Abstract
The cerebellum undergoes major rapid growth during the third trimester and early neonatal stage in humans, making it vulnerable to injuries in pre-term babies. Experiments in mice have revealed a remarkable ability of the neonatal cerebellum to recover from injuries around birth. In particular, recovery following irradiation-induced ablation of granule cell precursors (GCPs) involves adaptive reprogramming of Nestin-expressing glial progenitors (NEPs). Sonic hedgehog signaling is required for the initial step in NEP reprogramming; however, the full spectrum of developmental signaling pathways that promote NEP-driven regeneration is not known. Since the growth regulatory Hippo pathway has been implicated in the repair of several tissue types, we tested whether Hippo signaling is involved in regeneration of the cerebellum. Using mouse models, we found that the Hippo pathway transcriptional co-activator YAP1 (Yes-associated protein 1) but not TAZ (transcriptional coactivator with PDZ binding motif, or WWTR1) is required in NEPs for full recovery of cerebellar growth following irradiation one day after birth. Although Yap1 plays only a minor role during normal development in differentiation of NEPs or GCPs, the size of the cerebellum, and in particular the internal granule cell layer produced by GCPs, is significantly reduced in Yap1 mutants after irradiation, and the organization of Purkinje cells and Bergmann glial fibers is disrupted. The initial proliferative response of Yap1 mutant NEPs to irradiation is normal and the cells migrate to the GCP niche, but subsequently there is increased cell death of GCPs and altered migration of granule cells, possibly due to defects in Bergmann glia. Moreover, loss of Taz along with Yap in NEPs does not abrogate regeneration or alter development of the cerebellum. Our study provides new insights into the molecular signaling underlying postnatal cerebellar development and regeneration.
Keywords: Hippo signaling, adaptive reprogramming, Nestin-expressing progenitors, Yes-associated protein, Taz
INTRODUCTION
The cerebellum not only has a principal role in motor coordination and balance control, but also is linked to a wide range of higher order cognitive and social functions (Fatemi et al., 2012; Marek et al., 2018; Stoodley et al., 2017; Tsai et al., 2012; Tsai et al., 2018; S. S. Wang, Kloth, & Badura, 2014). Development of the cerebellum in both human and mouse is a protracted process, much of which spans from late embryonic to early postnatal stages (Altman & Bayer, 1997; Dobbing & Sands, 1973; Rakic & Sidman, 1970). Therefore, the cerebellum is particularly vulnerable to clinical and environmental insults around birth. Indeed, cerebellar injury is a frequent complication of premature birth and can lead to reduced cerebellar size (Biran, Verney, & Ferriero, 2012; Steggerda et al., 2009) and to long-term neurodevelopmental problems including in motor, cognitive, social and language development (Brossard-Racine, du Plessis, & Limperopoulos, 2015; Hortensius et al., 2018; Limperopoulos et al., 2007; Messerschmidt et al., 2008; S. S. Wang et al., 2014).Thus it is crucial to dissect the molecular mechanisms that underlie processes of repair in the neonatal cerebellum.
The mouse cerebellum originates from the anterior hindbrain and undergoes substantial growth in the first two postnatal weeks. Prior to birth (embryonic day (E) 13.5–15.5), the Atoh1-expressing granule cell precursors (GCPs) migrate over the surface of the cerebellum. After birth, GCPs rapidly proliferate in the external granule cell layer (EGL) in response to Sonic Hedgehog (SHH) secreted by Purkinje cells (PCs) (Corrales, Blaess, Mahoney, & Joyner, 2006; Lewis, Gritli-Linde, Smeyne, Kottmann, & McMahon, 2004; Sillitoe & Joyner, 2007). Until approximately postnatal day (P) 15, post-mitotic GCPs migrate down Bergmann glial fibers and past the Purkinje cell layer (PCL) to populate the internal granule cell layer (IGL) and complete differentiation and maturation (Sillitoe & Joyner, 2007). By following up on an earlier finding in infant rats that the EGL is rapidly reconstituted several days after depletion by irradiation (Altman, Anderson, & Wright, 1969), we found that the cerebellum of the neonatal mouse is capable of substantial recovery of growth after ablation of most GCPs in the EGL by irradiation at P1 (Wojcinski et al., 2017). Furthermore, PCs are completely replenished when −50% are killed at P1, in an age-dependent process that involves a rare immature PC population proliferating and producing new PCs (Bayin et al., 2018). Thus, at least two cell types in the cerebellum are effectively replenished when ablated soon after birth, and each involves a distinct cellular process.
The regeneration of GCPs after their depletion is dependent on a proliferative subpopulation of Nestin-expressing progenitors (NEPs), which are derived from the ventricular zone during late-embryogenesis (Buffo & Rossi, 2013; Fleming et al., 2013; Milosevic & Goldman, 2004). There are two main subpopulations of NEPs, one that resides in the PCL and gives rise to astrocytes and Bergmann glia and the other in the white matter (WM) produces interneurons and astrocytes (Cerrato et al., 2018; Fleming et al., 2013; Li et al., 2013; Parmigiani et al., 2015; Wojcinski et al., 2017). Furthermore, both populations rely on SHH for their proliferation. When GCPs in the EGL are depleted genetically or by irradiation at P1, NEPs in the PCL sense the injury and respond by increasing cell proliferation and then migrating into the EGL where they switch their fate to GCPs and produce granule cells that contribute to the IGL (Andreotti et al., 2018; Jaeger & Jessberger, 2017; Wojcinski et al., 2017; Wojcinski, Morabito, Lawton, Stephen, & Joyner, 2019). At the same time, NEPs in the WM transiently reduce their production of interneurons and astrocytes. Although SHH signaling is required for NEPs to replenish the injured EGL (Wojcinski et al., 2017), the full molecular repertoire underlying the regenerative capacity of NEPs, however remains to be determined.
The Hippo signaling pathway is a key regulator of size control of many organs (Haider & Johnson, 2011; Pan, 2010) through regulating cell proliferation and cell survival (Ardestani & Maedler, 2018; Emoto, 2011; Ikeda & Sadoshima, 2016; M. Lee, Goraya, Kim, & Cho, 2018; Udan, Kango-Singh, Nolo, Tao, & Haider, 2003; Wu, Huang, Dong, & Pan, 2003). In mammals, upon activation of a conserved kinase cascade consisting of the serine/threonine kinases MST1/2 (mammalian Ste2-like kinases) and LATS1/2 (large tumor suppressor kinase 1/2), the transcriptional cofactors Yes-associated protein 1 (YAP1) and transcriptional coactivator with PDZ binding motif (TAZ, also called WWTR1, for WW-domain containing transcription regulator 1) are phosphorylated, sequestered by 14–3-3 in the cytoplasm, and targeted for degradation in a ubiquitin-proteasome-dependent manner (Callus, Verhagen, & Vaux, 2006; Chan et al., 2005; Y. Liu & Deng, 2019; Nguyen & Kugler, 2018; Wu et al., 2003; Zhao, Li, Tumaneng, Wang, & Guan, 2010). Conversely, in the absence of Hippo signaling, dephosphorylated YAP1 and TAZ translocate into the nucleus and form complexes with the TEAD/TEF family of transcription factors to activate downstream transcriptional programs that include promotion of cell proliferation and organ growth (K. Wang, Degerny, Xu, & Yang, 2009). Although YAP is known to regulate the renewal of many tissue types after injury, including liver, incisors, and the colonic epithelium (Hu et al., 2017; Lu, Finegold, & Johnson, 2018; Yui et al., 2018), the role of YAP1/TAZ in mammalian brain development and regeneration remains poorly studied. Conditional genetic ablation of Yap1 (referred to as Yap) in radial glial progenitor cells (using Nestin-Cre) was found to cause hydrocephalus and a subtle defect in the proliferation of cortical neural progenitors, but no major anatomical changes in the brain (Park et al., 2016). On the other hand, inactivation of LATS1/2 from the same (Nestin+) neural progenitor population during brain development in mouse was shown to result in YAP/TAZ-driven global hypertranscription with upregulation of many target genes related to cell growth and proliferation (Lavado et al., 2018). The gene expression changes are thought to inhibit the differentiation of neural progenitors and promote transient over-proliferation of neural progenitors. In an ex vivo model, it was shown that Yap over-expression increases the proliferation of cultured GCPs, while shRNA knockdown of Yap decreases proliferation (Fernandez et al., 2009). Moreover, Yap over-expression was reported to protect cultured GCPs from irradiation-induced damage by sustaining their proliferation and survival (Fernandez et al., 2012). However, the potential function of YAP/TAZ in development and regeneration of the neonatal cerebellum has not been tested in vivo.
Here we utilized genetic deletion of Yap and/or Taz in mice and revealed an essential role for YAP in mammalian cerebellar regeneration following irradiation, but only a minor role in inhibiting differentiation during cerebellar development. Loss of Yap at P0 from NEPs disrupted restoration of cerebellar size following irradiation with pronounced reduction in the IGL and disorganization of PCs and Bergmann glial fibers. The poor recovery of the cerebellum was associated with elevated death of the NEPs in the PCL one day after irradiation and later death of GCPs in the EGL, and defective migration of the granule cells into the IGL potentially due to a defect in Burgmann glia. In contrast, loss of Yap at P0 in NEPs or GCPs in conditional knockout (cKO) mice resulted in only mild enhancement of differentiation of NEPs and GCPs and no alteration of the size or morphology of the adult cerebellum. Surprisingly, loss of Taz in addition to Yap at P0 in NEPs did not alter cerebellar development or reduce the recovery of the IGL after irradiation. Our study identifies Hippo signaling as a key molecular signaling pathway underlying the late stage of regeneration of the postnatal EGL following injury. Our discovery also raises the possibility that inhibiting Hippo signaling could reduce cerebellar hypoplasia after injury through enhancing recovery.
Materials and Methods
Mice
The following mouse lines were used: Nes-CFP (Mignone, Kukekov, Chiang, Steindler, & Enikolopov, 2004), Atoh1-GFP (Chen, Johnson, Zoghbi, & Segil, 2002), Nestin-FlpoER (Wojcinski et al., 2017), Atoh1-FlpoER (Wojcinski et al., 2019), Rosa26MASTR(frt-STOP-frt-GFPcre) (Lao, Raju, Bai, & Joyner, 2012), Yapflox/flox and Tazflox/flox (Reginensi et al., 2013), and Atoh1-Cre (Matei et al., 2005). Tamoxifen (Sigma, T5648) was dissolved in corn oil at 20 mg/mL and a single dose of 200 μg/g was administered to P0 animals by subcutaneous injection. Mouse husbandry and all experiments were performed in accordance with MSKCC lACUC-approved protocols.
