditions that often affect men and women at a younger age than other rheumatic conditions. Many of the women affected by these conditions are of child-bearing age and consider planning a family. Unfortunately, data is lacking on pregnancy outcomes, often making it difficult for rheumatologists and obstetricians to counsel their patients effectively.
To our knowledge, only one prospective cohort study dedicated to pregnant women with PsA exists in the literature which analyzed disease activity in pregnancy(1). The results were generally encouraging, with overall disease activity improving during pregnancy, although worsening postpartum. Previous case series and retrospective analyses regarding disease activity of PsA during pregnancy have not been consistent, with some reporting improvement and others reporting either no change or worsening disease activity during pregnancy(2–4). There are currently no prospective cohort studies in the literature assessing the risk for adverse pregnancy outcomes in PsA alone.
There is one major population-based case control study dedicated to pregnancy outcomes in AS(5). This Swedish study included 301 pregnant women with AS and 1082 controls and found a higher risk for preterm birth, both moderate preterm (32–36 weeks) and very preterm (<32 weeks), as well as higher rates of Caesarean deliveries, both emergent and elective, among AS women as compared to controls(5). Another smaller case control study found no increased risk for adverse pregnancy outcome among 20 AS women and 40 controls (6), and a small prospective cohort analyzed by Ostensen and Husby in 1983 found no adverse pregnancy outcome among 13 AS women compared to 31 controls(7). Two previous Norwegian cohort studies found an increased risk for Caesarean deliveries, growth restriction, and preterm birth among women with chronic arthritis, of which AS was only a small component and not analyzed separately(8,9).
The goal of our prospective cohort trial is to add to the very limited amount of robust data on pregnancy outcomes and disease activity in these two chronic inflammatory diseases.
Materials and Methods
Source of the sample
Data were obtained from the Organization of Teratology Information Specialists (OTIS) Autoimmune Disease in Pregnancy Project, a prospective cohort study among women in the U.S. and Canada. Participants were recruited from pregnant callers who initiated contact with an OTIS service. Mail, professional meetings, social media and the OTIS MotherToBaby study website were also used to recruit participants through direct marketing to health care professionals and specialists. Women were eligible for the cohort study if they enrolled before 20 weeks’ gestation and had not enrolled with a previous pregnancy. Delivery of at least one live-born infant and enrollment in the study between 2004 and 2018 were eligibility criteria for analysis. The protocol was approved by the institutional review board at the University of California, San Diego. Women in the study provided oral consent for interview data and written consent for release of medical records.
Study design and data collection
Women who consented to participate were interviewed by telephone two to three times during pregnancy using a standard questionnaire about their personal medical history, prescription and non-prescription medication exposures during pregnancy, history of previous pregnancies, family medical history, pre-pregnancy body mass index (BMI), and socioeconomic and demographic characteristics of the woman and her partner. Exposure history included start and stop dates of each prescription and over-the-counter medication, as well as indications, dosage changes and frequencies, use of caffeine, dietary supplements, occupational exposures, infections, prenatal testing or other medical procedures, and use of recreational drugs, tobacco, and alcohol.
Birth outcomes were obtained using a standard interview form completed by telephone shortly after delivery. Women were asked about exposure information during pregnancy, gestational age at delivery, mode of delivery, and any pregnancy complications. Medical records from the prenatal care provider, delivery hospital, any specialty providers that managed the woman’s care in pregnancy, and the pediatrician were collected and data abstracted for additional exposure and outcome information, including validation of maternal self-report of autoimmune disease diagnosis. If a discrepancy existed between information in the medical record and maternal report, the medical record data was used in the analysis.
Classification of Exposure Groups
Inflammatory diseases considered in the analysis included PsA and AS. Maternal report was used to classify maternal autoimmune disease and was validated by medical record. Women who were enrolled in the study, met criteria for inclusion in this analysis, and had no history of any autoimmune diseases or any other chronic disease were selected as a comparison cohort.
Medication treatments for autoimmune diseases were grouped by class and defined as treatment at any dose for any length of time in pregnancy. The specific classes of medications considered included non-biologic disease modifying anti-rheumatic drugs (DMARDs), biologic DMARDs, oral corticosteroids, and non-steroidal anti-inflammatory drugs (NSAIDs).
Disease activity for exposed subjects was documented at intake and 32 weeks’ gestation using patient-reported assessments, including the Health Assessment Questionnaire (HAQ) on a scale from 0–3, as well as pain score and patient global disease activity assessment on a scale from 0–100. The pain and patient global scores were then each divided by 10 for a range of 0–10. These three markers of disease activity were then added together to calculate the cumulative Routine Assessment of Patient Index Data 3 (RAPID3), with resulting values ranging from 1–30, with active disease defined as RAPID3 score ≥7(10). Active disease by HAQ was defined as score >0.5.
Covariates and Outcomes
Baseline covariates considered in the analysis included the following: maternal age in years at the estimated due date; parity at the time of conception; pre-pregnancy body mass index (BMI); maternal race; tobacco use; prior adverse pregnancy outcome such as preterm delivery, preeclampsia, or intrauterine growth restriction; and comorbid conditions such as thyroid dysfunction, diabetes mellitus type 1 and 2, asthma, or hypertension, and socioeconomic status (SES) as defined by Hollingshead categories(11).
