Abstract
Arrestins control signaling via the G protein coupled receptors (GPCRs), serving as both signal terminators and transducers. Previous studies identified several structural elements in arrestins that contribute to their functions as GPCR regulators. However, the importance of these elements in vivo is unclear, and the developmental roles of arrestins are not well understood. We carried out an in vivo structure-function analysis of Kurtz (Krz), the single ortholog of mammalian β-arrestins in the Drosophila genome. A combination of Krz mutations affecting the GPCR-phosphosensing and receptor core-binding (“finger loop”) functions (Krz-KKVL/A) resulted in a complete loss of Krz activity during development. Endosome recruitment and bioluminescence resonance energy transfer (BRET) assays revealed that the KKVL/A mutations abolished the GPCR-binding ability of Krz. We found that the isolated “finger loop” mutation (Krz-VL/A), while having a negligible effect on GPCR internalization, severely affected Krz function, suggesting that tight receptor interactions are necessary for proper termination of signaling in vivo. Genetic analysis as well as live imaging demonstrated that mutations in Krz led to hyperactivity of the GPCR Mist (also known as Mthl1), which is activated by its ligand Folded gastrulation (Fog) and is responsible for cellular contractility and epithelial morphogenesis. Krz mutations affected two developmental events that are under the control of Fog-Mist signaling: gastrulation and morphogenesis of the wing. Overall, our data reveal the functional importance in vivo of direct β-arrestin/GPCR binding, which is mediated by the recognition of the phosphorylated receptor tail and receptor core interaction. These Krz-GPCR interactions are critical for setting the correct level of Fog-Mist signaling during epithelial morphogenesis.
Keywords: arrestin, G protein coupled receptor, Drosophila, epithelial morphogenesis, Kurtz, gastrulation
Introduction
β-arrestins 1 and 2 (also called arrestins 2 and 3) and related visual arrestins were initially characterized as factors necessary for the desensitization of activated G protein coupled receptors (GPCRs) (Benovic et al., 1987; Lohse et al., 1990). In addition to this role, β-arrestins mediate internalization of GPCRs through binding to clathrin and other components of the endocytic machinery (Goodman et al., 1996; Kang et al., 2014; Lefkowitz and Shenoy, 2005). β-arrestins also act as signal transducers and scaffold proteins in several other developmentally important signaling pathways (Kovacs et al., 2009; Peterson and Luttrell, 2017), such as the receptor tyrosine kinase (RTK)/mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) (DeFea et al., 2000; Luttrell et al., 1999; Tipping et al., 2010), Hedgehog/Smoothened (Chen et al., 2004; Kovacs et al., 2008; Li et al., 2012; Molnar et al., 2011), Wnt/β-catenin (Bryja et al., 2007; Chen et al., 2001; Chen et al., 2003), Notch (Mukherjee et al., 2005; Puca et al., 2013) and Toll/NF-κB (Anjum et al., 2013; Gao et al., 2004; Tipping et al., 2010; Witherow et al., 2004) pathways. Despite this knowledge, the in vivo functions of β-arrestins are not well understood. Pharmacological importance of the GPCR signaling pathways (Hauser et al., 2018) and a growing appreciation for using β-arrestins as possible therapeutic targets (Peterson and Luttrell, 2017) warrant further investigation of their roles in organism physiology and development.
Studies in mammalian systems have identified numerous regions and individual residues in the visual arrestins and β-arrrestins that are important for GPCR regulation (reviewed in (Gurevich and Gurevich, 2012; Peterson and Luttrell, 2017; Scheerer and Sommer, 2017)). Two categories of important motifs in arrestins mediate receptor-proximal signaling events: residues that directly bind to GPCRs and residues that bind to endocytosis-related proteins (reviewed in (Peterson and Luttrell, 2017)). Phosphate-sensor residues K14 and K15 (Fig. 1A, red) are required for the bovine visual arrestin (arrestin-1, also called S-antigen visual arrestin, or SAG) binding to light-activated, phosphorylated rhodopsin in vitro (Vishnivetskiy et al., 2000; Zhou et al., 2017), and homologous residues also mediate interactions of β-arrestins with cognate GPCRs (Gimenez et al., 2012). Crystallographic studies confirmed that these residues are involved in electrostatic interactions of β-arrestin-1 (arrestin 2) with the phosphorylated C terminus of the V2 vasopressin receptor (Shukla et al., 2013). Residue R29 (Fig. 1A, orange) in bovine visual arrestin-1 binds to arrestin’s own C tail in an inactive (closed) state, but associates with the phosphorylated C terminus of rhodopsin in the activated state (Ostermaier et al., 2014). Interestingly, the R29A mutant showed the strongest reduction in binding to phosphorylated rhodopsin, compared to other single amino acid mutations in bovine visual arrestin-1 (Ostermaier et al., 2014). The “finger loop” of arrestins (Fig. 1A, yellow) significantly changes its conformation when the interaction between arrestin and phosphorylated GPCR is established (Hanson et al., 2006; Kang et al., 2015; Szczepek et al., 2014). This conformational change allows for the hydrophobic interaction of finger loop residues with the receptor core and prevents the interaction of the receptor with G proteins (Cahill et al., 2017; Chen et al., 2017; Shukla et al., 2014; Szczepek et al., 2014; Thomsen et al., 2018). This interaction appears to be important for the GPCR desensitization function of β-arrestins.
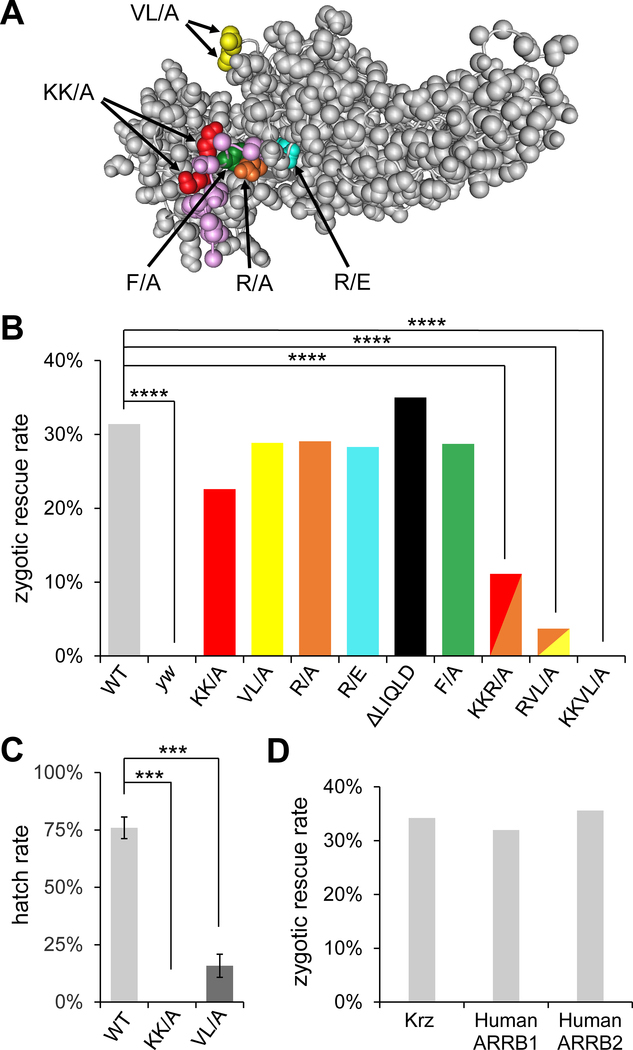
Fig. 1. Phospho-sensing motif and the finger loop are important for Krz function in vivo.
(A) A molecular model of bovine β-arrestin-1 (PDB accession 1G4R), with the corresponding mutations in Krz from this study indicated and highlighted in color. The C terminal tail of β-arrestin-1 is shown in magenta. The region corresponding to the LIQLD motif is disordered in the structure and not shown. (B) Results of the zygotic rescue experiments. Percentages of rescued adults in the krz1 homozygous mutant background are shown for the wild type (WT) genomic rescue construct krz5.7-SBP and the derived constructs carrying the indicated mutations. ****, p<0.0001 in chi-squared test. Expected maximal rescue rate by the WT construct is 33% (see Fig. S2 for details of the genetic crosses). Numbers of adult flies scored for each condition were: WT=156; yw=146; KK/A=202; R/A=289; VL/A=132; R/E=106; ΔLIQLD=125; F/A=101; KKR/A=198; RVL/A=216; KKVL/A=598. (C) Hatch rates of embryos obtained from homozygous krz1 females carrying the indicated krz5.7-SBP rescue constructs. ***, p<0.001 in chi-squared test. (D) da-GAL4 driven expression of krz as well as human β-arrestin-1 (ARRB1) and β-arrestin-2 (ARRB2) can rescue homozygous krz1 animals to adulthood.
Among the residues involved in interactions with endocytic components, the conserved LIE(F/L)(E/D) motif in the C terminus of β-arrestins is necessary for their interactions with clathrin (Kang et al., 2009; Krupnick et al., 1997). A component of the AP2 complex, β2-adaptin colocalizes with clathrin-coated pits on the cell surface and is involved in endocytosis (Kirchhausen, 2000). The F391A mutant (Fig. 1A, green) in bovine β-arrestin-1 abolished its interaction with AP2, but retained its ability to bind to β2-adrenergic receptor (β2AR) in vitro (Kim and Benovic, 2002). The F391 residue directly contacts the appendage domain of β2-adaptin (Edeling et al., 2006).