Irradiation
P1 mice were anesthetized by hypothermia and received a single dose of 4 Gy irradiation in an X-RAD 225Cx (Precision X-ray) Microirradiator in the MSKCC Small-Animal Imaging Core Facility. A 5-mm diameter collimator was used to target the cerebellum from the left side of the animal.
Tissue Processing
Animals younger than P4 were sacrificed and then brains were dissected and fixed in 4% paraformaldehyde overnight at 4°C. Animals P4–30 were anesthetized and transcardially perfused with PBS followed by chilled 4% paraformaldehyde. Brains were dissected and post-fixed overnight and cryoprotected in 30% sucrose before freezing in Cryo-OCT. Frozen brains were cryosectioned sagittally at 12 μm. Midline cerebellar sections were used for all analyses.
Immunofluorescent staining
Cryosections were stained overnight at 4 °C with the following primary antibodies: mouse anti-YAP (Abeam, AB56701), rabbit anti-TAZ (Santa Cruz, sc-48805), rat anti-GFP (1:1,000; Nacalai Tesque; 0440484), mouse anti-NeuN (Millipore, MAB377), rabbit anti-Calbindin D-28K (Swant, CB38), rabbit anti-GFAP (Dako, Z0334), rabbit anti-S100p (Dako, Z0311), rabbit anti-PAX2 (Invitrogen, 71600), goat anti-SOX2 (R&D System, AF2018), and rabbit anti-cleaved Caspase3 (Cell Signaling Technology, 9664S). Secondary antibodies for double labeling were donkey anti-species conjugated with Alexa Fluor 488 or 555 (1:1,000; Molecular Probes). Nuclei were counterstained with Hoechst 33258 (Invitrogen, H3569).
Microscopy
Images were collected either on a DM6000 Leica microscope using Zen software (Zeiss), or a NanoZoomer 2.0 HT slide scanner (Hamamatsu Photonics) using NDP.scan software. All images were taken with 20X objectives, and processed using NDP.view2 or Photoshop softwares.
Flow Cytometry
Cerebella of Atoh1-GFP and Nestin-CFP mice were dissected out under a dissection microscope. Tissues were digested by Trypsin/DNase, and then subject to FACS (fluorescence activated cell sorting) to isolate GFP+ or CFP+ cells.
RT-qPCR
RNA was isolated from FACS-isolated GFP+ cells from P4 Atoh1-GFP mice and FACS-isolated CFP+ cells from Nestin-CFP mice using a miRNeasyMicro Kit (Qiagen) according to the manufacturer’s protocol. cDNA was prepared using ¡Script cDNA synthesis kit (Bio-Rad). RT-qPCR was performed using PowerUp Sybr Green Master Mix (Applied Biosystems). Fold changes in expression were calculated using the ΔΔCt method. The Gapdh gene was used to normalize the results. The following primer pairs were used: Yap forward 5′-ACCCTCGTTTTGCCATGAAC-3′, Yap reverse 5′-TGTGCTGGGATTGATATTCCGTA-3′, Taz forward 5′-CATGGCGGAAAAAGATCCTCC-3′, Taz reverse 5′-GTCGGTCACGTCATAGGACTG-3′, Gapdh forward 5′-CCAAGGTGTCCGTCGTGGATCT-3′, and Gapdh reverse 5′-GTTGAAGTCGCAGGAGACAACC-3’.
Quantification and statistical analysis
ImageJ software was used to measure the area (mm2) of the cerebellum on sections near the midline. Cell counts from IF staining were obtained using Stereo Investigator Software. Four sections per animal for the TUNEL quantifications at P12 cerebella, or three sections per animal for all the other quantifications were analyzed on at least three animals. Statistical analyses were performed using Prism software (GraphPad) and significance was determined at P < 0.05. All statistical analyses were two-tailed. For two-group comparisons with equal variance as determined by the F-test, an unpaired Student’s t test was used. For comparisons among four independent groups with equal variance, an unpaired one-way ANOVA was used. For comparisons between groups that are split with two independent variables, an unpaired two-way ANOVA was used. P values and degrees of freedom are given in the figures and legends. Data are presented as mean ± S.D. (standard deviation). No statistical methods were used to predetermine the sample size, but our sample sizes are similar to those generally employed in the field. No randomization was used. Data collection and analysis were not performed blind to the conditions of the experiments.
EdU (5-ethynyl-2’-deoxyuridine) Injection and Staining
For assessing cell proliferation, EdU (Invitrogen, E10187) was given at 100 mg/g by i.p. injection 1 h before euthanasia. Click-it EdU assay with Sulfo-Cyanine5 azide (Lumiprobe corporation, A3330) was used according to the protocol of the manufacturer.
TUNEL Staining
For TUNEL staining, slides were permeabilized with 0.5% TritonX-100, pre-incubated with Tdt buffer (30 mM Tris HCI, 140 mM sodium cacodylate and 1 mM CoCI2) for 15 min at room temperature, and incubated for I h at 37 °C in TUNEL reaction solution containing Terminal Transferase (Roche, 03333574001) and Digoxigenin-11-dUTP (Sigma-Aldrich, 11093088910). Slides were then incubated with anti-digoxigenin-rhodamine (Sigma-Aldrich, 11207750910) for 1 h.
RESULTS
YAP and TAZ expression are enriched in NEPs in the neonatal cerebellum
As a first step in studying the functions of YAP and TAZ in the developing and regenerating cerebellum, we characterized the expression of the proteins in Nestin-CFP reporter mice using immunofluorescence (IF). It was previously reported that YAP is expressed in GCPs (Fernandez et al., 2009), but expression in NEPs was not addressed. In the cerebella of normal P4 mice, a low level of nuclear YAP was detected in some NEPs (CFP+ cells) whereas in GCPs in the EGL little or no YAP was detected (Fig. 1A,A’,A”). Since NEPs contribute to regeneration of the irradiated (IR) mouse cerebellum, we asked whether YAP expression changes in NEPs during their adaptive reprogramming following irradiation of the cerebellum. When the cerebella of Nestin-CFP transgenic mice were irradiated at P1 (IR mice) and analyzed at P4, nuclear YAP was detected mainly in NEPs (CFP+ cells) with little expression in GCPs (Fig. 1B,B’,B”). Of possible significance, in IR mice most of the CFP+ NEPs in the EGL showed nuclear expression of YAP (Fig. 1B,B’,B”), indicating that YAP could play a role in the adaptive reprogramming response of NEPs to EGL ablation. Nuclear located TAZ expression was more obvious than YAP in NEPs of Non-IR and IR cerebella, with similar expression in the two conditions. TAZ was also present in most NEPs in the EGL of IR mice and not detected in GCPs (Fig. 1C–D,C’–D’,C”–D”).
Figure 1. Nuclear YAP and TAZ are mainly detected in NEPs in the postnatal CB.
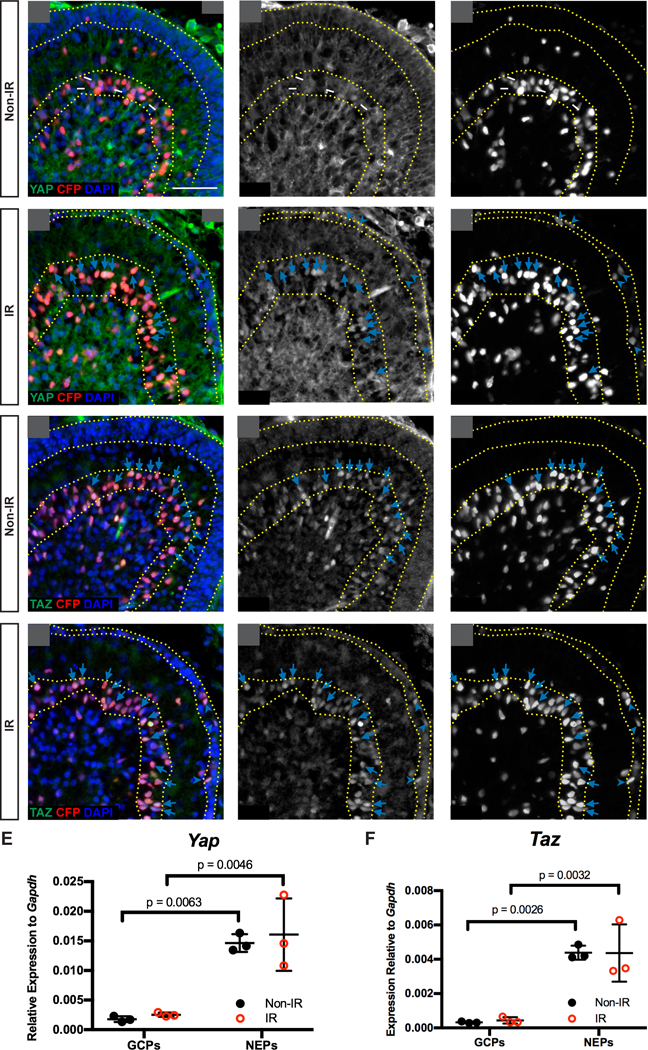
(A-D) Immunofluorescence (IF) detection of YAP (A-B) and TAZ (C-D), co-stained for CFP and DAPI, on midsagittal sections of cerebella from Non-IR and IR Nestin-CFP reporter mice at P4. Arrows and arrowheads indicate Nest in (CFP) positive cells in the Purkinje cell layer (PCL) or EGL, respectively that also have nuclear YAP or TAZ. Yellow dotted lines indicate the EGL and PCL. Scale bar, 50 μm. (E-F) RT-qPCR analysis of the mRNA expression of Yap (E) and Taz (F) relative to Gapdh in FACS isolated NEPs (Nestin-CFP+) and GCPs (Atoh1-GFP+) from Non-IR and IR mice at P4. Data are presented as mean ± S.D., and statistical analysis by two-way ANOVA. Each data point represents one animal.
To confirm that Yap and Taz are expressed at higher levels in NEPs than GCPs, we carried out quantitative RT-PCR (RT-qPCR) of mRNA from FACS-sorted Nestin-CFP+ NEPs and Atoh1-GFP+ GCPs from P4 IR and Non-IR transgenic mice. Indeed, Yap and Taz mRNA were significantly lower in GCPs than in NEPs of Non-IR and IR mice, and there were no significant changes after irradiation (Fig. 1E,F). Consistent with the antibody staining and RT-qPCR results, analysis of RNA-seq data from Nestin-CFP+ NEPs isolated by FACS from P5 IR and Non-IR mice (Wojcinski et al., 2017) showed the Yap and Taz transcripts were present in NEPs, with no significant changes after irradiation and a lower numbers of reads for Taz (Table. S1). The transcripts for Tead1 and Tead2 were abundant while Tead3 was minimal (Table. S1), indicating that TEAD1/2 are the main binding partners for YAP/TAZ in neonatal NEPs. The Hippo target gene Birc5 was present and appeared to be slightly upregulated in IR NEPs, but a second target gene Ctgf had little expression (Table. S1). Together, these data demonstrate that the cerebellar cell type that predominantly expresses Yap and Taz is NEPs, and thus YAP and TAZ could play a role in the regeneration of the EGL by NEPs following irradiation.