Pregnancy outcomes considered in the analysis included the following obstetric complications: preeclampsia, pregnancy-induced hypertension (PIH), placenta previa and/or placental abruption, gestational diabetes mellitus (GDM), fever anytime during pregnancy, oligohydramnios, and preterm labor. Fever during pregnancy was defined to include any documented fever over the course of the pregnancy, and preterm labor was defined as the onset of labor prior to 37 weeks’ gestation. Preterm delivery as an outcome was defined as delivery at less than 37 completed weeks’ gestation regardless of mode of delivery or indication. Moderate preterm delivery was defined as 32 weeks to less than 37 weeks’ completed gestation; very preterm was defined as delivery at less than 32 weeks’ completed gestation; and early term was defined as delivery at less than 39 weeks and at least 37 weeks’ completed gestation. Multiple pregnancy was defined as twin or higher order multiple with more than one fetus identified during prenatal care, regardless of whether all fetuses resulted in live births. In the cases of multiple gestation pregnancy, only the infant with the most severe outcome was included for analysis. Low birth weight (LBW) was defined as infant weight less than 2500 g, and very low birth weight (VLBW) was defined as less than 1500 g. Additional pregnancy outcomes included delivery by Caesarean delivery and infant hospitalization in the neonatal intensive care unit (NICU) for any duration of time.
Statistical analyses
Maternal and obstetric characteristics were compared among those with inflammatory disease to those in the comparison group using two-tailed univariate comparisons with Student’s t-test for continuous variables and Fisher’s Exact Test or chi-square tests for categorical variables, depending on the cell size. Poisson regression with robust standard errors was utilized to calculate unadjusted risk ratios (RR), multivariable adjusted risk ratios (aRR), and 95% Confidence Intervals (CI) for obstetric outcomes and pregnancy complications among women with PsA and AS versus women in the comparison group. Multivariable adjustment was made for maternal factors including age, White race, high SES, pre-pregnancy BMI, smoking, parity, pre-existing medical disease and previous adverse pregnancy outcome. Poisson regression with robust standard errors was then utilized to determine the effect of active disease at intake and at 32 weeks, as defined by HAQ or RAPID3, on those pregnancy outcomes that were found to be of significantly higher risk in the autoimmune conditions as compared to controls. Additional analysis was performed to determine the effect of medication exposure by trimester of use on preterm delivery. Multivariable adjustment was made for the same maternal factors.
A p-value cut-off of 0.05 was considered statistically significant for all analyses. All analyses were conducted using Statistical Package for the Social Science (SPSS) statistical software Version 25.0, 2017, Armonk, NY.
Results
A total of 963 women were eligible for analysis, with 117 in the PsA group, 129 in the AS group, and 717 in the comparison group. Baseline characteristics of the study population are outlined in Table 1. Women with PsA were older in age with a higher BMI than healthier comparison women. There was a higher percentage of women with at least three prior spontaneous abortions in the PsA group. Women with AS were more likely to be primigravid and less likely to have parity of at least two versus the comparison group. As expected, women in both disease groups had a higher proportion of comorbid conditions, including asthma, diabetes, hypertension, depression, anxiety or other psychiatric illness, as well as tobacco use in both groups and alcohol use in PsA (Table 1). Women in both disease groups were also of higher socioeconomic status than the comparison group in this study.
Table 1.
Maternal characteristics and exposures among women with psoriatic arthritis (PsA) and ankylosing spondylitis (AS) compared to women without inflammatory arthritis in OTIS cohort 2004–2018 (n = 963)
| COMPARISON GROUP | PsA | P valuea | AS | P valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| N = 717 | N = 117 | N = 129 | |||||||
| Maternal age at estimated due date, years, mean (SD) | 32.20 (4.94) | 34.3 (5.2) | <0.001 | 32.6 (5.0) | 0.379 | ||||
