Abstract
Objectives:
Amish children raised on traditional farms have lower atopy and asthma risk than Hutterite children raised on modern farms. In our previous study, we established that the Amish environment affects the innate immune response to lower asthma and atopy risk. Herein we investigated the T cell phenotypes in the same Amish and Hutterite children as in our earlier study to elucidate how this altered innate immunity effects adaptive T cells.
Methods:
Blood was collected from 30 Amish and 30 Hutterite age and sex-matched children; cells were cryopreserved until analysis. Flow cytometry was used to analyze cell subsets. Atopy was determined by allergen-specific and total IgE levels.
Results:
Children exposed to Amish farms had increased activated Treg phenotypes, while conventional CD4 T cell expressed lower levels of co-stimulation molecules and other activation markers. The increase in circulating activated Tregs was associated with increase in inhibitory receptors on monocytes in Amish, but not Hutterite, children. Strikingly, the Amish children had a higher proportion of CD28null CD8 T cells than Hutterite children (non-parametric t test p<0.0001), a difference that remained even after accounting for the effects of age and sex (conditional log regression exponential β=1.08, P=0.0053). The proportion of these cells correlated with high T cell IFNγ production (rs=0.573, P=0.005) and low serum IgE (rs=−0.417, P=0.025). Further, CD28null CD8 T cells were increased in Amish children with high expression of the innate genes TNF and TNFAIP3 in peripheral blood leukocytes.
Conclusion:
Amish children’s blood leukocytes are not only altered in their innate immune status, but additionally have distinct T cell phenotypes that are often associated with increased antigenic exposure.
Keywords: Asthma, atopy, adaptive immunity, T cell activation, CD4 T cells, CD8 T cells
Graphical Abstract

Capsule Summary:
Amish farm children, who are low-risk for asthma/atopy, have distinct T cell phenotypes including CD28-null CD8 cells and activated Tregs, suggesting that the Amish environment contributes to decreased atopy through both innate and adaptive immunity.
Introduction
Asthma is a complex disease with a strong environmental component. Asthma and allergic sensitization are reduced among children raised on farms compared to those from non-farming and urban environments (1). Moreover, our previous work demonstrated that Amish school-age children raised on traditional farms had a 4-fold lower risk of asthma compared to Hutterite children raised on modern, mechanized farms (2–4), despite having otherwise similar lifestyles and ancestry. Notably, this difference in asthma prevalence was paralleled by differences in the proportions and phenotype of innate peripheral blood leukocytes (PBLs) from these children. In particular, we showed that Amish children had significantly more monocytes with an inhibitory phenotype and more immature neutrophils compared to Hutterite children. In contrast, the proportion of Tregs were not different between Amish and Hutterite children. We therefore speculated that the profound differences in innate immunity in Amish children may impact other T cell phenotypes.
Continuous exposure to microbial products impacts innate immune function. Repeated exposures to LPS induce “endotoxin tolerance” or anergy in macrophages and monocytes that renders these cells unresponsive to further stimulation (5–8). This phenomenon is detrimental in patients with chronic infections or sepsis. It is also associated with reduced T cell responses, possibly due to low HLA-DR expression (9). Monocytes from Amish children also had suppressed HLA-DR levels and increased inhibitory receptor expression (2), but how this phenotype correlated with changes in adaptive T cell immunity in these children had not been explored.
In this study, we investigated whether T cell populations were phenotypically distinct between the same 30 Amish and 30 Hutterite schoolchildren who participated in our earlier study. We found many differences in the co-stimulatory molecules expressed by Amish and Hutterite children: Amish conventional CD4+ T cells expressed less CD28 or ICOS; and Amish CD4+ regulatory T cells (Tregs) expressed significantly higher levels of CD45RO and ICOS, a phenotype consistent with enhanced suppressive function (10, 11). Additionally, Amish children carried a significantly expanded, distinctive CD28null CD8+ T cell population, and the proportions of these cells correlated positively and negatively with IFNγ secretion by PBLs and serum IgE levels, respectively. Within the Amish, CD28null CD8+ T cells were increased in children with high expression of the innate genes IRF7, TNF, and TNFAIP3 (A20) in their peripheral blood leukocytes. Overall these results suggest that profound differences in T cell immunity between Amish and Hutterite children may contribute to their distinct asthma and atopy risk.