Loss of Yap in the NEP lineage hampers postnatal cerebellar regeneration
Given the potential role of YAP in NEPs for cerebellar regeneration, we determined whether Yap is required for injury-induced regeneration of the cerebellum by mutating Yap specifically in NEPs at P0 using a mosaic mutant analysis approach (MASTR, Mosaic mutant Analysis with Spatial and Temporal control of Recombination) (Lao et al., 2012; Wojcinski et al., 2017) (Fig. 2A). An inducible FLP site-specific recombinase expressed from a Nestin transgene was used to induce sustained expression of a protein fusion between GFP and CRE (referred to as GFPcre) following injection of tamoxifen (Tm), which then induces recombination of a Yap floxed allele (Reginensi et al., 2013), resulting in visualization of the mutant cells and their descendants based on GFP expression (Fig. 2A). Tm was administered to Nes-FlpoER/+;R26FSF-GFPcre/+;Yapflox/flox mice (Nes-mYap cKOs) and controls (Nes-FlpoER/+;Yapflox/flox, or R26FSF-GFPcre/+;yapflox/flox, or yapflox/flox at p0, X-ray irradiation was conducted at P1, and the size of cerebellum was measured at P30 (Fig. 2B). Notably, Nes-mYap cKOs showed a reduction in cerebellar size after irradiation compared to IR controls (Fig. 2E,F; Fig. 2S1A). The sizes of the cerebella (area at the midline) of IR mutants were reduced to 41.3 ± 2.10% of Non-IR mutants compared to 50.4 ± 2.75% for IR controls compared to Non-IR controls (Fig. 2G). Even more prominent was a significant reduction in the area of the IGL and ratio of IGL/cerebellum area in Nes-mYap cKOs compared to controls following irradiation (Fig. 2H–M; Fig. 2S1B,C). The reduced recovery of the IGL in mutants was most pronounced in lobules 1–5 and 10 (Fig. 2H–K; Fig. 2S1A–C). Furthermore, as is seen in mutant mice with a depleted IGL (Corrales et al., 2006; Corrales, Rocco, Blaess, Guo, & Joyner, 2004), the Calbindin+ PCs failed to form a single cell layer in the Nes-mYap cKOs; instead, the individual PCs were more disorganized than in controls and dispersed throughout the entire WM-IGL-ML layers (Fig. 2S1D–G,D’–G’). The GFAP+ Bergmann glial fibers also appeared more disorganized in the Nes-mYap cKO cerebella (Fig. 2S1H–K, H’–K’). Curiously, the disorganization of the PCs and Bergmann glia in Nes-mYap cKO cerebella was more extensive in lobules 1–5 and lobule 10, with lobule 9 being most similar to irradiated controls (Fig. 2S2). Surprisingly, when the areas of the cerebella and IGLs were measured at P12 and P16, we found that IR Nes-mYap cKOs and controls had similar IGL/cerebellum ratios at P12 but a significant reduction at P16 (Fig. 2S3). Together, these data indicate a crucial role of YAP in the NEP lineage at a late stage in the recovery of postnatal cerebellar growth after irradiation-induced injury to the EGL.
Figure 2. Mutation of Yap in the NEP lineage hampers postnatal cerebellar regeneration.
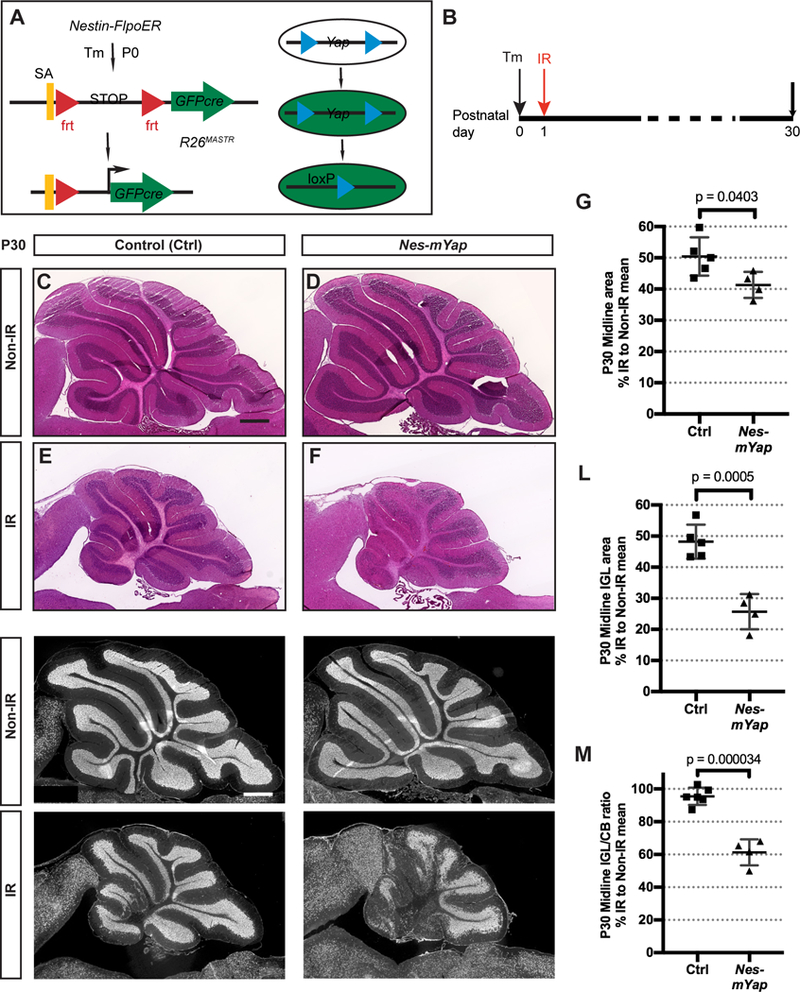
(A) Schematic of the MASTR mosaic mutant technique. (B) Schematic showing experimental design. (C-F) H&E staining of midsagittal sections of the cerebella from Non-IR and IR Nes-mYap cKO (Non-IR, n = 6; IR, n = 4) and control mice (Non-IR, n = 4; IR, n = 5) at P30. Scale bar, 500 μm. (G) Graph of the areas of midline sections of the cerebella of IR mice as a percentage of Non-IR animals of the same genotype. (H-K) IF detection of NeuN on midsagittal sections of the cerebella from Non-IR and IR Nes-mYap cKOs and controls at P30. Scale bar, 500 μm. (L-M) Graphs of the IGL areas (L) and IGL/cerebellum ratios (M) of midline cerebellar sections of IR Nes-mYap cKOs as a percentage of Non-IR mice of the same genotype (Non-IR, n = 6; IR, n = 4) and controls (Non-IR, n = 4; IR, n = 5) at P30. Data are presented as mean ± S.D., and statistical analysis by unpaired t test. Each data point represents one animal, and is calculated as the value for each IR mouse (average of 3 sections) divided by the mean for all the Non-IR mice of the same genotype, X 100.
Loss of Yap in NEPs results in an increase in cell death one day following irradiation at P1
Since over-expression of YAP in GCPs was shown to protect that cells from irradiation-induced cell death in culture (Fernandez et al., 2012), we examined whether YAP plays a role in maintaining the survival of NEPs in the PCL following irradiation. As expected, cell death (TUNEL+ particles) was minimal in the cerebella of P2 Non-IR Nes-mYap cKOs and controls given Tm at P0, and was prominent in the EGL of P2 IR mice (Fig. 3A–E). Interestingly, following irradiation Nes-mYap cKOs showed a 1.6-fold increase in the density of TUNEL+ particles within the PCL compared to controls (Fig. 3D,E,F). Since NEPs are the main proliferative cell type in the PCL, this result indicates that YAP protects NEPs from cell death following irradiation. Double labeling for TUNEL or cleaved Caspase3 (CC3) and GFP in Nes-mYap cKOs detected only rare TUNEL/GFP double positive cells (−5% of TUNEL+) and a higher percentage of CC3/GFP double positive cells (−15%) (Fig. 3S1). A similar or slightly lower percentage of TUNEL+ or CC3+ cells were labeled with GFP in Nes-FlpoER/+;R26FSF-GFPcre/+ (Nes-m) wild type IR an nonIR controls. The low double labeling of GFP and CC3 or TUNEL likely is because GFP degrades rapidly after cell death is initiated. It is therefore not clear whether the cell death is specific to NEPs.
Figure 3. Deletion of Yap in NEPs at P0 results in an increase in cell death in the PCL after irradiation-induced injury at P1.
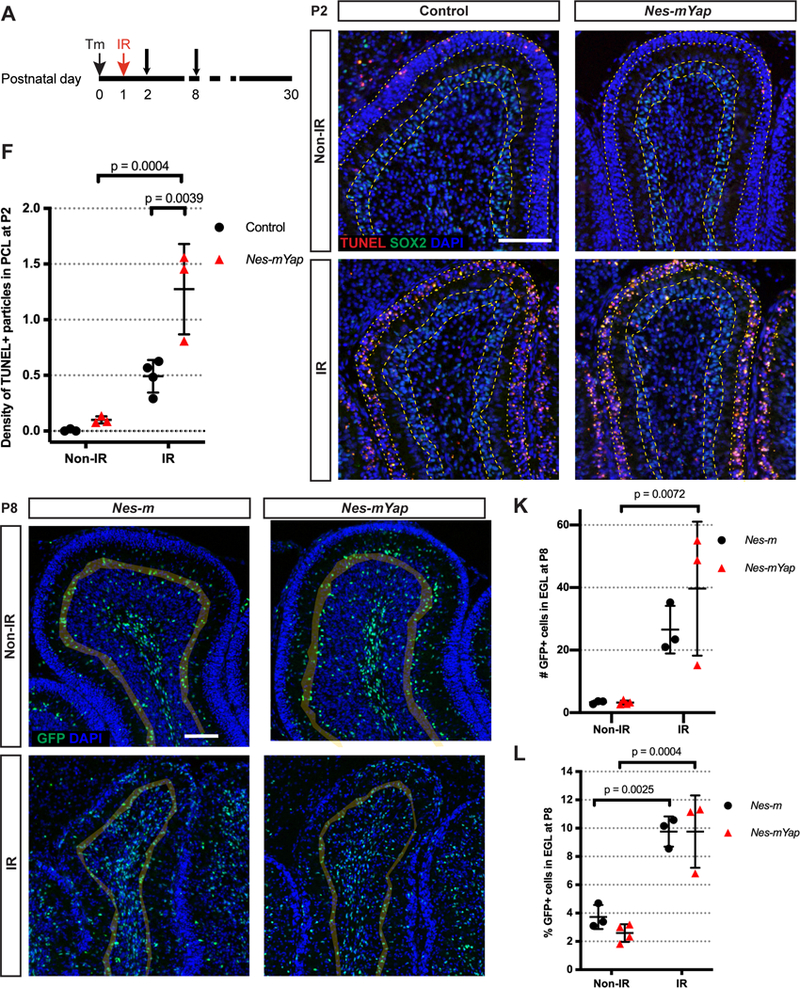
(A) Schematic showing experimental design. (B-E) Representative images from lobule 4/5 showing IF staining for TUNEL, SOX2, and DAPI on midsagittal sections from IR and Non-IR Nes-mYap cKOs and controls one day after IR at P1. Grey shadow highlights the PCL of the lobule. Arrows indicate TUNEL+ particles in the PCL. Scale bar, 100 μm. (F) Graph of the densities of TUNEL+ particles in the PCL of the midline cerebella from IR and Non-IR Nes-mYap cKOs (Non-IR, n = 3; IR, n = 3) and controls (Non-IR, n = 3; IR, n = 3) at P2. (G-J) Representative images from lobule 4/5 showing IF staining of GFP and DAPI on midsagittal cerebellar sections from IR and Non-IR Nes-m controls and Nes-mYap cKOs at P8. Grey shadow highlights the EGL of the lobule. Scale bar, 100 μm. (K-L) Graphs of the numbers of GFP+ cells normalized to total area measured in the EGL (K) and the percentages of GFP+ cells in the EGL among the total number of GFP+ cells (L) from Nes-m controls (Non-IR, n = 3; IR, n = 3) and Nes-mYap cKOs (Non-IR, n = 4; IR, n = 3) at P8. Data are presented as mean ± S.D., and statistical analysis by two-way ANOVA. Each data point represents one animal.