| Pre-pregnancy BMI, kg/m2 mean (SD) | 24.48 (5.72) | 27.0 (6.1) | <0.001 | 24.3 (4.9) | 0.688 | ||||
| Maternal weight gain during pregnancy, kg, mean (SD) | 14.52 (5.94) | 13.8 (6.6) | 0.249 | 14.6 (6.3) | 0.964 | ||||
| Race, n (%) | |||||||||
| White | 541 (75.5) | 104 (89.7) | 0.263 | 112 (86.8) | 0.354 | ||||
| Black | 43 (6.0) | 3 (2.6) | 6 (4.7) | ||||||
| Asian /Pacific Islander | 35 (4.9) | 6 (5.2) | 4 (3.1) | ||||||
| Native American | 8 (1.1) | 2 (1.7) | 1 (0.8) | ||||||
| Other | 21 (2.9) | 1 (0.9) | 1 (0.8) | ||||||
| SESb, n (%) | |||||||||
| High score (1–3) | 592 (86.9) | 109 (94.8) | 0.016 | 123 (96.1) | 0.003 | ||||
| Obstetric history, n (%) | |||||||||
| Primigravid | 247 (34.4) | 43 (36.8) | 0.628 | 66 (51.2) | <0.001 | ||||
| Parity, ≥2 | 139 (19.4) | 24 (20.5) | 0.776 | 13 (10.1) | 0.011 | ||||
| Prior SAB, ≥3 | 15 (2.1) | 8 (6.8) | 0.009 | 2 (1.6) | 1.000 | ||||
| Prior TAB, ≥1 | 77 (10.7) | 12 (10.3) | 1.000 | 16 (12.4) | 0.578 | ||||
| Prior preterm delivery | 58 (8.1) | 6 (5.1) | 0.265 | 6 (4.7) | 0.174 | ||||
| Prior preeclampsia | 22 (3.1) | 6 (5.1) | 0.265 | 4 (3.1) | 1.000 | ||||
| Comorbid pre-gestational conditions, n (%) | |||||||||
| Asthma | 0 (0) | 9 (7.7) | <0.001 | 32 (24.8) | <0.001 | ||||
| Thyroid dysfunction | 67 (9.3) | 14 (12.0) | 0.375 | 15 (11.6) | 0.420 | ||||
| Diabetes mellitus (type 1 and 2) | 0 (0) | 2 (1.7) | 0.020 | 3 (2.3) | 0.003 | ||||
| Hypertension | 12 (1.7) | 11 (9.4) | <0.001 | 7 (5.4) | 0.017 | ||||
| Depression | 42 (5.9) | 26 (22.2) | <0.001 | 21 (16.3) | <0.001 | ||||
| Anxiety or other psychiatric illness | 41 (5.7) | 16 (13.7) | 0.002 | 17 (13.2) | 0.002 | ||||
| Tobacco use, any in pregnancy, n (%) | 26 (3.6) | 14 (12.0) | <0.001 | 12 (9.3) | 0.004 | ||||
| Alcohol use, any in pregnancy, n (%) | 292 (40.7) | 62 (53.0) | 0.013 | 62 (48.1) | 0.120 | ||||
| Other autoimmune diseases, n (%) | |||||||||
| Additional diagnosis of seronegative SpA | NA | 5 (4.3) | 1 (0.8) | ||||||
| Psoriasis | NA | 90 (76.9) | 10 (7.8) | ||||||
| Inflammatory bowel disease | NA | 2 (1.7) | 7 (5.4) | ||||||
| Other connective tissue diseasec | NA | 2 (1.7) | 1 (0.8) | ||||||
| Disease characteristics | |||||||||
| Years since diagnosis, mean (SD) | NA | 7.8 (6.8) | 6.1 (4.9) | ||||||
| Active disease at enrollment, n (%) | |||||||||
| HAQ | NA | 23 (22.3) | 38 (34.5) | ||||||
| RAPID3 | NA | 42 (40.8) | 54 (49.1) | ||||||
| Medication use anytime during pregnancy, n (%) | |||||||||
| Biologic DMARDs | NA | 102 (87.2) | 105 (81.4) | ||||||
| 1st trimester | NA | 94 (80.3) | 101 (78.3) | ||||||
| 2nd trimester | NA | 72 (62.1) | 80 (63.0) | ||||||
| 3rd trimester | NA | 72 (62.6) | 75 (59.1) | ||||||
| DMARDs | NA | 14 (12.0) | 10 (7.8) | ||||||
| NSAIDs | NA | 20 (17.1) | 31 (24.0) | ||||||
| Corticosteroids | NA | 38 (32.5) | 49 (38.0) | ||||||
P values calculated by t-test for continuous variables and by chi-square test for categorical variables; Fisher’s exact test used for cells with expected count <5
SES defined by Hollingshead categories
Defined as lupus, antiphospholipid syndrome, Sjögren’s syndrome, or undifferentiated; SES = socioeconomic status; SAB = spontaneous abortion; TAB = terminal abortion; SpA = spondyloarthropathy; HAQ = Health Assessment Questionnaire; RAPID3 = Routine Assessment of Patient Index Data with 3 measures; DMARDs = Disease-Modifying Anti-Rheumatic Drugs; NSAIDs = Non-Steroidal Anti-Inflammatory Drugs; Race – data missing for 1 subject in PsA, 5 in AS; SES – data missing for 2 subjects in PsA, 1 in AS; Disease activity measures – data missing for 14 in PsA, 19 in AS; Active disease defined by HAQ score > 0.5 and RAPID3 score ≥7; Years since diagnosis – data missing for 2 PsA subjects; Biologic DMARD use in 2nd trimester – data missing for 1 subject in PsA, 2 subjects in AS; Biologic DMARD use in 3rd trimester – data missing for 2 subjects in PsA, 2 subjects in AS
After multivariable analysis, PsA was found to confer an increased risk for moderate preterm delivery (aRR 1.81, 95% CI 1.01–3.26), preterm labor (aRR 2.05, 95% CI 1.21–3.48), oligohydramnios (aRR 3.79, 95% CI 1.34–10.74), and Caesarean delivery (aRR 1.63, 95% CI 1.26–2.12) versus healthier comparison women. Women with AS had a 67% increased risk for infant hospitalization in NICU. An increased risk for very preterm delivery and VLBW was also noted for AS, although each of these events occurred in only 3 out of 129 AS subjects (Table 2).
Table 2.