A “constitutively active” mutant of bovine β-arrestin-1, R169E (Fig. 1A, magenta), bound to β2AR in a phosphorylation-independent manner and increased desensitization of unphosphorylated β2AR and the δ opioid receptor lacking G protein-coupled receptor kinase (GRK) phosphorylation sites (Kovoor et al., 1999). Despite its ability to bind the unphosphorylated receptors, the R169E mutant variant still required receptor activation for binding. Most of the structural arrestin elements described above are highly conserved across species (Fig. S1). However, the functional importance of these motifs in vivo is unknown, since their role in GPCR signaling has only been studied in vitro or in cultured cells.
Kurtz (Krz) is the only ortholog of mammalian β-arrestins in the Drosophila genome. Sequence similarity values between Krz and human β-arrestin-1 and β-arrestin-2 are 74% and 72%, respectively (Roman et al., 2000). A high degree of conservation of the overall sequence and individual structural motifs, combined with powerful genetic tools available in Drosophila, make Krz a good model protein to study β-arrestin structure-function relationships in vivo. We have previously shown that maternally contributed Krz plays critical roles in Drosophila embryonic development, including gastrulation (Tipping et al., 2010). During gastrulation, apical constriction of cells in the ventral midline is controlled by signaling downstream of the Folded gastrulation (Fog) ligand (Costa et al., 1994), which activates GPCRs Mist (also known as Mthl1) (Manning et al., 2013) and Smog (Kerridge et al., 2016). Fog signaling results in the activation of the Gα12/13 homolog Concertina (Cta) (Parks and Wieschaus, 1991), followed by activation of RhoGEF2 and Rho1 (Barrett et al., 1997), which transmit the signal to the kinase Rok that phosphorylates the regulatory light chain of non-muscle myosin II, Spaghetti squash (Sqh) (Karess et al., 1991), and causes apical constriction (Coravos and Martin, 2016; Dawes-Hoang et al., 2005; Kasza et al., 2014; Manning and Rogers, 2014; Martin et al., 2009; Mason et al., 2016; Morize et al., 1998). Fog signaling is also active in the larval wing discs, where overexpression of the Fog ligand can induce tissue misfolding and wing defects (Manning et al., 2013). Krz was recently shown to regulate the endocytosis of the Smog receptor (Jha et al., 2018).
Here, we took advantage of Drosophila as an experimental system to identify structural determinants that are critical for β-arrestin function in vivo. We identified the Krz-KKVL/A mutant, which affects both the phosphosensor and finger loop motifs, as a functional null. These mutations can also individually impair Krz function, suggesting that direct β-arrestin/GPCR binding is critical for β-arrestin activity in vivo. Furthermore, we show that mutations in Krz disrupt epithelial morphogenesis events in Drosophila via aberrant upregulation of the Fog-Mist signaling pathway. These findings advance our understanding of the molecular mechanisms of β-arrestin-mediated GPCR regulation that are important for organism development.
Results
Phospho-sensing domain and the finger loop are required for Krz function in vivo
To evaluate in vivo functional importance of structural elements in Krz, we made transgenic flies containing streptavidin binding peptide (SBP)-tagged Krz mutants, carried within a genomic rescue construct, krz5.7-SBP (see Materials and Methods). These transgenic lines were used in a genetic assay to test for their ability to rescue homozygous lethality of the krz1 allele, which eliminates Krz protein and mRNA expression (Roman et al., 2000; Tipping et al., 2010) (see Fig. S2 for a crossing scheme used in rescue experiments). We focused on several functional motifs that were previously shown to affect β-arrestin interactions with the activated GPCRs and endocytic components (Fig. 1A, Table 1, and Fig. S1). Expression of tagged proteins was verified by western blotting with arrestin and SBP antibodies (Fig. S3). Consistent with a previous study (Roman et al., 2000), the wild-type krz5.7-SBP construct completely rescued the lethality of the homozygous krz1 allele (Figs. 1B and S2; expected full zygotic rescue rate is 33%). Importantly, zygotic overexpression of HA-tagged human β-arrestin-1 or β-arrestin-2 using the ubiquitously expressed da-GAL4 driver also rescued the lethality of homozygous krz1 (Fig. 1D), indicating that the functionally important elements are conserved between Krz and its mammalian orthologs.
Table 1. Krz mutations used in this study, and their effects on viability.
Corresponding mutations in the mammalian orthologs were described in the following studies: phosphate sensor (KK/A) and phosphate binding (R/A) (Gimenez et al., 2012; Ostermaier et al., 2014; Shukla et al., 2013; Vishnivetskiy et al., 2000; Zhou et al., 2017), finger loop (VL/A) (Cahill et al., 2017; Hanson et al., 2006; Szczepek et al., 2014), phosphorylation-independent activated receptor binding (R/E) (Kovoor et al., 1999), clathrin binding (ΔLIQLD) (Kang et al., 2009; Krupnick et al., 1997), AP2 adaptor binding (F/A) (Edeling et al., 2006; Kim and Benovic, 2002).
| Location in Krz | Mutant abbr. | Location in bovine visual arrestin-1 (SAG) | Location in human β-arrestin-1 (ARRB1) | Affected function | Rescue of zygotic lethality | Maternal/zygotic phenotype |
|---|---|---|---|---|---|---|
| K51, K52 | KK/A | K14, K15 | K10, K11 | phosphate sensor | + | embryonic lethal |
| R66 | R/A | R29 | R25 | phosphate binding | + | adult viable |
| V111, L112 | VL/A | V74, M75 | V70, L71 | finger loop: receptor core binding | + | semi-lethal |
| R213 | R/E | R175 | R169 | phosphorylation-independent activated receptor binding | + | adult viable |
| L454-D458 | ΔLIQLD | N/A | L376-D380 | clathrin binding | + | adult viable |
| F472 | F/A | F380 | F391 | AP2 adaptor binding | + | adult viable |
| K51, K52, R66 | KKR/A | K14, K15, R29 | K10, K11, R25 | double: phosphate sensor/binding | + | embryonic lethal |
| K51, K52, V111, L112 | KKVL/A | K14, K15, V74, M75 | K10, K11, V70, L71 | double: phosphate sensor + finger loop | − | N/A |
| R66, V111, L112 | RVL/A | R29, V74, M75 | R25, V70, L71 | double: phosphate sensor + finger loop | +/− | N/A |
Unexpectedly, all constructs carrying individual motif mutations rescued krz1 homozygotes to adulthood (Fig. 1B and Table 1), suggesting that none of these mutations can independently disrupt Krz zygotic function. Since Krz is maternally expressed, and krz maternal mutant embryos have a stronger phenotype than zygotic mutants (Tipping et al., 2010), we tested for a maternal/zygotic effect of our mutations by analyzing the progeny of the rescued females (Fig. S2). Interestingly, flies rescued by the Krz-KK/A mutant construct laid eggs that did not develop beyond the embryonic stage (Fig. 1C and Table 1), and the progeny of the Krz-VL/A-rescued flies had a strongly reduced hatch rate (15.8%, compared to 75.6% for flies rescued with wild type Krz, Fig. 1C). Some of the progeny from the Krz-VL/A-rescued females survived to adulthood, but those flies invariably died within a few days (semi-lethal phenotype, Table 1). Therefore, mutations that impair direct interactions between β-arrestins and GPCRs (phospho-sensing, KK/A, and finger loop, VL/A) had the strongest effects on the ability of Krz to rescue, whereas other mutations did not appear to affect its developmental functions to the same extent.
Since the KK/A and VL/A mutations exhibited the strongest maternal effect, we hypothesized that a combination of these mutations would further impair Krz function. Indeed, the combined Krz-KKVL/A mutant failed to rescue krz1 homozygous mutants in a zygotic rescue assay (Fig. 1B, Table 1). We also generated two more double mutant combinations, Krz-KKR/A and Krz-RVL/A, that incorporated another predicted phosphate-binding residue, R66 (see Table 1). Since the R/A mutation alone did not have maternal effect (Table 1), we expected its effects in these mutant combinations to be weaker than those of KKVL/A, but stronger than KK/A or VL/A only. Indeed, constructs carrying Krz-KKR/A or Krz-RVL/A mutants rescued krz1 homozygotes to adulthood, but the rescue rates of both were significantly reduced (Fig. 1B). The progeny of KKR/A rescued flies were embryonic lethal, and RVL/A maternal effect could not be established because the rescued adults were semi-lethal (Table 1). Collectively, our analysis of the various mutant variants of Krz showed that the residues that are involved in direct β-arrestin/GPCR interactions (specifically, the phospho-sensing and finger loop motifs) are the ones that are most critical for developmental functions of Krz in vivo.