YAP is dispensable for the migration of NEPs into the EGL following irradiation-induced injury at P1
Given that the area of the IR cerebella of Nes-mYap cKOs at P12 was similar to IR controls, we examined whether NEPs are able to migrate into the EGL after irradiation when Yap is mutant. The distribution of GFP+ cells derived from NEPs labeled at P0 (and also mutated for Yap in Nes-mYap cKOs) in the different layers of Lobule 4/5 was quantified in both Nes-mYap cKOs and controls (Nes-m) at P8 (Fig. 3A). Consistent with the initial recovery of cerebellar area in Nes-mYap cKOs compared to IR controls, both the number and the percentage of GFP+ cells in the EGL of IR mice was similar between the two genotypes and much greater than in Non-IR mice (Fig. 3G–L). In addition, the numbers and percentages of NEPs in the molecular layer (ML) and IGL+WM were similar between the two genotypes after irradiation (Fig. 3S2). These results indicate that there is no cell autonomous requirement for YAP in NEPs for their migration from the PCL into the EGL.
YAP regulates differentiation of NEPs during normal cerebellum development, and the requirement is over-ridden following irradiation
We next examined whether Yap is required for the differentiation of NEPs during development of the cerebellum, by determining the distribution within the layers of the cerebellum of the descendants of NEPs labeled at P0 and analyzed at P8. Quantification of the GFPcre+ NEP-derived cells in lobule 4/5 of P8 cerebella demonstrated a significant increase in the number of GFP+ cells in the ML normalized to the area of the lobule analyzed, as well as the percentage of cells in the ML in Nes-mYap cKOs compared to Nes-m controls (Fig. 4A–E; Fig. 4S1A,B), but no significant change in the total number of mutant cells present at P8 (Fig. 4C; Fig. 4S1A,B). There was a concomitant decrease in the percentage of cells in the IGL+WM layers, but not the total number of cells in the IGL+WM layers, indicating that the significant change in Nes-mYap cKOs is an increase in the production of NEP-derived cells that populate the ML (Fig. 4E; Fig. 4S1A,B). Consistent with the GFP+ cells in the ML being the expected interneurons produced by WM-NEPs, there was also a significant increase in the number of cells in the ML that expressed PAX2, a marker of differentiating interneurons (Fig. 2F). The number of PAX2+ cells in the IGL+WM also was increased, but not significantly, indicating that in the absence of Yap WM NEPs increase their production of interneurons that migrate to the ML (Fig. 4G; Fig. 4S1C,D). Quantification of S100β+ astrocytes and Bergmann glia among the GFP+ populations in lobule 4/5 revealed an increase in the number of S100β+ cells in the PCL (Bergmann glia), but not S100p+ (astrocytes) in the IGL+WM (Fig. 4H; Fig. 4S1E,F). These data reveal that YAP normally plays a role in attenuating production of interneurons and Bergmann glia.
Figure 4. YAP attenuates the differentiation of NEPs during normal postnatal cerebellar development.
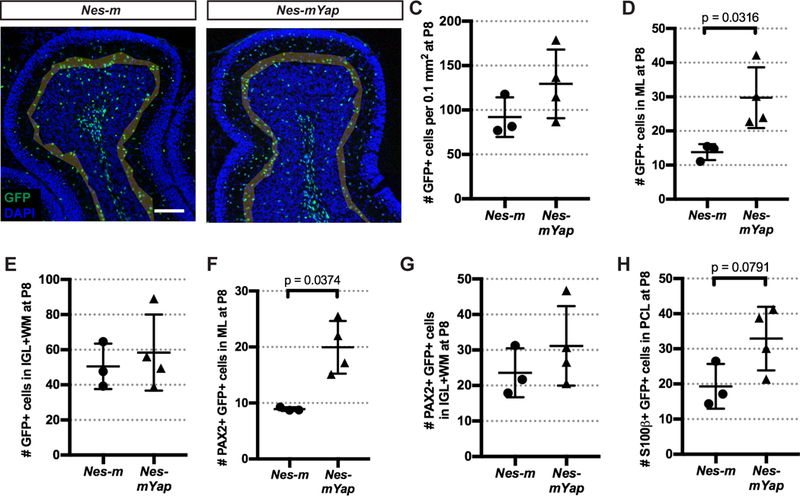
(A-B) Representative images from lobule 4/5 showing IF staining of GFP and DAPI on midsagittal sections from a Nes-m control and a Nes-mYap cKO at P8. Shading indicates the partitioning of the different layers (EGL, ML, PCL, IGL and WM) within the lobule. Scale bar, 100 μm. (C-H) Graphs of the numbers of GFP+ cells in all the layers (C) or in the ML (D) or IGL+WM (E), and the numbers of PAX2+ GFP+ cells in the ML (F) and IGL+WM (G), and the numbers of S100β+ GFP+ cells in the PCL (H), per 0.1 mm2 of the total area analyzed in lobule 4/5 from Nes-m controls (n = 3) and Nes-mYap cKOs (n = 4) at P8. (C) P = 0.1996; (E) P = 0.6065; (G) P = 0.3548. Statistical analysis is conducted by unpaired t test. Data are presented as mean ± standard deviation (S.D.), and each data point represents one animal.
Given that we did not observe a change in the distribution of Yap mutant GFP+ cells in the layers of the P8 cerebellum after irradiation, we next examined whether loss of YAP in NEPs alters their differentiation into interneurons (PAX2+) and astrocytes (S100β+) during irradiation-induced recovery of the cerebellum. No difference was observed in the number or percentage of PAX2+ cells in a specific layer (Fig. 4S2A,B), and there was no difference in the production of S100p+ astrocytes between Yap mutant and control mice after irradiation (Fig. 4S2C,D). These results indicate that the requirement for YAP in differentiation of NEPs is over-ridden when the EGL of the cerebellum is injured.
Loss of YAP results in an increase in cell death in the EGL and a defect in granule cell migration at P12 following irradiation-induced injury at P1
We next analyzed the cerebellum at P12, when the EGL is normally diminishing due to increased production of granule neurons. Quantification of the area and the thickness of the EGL showed no difference between IR Nes-mYap cKOs and controls, or in cerebellar IGL area (Fig. 5S1). Given the reduction in the size of the IGL in IR mutants compared to IR controls at P30, we asked whether YAP plays a role in cell survival or migration of granule cells. Strikingly, TUNEL staining in the EGL showed a significant increase in the density of TUNEL+ particles within the EGL (number of TUNEL+ particles per 0.1mm2) of Nes-mYap cKOs compared to controls after irradiation (Fig. 5A–F). In contrast, the density of TUNEL+ particles within the EGL at P8 was not different between controls and mutants (Fig. 5S2), consistent with the similar distributions and numbers of GFP+ cells between the cell layers in the two genotypes at P8. Furthermore, we examined PAX6 staining in the ML to determine whether there was a change in migration of granule cells from the EGL to the IGL, and found a higher density of PAX6+ cells in the ML in lobule 4/5 but not lobule 9 of Nes-mYap mutants compared to controls specifically after irradiation (Fig. 5G; Fig. 5S3). Since only rare PAX6+ cells in the ML were GFP+, one possibility is that there is a defect in the Yap mutant Bergmann glia that reduces migration of all granule cells (mutant and wild type)(Fig. 2S1H–K). Together, these results indicate that YAP is required for GCPs to maintain cell survival in the EGL and for granule cells to migrate along Bergmann glia through the ML into the IGL during injury-induced recovery.
Figure 5. Mutation of Yap in NEPs at P0 results in more cell death in the EGL and a defect in granule cell migration at P12 after irradiation-induced EGL injury at P1.
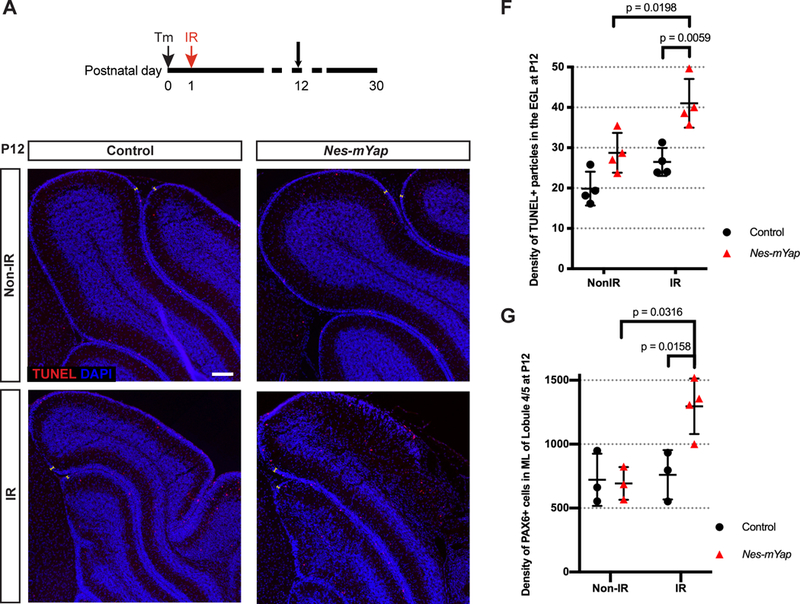
(A) Schematic showing experimental design. (B-E) Representative images showing IF staining for TUNEL and DAPI on midsagittal sections from IR and Non-IR Nes-mYap cKOs and controls at P12. Arrows indicate TUNEL+ particles in the EGL. Scale bar, 100 μm. (F) Graph of the densities of TUNEL+ particles in the EGL (the number of TUNEL+ particles per 0.1 mm2 of EGL area) in midline cerebellar sections from Nes-mYap cKOs (Non-IR, n = 3; IR, n = 4) and controls (Non-IR, n = 4; IR, n = 4) at P12. (G) Graph of the densities of PAX6+ cells in the ML (the number of PAX6+ cells per 1 mm2 of ML area) in midline cerebellar sections from Nes-mYap cKOs (Non-IR, n = 3; IR, n = 3) and controls (Non-IR, n = 3; IR, n = 3) at P12. Data are presented as mean ± S.D., and statistical analysis by two-way ANOVA. Each data point represents one animal.