Obstetric outcomes among women with psoriatic arthritis (PsA) and ankylosing spondylitis (AS) compared to women without inflammatory arthritis in OTIS cohort 2004–2018 (n = 963)
| COMPARISON GROUP (N = 717) |
PsA (N = 117) |
AS (N = 129) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | UNADJUSTED RISK RATIO (95% CI) | ADJUSTED RISK RATIOa (95% CI) | N (%) | UNADJUSTED RISK RATIO (95% CI) | ADJUSTED RISK RATIOa (95% CI) | |||
| Gestational age at delivery | |||||||||
| Preterm (<37 weeks’ gestation) | 61 (8.5) | 16 (13.7) | 1.61 (0.96–2.69) | 1.69 (0.94–3.03) | 14 (10.9) | 1.28 (0.74–2.21) | 1.51 (0.81–2.82) | ||
| Very preterm (<32 weeks)b | 6 (0.8) | 0 (0) | -- | -- | 3 (2.3) | 2.81 (0.71–11.06) | 10.19 (2.09–49.78) | ||
| Moderate preterm (≥32 weeks and <37 weeks)b | 55 (7.7) | 16 (13.7) | 1.77 (1.05–2.98) | 1.81 (1.01–3.26) | 11 (8.5) | 1.13 (0.61–2.10) | 1.22 (0.61–2.45) | ||
| Early term (≥37 weeks and <39 weeks)c | 192 (26.8) | 44 (37.6) | 1.49 (1.16–1.92) | 1.28 (0.97–1.70) | 35 (27.1) | 1.04 (0.77–1.41) | 1.03 (0.75–1.41) | ||
| Multiple pregnancy | 31 (4.3) | 6 (5.1) | 1.19 (0.51–2.78) | 0.92 (0.38–2.24) | 3 (2.3) | 0.54 (0.17–1.73) | 0.47 (0.14–1.65) | ||
| Obstetric complications | |||||||||
| Preeclampsia | 27 (3.8) | 10 (8.5) | 2.26 (1.12–4.54) | 2.22 (0.98–5.04) | 4 (3.1) | 0.82 (0.29–2.30) | 0.63 (0.19–2.04) | ||
| PIH without preeclampsia | 31 (4.3) | 11 (9.4) | 2.18 (1.12–4.21) | 1.11 (0.53–2.32) | 4 (3.1) | 0.72 (0.26–2.00) | 0.48 (0.14–1.70) | ||
| Placenta previa and/or abruption | 10 (1.4) | 0 (0) | -- | -- | 2 (1.6) | 1.11 (0.25–5.02) | 2.05 (0.44–9.55) | ||
| Preterm labor | 59 (8.4) | 19 (16.2) | 1.94 (1.20–3.13) | 2.05 (1.21–3.48) | 13 (10.2) | 1.21 (0.69–2.15) | 1.32 (0.72–2.44) | ||
| Gestational diabetes mellitus | 43 (6.1) | 11 (9.4) | 1.54 (0.82–2.90) | 1.41 (0.70–2.87) | 4 (3.1) | 0.51 (0.19–1.40) | 0.49 (0.18–1.34) | ||
| Fever during pregnancy | 137 (19.1) | 21 (17.9) | 0.94 (0.62–1.42) | 0.90 (0.58–1.41) | 29 (22.5) | 1.18 (0.83–1.68) | 1.08 (0.74–1.57) | ||
| Oligohydramnios | 11 (7.0) | 5 (25.0) | 3.59 (1.39–9.28) | 3.79 (1.34–10.74) | 1 (9.1) | 1.31 (0.19–9.21) | 2.69 (0.53–13.60) | ||
| Delivery Mode | |||||||||
| Caesarian delivery | 188 (26.2) | 57 (48.7) | 1.86 (1.49–2.32) | 1.63 (1.26–2.12) | 43 (33.3) | 1.27 (0.97–1.67) | 1.19 (0.88–1.61) | ||
| Adverse infant outcomes | |||||||||
| LBW (<2500 g) | 48 (6.7) | 11 (9.4) | 1.40 (0.75–2.63) | 1.52 (0.76–3.04) | 9 (7.0) | 1.04 (0.52–2.07) | 1.06 (0.50–2.27) | ||
| VLBW (<1500 g)d | 2 (0.3) | 1 (0.9) | -- | -- | 3 (2.3) | 8.18 (1.38–48.47) | 11.02 (2.24–54.12) | ||
| Hospitalization in NICU | 85 (11.9) | 14 (12.0) | 1.00 (0.59–1.71) | 1.06 (0.60–1.87) | 22 (17.2) | 1.44 (0.94–2.22) | 1.67 (1.05–2.67) | ||
Risk ratio computed using Poisson regression
Multivariable adjusted risk ratio computed using Poisson regression with robust standard errors for: Maternal age, Race (white), SES (high), Pre-pregnancy BMI, Smoking, Parity, Pre-existing medical disease (HTN, thyroid disease, diabetes, asthma), and previous adverse pregnancy outcome (preeclampsia, preterm, IUGR)
calculated with term pregnancy (≥37 weeks) as reference group
calculated with delivery ≥39 weeks as reference group
calculated with normal birth weight (≥2500 g) as reference group; PIH = pregnancy-induced hypertension; LBW = low birth weight; VLBW = very low birth weight; NICU = neonatal intensive care unit
Further analyses were performed on those increased risks seen in Table 2 to determine the effect of disease activity and medication use on those risks. Preterm delivery, preterm labor, Caesarean delivery, and NICU hospitalization were analyzed further among women with PsA and AS. Due to low numbers, subgroup analyses were unable to be performed for oligohydramnios or for VLBW. Active disease at 32 weeks by both HAQ and RAPID3 scores was found to increase the risk for preterm delivery in PsA, although estimates were imprecise. Only HAQ score at 32 weeks was significantly associated with preterm labor in PsA, whereas score by RAPID3 did not reach statistical significance. There was no association between disease activity and Caesarean delivery or NICU hospitalization in PsA (Table 3). In AS, active disease by RAPID3 at intake was associated with an increased risk of Caesarian delivery, and active disease by RAPID3 at 32 weeks was associated with an increased risk for preterm labor, although estimates again were quite imprecise. Disease activity was not significantly associated with preterm delivery or NICU hospitalization in AS (Table 3). Medication use including corticosteroids, non-biologic DMARDs, biologic DMARDs, and NSAIDs in the first or second trimester was not associated with an increased risk of preterm delivery among women with PsA (Table 4). Corticosteroid use in the second trimester, however, was significantly associated with preterm delivery in AS, although this estimate was limited by relatively small numbers (Table 4).
Table 3.