Phospho-sensing domain and the finger loop are required for Krz recruitment to GPCRs and formation of endosomes
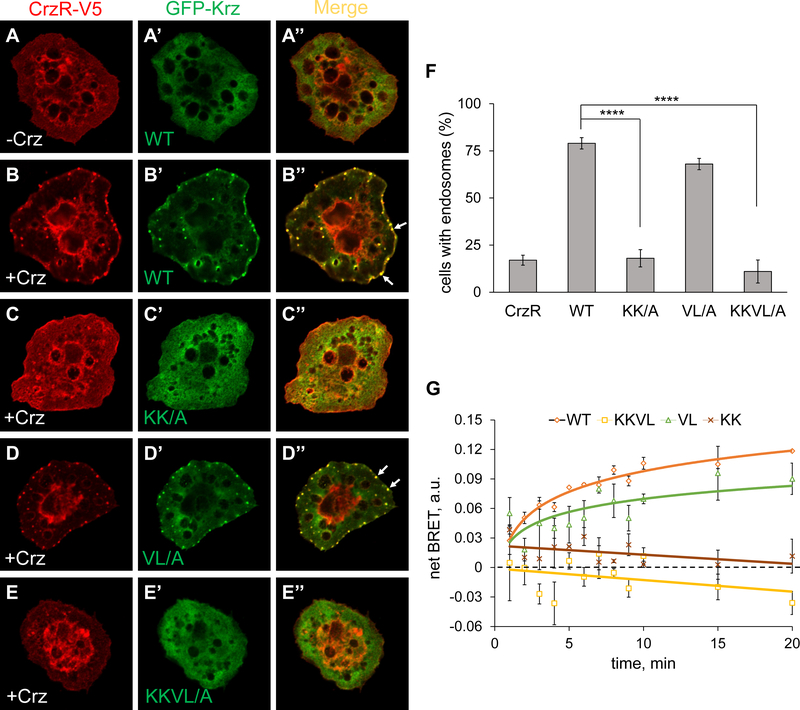
Based on the results of genetic rescue experiments, we hypothesized that the Krz-KKVL/A mutant variant was defective in receptor interactions, which resulted in a complete loss of Krz function. To investigate this possibility, we utilized two cell-based assays. First, we used a neuronal GPCR, Corazonin (Crz) receptor (CrzR), that can be activated with its respective ligand Crz in Drosophila cultured S2 cells (Johnson et al., 2008), to study the recruitment of GFP-Krz and the receptor to intracellular endosomes. CrzR is a Class B GPCR which readily forms endosomes with β-arrestins upon ligand stimulation, visible as puncta around the cell periphery (Johnson et al., 2008; Oakley et al., 2000). GFP-Krz showed diffused cytoplasmic localization in the absence of Crz (Fig. 2A–A’’), but robustly colocalized with CrzR-V5 in endosomes upon stimulation (Fig. 2B–B’’). In contrast, GFP-Krz-KKVL/A did not colocalize with CrzR and retained cytoplasmic distribution (Fig. 2E–E’’). Interestingly, GFP-Krz-KK/A also remained in the cytoplasm upon stimulation (Fig. 2C–C’’), while GFP-Krz-VL/A colocalized with the receptor, with a slightly but not significantly reduced number of cells containing endosomes (Figs. 2D–D’’ and 2F). Therefore, loss of interactions with the phosphorylated receptor, represented by the KK/A mutation, had a stronger effect on the ability of Krz to be recruited to the endocytic structures upon receptor activation, compared to the finger loop mutation (VL/A). The essentially normal endocytosis capability of the Krz-VL/A mutant observed in this assay was surprising, given the fact that the maternal rescue capability of this mutant was impaired (see Table 1).
Fig. 2. Phospho-sensing motif is required for Krz recruitment to the Corazonin receptor.
Localization of CrzR-V5 (red) and GFP-Krz (green, wild type or indicated mutants) in transfected S2 cells, stained with anti-V5 and anti-GFP antibodies. (A-A’’) Without agonist (corazonin, Crz), CrzR and Krz and diffusely cytoplasmic. (B-E’’). Localization of CrzR and GFP-Krz 10 min after addition of corazonin. Krz-KK/A and Krz-KKVL/A did not induce CrzR relocalization into endosomes. Arrows indicate representative endosomes in wild type Krz and Krz-VL/A transfections. (F) Quantification of cells containing endosomes. A cell was counted as positive if it contained 3 or more endosomes. ****, p<0.0001 in chi-squared test. (G) Interactions between wild type (WT) Krz-mVenus (or an indicated mutant) and CrzR-Rluc8 analyzed by a BRET assay. Net BRET is shown as a function of time upon stimulation of cells with 1 μM corazonin at 25 °C. The data represent the mean ±SEM of three independent experiments.
To obtain a more direct measure of interactions between Krz mutants and activated CrzR, we used a bioluminescence resonance energy transfer (BRET) assay (Donthamsetti et al., 2015), in which the receptor is tagged with luciferase (RLuc8) and Krz is tagged with Venus. Light transfer from RLuc8 to Venus can only occur when the two molecules are in close proximity, which requires a direct β-arrestin/GPCR interaction. Upon ligand addition, wild type Krz-Venus translocated to the receptor, increasing the BRET signal, whereas Krz-KK/A-Venus and Krz-KKVL/A-Venus failed to respond to ligand stimulation (Fig. 2G). The VL/A mutation did not significantly affect the ability of Krz-VL/A-Venus to interact with CrzR (Fig. 2G). In summary, endosome recruitment and BRET assays confirmed that the Krz-KKVL/A mutant lost its ability to interact with activated GPCRs, and that the phospho-sensing KK/A mutation had a stronger effect on Krz-receptor interactions than the finger loop VL/A mutant. These results generally agreed with the rescuing ability of these mutants (Fig. 1), with the KK/A-bearing constructs being most severely impaired.
Krz controls Fog signaling during gastrulation
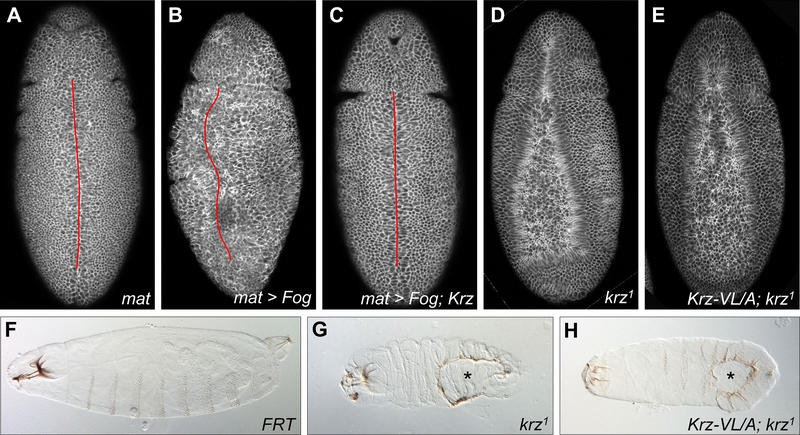
We then asked which developmental processes were affected by Krz mutations. During gastrulation in Drosophila, apical constriction of the ventral epithelial cells is initiated by the Fog signaling pathway, in which the secreted Fog ligand signals through GPCRs Mist and Smog (Kerridge et al., 2016; Manning et al., 2013). Overexpression of Fog using the maternal driver matα4-GAL4-VP16 impaired the formation of the ventral midline during gastrulation, resulting in embryo twisting and an incomplete midline closure (Figs. 3A and 3B). Co-expression of Fog with Krz restored this phenotype to wild-type (Fig. 3C). These findings suggest that higher Krz levels can suppress overactive Fog signaling during gastrulation.
Fig. 3. Krz limits Fog pathway activity in the embryo.
Embryos in (A-E) were stained with anti-Dlg antibody. (A-C) Overexpression of Krz suppressed the defective ventral midline phenotype resulting from Fog overexpression. Twisting and abnormal cell constrictions are visible in (B). Ventral midline is indicated by a red line. (D-E) krz1 and krz5.7-VL/A; krz1 maternal mutant embryos showed a similar delayed ventral furrow formation phenotype. (F-H) Cuticular preparations of (F) FRT control, (G) krz1 and (H) krz5.7-VL/A; krz1 maternal mutants. Ventral holes in the mutants (G-H) are indicated with asterisks.
We then asked whether Krz is required for limiting Fog pathway activity during gastrulation. Fog signaling induces the constriction of apical cells through the phosphorylation of the regulatory light chain of non-muscle myosin II, Spaghetti squash (Sqh) (reviewed in (Manning and Rogers, 2014)). Previous studies showed that overexpression of Fog delayed ventral furrow formation due to aberrant myosin localization and dynamics (Dawes-Hoang et al., 2005; Fuse et al., 2013; Morize et al., 1998). To obtain a dynamic view of the consequences of krz loss during gastrulation, we performed time-lapse imaging of gastrulating embryos carrying Sqh-GFP. As previously described (Martin et al., 2009), wild type control embryos showed pulses of Sqh-GFP concentrated in the medial-apical regions in cells along the ventral midline (Video 1). These cells were subsequently internalized into the embryo, resulting in a properly closed ventral furrow after approximately 15 min. In contrast, maternal krz1 mutants showed persistent localization of Sqh-GFP in the medial-apical regions of cells along the ventral midline, and this pattern persisted without a proper invagination for over 30 min (Video 2). In severe cases, persistent cellular contractility resulted in tissue rupturing and loss of epithelial organization (Video 2). These results suggest that Krz is required to limit the activity of the Fog signaling pathway and prevent abnormal cellular contractility in Drosophila gastrulation.
Finger loop is required for Krz function during gastrulation
To test whether Krz’s ability to interact with GPCRs is required for its function during gastrulation, we characterized maternal effects of GPCR binding-defective Krz mutations. Since the combined Krz-KKVL/A mutant cannot rescue krz1 homozygotes, we analyzed embryos from Krz-KK/A or Krz-VL/A rescued krz1 homozygous females crossed with wild type males. Embryos from Krz-KK/A rescued females died before cellularization and were not analyzed further. Embryos from Krz-VL/A rescued females showed delayed ventral furrow formation (Fig. 3E) which was similar to the defects in krz1 maternal mutants (Figs. 3D and S4). Cuticle preparations of embryos from Krz-VL/A rescued females revealed large ventral holes, again resembling cuticular defects in krz1 maternal mutants (Fig. 3F–H and (Tipping et al., 2010)). These results demonstrate that the ability of Krz to directly interact with the GPCR core (specifically, via the finger loop) is critical for its function as an inhibitor of Fog signaling during early embryonic development.