Methods
Study participants and sample collection
The 30 Amish and 30 Hutterite schoolchildren (6–14 years old) were age- and sex-matched as previously described (2). None of the 30 Amish children had asthma, while 6 of the 30 Hutterite children had asthma. Written consent was obtained from the parents and written assent was obtained from the children. The study was approved by the institutional review boards at the University of Chicago and St. Vincent Hospital, Indianapolis. Blood was collected for cell analyses and serum IgE measurements as previously reported (2). To obtain PBLs, whole blood was lysed with ACK lysis buffer (150mM ammonium chloride, 10mM potassium carbonate, 0.1mM EDTA) and the remaining leukocytes were cryopreserved in 90% FBS/10% DMSO. Cells were kept in liquid nitrogen storage for approximately 6 months prior to thawing for flow cytometry experiments.
Flow cytometry
Frozen PBLs were thawed, washed in RPMI containing Deoxyribonuclease I (0.02 mg/mL), and resuspended in FACS buffer (PBS containing 0.1% sodium azide and 1% BSA). Approximately 3×105 cells in 100 μL per sample were incubated for 10 min with pooled human IgG (FcX, Biolegend, San Diego, CA) to block non-specific antibody binding before staining with fluorescently conjugated antibodies (Table S1). For surface phenotyping, flow cytometry data were acquired immediately after staining on an LSRFortessa (BD Biosciences, San Jose, CA), and the data were analyzed with FlowJo software (Tree Star, Inc., Ashland, Oregon). For FoxP3 staining, cells were surface stained as described above before performing the FoxP3 staining according to manufacturer’s instructions (FoxP3 Fix/Perm Kit, eBioscience). T cell subsets were gated as shown in Supplemental Figure 1 and Supplemental Figure 2.
IFNγ Measurement
Whole blood was drawn directly into TruCulture® (Myriad RBM) collection tubes. One ml of whole blood was drawn into two different tubes: one containing TruCulture® media + anti-CD3 and anti-CD28 antibodies, and one containing TruCulture® media alone. Whole blood samples in the TruCulture tubes were incubated upright in a dry heat block at 37C for 30 hours.
After incubation, supernatant from the TruCulture® tubes was flash frozen for cytokine studies using the provided Seraplus valve. Amish cell samples were processed in the laboratory at the University of Chicago and Hutterite samples were processed on site in a makeshift lab in the Oaklane colony. The same individuals processed both sets of samples. Supernatants from both Amish and Hutterites were thawed on the same day and IFNγ was quantified using a multiplex assay (Millipore Sigma, Burlington, MA). T cell IFNγ was defined as the difference between IFNγ measured in the anti-CD3/CD28 sample and the control media-alone sample for each child.
Statistical analyses
Two group comparisons of continuous variable data were analyzed using a non-parametric Mann-Whitney test. A Bonferroni-corrected p value for t tests was calculated based on the number of comparisons that were generated. Correlations were computed using a linear regression followed by a non-parametric Spearman test for significance. Additional details for each figure can be found in the corresponding legend. All analyses and graphs were generated using Prism graphing software (Graphpad Software, La Jolla, CA).
Results
Amish children have similar proportions of circulating T cells compared to Hutterite children.