YAP is not required in GCPs for cerebellar growth during normal development
Given that when Yap is deleted in NEPs there is an increase in cell death of GCPs at P12 in IR cerebella, one possible explanation is that YAP is required for GCP proliferation/differentiation or survival. In order to determine whether YAP plays a role in GCPs during normal development of the cerebellum, we generated two mutants. First, we deleted Yap from the ATOH1-expressing rhombic lip lineage when GCPs are generated in the embryo and analyzed the size of the cerebellum at P30. Similar to mice lacking Yap in NEPs (Fig. 2C,D), P30 Atoh1-Cre/+;Yapflox/flox (Atoh1-Yap cKO) mice showed no reduction in the area of the midline cerebellum (Fig. 6A,B), or the IGL area or IGL/cerebellum ratio compared to Yapflox/flox littermate controls (Fig. 6C–G). This lack of a growth phenotype shows that YAP does not play a major role in the Atoh1-lineage for growth of the cerebellum during development.
Figure 6. YAP attenuates differentiation of GCPs during postnatal development of the cerebellum.
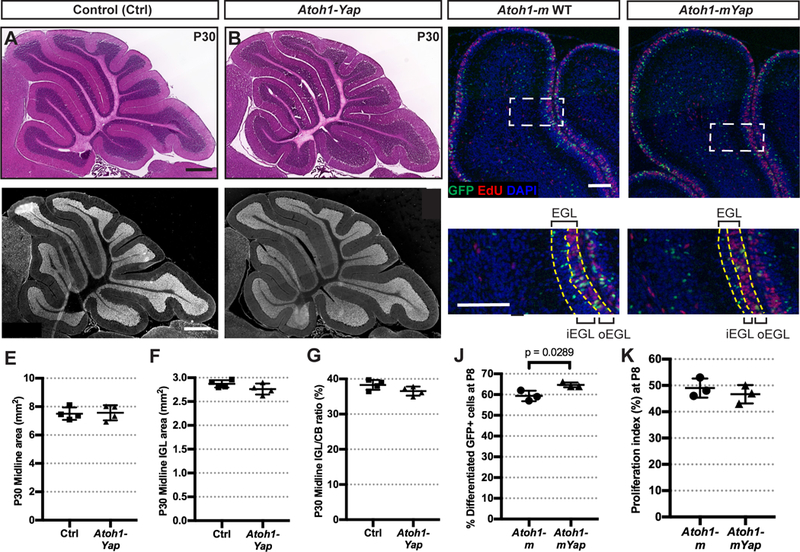
(A-D) H&E staining (A-B) and IF detection of the granule cell marker NeuN (C-D) on midsagittal sections of cerebella from Atoh1-Yap cKOs and control (Ctrl) mice at P30. Scale bars, 500 μm. (E-G) Graphs of the areas of the midline of the cerebellum (E), the areas of the IGL (F), and IGL/cerebellum area ratios (G) of Atoh1-Yap cKO (n = 4) and littermate controls (n = 4) at P30. (E) p = 0.8554; (F) p = 0.1727; (G) p = 0.1155. (H-l) Representative images from lobule 4/5 showing IF staining for GFP, EdU, and DAPI on midsagittal sections from an Atoh1-m control and Atoh1-mYap cKO at P8 after TM at P0. (H’-l’) Magnification of areas within dotted lines in H-l. Yellow dashed lines indicate the EGL and the border of the outer-and inner-EGL. Scale bars, 100 μm. (J-K) Graphs of the percentages of GFP+ differentiating cells (in all layers except the proliferating oEGL) among all GFP+ cells (J) and proliferation indices (G) in the midline of the cerebella in Atoh1-m controls (n = 3) and Atoh1-mYap cKOs (n = 3) at P8. (K) P = 0.4670. Data are presented as mean ± S.D., and statistical analysis by unpaired t test. Each data point represents one animal.
We next used the MASTR mosaic mutant approach with an Atoh1-FlpoER transgene (Wojcinski et al., 2019) as a sensitive assay for a mild change in differentiation of GCPs when Yap is removed. Tm was administered at P0 to Atoh1-FlpoER/+;R26FSF-GFPcre/+;Yapflox/flox (Atoh1-mYap cKO) mice and controls (Atoh1-FlpoER/+; R26FSF-GFPcre/+ or Atoh1-m), and then EdU was injected 1 hour prior to sacrifice at P8. The EdU+ outer EGL (oEGL) consists of actively proliferating GCPs. The percentage of post-mitotic GFP+ granule cells was quantified in the EdU-negative “inner layers” including the inner EGL (iEGL), ML, IGL, and WM as a percentage of all GFP+ cells (Fig. 6H–I, H’–l’). Interestingly, we found that the percentage of GFP+ postmitotic cells in the “inner layers” was slightly but significantly higher in Atoh1-mYap cKOs compared to Atoh1-m controls (Fig. 6J), indicating an increase in differentiation, or decrease in self-renewal of GCPs. The proliferation index (percent of EdU+ GFP+ cells among all GFP+ cells in the oEGL) was similar between GFP+ Atoh1-mYap cKO cells and Atoh1-m control GCPs (Fig. 6K), showing that Yap does not regulate the proliferation rate. Taken together, these data indicate that YAP plays only a minor role in attenuating differentiation of GCPs into granule neurons.
Loss of Taz in Yap mutant NEPs does not abrogate recovery after irradiation
Since TAZ and YAP have been found to have both similar and distinct requirements in the development or regeneration of various organs (Deng et al., 2016; Hossain et al., 2007; Makita et al., 2008; Morin-Kensicki et al., 2006; Reginensi et al., 2013; Tian et al., 2007), and we detected nuclear TAZ in NEPs (Fig. 1C–D,C’–D’, C”–D”), we asked whether TAZ contributes to the regenerative response following irradiation in Nes-mYap cKOs. We first tested whether Taz alone is required for development and replenishment of the cerebellum after irradiation by administering Tm at P0 to Nes-FlpoER/+;R26FSF-GFPcre/+;Tazflox/flox mice (Nes-mTaz cKOs) and controls (Nes-FlpoER/+;Tazflox/flox, or R26FSF-GFPcre/+;Tazflox/flox, or Tazflox/flox). Similar to Nes-mYap cKOs, conditional deletion of Taz did not alter cerebellar size at P30 (Fig. 7; Fig. 7S). However, unlike Nes-mYap cKOs, Nes-mTaz cKOs recovered almost as well as control mice after irradiation (Fig. 7; Fig. 7S).
Figure 7. Mutation of Taz in NEPs at P0 does not alter cerebellar growth during development and regeneration after irradiation at P1.
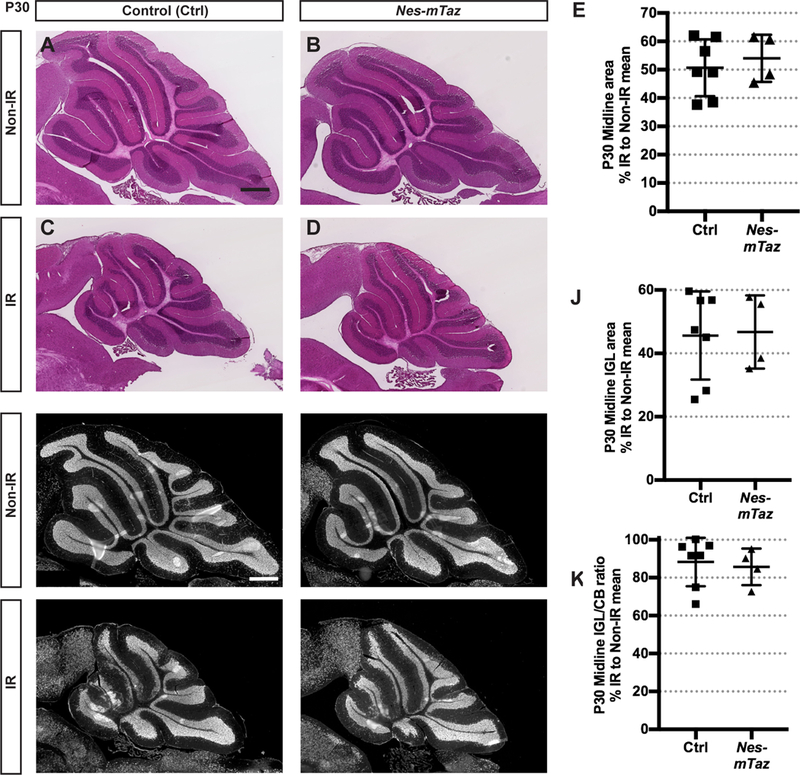
(A-D) H&E staining of midsagittal sections of cerebella from Non-IR and IR of Nes-mTaz cKOs and controls at P30. Scale bars, 500 μm. (E) Graph of the areas of the cerebella of IR animals as a percentage of Non-IR mice of the same genotype in midline sections, p = 0.5866. (F-l) IF detection of NeuN on midsagittal sections of the cerebella from Non-IR and IR Nes-mTaz cKOs and controls at P30. Scale bar, 500 μm. (J,K) Graphs of the IGL areas (J) and IGL/cerebellum ratios (K) of IR Nes-mTaz cKOs (Non-IR, n = 6; IR, n = 4) and controls (Non-IR, n = 4; IR, n = 7) at P30 as a percentage of Non-IR same genotype. (J) p = 0.8945; (K) p = 0.7335. Data are presented as mean ± S.D., and statistical analysis by unpaired t test. Each data point represents one animal, and is calculated using each IR measurement divided by the mean of Non-IR mice of the same genotype, X 100.