Association of active disease versus inactive disease on pregnancy outcomes among women with psoriatic arthritis (PsA) (n = 117) and AS (n = 129) in OTIS cohort 2004–2018
| PsA (N = 117) |
AS (N = 129) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Active Disease by HAQb | Active Disease by RAPID3b | Active Disease by HAQb | Active Disease by RAPID3b | |||||
| At intake (n = 23) |
At 32 weeks (n = 26) |
At intake (n = 42) |
At 32 weeks (n = 27) |
At intake (n = 38) |
At 32 weeks (n = 41) |
At intake (n = 54) |
At 32 weeks (n = 46) |
|
| Preterm Delivery | ||||||||
| Number of events, n (%) | 3 (13.0) | 8 (30.8) | 8 (19.0) | 8 (29.6) | 5 (13.2) | 4 (9.8) | 7 (13.0) | 4 (8.7) |
| Adjusted Risk Ratioa (95% CI) | 0.73 (0.18–3.00) | 3.82 (1.51–9.67) | 1.60 (0.57–4.48) | 3.75 (1.20–11.67) | 1.81 (0.52–6.29) | 1.45 (0.38–5.49) | 1.63 (0.61–4.33) | 1.59 (0.43–5.93) |
| Preterm Labor | ||||||||
| Number of events, n (%) | 5 (21.7) | 8 (30.8) | 10 (23.8) | 8 (29.6) | 7 (18.9) | 3 (7.3) | 7 (13.2) | 4 (8.7) |
| Adjusted Risk Ratioa (95% CI) | 0.94 (0.32–2.73) | 2.97 (1.20–7.34) | 1.81 (0.73–4.50) | 3.24 (0.96–10.98) | 2.93 (0.87–9.91) | 1.63 (0.29–9.27) | 1.60 (0.51–5.02) | 5.82 (1.06–31.78) |
| Caesarian Delivery | ||||||||
| Number of events, n (%) | 10 (43.5) | 0.66 (0.37–1.19) | 19 (45.2) | 14 (51.9) | 18 (47.4) | 14 (34.1) | 26 (48.1) | 17 (37.0) |
| Adjusted Risk Ratioa (95% CI) | 16 (61.5) | 0.96 (0.64–1.44) | 0.99 (0.60–1.62) | 0.82 (0.54–1.23) | 1.38 (0.78–2.43) | 1.01 (0.54–1.88) | 1.97 (1.12–3.47) | 1.58 (0.87–2.87) |
| NICU Hospitalization | ||||||||
| Number of events, n (%) | 4 (17.4) | 1.16 (0.37–3.66) | 5 (11.9) | 4 (14.8) | 7 (18.9) | 9 (22.0) | 11 (20.8) | 8 (17.4) |
| Adjusted Risk Ratioa (95% CI) | 5 (19.2) | 2.01 (0.50–7.99) | 1.00 (0.28–3.48) | 1.40 (0.24–8.05) | 1.19 (0.49–2.91) | 1.97 (0.75–5.15) | 1.41 (0.66–2.99) | 0.96 (0.36–2.54) |
Multivariable adjusted risk ratio computed using Poisson regression with robust errors for: Maternal age, Race (white), SES (high), Pre-pregnancy BMI, Smoking, Parity, Pre-existing medical disease (HTN, thyroid disease, diabetes, asthma), and previous adverse pregnancy outcome (preeclampsia, preterm, IUGR)
Active disease defined by HAQ score > 0.5 and RAPID3 score ≥7 and inactive disease defined as HAQ score ≤0.5 and RAPID3 score ≤6; Disease activity measures missing for 14 subjects in PsA and 19 subjects in AS at intake and 34 subjects in PsA and 38 subjects in AS at 32 weeks. HAQ = health assessment questionnaire; RAPID3 = Routine Assessment of Patient Index Data 3.
Table 4.
Association of medications with preterm delivery among women with psoriatic arthritis (N = 117) and ankylosing spondylitis (N = 129) by trimester of use in OTIS cohort 2004–2018
| Biologic DMARDsa | Non-biologic DMARDsa | NSAIDsa | Corticosteroidsa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anyb | 1T | 2T | Anyb | 1T | 2T | Anyb | 1T | 2T | Anyb | 1T | 2T | |
| PsA | ||||||||||||
| N = 101 | N = 94 | N = 72 | N = 14 | N = 12 | N = 11 | N= 20 | N = 14 | N = 9 | N = 33 | N = 26 | N = 25 | |
| Preterm Delivery, n (%) | 14 (13.9) | 12 (12.8) | 12 (16.7) | 1 (7.1) | 1 (8.3) | 1 (9.1) | 5 (25.0) | 2 (14.3) | 3 (33.3) | 6 (18.2) | 5 (19.2) | 5 (20.0) |