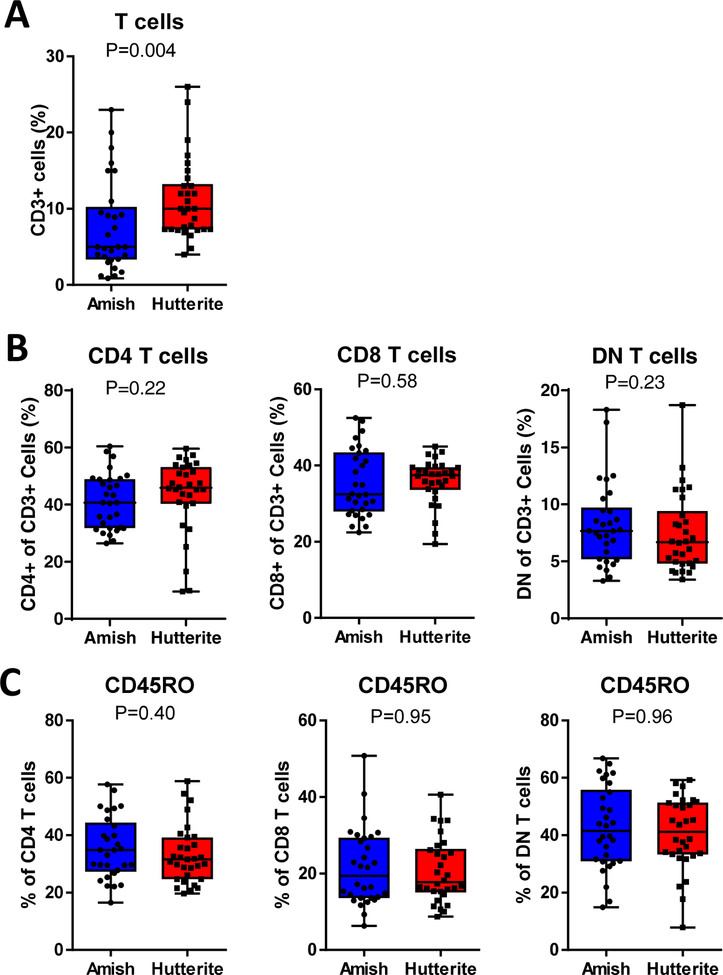
We examined cell phenotypes in a cohort of children from two farming communities: the Amish, who have a low incidence of asthma and atopy; and the Hutterites, who have a higher incidence (3, 12). The overall prevalence of asthma in Amish children ages 6–12 is 5.2%, while in Hutterite children ages 6–10 is 21.3%. Allergic sensitization defined by skin prick tests is 7.2% and 33.3% in Amish and Hutterite children, respectively. In our study, none of the 30 Amish children and 6 of the 30 Hutterite children (20%) had asthma. Two Amish children and nine Hutterite children had allergen-specific IgE above a 3.5kUA/L threshold. Demographic information was previously published (2) and is shown in Supplemental Table 1. Amish children had reduced total IgE and eosinophil percentages compared to Hutterite children. Total IgE was correlated with the sum of specific IgE levels as well as eosinophil percentages in all children, although significance was greater in Hutterite children who were more likely to have elevated IgE and eosinophils (Supplemental Fig 3). Amish children had a trend of a reduction in the proportion of CD3+ T cells of total PBLs compared to Hutterite children (P=0.004 with P<0.0025 required for Bonferonni-corrected significance) (Fig 1A). No differences were found in the percent of T cells defined as CD4+, CD8+, or double negative for both markers (DNs) (Fig 1B), or in the percentage of each T cell subset expressing the effector/memory marker CD45RO (Fig 1C).
Figure 1. T cell proportions in Amish and Hutterite children.
(A) The percent of CD3+ cells of total peripheral blood leukocytes. (B) The proportion of CD3+ cells that are CD4+, CD8+, or double negative (DN). (C) CD45RO expression on each subset was measured as a marker of effector and/or memory T cells. Value shown on each graph is p value from non-parametric Mann-Whitney test with p<0.0025 required for Bonferonni-corrected significance.
Tregs from Amish children have an activated phenotype that correlates with upregulation of ILT5 on monocytes.
The percentage of Tregs (CD3+ CD4+ FoxP3+ CD127−) was similar in the Amish and Hutterite children despite their disparate asthma and atopy risk ((2) and Fig 2A). However, the percent of CD45RO+ ICOS+ Tregs was significantly increased in Amish children compared to Hutterite children (36.8±16.2% versus 24.2±11.8%, respectively, P=0.0009) (Fig. 2B–C). These markers are characteristic of activated Tregs with more suppressive function in both humans and mice (10, 13, 14). Higher levels of the inhibitory molecule PD-1 were also observed on the Amish Tregs (P=0.001) (Fig 2D).
Figure 2. Amish and Hutterite schoolchildren have a similar proportion of Tregs, but Amish Tregs express increased markers of activation.
Tregs were identified by size, negative for a dead cell stain, and CD3+CD4+FoxP3+CD127lo. (A) The proportion of Tregs in Amish and Hutterite of total CD4 T cells. (B,C) The percentage of activated Tregs were identified as CD45RO+ICOS+ of total Tregs in the two groups. (D) PD-1 expression on Tregs. Value shown on each graph is p value from non-parametric Mann-Whitney test with p<0.0025 required for Bonferonni-corrected significance. Significant p values are denoted with as asterisk.