Krz controls the Fog-Mist pathway during wing development
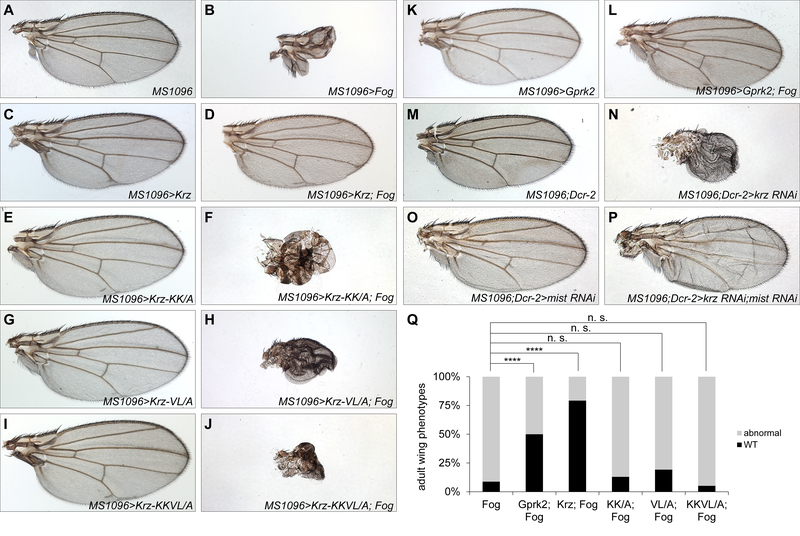
Since the Fog-Mist signaling pathway also controls the folding of wing imaginal discs (Manning et al., 2013), we asked whether Krz functions as an inhibitor of this pathway during wing development. Overexpression of Fog resulted in a severe phenotype in which most of the surface of the wing blade was missing (Fig. 4A, B). It is likely that this phenotype results from abnormal cellular constrictions and folding defects in wing imaginal discs that are due to hyperactivity of Mist. Remarkably, co-expression of Fog with GFP-Krz strongly suppressed this phenotype (Fig. 4C, D, Q). Co-expression of Gprk2, the Drosophila ortholog of human G protein-coupled receptor kinases (GRKs), also suppressed Fog gain of function wing phenotype (Fig. 4K, L, Q). In contrast, co-expression of GFP-Krz-KK/A, GFP-Krz-VL/A, or GFP-Krz-KKVL/A with Fog could not restore the adult wing phenotype (Fig. 4E–J, Q). These experiments show that overexpression of Krz is sufficient to inhibit overactive Fog-Mist signaling in the Drosophila wing, and both of the GPCR-interacting motifs in Krz are required for this function.
Fig. 4. Genetic interactions between Krz and the Fog-Mist signaling pathway in the wing.
(A-P) Adult wing phenotypes resulting from overexpression of the indicated UAS transgenes under the control of the MS1096-GAL4 driver. (A) MS1096-Gal4. (B) A major wing defect observed with Fog overexpression. (C, E, G, I) Overexpression of wild type Krz or the indicated mutants did not result in abnormal wing development. (D) Overexpression of wild type Krz suppressed Fog-induced abnormal wing development, however mutant Krz variants failed to suppress those defects: (F) Krz-KK/A, (H) Krz-VL/A, (J) Krz-KKVL/A. (K, L) Co-erexpression of Gprk2 suppressed wing defects associated with Fog overexpression. (M-P) Genetic interaction between krz and mist. (M, N) Knockdown of krz by RNAi resulted in unexpanded and misfolded wings. (O, P) Simultaneous knockdown of krz and mist partially restored wing development. (Q) Quantification of wing phenotypes shown in (A-L). ****, p<0.0001 in chi-squared test; n. s., not significant; WT, wild type.
To test whether Krz is required for limiting the activity of Fog-Mist signaling in the wing, we used RNAi knockdown of krz in combination with mist. Knockdown of krz alone using krz RNAi co-expressed with Dicer-2 (Dcr-2) resulted in un-expanded, misfolded adult wings (Fig. 4M, N). Whereas knockdown of mist alone had a minor effect (Fig. 4O), a joint knockdown of krz together with mist resulted in a restoration of wing expansion and proper folding, compared to the effects of krz alone (Fig. 4N, P). Collectively, our studies of genetic interactions between Krz and the Fog signaling pathway in the wing show that Krz limits the activity of Fog-Mist signaling during wing development, and suggest that this activity of Krz relies on its ability to interact with the activated Mist receptor.
Discussion
Our study reveals the developmentally important structural elements in the Drosophila β-arrestin Krz. Several lines of evidence, obtained in vivo and in cultured Drosophila cells, support a view that the residues most critical for Krz function during development include the phosphate-sensing region (disrupted by the KK/A mutation) and the finger loop (disrupted by the VL/A mutation) (Fig. 5). These motifs are engaged in direct interactions with the phosphorylated GPCR tail and the receptor core region, respectively, and both contribute to the tight binding of β-arrestins to the receptor (Cahill et al., 2017; Thomsen et al., 2018). Since such interactions mediate efficient uncoupling of the receptor from G proteins, it appears that the primary function of Krz in development is to inhibit GPCR signaling via receptor desensitization.
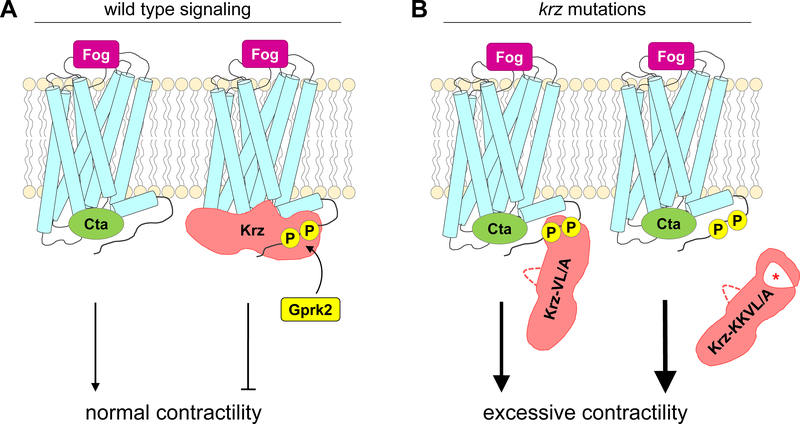
Fig. 5. A model summarizing the effects of the VL/A and KKVL/A mutations on Krz-GPCR interactions and cellular contractility.
(A) In wild type Fog-Mist signaling, the G protein Cta transmits the signal from the Mist receptor activated by its ligand Fog. Mist is then phosphorylated by Gprk2, followed by an interaction with Krz that properly terminates signaling. (B) The finger loop mutation (VL/A) interferes with the ability of Krz to uncouple Mist from Cta, while still allowing partial association with the Gprk2-phosphorylated residues in the receptor tail. Combination of the finger loop and phosphate sensor mutations (KKVL/A) leads to inability of Krz to associate with the receptor. Both the VL/A and KKVL/A mutations result in hyperactivity of the Fog-Mist signaling pathway, leading to excessive cellular contractility.
The combined mutation Krz-KKVL/A resulted in a complete inactivation of Krz as a developmental regulator, however the disruption of the two key phosphate-sensing residues together with an additional phosphate-binding residue (KKR/A), leaving the finger loop intact, was not sufficient to eliminate Krz zygotic function (Table 1). Structural studies suggest that the G proteins and the finger loop of arrestins make contacts with the same region in GPCRs (Cahill et al., 2017; Kang et al., 2015; Shukla et al., 2014; Szczepek et al., 2014; Thomsen et al., 2018). Therefore, the binding of arrestins’ finger loop to the receptor directly interferes with the GPCR/G protein interactions, and mutations of this motif are expected to disrupt the ability of arrestins to uncouple GPCRs from G proteins. In support of this view, we found that embryos from Krz-VL/A-rescued females showed gastrulation defects and cuticular phenotypes that were similar to the ones observed for krz1, the strongest loss of function allele of krz. These findings suggest that the finger loop region of Krz is critical for its in vivo functions. We noted that embryos obtained from Krz-KK/A-rescued females aborted development before cellularization, which is a phenotype that is even stronger than that observed in maternal krz1 mutants. The KK/A mutation may thus have a dominant-negative effect, possibly due to a global hyperactivity and dysregulation of multiple GPCRs.