| Adjusted Risk Ratio (95% CI)c | 0.92 (0.23–3.74) | 0.49 (0.18–1.36) | 1.89 (0.59–6.01) | 0.50 (0.07–3.77) | 0.55 (0.07–4.11) | 0.69 (0.08–5.60) | 2.34 (0.79–6.94) | 0.96 (0.24–3.90) | 2.73 (0.71–10.52) | 1.45 (0.47–4.48) | 1.58 (0.53–4.77) | 1.19 (0.41–3.49) |
| AS | ||||||||||||
| N = 105 | N = 101 | N = 80 | N = 10 | N = 9 | N = 4 | N = 30 | N = 26 | N = 11 | N = 62 | N = 20 | N = 28 | |
| Preterm delivery, n (%) | 11 (10.5) | 10 (9.9) | 7 (8.8) | 1 (10.0) | 1 (11.1) | 0 (0) | 1 (3.3) | 1 (3.8) | 0 (0) | 8 (12.9) | 2 (10.0) | 7 (25.0) |
| Adjusted Risk Ratio (95% CI)c | 1.29 (0.23–7.25) | 1.03 (0.22–4.78) | 0.74 (0.25–2.19) | 0.92 (0.28–2.99) | 0.92 (0.28–2.99) | -- | 0.36 (0.05–2.70) | 0.45 (0.06–3.28) | -- | 2.22 (0.71–6.92) | 0.98 (0.22–4.25) | 4.41 (1.57–12.41) |
Reference group for analysis is all other subjects, including all other medication use
Any use in either the 1st or 2nd trimester (3rd trimester use excluded)
Multivariable adjusted risk ratio computed using Poisson regression with robust errors for: Maternal age, Race (white), SES (high), Pre-pregnancy BMI, Smoking, Parity, Preexisting medical disease (HTN, thyroid disease, diabetes, asthma), previous adverse pregnancy outcome (preeclampsia, preterm, IUGR), and disease activity defined by HAQ at intake for PsA and RAPID3 at intake for AS; DMARDs = Disease-Modifying Anti-Rheumatic Drugs; NSAIDs = Non-Steroidal Anti-Inflammatory Drugs
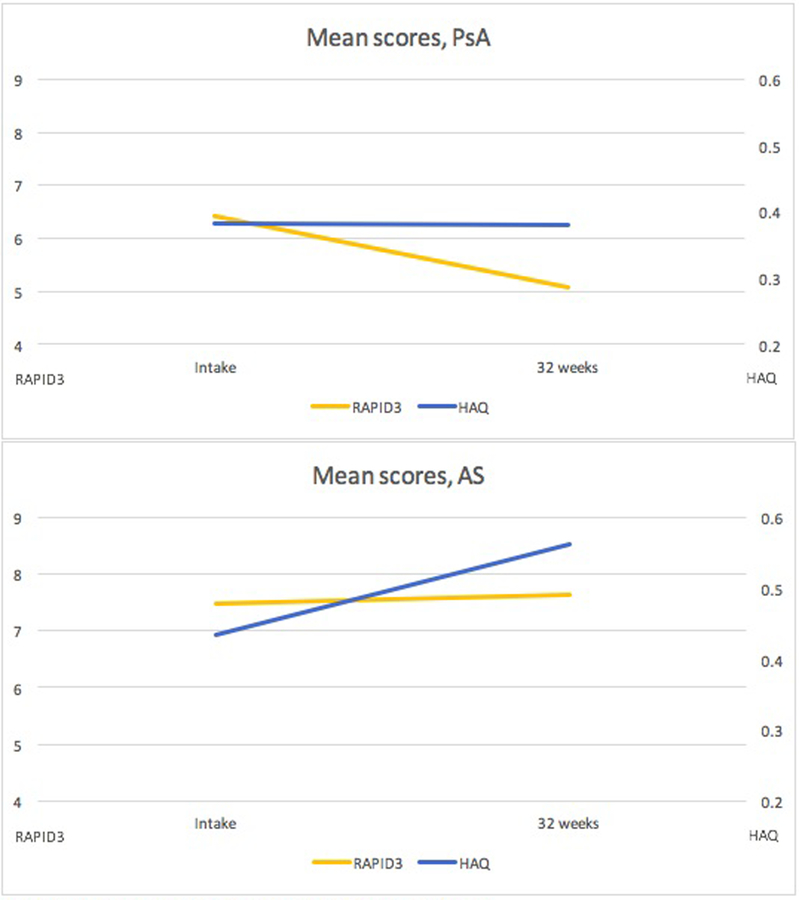
In PsA, 22.3% (23 of 103) were defined as active at intake by HAQ, whereas a much higher percentage of 40.8% (42 of 103) were considered active by RAPID3. Similarly, in AS, 34.5% (38 out of 110) were defined as active at intake by HAQ, whereas 49.1% (54 out of 110) were defined as active at intake by RAPID3 (Table 1). Mean disease activity scores over the course of pregnancy were either stable (by HAQ) or improved (by RAPID3) among women with PsA, whereas among women with AS, scores slightly worsened with both measures, although changes were modest in both disease groups (Figure 1).
Figure1.
Disease activity at intake and at 32 weeks’ gestation as measured by the RAPID3 or HAQ
HAQ score ranging 0–3 on secondary axis, scale magnified for readability
Discussion
The results of our study indicate that women with PsA and AS have generally favorable pregnancy outcomes. Although women with PsA were not shown to have an increased risk for overall preterm birth in this study, there was an increased risk for preterm labor, and when examined by gestational week, for moderate preterm birth. No previous evidence exists to support the higher risk of moderate preterm birth seen in PsA in this study, but to our knowledge no other prospective study has analyzed pregnancy outcomes in this disease. The association between preterm birth and other inflammatory conditions such as RA has been demonstrated in multiple studies; however, the individual contributions of medication use, disease activity, and the condition itself on that risk have remained unclear. A recent analysis also from the OTIS group suggested that the increased risk for preterm delivery in RA may be driven by corticosteroid use and high disease activity(12). Our study suggests that similar trends may exist in PsA and AS, as active disease later in pregnancy was found to increase the risk for preterm delivery in PsA, and second trimester corticosteroid use was found to increase the risk for preterm delivery in AS.