We next ablated both Yap and Taz from NEPs using Nes-FlpoER/+;R26FSF-GFPcre/+;yapflox/flox;Tazflox/flox mice (Nes-mYapTaz cKOs) and controls (Nes-FlpoER/+; Yapflox/flox;Tazflox/flox or R26FSF-GFPcre/+;Yapflox/flox;Tazflox/flox or Yapflox/flox;Tazflox/flox) given Tm at P0. Surprisingly, ablation of both Yap and Taz did not result in a worse recovery of the cerebellum after injury compared to Nes-mYap cKOs. On the contrary, Nes-mYapTaz cKOs had no significant reduction in the area of the midline cerebellum or IGL, although they had a small but significant reduction in the IGL/cerebellum ratio compared to controls (Fig. 8; Fig. 8S1A–C). The genetic loss of Yap and Taz was confirmed via RT-qPCR analysis of the Yap and Taz mRNA levels in GFPcre+ NEPs isolated by FACS from Nes-mYapTaz cKO compared to Nes-m control mice at P8 (Fig. 8S2). Significantly, the mRNA expression of both genes was greatly reduced (Fig. 8S2). The apparent better recovery of Nes-mYapTaz cKOs compared to Nes-mYap cKOs could indicate that loss of Taz partially rescues the poor late recovery observed in Nes-mYap cKOs (Fig. 8S1D–F).
Figure 8. Loss of Taz in NEPs lacking Yap at P0 does not abrogate recovery of the IGL after irradiation at P1.
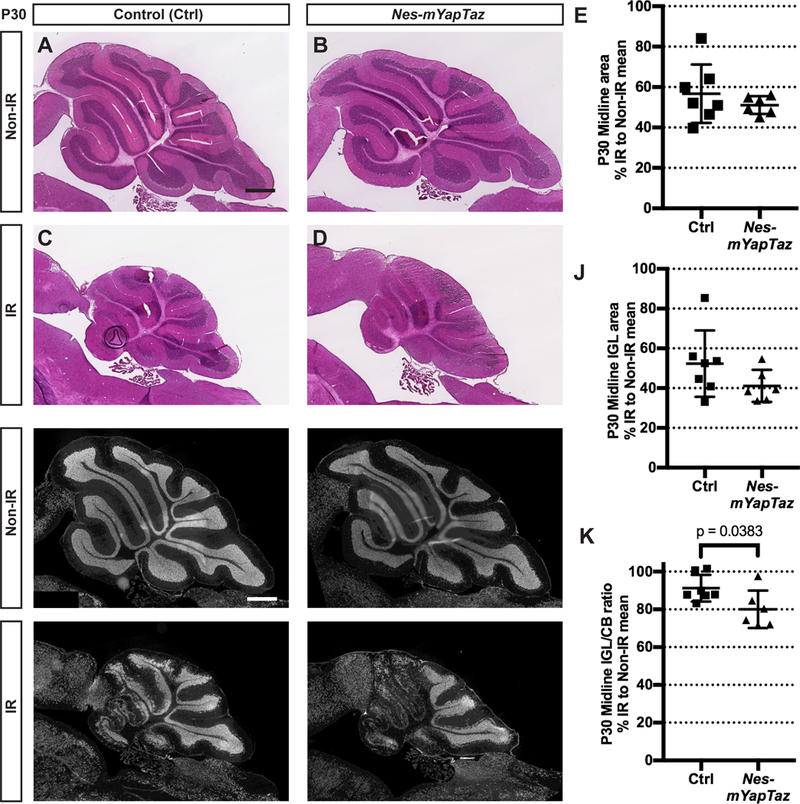
(A-D) H&E staining of midsagittal sections of the cerebella from Non-IR and IR Nes-mYapTaz cKOs and controls at P30. Scale bars, 500 μm. (E) Graph of the midline cerebellar areas of IR mice as a percentage of Non-IR animals of the same genotype. P = 0.3815. (F-l) IF detection of NeuN on midsagittal sections of the cerebella from Non-IR and IR animals at P30. Scale bar, 500 μm. (J,K) Graphs of the areas of the IGL (J) and IGL/cerebellum ratios (K) of IR mice as a percentage of Non-IR animals of the same genotype on midsaggital sections at P30. Nes-mYapTaz cKOs (Non-IR, n = 7; IR, n = 6) and controls (Non-IR, n = 4; IR, n = 7). (J) p = 0.1637; (K) p = 0.0383. Data are presented as mean ± S.D., and statistical analysis by unpaired t test. Each data point represents one animal, and is calculated using the average for each IR mouse divided by the mean of the Non-IR mice of the same genotype, X 100.
In order to examine whether the apparent better recovery in Nes-mYapTaz cKOs compared to Nes-mYap cKOs correlates with better survival of GCPs in the EGL at P12, we quantified cell death by TUNEL staining of cerebellar sections. Unlike the increase in the density of TUNEL+ particles within the EGL of P12 Nes-mYap cKOs compared to controls after irradiation (Fig. 5F), IR Nes-mYapTaz cKOs did not have a significant increase in cell death in the EGL compared to controls (Fig. 8S3). Such an attenuation of cell death could contribute to the slightly better regeneration observed in Nes-mYapTaz cKOs compared to Nes-mYap cKOs. Furthermore, the differentiation of NEPs was not altered in Nes-mYapTaz cKOs compared to controls at P8 in nonIR mice (Fig. 8S4).
DISCUSSION
We have demonstrated that YAP is required for postnatal regeneration of the cerebellum following irradiation at P1. In particular, mutation of Yap impairs the partial recovery of cerebellar size and formation of the IGL following irradiation, but not the adaptive reprogramming of NEPs (Fig. 9). The defect in regeneration of the IGL in Nes-mYap cKOs is accompanied by an increase in cell death in the EGL and accumulation of granule cells in the ML at P12, following normal initial expansion and migration of NEPs to the EGL in response to the injury (Fig. 9). The organizations of Purkinje cells and Bergmann glial fibers also is disrupted in Yap mutants. It is possible that this aspect of the phenotype is a secondary result of the failure of the GCPs to generate an IGL in IR Yap mutants. Alternatively, Yap depletion in NEPs could result in a cell autonomous defect in Bergmann glia that is only critical following irradiation, and that cell non-autonomously reduces survival of GCPs and/or hinders the migration of granule cells into the IGL resulting in a depletion of the EGL. Consistent with the latter, granule cells only accumulate in lobules where recovery of the IGL is very poor and Bergmann glia are the most abnormal in mutants after irradiation (lobules 4/5 and not 9).
Figure 9. Model of the cellular responses during the development and regeneration of neonatal cerebellum in normal and Nes-mYap mice after irradiation at P1.
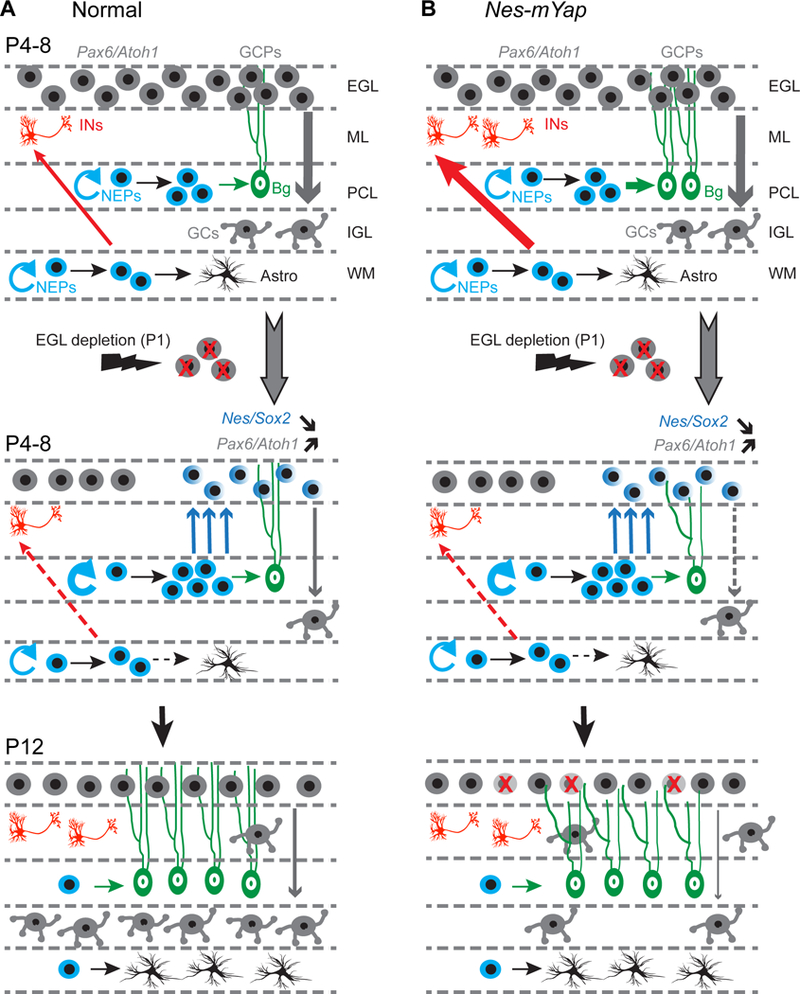
(A) Depletion of the External Granule Layer (EGL) during the first days of postnatal cerebellar development results in an expansion and migration of Nestin-Expressing Progenitors (NEPs) in the Purkinje Cell Layer (PCL) that normally produce Astrocytes (Astro) and Bergmann Glia (Bg). Once in the EGL, NEP-derived cells initiate expression of Granule Cell lineage-specific genes (Pax6 and Atoh1) and expand to replenish the EGL. Concomitantly, white matter (WM) NEPs have a transient reduction in production of interneurons (INs) and astrocytes.
(B) In Non-IR Nes-mYap mice, the growth of cerebella is not altered during development, with normal cerebellar size and internal granule cell layer (IGL). Genetic ablation of Yap from NEPs promotes the differentiation of WM-NEPs into interneurons in the molecular layer (ML), and the differentiation of PCL-NEPs into astrocytes. In IR Nes-mYap mice, depletion of the EGL at P1 leads to the expansion of NEPs in the PCL and migration to the EGL. GCPs in Nes-mYap mice have increased cell death and defective migration into the IGL compared to that in control mice, possibly because the Bergmann glia are defective. These defects in the granule cell lineage contribute to the poor recovery of the IGL that has much fewer granule cells (GCs). The slight enhancement of differentiation of Yap mutant NEPs, however, is overridden in IR cerebella.