A major GPCR system involved in early Drosophila embryogenesis is the Fog signaling pathway. After several steps of signal transduction, activation of Fog signaling culminates in phosphorylation of Sqh (reviewed in (Manning and Rogers, 2014)), which controls acto-myosin contractility and mediates apical constrictions. Setting a proper level of cellular contractility is required for gastrulation movements. Consistent with a recent report (Jha et al., 2018), we found that loss of krz resulted in excessive accumulation of Sqh-GFP in the mid-apical region of ventral cells, indicating overactivation of Fog signaling (Fig. S4). Live imaging showed that mid-apical accumulation of Sqh-GFP persists much longer in krz maternal mutants than in wild type embryos, resulting in a stalled and aberrant gastrulation (Videos 1 and 2). We noted that the area of cells undergoing apical constriction was wider in krz1 maternal mutants, which may be explained in part by ectopic activity of the Toll signaling pathway, that may lead to an expansion of Fog and Mist expression domains downstream of Twist activation (Manning and Rogers, 2014; Tipping et al., 2010). However, medial-apical Sqh-GFP localization persisted in krz1 mutants even in cells along the ventral midline (Video 2), suggesting that Krz is required to limit the activity of the Fog pathway within the normal domain of its activation. In support of this view, maternal knockdown of krz by RNAi did not result in an expansion of Twist expression, yet led to abnormal mid-apical accumulation of Sqh (Jha et al., 2018).
Fog signals through its receptors Mist and Smog (Kerridge et al., 2016; Manning et al., 2013), and Krz may control Smog signaling at the level of endocytosis (Jha et al., 2018). Our analysis of Krz mutants that were expected to disrupt clathrin and AP2 interactions (Krz-ΔLIQLD and Krz-F/A, respectively) showed that neither of these mutations affected Krz functions (Fig. S5). Both Krz variants rescued krz1 homozygotes to adulthood, did not have maternal effect (Table 1), and internalized into endosomes with CrzR upon ligand activation, though Krz-ΔLIQLD and the double Krz-ΔLIQLD+F/A mutant did show a mild reduction in the percentage of cells containing endosomes (Fig. S5). It is possible that Krz relies on other structural elements to mediate GPCR endocytosis that were not included in our analysis, such as a short motif present in Krz that resembles the second clathrin-binding region in the long splice variant of β-arrestin-1 (Kang et al., 2009; Sterne-Marr et al., 1993). However, it is also possible that the endocytic function of Krz is secondary to the role of direct receptor engagement. Consistent with this view, we found that mutation of the finger loop (Krz-VL/A) resulted in a strong reduction in Krz function (Table 1 and Fig. 3), without affecting endocytosis (Fig. 2). Deletion of the finger loop region in rat β-arrestin-1 also did not affect its ability to internalize GPCRs but impaired its ability to uncouple the receptor from G proteins (Cahill et al., 2017). These results suggest that Krz-mediated attenuation of GPCR and G protein signaling via direct receptor interactions is critical during early development in Drosophila, though receptor internalization may also be involved as an additional regulatory step.
In addition to controlling gastrulation, the Fog-Mist signaling is involved in the development of the wing, and our data show that Krz-mediated regulation of this pathway also occurs in this tissue. The effects of the phospho-sensing and finger loop mutations were even more pronounced in the wing, as neither Krz-KK/A nor Krz-VL/A were capable of effectively suppressing the gain of function Fog phenotype (Fig. 4). Thus, Krz controls Fog-Mist signaling in the embryo and in the wing, and perhaps in other tissues where this pathway guides epithelial morphogenesis. It was previously reported that loss of krz had no significant effect on wing development (Molnar et al., 2011). It is possible that the discrepancy with our results was due to the use of the specific GAL4 drivers used in that study (638-GAL4 and nub-GAL4), that may be expressed in a more restricted pattern or at a lower level than the MS1096-GAL4 driver that we used. Consistent with our observation of Krz requirement in wing development, homozygous mutant krz1 flies that are rescued with the elavC155-GAL4 driver, which is primarily expressed in the nervous system, have abnormal wings (Roman et al., 2000) – a result we confirmed (data not shown).
Phosphorylation of GPCRs by G protein-coupled receptor kinases (GRKs in mammals, Gprk2 in Drosophila) is a required step for the recognition of the activated receptor by arrestins (Gurevich et al., 2012). Previously, mutations in Gprk2 were shown to result in gastrulation defects that are remarkably similar to the phenotypes we observed in krz maternal mutants ((Fuse et al., 2013) and Fig. 3D), suggesting that phosphorylation of GPCRs by Gprk2 is required for the binding of Krz to the receptors. In support of this view, the Krz-KK/A mutant, which disrupts the phosphate-sensing residues, lost its ability to suppress the Fog overexpression phenotype in the wing and exhibited a strong maternal effect. We also found that overexpression of Gprk2 itself could suppress overactive Fog signaling in the wing (Fig. 4K, L). Gprk2 and Krz thus operate in concert to inhibit GPCR signaling (Fig. 5).
While Krz has been implicated in the regulation of several signaling pathways (Anjum et al., 2013; Johnson et al., 2008; Li et al., 2012; Molnar et al., 2011; Mukherjee et al., 2005; Tipping et al., 2010), this study highlights the developmental importance of Krz in controlling its prototypical targets, GPCRs. In all contexts examined here, Krz plays an inhibitory role, which is likely mediated by direct engagement of the activated GPCRs via the two-part recognition of the phosphorylated receptor tail and its core (Fig. 5 and (Cahill et al., 2017; Thomsen et al., 2018)). It will be of interest to examine how Krz coordinates its involvement in the various pathways active in the early embryo, such as the GPCR, ERK, and Toll signaling pathways, and whether these pathways are differentially affected by the mutations studied here. Of note, we found that the pre-activating mutation Krz-R/E (Table 1) did not affect Krz interaction with the Toll pathway regulator Cactus, but did increase the association of Krz with ERK (Tipping et al., 2010). With regard to ERK signaling, we previously showed that Krz inhibits ERK activation downstream of receptor tyrosine kinases in Drosophila development (Tipping et al., 2010). One question for future studies is to determine whether Krz is involved in the positive signaling events downstream of GPCRs, given the recent interest in developing G protein- and β-arrestin-biased ligands that modulate ERK activation and can increase the specificity and efficacy of GPCR-directed therapies (Wootten et al., 2018). Rescue of krz1 homozygous animals by both human β-arrestins (Fig. 1D) shows significant functional conservation and suggests that Drosophila Krz can serve as a platform for modeling mammalian β-arrestin functions.
Conclusions
In this work, we have used the Drosophila β-arrestin Kurtz (Krz) to identify conserved structural elements in the β-arrestin molecule that are functionally important in vivo. We found that some of the previously identified elements appear to be dispensable. Nonetheless, we revealed that two regions in Krz are critical for its activity: a phosphate sensing motif which interacts with the phosphorylated GPCR tail, and a “finger loop” region that directly contacts the GPCR core. The finger loop mutation (VL/A) is notable because it severely affected Krz function without having a significant effect on its ability to internalize a GPCR in endosomes. We showed that the GPCR-binding Krz mutations disrupt its ability to limit the activity of the Fog-Mist signaling pathway that plays a key role during epithelial morphogenesis events, such as embryo gastrulation and folding of the wing epithelium. Our studies thus uncovered the structural motifs in β-arrestins that are critical for their developmental functions as GPCR regulators in vivo.
Materials and Methods
Plasmid construction
Drosophila Krz and Corazonin receptor (CrzR), Mist (Mthl1), mVenus, Rluc8 (Addgene) open reading frames were amplified by PCR using tag and/or restriction site-containing primers and cloned into pMT/V5-HisB vector (Invitrogen) to generate carboxy-terminally tagged CrzR-V5, CrzR-Rluc8-V5, Mist-V5 and amino-terminally tagged GFP-Krz and mVenus-Krz. pMT-GFP-Krz or pMT-mVenus-Krz plasmids were then used as templates to make tagged Krz mutants by overlap PCR. GFP-Krz mutants were cloned into the pUAST-attB vector (Bischof et al., 2007) for transgenic expression in flies. An SBP tagged genomic krz rescue construct (krz5.7-SBP) (Tipping et al., 2010) was used as a template to make SBP-tagged genomic Krz mutants by overlap PCR. Krz-SBP mutants were cloned into the pattB vector (Potter et al., 2010) for transgenic expression in flies. Human ß-arrestin-1 and ß-arrestin-2 cDNAs were obtained from Invitrogen/ResGen, amplified by PCR with an HA tag at the amino terminus, and inserted into the pUAST vector for transgenic expression in flies.
Drosophila melanogaster stocks
All Drosophila stocks were maintained on standard yeast-cornmeal-agar medium at 25 °C krz1 allele (Roman et al., 2000), MS1096-GAL4, matα4-GAL4-VP16, UAS-Dcr-2 (Dietzl et al., 2007) were obtained from the Bloomington Drosophila Stock Center. UAS-krz RNAi (GD #8470), UAS-mist RNAi (GD #33135) were from the Vienna Drosophila Resource Center (VDRC). Transgenic lines were generated by Rainbow Transgenic Flies. The sqh-GFP line was a gift from Adam Martin. The UAS-fog line was a gift from Eric Wieschaus. krz1 maternal mutant embryos were generated by crossing FRT82B krz1/TM6B males with HS-FLP22; sqh-GFP; FRT82B ovoD1/TM6B females and heat shocking the progeny larvae twice for 2 hrs at 37 °C.
To test for the ability of various Krz mutants to rescue krz1 lethality, crosses were set up using wild type and mutant SBP-krz5.7 genomic transgenes, as shown in Fig. S2. The yw line was used as a negative control. The zygotic rescue rate was calculated as the percentage of non-Hu (i.e., krz1 homozygous) flies among all progeny of the F2 self-cross (Fig. S2). Based on the law of independent assortment, the theoretical maximal rescue rate with the wild type SBP-krz5.7 transgene is 33% (1/3 of all progeny), given the homozygous lethality of the TM6B balancer.