In PsA we additionally found an increased risk for Caesarean delivery that was not explained by disease activity. The indications for Caesarian delivery were not specifically studied in this analysis (i.e. elective or emergent), and therefore it is unclear if the increased risk was driven by patient or physician preference, or other medical indication. A higher risk of oligohydramnios for PsA women was also noted in our study. The mechanism for this is unclear, although medication use and other maternal factors or comorbidities that were not noted or analyzed specifically may play a role.
Despite the concern that women with AS may have anatomical reasons to elect for Caesarean delivery over spontaneous vaginal delivery such as spondyloarthritis, sacroiliitis, or hip arthritis, we reassuringly did not find an overall increased risk for Caesarean delivery among women with AS in our study. We did, however, find that high disease activity at intake was associated with an increased risk for Caesarean delivery in this group, although it is unclear how many of these deliveries were elective versus emergent. Jakobsson et al noted an increased risk for Caesarean delivery among women with AS in their Swedish study (5), but other studies have not reproduced these findings(6,7). In addition, the contribution of disease activity on this outcome was not measured. One must consider differing practices in the various countries in which these studies are conducted with regards to elective Caesarean deliveries, as well as the contribution of disease activity, medication use, and other pertinent factors behind the Caesarean deliveries.
An increased risk for infant hospitalization in the NICU was seen with AS in our study. This may have been influenced by the higher incidence of VLBW and very preterm infants in the AS group. The increased risk for the latter two outcomes seen in AS, however, was based on only three AS cases for each outcome. When analyzing these cases in more depth, we found that two of the VLBW cases and one of the very preterm cases were twin pregnancies. Given the incredibly high risk for preterm delivery and LBW among all twin pregnancies (reported in up to 60%) (13), the few cases in our study may be explained at least in part by this fact. Interestingly, Jakobsson et al also found an increased risk for very preterm delivery in AS, with subanalyses indicating potential mediations by preeclampsia(5). Of note, none of the AS cases of VLBW or very preterm delivery developed preeclampsia in our study.
There was a surprisingly high rate of corticosteroid use in the AS patient group, with 38% exposed to corticosteroids at some point during pregnancy. The 2016 American College of Rheumatology guidelines in fact recommend against the use of systemic corticosteroids for the treatment of AS, with the exception of short-term treatment with rapid tapering in circumstances such as flares during pregnancy, flares of concomitant inflammatory bowel disease, or flare of peripheral arthritis(14). Our study found that second trimester corticosteroid use in AS was associated with an increased risk for preterm delivery in multivariable analysis, although based on small numbers. This association remained after adjustment for disease activity, although it is possible that there may have been active disease in domains incompletely captured by the outcomes collected. These findings from our study imply that systemic corticosteroid treatment for flares during pregnancy may not be without risk, and perhaps alternative medications should be entertained first, if clinically appropriate.
When looking at disease activity in PsA pregnancies, we found that the mean disease activity score remained unchanged by HAQ and improved by RAPID3 from intake to 32 weeks. Despite the RAPID3 being more sensitive than the HAQ at detecting active disease at intake (41% versus 22%), our study suggests that the HAQ may be more useful in terms of predicting outcomes for women with PsA. While there has been concern that pregnancy itself may alter the scoring of the HAQ, making it perhaps a less reliable measure in pregnancy (15), this may not be as much of a concern when analyzing how well the score predicts pregnancy outcomes. Another cohort study from our group on pregnancy outcomes in RA demonstrated that HAQ score predicted preterm birth(16). In this study, while the HAQ was perhaps more predictive in PsA, the RAPID3 was more predictive of obstetric outcomes in the AS group. To our knowledge, no other studies have analyzed the use of the RAPID3 to assess rheumatic diseases during pregnancy.
While PsA disease activity in pregnancy may vary across studies depending on which measure is used, the data has been relatively consistent in AS, although limited. Similarly to our study, Ostensen found that as much as 80% of AS pregnant patients had either unchanged or worsened disease symptoms during pregnancy(17). It is unclear how much of this is driven by medication discontinuation, as a recent study by van den Brandt et al found that discontinuation of an anti-TNF medication during pregnancy increased the risk for flare in axial spondyloarthropathy, particularly in the second trimester(18). Further studies are needed to tease out the association of medication use, or discontinuation, on disease activity measures.
Strengths of this study include the multivariable adjustment analyses as well as the prospective cohort design, as other trials on pregnancy outcomes in these two diseases have predominantly been retrospective or case control in nature. One limitation is that our cohort consists of predominantly White women with a high SES level, and a significant proportion of women in the disease groups were on a biologic DMARD at some point during their pregnancy (greater than 80% in both disease groups). These numbers are likely to be higher in our cohort than would be seen globally, as patients in other countries may not have easy access to these medications. In addition, because the exposed women may have chosen to participate in the study as a result of their disease, the sample may have included women with more severe disease, more medication use, or a higher number of comorbid conditions than is typical for other studies. Lastly, the volunteers in the comparison cohort may have been healthier than the general population, resulting in stronger effect estimates than what would be observed from other types of studies.
Future studies are needed to confirm the novel findings seen in our study, as well as to continue to analyze the effect of different disease activity measures and medication use on pregnancy outcomes in these two chronic conditions.
Significance and Innovations.
Pregnancy outcomes are generally favorable for women with psoriatic arthritis and ankylosing spondylitis.
Disease activity remains relatively stable over pregnancy in both conditions.
High disease activity in pregnancy may increase the risk for preterm labor and preterm delivery among women with psoriatic arthritis, and for preterm labor and Caesarian delivery among women with ankylosing spondylitis.