Inducible Tregs are polarized from naive T cells through interactions with specific antigen-presenting cell (APC) populations. Monocytes are one potential APC that can drive Treg expansion. Similar to the observed proportions of Tregs, the overall proportion of monocytes (CD14+CD66b− cells) did not differ between the Amish and Hutterite children (2). As previously reported, monocytes from the Amish children had decreased surface expression of HLA-DR (P<0.001) and increased surface expression of the inhibitory molecules ILT3 (P=0.003) and ILT5 (P=0.005) compared to monocytes from Hutterite children (2). Little is known about the function of ILT5, but it can be expressed by both antigen presenting cells and granulocytes. In Amish children ILT5 was expressed at a significantly higher level on monocytes compared to Hutterite children (P<0.001) (Fig 3A), while neutrophils from both groups of children expressed similar levels (P=0.69) (Fig 3B). In the Amish, monocyte ILT5 expression positively correlated with CD45+ICOS+ Tregs (rs =0.4696, P=0.0088) (Fig 3C–D). No association was observed in Hutterite children (rs =−0.26, P=0.16). Although ILT3 has been associated with the induction of Tregs (15, 16), we found a negative correlation between ILT3 expression on monocytes and activated CD45RO+ICOS+ Tregs in Amish children (rs=−0.421, P=0.021), and no correlation between these cell phenotypes in Hutterite children (rs=0.041, P=0.8276) (Supplemental Fig 4).
Figure 3. Activation of Tregs correlates with expression of the inhibitory receptor ILT5 on monocytes.
(A) Expression of ILT5 on monocytes (left panel) and neutrophils (right panel), in Amish children. Value shown on each graph is p value from non-parametric Mann-Whitney test with p<0.0025 required for Bonferonni-corrected significance. Significant p values are denoted with as asterisk. (B) Correlation of ILT5 expression with the percent of activated CD45RO+ICOS+ Tregs. R value is Spearman’s rho correlation coefficient and associated P value.
Amish CD4 Tconv cells express a distinct co-stimulatory molecule profile indicative of reduced activation.
The suppressive phenotypes identified in Amish monocytes and Tregs may function to prevent effector T cell responses. Therefore, we examined the activation state of Tconv CD4 T cells in Amish and Hutterite children. CD28 expression, a critical co-stimulatory molecule expressed by T cells to facilitate T cell proliferation and cytokine secretion, was similar in both groups (Fig 4A). The IL-7 receptor (CD127), which promotes memory T cell proliferation, showed a trend of lower expression on Amish CD4 T cells (Fig 4B). We next examined cell surface expression of ICOS and PD-1, two CD28 family members with costimulatory and co-inhibitory function, respectively. A dramatic reduction in ICOS+ CD4 T cells was present in Amish children (10.7±5.1% ICOS+ CD4 T cells in Amish compared to 18.7±7.6% in Hutterite, P<0.0001) (Fig 4C) while a non-significant trend was observed for higher PD-1 expression on Amish CD4 T cells (Fig 4D). A similar phenotype was observed in DN T cells (Supplemental Fig 5).
Figure 4. Conventional (non-Treg) CD4 T cells in the Amish exhibit low activation with a specific reduction in ICOS.
(A) Expression of CD28, (B) CD127, (C) ICOS, and (D) PD-1 expression on CD4 T cells from Amish and Hutterite children. Value shown on each graph is p value from non-parametric Mann-Whitney test with p<0.0025 required for Bonferonni-corrected significance. Significant p values are denoted with as asterisk. (E) ICOS expression on CD4 T cells was correlated with circulating IgE in either Amish (left panel) or Hutterite (right panel) children. R value is Spearman’s rho correlation coefficient and associated P value.
Our previous studies in a cohort that included both Hutterites and non-Hutterites revealed that SNPs in the ICOS promoter were associated with allergic sensitization and IgE levels (17), and ICOS expression is positively correlated with Th2 responses in mice (18). Consistent with the role of ICOS in Th2 responses, the percent of ICOS+ CD4 T cells positively correlated with total IgE levels in Hutterite children, but not in the Amish children, who had both low ICOS and IgE (Fig 4E).
CD8 T cells from Amish children are characterized by the presence of CD25hi cells and increased CD28null cells
Like CD4 T cells, CD8 T cells also play an important role in asthma pathogenesis. CD8 T cell expression of leukotriene B4 receptor 1 is associated with asthma severity, and bronchial CD8 T cells from patients with asthma produce IL-13 (19, 20). We examined the phenotype of CD8 T cells in Amish children. Examination of the total CD8 T cell population demonstrated that Amish CD8 T cells expressed higher ICOS and lower CD127 than Hutterite CD8 T cells, a phenotype characteristic of effector and/or memory cells (Supplemental Fig 6A,B) (21, 22). No difference was observed in PD-1 expression (Supplemental Fig 6C).