Time lapse imaging
Control (FRT82B) or krz1 maternal mutant embryos carrying the sqh-GFP transgene were dechorionated, mounted under halocarbon 27 oil as described in (Mason et al., 2016), and imaged using the 40x/1.3 oil immersion objective on a Zeiss LSM 880 confocal microscope. Continuous z stacks were acquired at 10 μm total thickness (11 frames), at ~10 sec/stack. Each individual stack was processed using the maximum intensity projection function in Zen software, to generate a single frame for the video. Videos were then exported at 24 frames/sec.
Antibodies and immunostaining
Antibodies used for cell and embryo immunostaining were as follows: mouse anti-Dlg (1:50, Developmental Studies Hybridoma Bank), rabbit anti-GFP (1:500, Molecular Probes) and mouse anti-V5 (1:500, Sigma). For Western blotting, the following antibodies were used: rabbit anti-pan-arrestin (1:1000, Affinity Bioreagents) and mouse anti-SBP (1:200, Santa Cruz). Secondary antibodies were from Invitrogen (immunofluorescence) and LI-COR (Westerns). For immunofluorescence staining, embryos were collected on apple juice/agar plates with yeast paste at 25 °C and fixed in 4% formaldehyde in PBS/heptane, then devitellinized in methanol. Embryos were rehydrated in PBT (1 × PBS with 0.1% Tween-20, Sigma) and incubated for 2 hrs at room temperature in blocking buffer (1:1 of PBT and Roche blocking buffer, Sigma), then incubated with primary antibody diluted in blocking buffer overnight at 4 °C. Embryos were washed with PBT containing 0.1% IgG-free BSA, re-blocked with blocking buffer and incubated with fluorescent secondary antibodies (Invitrogen) diluted in blocking buffer for 1 hr at room temperature. After washes in PBT, embryos were mounted with Prolong Gold anti-fade mounting reagent with DAPI (Invitrogen), and images were acquired with Zeiss LSM 880 confocal microscope.
Endosome recruitment assay
Drosophila Schneider 2 (S2) cells were maintained at 25 °C in Schneider’s Drosophila medium (Gibco) with 10% heat-inactivated FBS (Invitrogen) and Penicillin-Streptomycin (1:1000, Invitrogen). 0.5 ml S2 cells were seeded with 1.5 ml media in 6-well plates, then transfected with a 1:1 ratio of pMT-CrzR-V5 DNA and pMT-GFP-Krz wild type or mutant DNAs using Effectene transfection reagent (Qiagen). 24 hrs after transfection, 0.8 ml transfected cells were mixed with 1 ml media and induced with 0.35 mM CuSO4 in a 6-well plate containing a coverslip treated with concanavalin A, and incubated at 25 °C overnight. 10 μl of 1 mM Corazonin (Abbiotec) stock was diluted 100-fold to a volume of 1 ml in complete S2 media, to obtain a 10 μM solution. 16 hrs after CuSO4 induction, 200 μl of 10 μM Corazonin solution in media was added to each well (final volume 1 ml) and mixed. Cells were incubated in the dark for 15 min. The media were aspirated, and coverslips were briefly rinsed with PBS and fixed in 4% formaldehyde for 15 min. After fixation, coverslips were washed in PBT and blocked with blocking buffer (as above) for 1 hr at room temperature. Coverslips were then incubated with primary antibodies diluted in blocking buffer for 1.5 hours at room temperature then washed with PBT containing 0.1% IgG-free BSA (Rockland). Cells were then re-blocked for 30 min and incubated with fluorescent secondary antibodies diluted in blocking buffer for 1 hr at room temperature. After washes in PBT, coverslips were mounted with Prolong Gold anti-fade mounting reagent with DAPI (Invitrogen), and images were acquired with Zeiss LSM 880 confocal microscope.
Bioluminescence resonance energy transfer (BRET) assay
0.5 ml of dense S2 cell culture were seeded with 1.5 ml media in a 6-well plate, then transfected with a 1:10 ratio of CrzR-Rluc8-V5 DNA and mVenus-Krz (wild type or mutant) DNAs using Effectene transfection reagent (Qiagen). 24 hrs after transfection, transfected cells were induced with 0.35 mM CuSO4. 24 hrs after induction, transfected cells were collected and resuspended in 1 ml DPBS with 5 mM glucose. A white 96-well flat bottom plate (Corning) was used for BRET assay. First, 5 μl of Concanavalin A was added to wells and air-dried. 50 μl of suspended cells were added to treated wells and allowed to settle for 2 hrs. To blank, all media were removed by pipetting then 80 μl of DPBS was added to the wells. After blanking the plate reader, 10 μl of 50 μM coelenterazine H substrate was added into each well and incubated in the dark for 8 minutes, then 10 μl of 5 mM Crz ligand was added into experimental wells while 10 μl of DPBS was added into control wells. All reagents were added simultaneously using a multi-channel pipet. Signals were acquired on POLARstar Omega multifunction microplate reader (BMG Labtech). A total of 12 measurements were taken up to 20 min after ligand addition, in biological triplicates. 0–10 min measurements were taken every minute, followed by a measurement at 15 min and 20 min. The formula, (ligand at 526 nm / ligand at 488 nm) – (control at 526 nm / control at 488 nm) was used to calculate the net BRET value.
Statistical analysis was performed in R. To evaluate the effect of Krz mutations (genotype), time, and their interaction on BRET, we fit a linear mixed effects model with log(x+1) BRET as the response variable. In order to properly evaluate the fixed effects, we fit the model using maximum likelihood instead of restricted maximum likelihood (REML). To determine which genotypes perform differently than the wild type, t-tests at α = 0.05 were used to see which time by genotype interaction coefficients differed from 0. We removed one outlier replicate point (VL/A, 7 min) due to it being of opposite sign and over 4 standard deviations away from the other two replicates at this time point, thus likely representing instrument error.
Wing and cuticle preparation
Wings were dissected from adult Drosophila flies and placed on a slide. 20 μl of isopropanol was added to the wings. After isopropanol evaporated, 15 μl of mounting media (CMCP and lactic acid 3:1) was distributed on the slide and wings were covered with a coverslip. For cuticular preparations, embryos were collected on apple juice agar plates with yeast paste and aged at 25 °C for 24 hr s, then dechorionated in 50% bleach. Embryos were devitellinized in methanol then incubated in a mixture of glycerol and acetic acid (1:4) for 1 hr at 65 °C. Cuticles were then incubated at 25 °C for 24 hours then transferred to slides. Cuticles were mounted in 15 μl of mounting media (see above) and flattened with a weight placed on the coverslip.
Supplementary Material
Video 1. Gastrulation in a control FRT92B embryo. Ventral view, anterior is left. Pulses of Sqh-GFP mid-apical localization were visible in cells along the ventral midline, leading to a proper mesoderm invagination and formation of a closed ventral furrow after approximately 15 min. Time is indicated in minutes:seconds. Video 1 hyperlink.
Video 2. Gastrulation in a krz1 maternal mutant embryo, representing a severe phenotype. Ventral view, anterior is left. Sqh-GFP mid-apical localization persisted much longer in the ventral midline cells (over 30 min), resulting in stalled mesoderm invagination and disrupted ventral furrow formation. Aberrant contractility also led to tissue rupturing. Time is indicated in minutes:seconds. Video 2 hyperlink.
Fig. S1. Alignment of protein sequences for D. melanogaster Krz, bovine visual arrestin-1 (S-antigen visual arrestin, or SAG), and human β-arrestin-1 (ARRB1, also called arrestin 2) and β-arrestin-2 (ARRB2, also called arrestin 3). Functional elements mutated in this study are indicated and color-coded as in Fig. 1.
Fig. S2. Crossing scheme for zygotic rescue experiments. Crosses are shown for the wild type genomic transgene carrying SBP-tagged Krz (SBP-krz5.7). Mutations were introduced into this construct and used in the same crossing scheme. All transgenes were inserted in the same location on the second chromosome (attP40). For zygotic rescue, the expected maximal rescue rate is 33% (or 1/3 of all progeny from the F2 self-cross). If zygotic rescue was successful, rescued females were tested for maternal effect, as indicated, and the results are shown in Table 1.
Fig. S3. Expression of SBP-tagged Krz and endogenous Krz in transgenic flies. (A) Western blot using anti-arrestin antibody using protein extracts from the indicated fly lines. The locations of endogenous Krz and Krz-SBP are indicated. It is likely that the F/A mutant impairs the arrestin antibody epitope, resulting in loss of signal for Krz-SBP. (B) The expression of transgenic Krz-F/A-SBP was confirmed by using anti-SBP antibody.
Fig. S4. Localization of Sqh-GFP in krz1 mutant embryos. Cell boundaries were visualized by anti-Dlg staining (red), and Sqh-GFP is shown as green signal. (A-A’’’) Control FRT82B embryos have completed mesoderm invagination and formed a properly closed ventral furrow. Sqh-GFP is no longer concentrated in the mid-apical regions. (B-B’’’) In krz1 maternal mutant embryos, gastrulation was delayed or abolished, and the cells along the ventral midline showed persistent mid-apical Sqh-GFP localization (arrows). Embryo in B-B’’’ is an example of a mild phenotype. See Videos 1 and 2 for a time-lapse visualization of Sqh-GFP dynamics in krz mutants and controls.