Corticosteroid use later in pregnancy may increase the risk for preterm delivery in ankylosing spondylitis, independent of disease activity.
Controlling disease activity and minimizing use of corticosteroids may contribute to better obstetric outcomes among women with these conditions.
Acknowledgements
The authors would like to thank Alan Wells, Yunjun Luo, Diana Johnson, and Robert Terkeltaub, MD. The OTIS Collaborative Research Group receives research funding from the following industry sponsors: AbbVie; Amgen Inc.; Apotex, Barr Laboratories, Inc.; Bristol-Myers Squibb; Celgene; Janssen Pharmaceuticals; Kali Laboratories, Inc.; Pfizer, Inc.; Hoffman La Roche-Genentech; Sandoz Pharmaceuticals; Genzyme Sanofi-Aventis; Takeda Pharmaceutical Company Limited; Teva Pharmaceutical Industries Ltd.; UCB, USA; Seqirus; Regeneron; Glaxo Smith Kline; and Astra Zeneca Medimmune.
Funding: Chelsey Smith is supported by the National Institutes of Health, Grant T32 AR064194. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures: The authors report no conflicts of interest or financial disclosures.
Conflicts of Interest
The authors do not have any conflicts of interest to declare.
References
- 1.Ursin K, Lydersen S, Skomsvoll JF, Wallenius M. Disease activity of psoriatic arthritis during and after pregnancy: A prospective multicenter study. Arthritis Care Res (Hoboken) 2018; September 7 [Epub ahead of print] [DOI] [PubMed]
- 2.Polachek A, Polachek Shlomi I, Spitzer K, Pereira D, Ye JY, Chandran V, et al. Outcome of pregnancy in women with psoriatic arthritis compared to healthy controls. Clin Rheumatol 2018; December 7 [Epub ahead of print] [DOI] [PubMed]
- 3.Berman M, Zisman D, Wollman J, Levartovsky D, Rimon E, Elkayam O, et al. The effect of pregnancy on disease activity in patients with psoriatic arthritis. J Rheumatol 2018;45:1651–1655. [DOI] [PubMed] [Google Scholar]
- 4.Mouyis MA, Thornton CC, Williams D, Giles IP. Pregnancy Outcomes in Patients with Psoriatic Arthritis. J Rheumatol 2017;44:128–129. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsson GL, Stephansson O, Askling J, Jacobsson LT. Pregnancy outcomes in patients with ankylosing spondylitis: a nationwide register study. Ann Rheum Dis 2016;75:1838–42. [DOI] [PubMed] [Google Scholar]
- 6.Timur H, Tokmak A, Türkmen GG, Ali İnal H, Uygur D, Danışman N. Pregnancy outcome in patients with ankylosing spondylitis. J Matern Fetal Neonatal Med 2016;29:2470–4. [DOI] [PubMed] [Google Scholar]
- 7.Ostensen M, Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum 1983;26:1155–9. [DOI] [PubMed] [Google Scholar]
- 8.Skomsvoll JF, Ostensen M, Baste V, Irgens LM. Number of births, interpregnancy interval, and subsequent pregnancy rate after a diagnosis of inflammatory rheumatic disease in Norwegian women. J Rheumatol 2001;28:2310–4. [PubMed] [Google Scholar]
- 9.Wallenius M, Skomsvoll JF, Irgens LM, Salvesen KÅ, Nordvåg BY, Koldingsnes W, et al. Pregnancy and delivery in women with chronic inflammatory arthritides with a specific focus on first birth. Arthritis Rheum 2011;63:1534–42. [DOI] [PubMed] [Google Scholar]
- 10.Pincus T, Swearingen CJ, Bergman M, Yazici Y. RAPID3 (Routine Assessment of Patient Index Data 3), a rheumatoid arthritis index without formal joint counts for routine care: proposed severity categories compared to disease activity score and clinical disease activity index categories. J Rheumatol 2008;35:2136–47. [DOI] [PubMed] [Google Scholar]
- 11.Hollingshead AB. Four factor index of social status. Yale J Sociol 2011;8:21–51. [Google Scholar]
- 12.Smith CJF, Förger F, Bandoli G, Chambers CD. Factors associated with preterm delivery among women with rheumatoid arthritis and juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2018; August 21. [DOI] [PMC free article] [PubMed]
- 13.Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol 2010;203:305–15. [DOI] [PubMed] [Google Scholar]
- 14.Ward MM, Deodhar A, Aki EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Man YA, Hazes JM, van de Geijn FE, Krommenhoek C, Dolhain RJ. Measuring disease activity and functionality during pregnancy in patients with rheumatoid arthritis. Arthritis Rheum 2007;57:716–22. [DOI] [PubMed] [Google Scholar]
- 16.Bharti B, Lee SJ, Lindsay SP, Wingard DL, Jones KL, Lemus H, et al. Disease Severity and Pregnancy Outcomes in Women with Rheumatoid Arthritis: Results from the Organization of Teratology Information Specialists Autoimmune Diseases in Pregnancy Project. J Rheumatol 2015;42:1376–82. [DOI] [PubMed] [Google Scholar]
- 17.Ostensen M The effect of pregnancy on ankylosing spondylitis, psoriatic arthritis, and juvenile rheumatoid arthritis. Am J Reprod Immunol 1992;28:235–7. [DOI] [PubMed] [Google Scholar]
- 18.van den Brandt S, Zbinden A, Baeten D, Villiger PM, Østensen M, Förger F. Risk factors for flare and treatment of disease flares during pregnancy in rheumatoid arthritis and axial spondyloarthritis patients. Arthritis Res Ther 2017;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]