The pivotal role of Hippo signaling in brain development has been extensively studied in Drosophila, a model organism in which the Hippo pathway itself was first discovered. Drosophila lacking the YAP homolog Yorkie display semi-lethality and impaired proliferation and growth of neural stem cells at the larval stage (Ding, Weynans, Bossing, Barros, & Berger, 2016). In a complementary manner, inhibition of Hippo signaling, through either over-expression of Yorkie or loss-of-function mutations in several core kinases that activate Yorkie function, results in increased neuroblast proliferation and substantial brain overgrowth (Poon, Mitchell, Kondo, Cheng, & Harvey, 2016). In the mouse, genetic ablation of Yap in radial glial progenitors leads to only subtle proliferation defects during cortical development (Park et al., 2016), whereas loss of both Yap and Taz results in a loss of ependymal cells that line the ventricle in the postnatal brain (W. A. Liu et al., 2018). Consistent with the subtle requirement for YAP in cortical development (Park et al., 2016), we found that Yap ablation from NEPs or GCPs does not significantly reduce the overall size of the adult cerebellum. We nevertheless observed mild increases in differentiation of Yap mutant NEPs and GCPs. In particular, removal of Yap from NEPs or GCPs in a mosaic manner in the cerebellum between P1 and P8 results in an increase in differentiation of NEPs (PCL-NEPs to produce Bergmann glia and WM-NEPs to produce interneurons) and GCPs (to produce granule cells) possibly at the expense of self-renewal. YAP seems to have a similar function in progenitor proliferation/differentiation in other organs. For example, YAP over-activation in mouse intestine or chick neural tube leads to expansion of progenitor cells and loss of differentiated cells (Camargo et al., 2007; Cao, Pfaff, & Gage, 2008). It also has been shown that over-expression of YAP in cultured myoblasts inhibits differentiation and formation of myotubes (Watt et al., 2010), and reduced Hippo signaling in mouse epithelial cells results in hyper-proliferation of progenitors and failure of differentiation into multiple epithelial cell types (J. H. Lee et al., 2008). Thus, a consistent finding from our current study in the cerebellum and work of others in distinct organs is that YAP modulates a timely transition of progenitors from cell proliferation into differentiation.
Several lines of evidence suggest an intersection or coupling of the Hedgehog (HH) and Hippo pathways in Drosophila and mice (Akladios et al., 2017; Fernandez et al., 2009; Hsu et al., 2017; Huang & Kalderon, 2014; Lin et al., 2012). For example, in flies elevated Hh signaling (ptc mutants) induces Yorkie target gene transcription, whereas additional deletion of Yorkie abolishes the effects of excess Hh signaling on cell proliferation and survival (Huang & Kalderon, 2014). In cultured mouse GCPs, SHH treatment was reported to increase the transcription and nuclear localization of YAP protein (Fernandez et al., 2009). Our previous study showed that SHH signaling is required for NEPs to regenerate the IR mouse cerebellum, particularly at the initial stage of recovery (Wojcinski et al., 2017). Thus it is possible that SHH signaling after irradiation activates YAP function and this promotes survival of NEPs immediately after irradiation, since we observed an increase in cell death after irradiation in the PCL of mutants where NEPs are the main proliferating cell type. However, comparison of Nes-mSmo cKO (Wojcinski et al., 2017) and Nes-mYap cKO phenotypes after irradiation of the cerebellum reveals additional requirements for SHH signaling that are independent of Yap. In Nes-mSmo cKOs there is an immediate defect in expansion of NEPs and their migration to the EGL after irradiation (Wojcinski et al., 2017), whereas these cellular responses are intact in Nes-mYap cKOs. Taken together, the results provide evidence that SHH has critical target genes other than Yap that are required for proliferation and migration of NEPs.
YAP and TAZ have often been found to have overlapping functions, consistent with their significant homology and common binding partners, but some recent studies point to distinct roles in addition to overlapping functions of YAP and TAZ that appear to be context dependent. For example, Yap null mutant mice are embryonical lethals (Morin-Kensicki et al., 2006), whereas Taz nulls only show partial embryonic lethality and mice that survive have lung defects and kidney disease (Hossain et al., 2007; Makita et al., 2008; Tian et al., 2007). It has also been shown that TAZ competes with YAP in an interaction with a regulator protein that modulates the maturation of chondrocytes, a critical step for skeletal development and bone repair (Deng et al., 2016). Similarly we found that Yap ablation from NEPs leads to poor recovery of the postnatal cerebellum following acute injury, but deletion of Taz does not abrogate regeneration. Furthermore, double conditional Yap and Taz mutants appear to have a milder phenotype than Yap cKOs, indicating that loss of Taz partially rescues the Yap mutant phenotype. This finding raises the possibility of antagonistic functions between YAP and TAZ in NEPs, as in the bone, compared to overlapping functions under other circumstances. It is also possible that differences in the genetic backgrounds of the single and double mutants contribute to the degree of recovery of Yap mutant NEPs after irradiation. Thus it will be important to dissect the distinct downstream molecular pathways of the two co-activators to reveal their context-dependent functions in the Hippo pathway. Leveraging this knowledge will pave the road for potential therapeutic intervention for cerebellar hypoplasia caused by injury.
CONCLUSIONS
YAP but not TAZ is required for postnatal regeneration of the cerebellum following irradiation at P1 at the level of promoting production of the granule cells that make up the IGL. YAP is not required for the initial adaptive reprogramming of NEPs or migration of NEP-derived cells to the EGL following irradiation. YAP plays a role in cell survival following irradiation, first in the PCL at P2 and then in the EGL at P12. YAP also seems to promote migration of granule cells from the EGL to the IGL at P12 in IR mice. YAP normally plays only a minor role during development in attenuating differentiation of NEPs and GCPs. Finally, TAZ does not appear to have an overlapping function with YAP in NEPs.
Supplementary Material
Highlights.
YAP1 but not TAZ is required in NEPs for regeneration of the perinatal cerebellum.
YAP1 is not required for adaptive reprogramming of NEPs into GCPs.
YAP1 is required in NEPs for survival and migration of GCPs after irradiation.
YAP1 plays a minor role in attenuating differentiation of NEPs and GCPs.
Acknowledgements
We thank Dr. Jeff Wrana for providing the Yap and Taz conditional mutant mice via Dr. Kathryn Anderson’s lab. We thank Dr. Alexandre Wojcinski for insightful suggestions on experimental design and interpretation of results, and Dr. I-Li Tan for performing one RT-qPCR experiment. We are grateful to Daniel Stephen and Zhimin Lao for technical assistance. We thank all the Joyner lab members for their helpful comments and discussions. We also thank the Flow Cytometry core and the Center for Comparative Medicine and Pathology of MSKCC for outstanding technical support. We gratefully acknowledge P. Zanzonico for his help with mouse irradiation; Q. Chen and the MSKCC Small-Animal Imaging Core Facility for technical assistance; and a Shared Resources Grant from the MSKCC Geoffrey Beene Cancer Research Center, which provided funding support for the purchase of the XRad 225Cx Microirradiator.
Funding
This work was supported by a grant from the National Institutes of Health to A.L.J. [NS092096], and a National Cancer Institute Cancer Center Support Grant [P30 CA008748-48].
Footnotes
Competing interests
The authors declare no competing or financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akladios B, Mendoza Reinoso V, Cain JE, Wang T, Lambie DL, Watkins DN, & Beverdam A (2017). Positive regulatory interactions between YAP and Hedgehog signalling in skin homeostasis and BCC development in mouse skin in vivo. PLoS One, 12(8), e0183178. doi: 10.1371/journal.pone.0183178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Anderson WJ, & Wright KA (1969). Early effects of x-irradiation of the cerebellum in infant rats: decimation and reconstitution of the external granular layer. Exp Neurol, 24(2), 196–216. [DOI] [PubMed] [Google Scholar]
- Altman J, & Bayer SA (1997). Development of the cerebellar system in relation to its evolution, structure, and functions. Boca Raton: CRC Press. [Google Scholar]
- Andreotti JP, Prazeres P, Magno LAV, Romano-Silva MA, Mintz A, & Birbrair A (2018). Neurogenesis in the postnatal cerebellum after injury. Int J Dev Neurosci, 67, 33–36. doi: 10.1016/j.ijdevneu.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani A, & Maedler K (2018). The Hippo Signaling Pathway in Pancreatic beta-Cells: Functions and Regulations. Endocr Rev, 39(1), 21–35. doi: 10.1210/er.2017-00167 [DOI] [PubMed] [Google Scholar]
- Bayin NS, Wojcinski A, Mourton A, Saito H, Suzuki N, & Joyner AL (2018). Age-dependent dormant resident progenitors are stimulated by injury to regenerate Purkinje neurons. Elife, 7. doi: 10.7554/eLife.39879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran V, Verney C, & Ferriero DM (2012). Perinatal cerebellar injury in human and animal models. Neurol Res Int, 2012, 858929. doi: 10.1155/2012/858929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard-Racine M, du Plessis AJ, & Limperopoulos C (2015). Developmental cerebellar cognitive affective syndrome in ex-preterm survivors following cerebellar injury. Cerebellum, 14(2), 151–164. doi: 10.1007/s12311-014-0597-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, & Rossi F (2013). Origin, lineage and function of cerebellar glia. Prog Neurobiol, 109, 42–63. doi: 10.1016/j.pneurobio.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Callus BA, Verhagen AM, & Vaux DL (2006). Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. Febs j, 273(18), 4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, & Brummelkamp TR (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol, 17(23), 2054–2060. doi: 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, & Gage FH (2008). YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev, 22(23), 3320–3334. doi: 10.1101/gad.1726608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerrato V, Parmigiani E, Figueres-Onate M, Betizeau M, Aprato J, Nanavaty I, … Buffo A (2018). Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity. PLoS Biol, 16(9), e2005513. doi: 10.1371/journal.pbio.2005513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, & Sillje HH (2005). The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene, 24(12), 2076–2086. doi: 10.1038/sj.onc.1208445 [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, & Segil N (2002). The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development, 129(10), 2495–2505. [DOI] [PubMed] [Google Scholar]
- Corrales JD, Blaess S, Mahoney EM, & Joyner AL (2006). The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development, 133(9), 1811–1821. doi: 10.1242/dev.02351 [DOI] [PubMed] [Google Scholar]
- Corrales JD, Rocco GL, Blaess S, Guo Q, & Joyner AL (2004). Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development, 131(22), 5581–5590. doi: 10.1242/dev.01438 [DOI] [PubMed] [Google Scholar]
- Deng Y, Wu A, Li P, Li G, Qin L, Song H, & Mak KK (2016). Yap1 Regulates Multiple Steps of Chondrocyte Differentiation during Skeletal Development and Bone Repair. Cell Rep, 14(9), 2224–2237. doi: 10.1016/j.celrep.2016.02.021 [DOI] [PubMed] [Google Scholar]