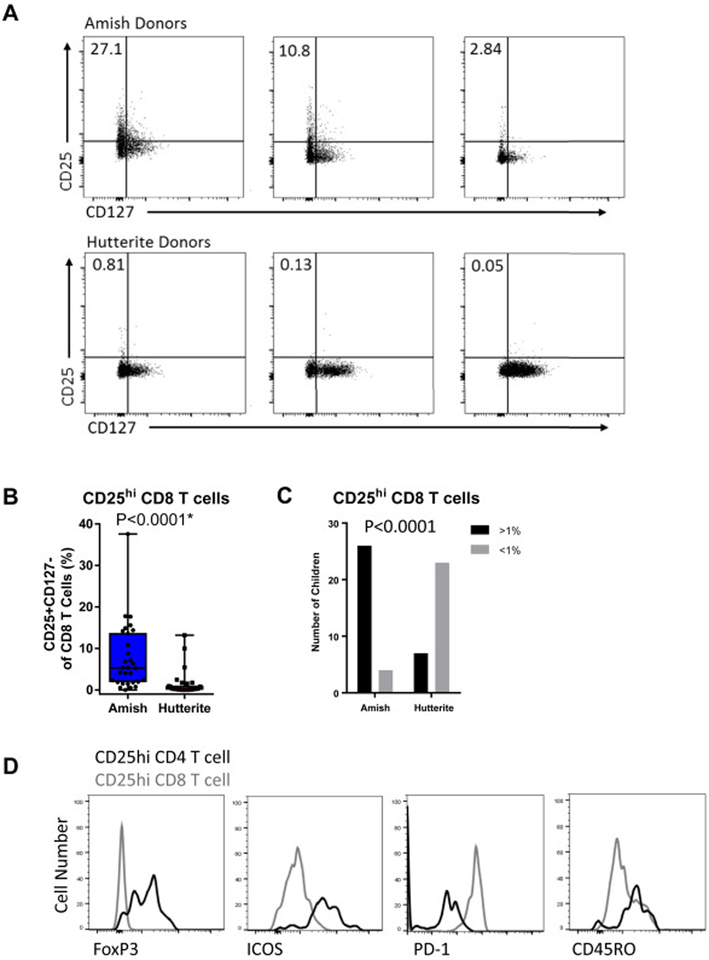
On further analysis of the CD8 T cells, we discovered that the Amish PBLs contained a unique subset of CD25hiCD127lo CD8 T cells (Fig 5A–C). This subset was found in 26 of the 30 Amish children (defined as having 1% or greater CD25hi of CD8 T cells), but only 7 of 30 Hutterite children. The medians of CD25hi CD8 T cells were 5.30 (IQR 1.89–13.75) and 0.26 (IQR 0.08–1.08) in the Amish and Hutterite children, respectively (P<0.0001). As CD25hi CD4 T cells are mainly composed of FoxP3+ Tregs, we assessed whether the CD25hi CD8 T cells had a similar regulatory phenotype. However, unlike CD25hi CD4 T cells, this subset did not express FoxP3 or ICOS, and instead expressed high PD-1, a marker of exhaustion (Fig 5D).
Figure 5. Amish children have a unique population CD8 T cells that express high levels of CD25.
(A) Example flow plots showing the expression of CD25 and CD127 on CD3+CD8+ T cells in three Amish and three Hutterite donors. (B) Percent of CD25hiCD127lo CD8 T cells in each group with p value from non-parametric Mann-Whitney test. Asterisk indicates a significant difference after multiple testing correction. (C) The proportion of Amish or Hutterite children that had <1% (grey bars) >1% (black bars) of CD8 T cells exhibiting the CD25hiCD127lo phenotype. P value from Fisher’s exact test. (D) The expression of FoxP3, ICOS, PD-1, and CD45RO was compared between CD25hiCD127lo CD8 T cells (gray line) and CD25hiCD127lo CD4 T cells (black line) from the same Amish donor.