Fig. S5. Localization of Krz and CrzR in endosomes. Localization of CrzR-V5 (red) and GFP-Krz (green, wild type or indicated mutants) in transfected S2 cells, stained with anti-V5 and anti-GFP antibodies. (A-C’’). Localization of CrzR and GFP-Krz 10 min after addition of corazonin. Krz-F/A, Krz-ΔLIQLD, and the double mutant were capable of inducing relocalization of CrzR and Krz into endosomes. Arrows indicate representative endosomes. (D) Quantification of cells containing endosomes. A cell was counted as positive if it contained 3 or more endosomes. *, p<0.05 in chi-squared test; n. s., not significant.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-Discs large (Dlg) | Developmental Studies Hybridoma Bank | 4F3; RRID: AB_528203 |
| Mouse monoclonal anti-HSP70 | Sigma-Aldrich | Cat#H5147 |
| Mouse monoclonal anti-V5 | Sigma-Aldrich | Cat#V8012 |
| Mouse monoclonal anti-SBP | Santa Cruz Biotechnology | Cat#sc-101595 |
| Rabbit monoclonal anti-GFP | Invitrogen | Cat#A-11122; RRID: AB 221569 |
| Rabbit polyclonal anti-pan-arrestin | Invitrogen | Cat#PA1-730; RRID: AB 2274371 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Corazonin peptide | Abbiotech | Cat#350130 |
| Experimental Models: Cell Lines | ||
| D. melanogaster: Cell line S2: S2-DRSC | Laboratory of Spyros Artavanis-Tsakonas | FlyBase: FBtc0000181 |
| Experimental Models: Organisms/Strains | ||
| D. melanogaster: MS-1096-GAL4 driver: w[1118]P{w[+mW.hs]=GawB}Bx[MS1096] | Bloomington Drosophila Stock Center | RRID:BDSC_8860 |
| D. melanogaster : matα4-GAL4-VP16 driver: w[*];P{w[+mC]=matalpha4-GAL-VP16}V2H | Bloomington Drosophila Stock Center | RRID:BDSC_7062 |
| D. melanogaster : RNAi of Mist: w[1118];P{GD727}v33135 | Vienna Drosophila Resource Center | VDRC: v33135 Flybase: FBst0459950 |
| D. melanogaster : RNAi of Krz: w[1118];P{GD8470}v41559 | Vienna Drosophila Resource Center | VDRC: v41559 FlyBase: FBst0464160 |
| Recombinant DNA | ||
| pattB vector | K. Basler lab | GenBank: KC896839.1 |
| BRAC/pcDNA3 (mVenus-Calmodulin-M13-Rluc8) | Addgene | Cat#51967 |
| Corazonin receptor (CrzR) cDNA RE51322 | Drosophila Genomics Resource Center | DGRC: 1122470; FlyBase: FBcl0239316 |
Krz limits Fog-Mist signaling during gastrulation and wing folding
Mutations in Krz result in excessive cellular contractility
Direct Krz-GPCR interactions are critical in vivo, during development
Key interacting residues in Krz include the phosphosensing and finger loop regions
Acknowledgments
Marla Tipping contributed to the generation of the mutant Krz constructs. We thank Melissa Brown and other members of the Veraksa lab for helpful comments on the manuscript, and Adam Martin and Eric Wieschaus for providing fly stocks used in this work. We thank the Bloomington Drosophila Stock Center, Vienna Drosophila Resource Center, and Developmental Studies Hybridoma Bank for their services. This work was supported by the grant from the National Institutes of Health GM097727 to A.V. Fei Chai was supported by Oracle Doctoral Fellowship at UMass Boston.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anjum SG, Xu W, Nikkholgh N, Basu S, Nie Y, Thomas M, Satyamurti M, Budnik BA, Ip YT, Veraksa A, 2013. Regulation of Toll Signaling and Inflammation by beta-Arrestin and the SUMO Protease Ulp1. Genetics 195, 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett K, Leptin M, Settleman J, 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell 91, 905–915. [DOI] [PubMed] [Google Scholar]
- Benovic JL, Kuhn H, Weyand I, Codina J, Caron MG, Lefkowitz RJ, 1987. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci U S A 84, 8879–8882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K, 2007. An optimized transgenesis system for Drosophila using germ-line-specific phi C31 integrases. Proceedings of the National Academy of Sciences of the United States of America 104, 3312–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Gradl D, Schambony A, Arenas E, Schulte G, 2007. Beta-arrestin is a necessary component of Wnt/beta-catenin signaling in vitro and in vivo. Proc Natl Acad Sci U S A 104, 6690–6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ, Lamerdin JE, Triest S, Shukla AK, Berger B, Little J.t., Antar A, Blanc A, Qu CX, Chen X, Kawakami K, Inoue A, Aoki J, Steyaert J, Sun JP, Bouvier M, Skiniotis G, Lefkowitz RJ, 2017. Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci U S A 114, 2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Perry NA, Vishnivetskiy SA, Berndt S, Gilbert NC, Zhuo Y, Singh PK, Tholen J, Ohi MD, Gurevich EV, Brautigam CA, Klug CS, Gurevich VV, Iverson TM, 2017. Structural basis of arrestin-3 activation and signaling. Nat Commun 8, 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hu LA, Semenov MV, Yanagawa S, Kikuchi A, Lefkowitz RJ, Miller WE, 2001. beta-Arrestin1 modulates lymphoid enhancer factor transcriptional activity through interaction with phosphorylated dishevelled proteins. Proc Natl Acad Sci U S A 98, 14889–14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ, 2004. Activity-dependent internalization of smoothened mediated by betaarrestin 2 and GRK2. Science 306, 2257–2260. [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ, 2003. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 301, 1391–1394. [DOI] [PubMed] [Google Scholar]
- Coravos JS, Martin AC, 2016. Apical Sarcomere-like Actomyosin Contracts Nonmuscle Drosophila Epithelial Cells. Dev Cell 39, 346–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Wilson ET, Wieschaus E, 1994. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76, 1075–1089. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF, 2005. folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178. [DOI] [PubMed] [Google Scholar]
- DeFea KA, Vaughn ZD, O’Bryan EM, Nishijima D, Dery O, Bunnett NW, 2000. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta - arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A 97, 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ, 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 12, 151–156. [DOI] [PubMed] [Google Scholar]
- Donthamsetti P, Quejada JR, Javitch JA, Gurevich VV, Lambert NA, 2015. Using Bioluminescence Resonance Energy Transfer (BRET) to Characterize Agonist-Induced Arrestin Recruitment to Modified and Unmodified G Protein-Coupled Receptors. Curr Protoc Pharmacol 70, 2 14 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM, 2006. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell 10, 329–342. [DOI] [PubMed] [Google Scholar]
- Fuse N, Yu F, Hirose S, 2013. Gprk2 adjusts Fog signaling to organize cell movements in Drosophila gastrulation. Development 140, 4246–4255. [DOI] [PubMed] [Google Scholar]
- Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G, 2004. Identification of beta-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell 14, 303–317. [DOI] [PubMed] [Google Scholar]
- Gimenez LE, Kook S, Vishnivetskiy SA, Ahmed MR, Gurevich EV, Gurevich VV, 2012. Role of receptor-attached phosphates in binding of visual and non-visual arrestins to G protein-coupled receptors. J Biol Chem 287, 9028–9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB Jr., Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL, 1996. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383, 447–450. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV, 2012. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther 133, 40–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV, 2012. Synthetic biology with surgical precision: targeted reengineering of signaling proteins. Cell Signal 24, 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Francis DJ, Vishnivetskiy SA, Kolobova EA, Hubbell WL, Klug CS, Gurevich VV, 2006. Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci U S A 103, 4900–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, Babu MM, 2018. Pharmacogenomics of GPCR Drug Targets. Cell 172, 41–54 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, van Zanten TS, Philippe JM, Mayor S, Lecuit T, 2018. Quantitative Control of GPCR Organization and Signaling by Endocytosis in Epithelial Morphogenesis. Curr Biol 28, 1570–1584 e1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Tift FW, McCauley A, Liu L, Roman G, 2008. Functional characterization of kurtz, a Drosophila non-visual arrestin, reveals conservation of GPCR desensitization mechanisms. Insect Biochem Mol Biol 38, 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DS, Kern RC, Puthenveedu MA, von Zastrow M, Williams JC, Benovic JL, 2009. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J Biol Chem 284, 29860–29872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DS, Tian X, Benovic JL, 2014. Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol 27, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, Xu Q, de Waal PW, Ke J, Tan MH, Zhang C, Moeller A, West GM, Pascal BD, Van Eps N, Caro LN, Vishnivetskiy SA, Lee RJ, Suino-Powell KM, Gu X, Pal K, Ma J, Zhi X, Boutet S, Williams GJ, Messerschmidt M, Gati C, Zatsepin NA, Wang D, James D, Basu S, Roy-Chowdhury S, Conrad CE, Coe J, Liu H, Lisova S, Kupitz C, Grotjohann I, Fromme R, Jiang Y, Tan M, Yang H, Li J, Wang M, Zheng Z, Li D, Howe N, Zhao Y, Standfuss J, Diederichs K, Dong Y, Potter CS, Carragher B, Caffrey M, Jiang H, Chapman HN, Spence JC, Fromme P, Weierstall U, Ernst OP, Katritch V, Gurevich VV, Griffin PR, Hubbell WL, Stevens RC, Cherezov V, Melcher K, Xu HE, 2015. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karess RE, Chang XJ, Edwards KA, Kulkarni S, Aguilera I, Kiehart DP, 1991. The regulatory light chain of nonmuscle myosin is encoded by spaghetti-squash, a gene required for cytokinesis in Drosophila. Cell 65, 1177–1189. [DOI] [PubMed] [Google Scholar]