- Ding R, Weynans K, Bossing T, Barros CS, & Berger C (2016). The Hippo signalling pathway maintains quiescence in Drosophila neural stem cells. Nat Commun, 7, 10510. doi: 10.1038/ncomms10510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, & Sands J (1973). Quantitative growth and development of human brain. Arch Dis Child, 48(10), 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto K (2011). The growing role of the Hippo--NDR kinase signalling in neuronal development and disease. J Biochem, 150(2), 133–141. doi: 10.1093/jb/mvr080 [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, … Welsh JP (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum, 11(3), 777–807. doi: 10.1007/s12311-012-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, … Kenney AM (2009). YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev, 23(23), 2729–2741. doi: 10.1101/gad.1824509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LA, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, … Kenney AM (2012). Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene, 31(15), 1923–1937. doi: 10.1038/onc.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JT, He W, Hao C, Ketova T, Pan FC, Wright CC, … Chiang C (2013). The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Dev Cell, 27(3), 278–292. doi: 10.1016/j.devcel.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider G, & Johnson RL (2011). Hippo signaling: growth control and beyond. Development, 138(1), 9–22. doi: 10.1242/dev.045500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortensius LM, Dijkshoorn ABC, Ecury-Goossen GM, Steggerda SJ, Hoebeek FE, Benders M, & Dudink J (2018). Neurodevelopmental Consequences of Preterm Isolated Cerebellar Hemorrhage: A Systematic Review. Pediatrics, 142(5). doi: 10.1542/peds.2018-0609 [DOI] [PubMed] [Google Scholar]
- Hossain Z, AN SM, Ko HL, Xu J, Ng CP, Guo K, … Hunziker W (2007). Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtrl Proc Natl Acad Sci USA, 104(5), 1631–1636. doi: 10.1073/pnas.0605266104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TH, Yang CY, Yeh TH, Huang YC, Wang TW, & Yu JY (2017). The Hippo pathway acts downstream of the Hedgehog signaling to regulate follicle stem cell maintenance in the Drosophila ovary. Sci Rep, 7(1), 4480. doi: 10.1038/S41598-017-04052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JK, Du W, Shelton SJ, Oldham MC, DiPersio CM, & Klein OD (2017). An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice. Cell Stem Cell, 21(1), 91–106.e106. doi: 10.1016/j.stem.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, & Kalderon D (2014). Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J Cell Biol, 205(3), 325–338. doi: 10.1083/jcb.201309141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, & Sadoshima J (2016). Regulation of Myocardial Cell Growth and Death by the Hippo Pathway. Circ J, 80(7), 1511–1519. doi: 10.1253/circj.CJ-16-0476 [DOI] [PubMed] [Google Scholar]
- Jaeger BN, & Jessberger S (2017). Unexpected help to repair the cerebellum. Nat Neurosci, 20(10), 1319–1321. doi: 10.1038/nn.4640 [DOI] [PubMed] [Google Scholar]
- Lao Z, Raju GP, Bai CB, & Joyner AL (2012). MASTR: a technique for mosaic mutant analysis with spatial and temporal control of recombination using conditional floxed alleles in mice. Cell Rep, 2(2), 386–396. doi: 10.1016/j.celrep.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Park JY, Pare J, Finkelstein D, Pan H, Xu B, … Cao X (2018). The Hippo Pathway Prevents YAP/TAZ-Driven Hypertranscription and Controls Neural Progenitor Number. Dev Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, … Lim DS (2008). A crucial role of WW45 in developing epithelial tissues in the mouse. Embo j, 27(8), 1231–1242. doi: 10.1038/emboj.2008.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Goraya N, Kim S, & Cho SH (2018). Hippo-yap signaling in ocular development and disease. Dev Dyn, 247(6), 794–806. doi: 10.1002/dvdy.24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, & McMahon AP (2004). Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol, 270(2), 393–410. doi: 10.1016/j.ydbio.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Li P, Du F, Yuelling LW, Lin T, Muradimova RE, Tricarico R, … Yang ZJ (2013). A population of Nestin-expressing progenitors in the cerebellum exhibits increased tumorigenicity. Nat Neurosci, 16(12), 1737–1744. doi: 10.1038/nn.3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr., Sullivan NR, Benson CB, … duPlessis AJ (2007). Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics, 120(3), 584–593. doi: 10.1542/peds.2007-1041 [DOI] [PubMed] [Google Scholar]
- Lin YT, Ding JY, Li MY, Yeh TS, Wang TW, & Yu JY (2012). YAP regulates neuronal differentiation through Sonic hedgehog signaling pathway. Exp Cell Res, 318(15), 1877–1888. doi: 10.1016/j.yexcr.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Liu WA, Chen S, Li Z, Lee CH, Mirzaa G, Dobyns WB, … Shi SH (2018). PARD3 dysfunction in conjunction with dynamic HIPPO signaling drives cortical enlargement with massive heterotopia. Genes Dev, 32(11–12), 763–780. doi: 10.1101/gad.313171.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, & Deng J (2019). Ubiquitinationdeubiquitination in the Hippo signaling pathway (Review). Oncol Rep, 41(3), 1455–1475. doi: 10.3892/or.2019.6956 [DOI] [PubMed] [Google Scholar]
- Lu L, Finegold MJ, & Johnson RL (2018). Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp Mol Med, 50(1), e423. doi: 10.1038/emm.2017.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, … Kurihara H (2008). Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol, 294(3), F542–553. doi: 10.1152/ajprenal.00201.2007 [DOI] [PubMed] [Google Scholar]
- Marek S, Siegel JS, Gordon EM, Raut RV, Gratton C, Newbold DJ, … Dosenbach NUF (2018). Spatial and Temporal Organization of the Individual Human Cerebellum. Neuron. doi: 10.1016/j.neuron.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, … Fritzsch B (2005). Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn, 234(3), 633–650. doi: 10.1002/dvdy.20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt A, Fuiko R, Prayer D, Brugger PC, Boltshauser E, Zoder G, … Birnbacher R (2008). Disrupted cerebellar development in preterm infants is associated with impaired neurodevelopmental outcome. Eur J Pediatr, 167(10), 1141–1147. doi: 10.1007/s00431-007-0647-0 [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, & Enikolopov G (2004). Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol, 469(3), 311–324. doi: 10.1002/cne.10964 [DOI] [PubMed] [Google Scholar]
- Milosevic A, & Goldman JE (2004). Potential of progenitors from postnatal cerebellar neuroepithelium and white matter: lineage specified vs. multipotent fate. Mol Cell Neurosci, 26(2), 342–353. doi: 10.1016/j.men.2004.02.008 [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, … Milgram SL (2006). Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol, 26(1), 77–87. doi: 10.1128/mcb.26.1.77-87.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, & Kugler JM (2018). Ubiquitin-Dependent Regulation of the Mammalian Hippo Pathway: Therapeutic Implications for Cancer. Cancers (Basel), 10(4). doi: 10.3390/cancers10040121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D (2010). The hippo signaling pathway in development and cancer. Dev Cell, 19(4), 491–505. doi: 10.1016/j.devcel.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R, Moon UY, Park JY, Hughes LJ, Johnson RL, Cho SH, & Kim S (2016). Yap is required for ependymal integrity and is suppressed in LPA-induced hydrocephalus. Nat Commun, 7, 10329. doi: 10.1038/ncomms10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani E, Leto K, Rolando C, Figueres-Onate M, Lopez-Mascaraque L, Buffo A, & Rossi F (2015). Heterogeneity and Bipotency of Astroglial-Like Cerebellar Progenitors along the Interneuron and Glial Lineages. J Neurosci, 35(19), 7388–7402. doi: 10.1523/jneurosci.5255-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CL, Mitchell KA, Kondo S, Cheng LY, & Harvey KF (2016). The Hippo Pathway Regulates Neuroblasts and Brain Size in Drosophila melanogaster. Curr Biol, 26(8), 1034–1042. doi: 10.1016/j.cub.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Rakic P, & Sidman RL (1970). Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J Comp Neurol, 139(4), 473–500. doi: 10.1002/cne.901390407 [DOI] [PubMed] [Google Scholar]
- Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, … McNeill H (2013). Yap-and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet, 9(3), e1003380. doi: 10.1371/journal.pgen.1003380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, & Joyner AL (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol, 23, 549–577. doi: 10.1146/annurev.cellbio.23.090506.123237 [DOI] [PubMed] [Google Scholar]
- Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, & van Wezel-Meijler G (2009). Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology, 252(1),190–199. doi: 10.1148/radiol.2521081525 [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, … Tsai PT (2017). Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci, 20(12), 1744–1751. doi: 10.1038/s41593-017-0004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, … Benjamin T (2007). TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol, 27(18), 6383–6395. doi: 10.1128/mcb.00254-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, … Sahin M (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature, 488(7413), 647–651. doi: 10.1038/nature11310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Rudolph S, Guo C, Ellegood J, Gibson JM, Schaeffer SM, … Sahin M (2018). Sensitive Periods for Cerebellar-Mediated Autistic-like Behaviors. Cell Rep, 25(2), 357–367.e354. doi: 10.1016/j.celrep.2018.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, & Haider G (2003). Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol, 5(10), 914–920. doi: 10.1038/ncb1050 [DOI] [PubMed] [Google Scholar]
- Wang K, Degerny C, Xu M, & Yang XJ (2009). YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol, 87(1), 77–91. doi: 10.1139/008-114 [DOI] [PubMed] [Google Scholar]
- Wang SS, Kloth AD, & Badura A (2014). The cerebellum, sensitive periods, and autism. Neuron, 83(3), 518–532. doi: 10.1016/j.neuron.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt KI, Judson R, Medlow P, Reid K, Kurth TB, Burniston JG, … Wackerhage H (2010). Yap is a novel regulator of C2C12 myogenesis. Biochem Biophys Res Commun, 393(4), 619–624. doi: 10.1016/j.bbrc.2010.02.034 [DOI] [PubMed] [Google Scholar]
- Wojcinski A, Lawton AK, Bayin NS, Lao Z, Stephen DN, & Joyner AL (2017). Cerebellar granule cell replenishment postinjury by adaptive reprogramming of Nestin(+) progenitors. Nat Neurosci, 20(10), 1361–1370. doi: 10.1038/nn.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski A, Morabito M, Lawton AK, Stephen DN, & Joyner AL (2019). Genetic deletion of genes in the cerebellar rhombic lip lineage can stimulate compensation through adaptive reprogramming of ventricular zone-derived progenitors. Neural Dev, 14(1), 4. doi: 10.1186/s13064-019-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, & Pan D (2003). hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with Salvador and warts. Cell, 114(4), 445–456. [DOI] [PubMed] [Google Scholar]
- Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, … Jensen KB (2018). YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell, 22(1), 35–49.e37. doi: 10.1016/j.stem.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, & Guan KL (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev, 24(1), 72–85. doi: 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