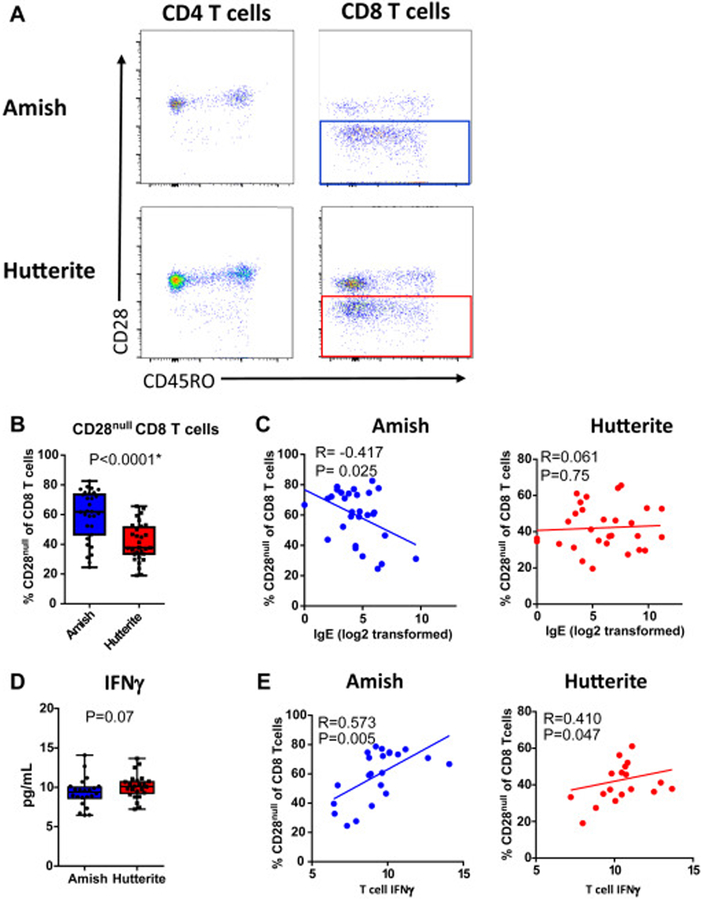
Strikingly, the majority of the CD8 T cells from the Amish children lacked the co-stimulatory molecule CD28 (CD28null CD8 T cells, P<0.0001) (Fig 6A,B). A significant difference remained in a conditional regression model to remove the effects of age and sex (conditional log regression exponential β=1.08, P=0.0053, Supplemental Table 1). The level of CD28null CD8 T cells negatively correlated with IgE in Amish children, but not Hutterite children (rs=−0.417, P=0.025 and rs=0.061, P=0.75 in Amish and Hutterite, respectively) (Fig 6C). CD28null CD8 T cells have been associated with chronic antigen exposure, CMV infection, aging, and cancer (23–25). As CD28null CD8 T cells are generally associated with IFNγ-mediated responses, we asked whether the proportion of these cells correlated with IFNγ production by T cells. No difference was observed between Amish and Hutterite IFNγ production after T cell stimulation (Fig 6D). Yet in both the Amish and Hutterite children, IFNγ levels were correlated with the percentage of CD28null CD8 T cells (rs=0.573, P=0.005 and rs=0.410, P=0.047 in Amish and Hutterite, respectively) (Fig 6E).
Figure 6. CD8 T cells from Amish children have low levels of the co-stimulatory molecule CD28 and correlate with increased IFNγ and low IgE.
(A) Example flow plots of the expression of CD28 and CD4 and CD8 T cells Amish and Hutterite children. (B) Proportion of CD28null CD8 T cells of total CD8 T cells with p value from non-parametric Mann-Whitney test. Asterisk indicates a significant difference after multiple testing correction. (C) Correlation of CD28null CD8 T cells and serum IgE as measured by ELISA. (D) IFNγ was measured in the supernatant of whole blood from 21 of the 30 Hutterite children and 22 of the 30 Amish children stimulated with anti-CD3 and anti-CD28 antibodies for 30 hours. The children who were omitted have a low affinity allele in FcγRIIA that reduces binding of the anti-CD3 and anti-CD28 antibodies (38). (E) Correlation of IFNγ and IgE in Amish and Hutterite children. R value is Spearman’s rho correlation coefficient and associated P value.