- Kasza KE, Farrell DL, Zallen JA, 2014. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc Natl Acad Sci U S A 111, 11732–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge S, Munjal A, Philippe JM, Jha A, de las Bayonas AG, Saurin AJ, Lecuit T, 2016. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat Cell Biol 18, 261–270. [DOI] [PubMed] [Google Scholar]
- Kim YM, Benovic JL, 2002. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem 277, 30760–30768. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, 2000. Three ways to make a vesicle. Nat Rev Mol Cell Biol 1, 187–198. [DOI] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ, 2009. Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 17, 443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ, 2008. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science 320, 1777–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV, 1999. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem 274, 6831–6834. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Goodman OB Jr., Keen JH, Benovic JL, 1997. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem 272, 15011–15016. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK, 2005. Transduction of receptor signals by beta-arrestins. Science 308, 512–517. [DOI] [PubMed] [Google Scholar]
- Li S, Chen Y, Shi Q, Yue T, Wang B, Jiang J, 2012. Hedgehog-regulated ubiquitination controls smoothened trafficking and cell surface expression in Drosophila. PLoS Biol 10, e1001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ, 1990. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science 248, 1547–1550. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, Caron MG, Lefkowitz RJ, 1999. Beta-arrestindependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283, 655–661. [DOI] [PubMed] [Google Scholar]
- Manning AJ, Peters KA, Peifer M, Rogers SL, 2013. Regulation of epithelial morphogenesis by the G protein-coupled receptor mist and its ligand fog. Sci Signal 6, ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AJ, Rogers SL, 2014. The Fog signaling pathway: insights into signaling in morphogenesis. Dev Biol 394, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF, 2009. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason FM, Xie S, Vasquez CG, Tworoger M, Martin AC, 2016. RhoA GTPase inhibition organizes contraction during epithelial morphogenesis. J Cell Biol 214, 603–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar C, Ruiz-Gomez A, Martin M, Rojo-Berciano S, Mayor F, de Celis JF, 2011. Role of the Drosophila non-visual ss-arrestin kurtz in hedgehog signalling. PLoS Genet 7, e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morize P, Christiansen AE, Costa M, Parks S, Wieschaus E, 1998. Hyperactivation of the folded gastrulation pathway induces specific cell shape changes. Development 125, 589–597. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S, 2005. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol 7, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS, 2000. Differential affinities of visual arrestin, beta arrestin1 and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275, 17201–17210. [DOI] [PubMed] [Google Scholar]
- Ostermaier MK, Schertler GF, Standfuss J, 2014. Molecular mechanism of phosphorylation-dependent arrestin activation. Curr Opin Struct Biol 29, 143–151. [DOI] [PubMed] [Google Scholar]
- Parks S, Wieschaus E, 1991. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell 64, 447–458. [DOI] [PubMed] [Google Scholar]
- Peterson YK, Luttrell LM, 2017. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol Rev 69, 256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter CJ, Tasic B, Russler EV, Liang L, Luo L, 2010. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141, 536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puca L, Chastagner P, Meas-Yedid V, Israel A, Brou C, 2013. Alpha-arrestin 1 (ARRDC1) and beta-arrestins cooperate to mediate Notch degradation in mammals. J Cell Sci 126, 4457–4468. [DOI] [PubMed] [Google Scholar]
- Roman G, He J, Davis RL, 2000. kurtz, a novel nonvisual arrestin, is an essential neural gene in Drosophila. Genetics 155, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P, Sommer ME, 2017. Structural mechanism of arrestin activation. Curr Opin Struct Biol 45, 160–169. [DOI] [PubMed] [Google Scholar]
- Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, Paduch M, Tripathi-Shukla P, Koide A, Koide S, Weis WI, Kossiakoff AA, Kobilka BK, Lefkowitz RJ, 2013. Structure of active beta-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Westfield GH, Xiao K, Reis RI, Huang LY, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, Dosey AM, Su M, Liang CR, Gu LL, Shan JM, Chen X, Hanna R, Choi M, Yao XJ, Klink BU, Kahsai AW, Sidhu SS, Koide S, Penczek PA, Kossiakoff AA, Woods VL Jr., Kobilka BK, Skiniotis G, Lefkowitz RJ, 2014. Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512, 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne-Marr R, Gurevich VV, Goldsmith P, Bodine RC, Sanders C, Donoso LA, Benovic JL, 1993. Polypeptide variants of beta-arrestin and arrestin3. J Biol Chem 268, 15640–15648. [PubMed] [Google Scholar]
- Szczepek M, Beyriere F, Hofmann KP, Elgeti M, Kazmin R, Rose A, Bartl FJ, von Stetten D, Heck M, Sommer ME, Hildebrand PW, Scheerer P, 2014. Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat Commun 5, 4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ARB, Jensen DD, Hicks GA, Bunnett NW, 2018. Therapeutic Targeting of Endosomal G-Protein-Coupled Receptors. Trends Pharmacol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping M, Kim Y, Kyriakakis P, Tong M, Shvartsman SY, Veraksa A, 2010. β-arrestin Kurtz inhibits MAPK and Toll signaling in Drosophila development. EMBO J 29, 3222–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Schubert C, Climaco GC, Gurevich YV, Velez MG, Gurevich VV, 2000. An additional phosphate-binding element in arrestin molecule. Implications for the mechanism of arrestin activation. J Biol Chem 275, 41049–41057. [DOI] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ, 2004. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A 101, 8603–8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM, 2018. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol 19, 638–653. [DOI] [PubMed] [Google Scholar]
- Zhou XE, He Y, de Waal PW, Gao X, Kang Y, Van Eps N, Yin Y, Pal K, Goswami D, White TA, Barty A, Latorraca NR, Chapman HN, Hubbell WL, Dror RO, Stevens RC, Cherezov V, Gurevich VV, Griffin PR, Ernst OP, Melcher K, Xu HE, 2017. Identification of Phosphorylation Codes for Arrestin Recruitment by G Protein-Coupled Receptors. Cell 170, 457–469 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Gastrulation in a control FRT92B embryo. Ventral view, anterior is left. Pulses of Sqh-GFP mid-apical localization were visible in cells along the ventral midline, leading to a proper mesoderm invagination and formation of a closed ventral furrow after approximately 15 min. Time is indicated in minutes:seconds. Video 1 hyperlink.
Video 2. Gastrulation in a krz1 maternal mutant embryo, representing a severe phenotype. Ventral view, anterior is left. Sqh-GFP mid-apical localization persisted much longer in the ventral midline cells (over 30 min), resulting in stalled mesoderm invagination and disrupted ventral furrow formation. Aberrant contractility also led to tissue rupturing. Time is indicated in minutes:seconds. Video 2 hyperlink.
Fig. S1. Alignment of protein sequences for D. melanogaster Krz, bovine visual arrestin-1 (S-antigen visual arrestin, or SAG), and human β-arrestin-1 (ARRB1, also called arrestin 2) and β-arrestin-2 (ARRB2, also called arrestin 3). Functional elements mutated in this study are indicated and color-coded as in Fig. 1.
Fig. S2. Crossing scheme for zygotic rescue experiments. Crosses are shown for the wild type genomic transgene carrying SBP-tagged Krz (SBP-krz5.7). Mutations were introduced into this construct and used in the same crossing scheme. All transgenes were inserted in the same location on the second chromosome (attP40). For zygotic rescue, the expected maximal rescue rate is 33% (or 1/3 of all progeny from the F2 self-cross). If zygotic rescue was successful, rescued females were tested for maternal effect, as indicated, and the results are shown in Table 1.
Fig. S3. Expression of SBP-tagged Krz and endogenous Krz in transgenic flies. (A) Western blot using anti-arrestin antibody using protein extracts from the indicated fly lines. The locations of endogenous Krz and Krz-SBP are indicated. It is likely that the F/A mutant impairs the arrestin antibody epitope, resulting in loss of signal for Krz-SBP. (B) The expression of transgenic Krz-F/A-SBP was confirmed by using anti-SBP antibody.
Fig. S4. Localization of Sqh-GFP in krz1 mutant embryos. Cell boundaries were visualized by anti-Dlg staining (red), and Sqh-GFP is shown as green signal. (A-A’’’) Control FRT82B embryos have completed mesoderm invagination and formed a properly closed ventral furrow. Sqh-GFP is no longer concentrated in the mid-apical regions. (B-B’’’) In krz1 maternal mutant embryos, gastrulation was delayed or abolished, and the cells along the ventral midline showed persistent mid-apical Sqh-GFP localization (arrows). Embryo in B-B’’’ is an example of a mild phenotype. See Videos 1 and 2 for a time-lapse visualization of Sqh-GFP dynamics in krz mutants and controls.
Fig. S5. Localization of Krz and CrzR in endosomes. Localization of CrzR-V5 (red) and GFP-Krz (green, wild type or indicated mutants) in transfected S2 cells, stained with anti-V5 and anti-GFP antibodies. (A-C’’). Localization of CrzR and GFP-Krz 10 min after addition of corazonin. Krz-F/A, Krz-ΔLIQLD, and the double mutant were capable of inducing relocalization of CrzR and Krz into endosomes. Arrows indicate representative endosomes. (D) Quantification of cells containing endosomes. A cell was counted as positive if it contained 3 or more endosomes. *, p<0.05 in chi-squared test; n. s., not significant.