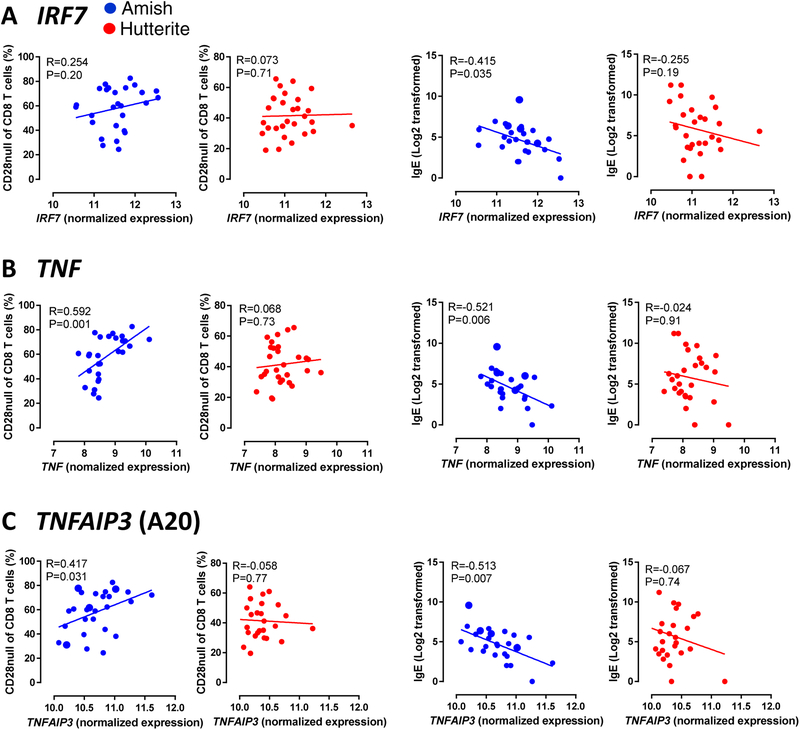
Previously, we found that gene expression of a module of co-expressed innate immune genes was higher in the PBLs of Amish compared to Hutterite children (2). To understand the relationship between these innate immune genes and allergic sensitization, we correlated serum IgE levels to three genes in the innate network identified from the module (IRF7, TNF, and TNFAIP3) that have significantly increased expression in Amish children ((2) and Supplemental figure 7). TNF and TNFAIP3 exhibited a positive correlation with the frequency of CD28null CD8 T cells, and all three genes exhibited a negative correlation with serum IgE, in Amish children (Fig 7). Of the three genes tested, TNF expression exhibited the strongest correlation with CD28null CD8 T cells (rs=0.592, P=0.001) and IgE (rs=−0.521, P=0.006). No significant correlations were found in Hutterite children. The Hutterite children with asthma were not driving these findings, as all comparisons were examined with the six asthmatic children exclusion (Supplemental Tables 2 and 3). A summary of our observations on immune activation in Amish children is shown in Figure 8.
Figure 7. High expression of innate immune genes correlates with increased CD28null CD8 T cells and low IgE in Amish children.
The association between normalized expression of an IRF7 (A), TNF (B), and TNFAIP3 (C) with CD28null CD8 T cells and IgE. Gene expression in Amish and Hutterite PBL samples was assessed by microarray after culture in media for 30hr. R value is Spearman’s rho correlation coefficient and associated P value.
Figure 8. Effects of Amish environment on circulating immune cells: Potential mechanisms for reduced asthma risk.
Our studies in Amish and Hutterite children support a model in which microbial exposures in the Amish drive neutrophil emigration from bone marrow and induce suppressive monocytes (previously published in NEJM). These monocytes are associated with activated Tregs that may be inhibiting effector T cells or antigen presenting cells. The Amish environment also leads to unique CD8 T cell phenotypes, including CD25hiCD127lo CD8 T cells whose function is unknown. Increased CD28null CD8 T cells were associated with increased IFNγ and decreased IgE. These cells may be directly or indirectly suppressing Th2 responses to reduce atopy and asthma risk.
Discussion
The Amish farm environment shapes the innate immune response in children (2), and we hypothesized that this would lead to altered adaptive T cell responses. Here we show that the Amish children had a lower proportion of T cells, including CD4, CD8 and DN T cell subsets compared to Hutterite children. All three T cell subsets in the Amish expressed less CD28, a co-stimulatory molecule critical for effector T cell functions. ICOS was lower on CD4 Tconv cells in the Amish compared to the Hutterites, suggesting less activation of CD4 T cells. Yet ICOS was expressed at a much higher level on Tregs in the Amish. These data indicate that Amish have specific activation of regulatory CD4 T cells that may contribute to their reduced levels of IgE. High ICOS expression on Amish Tregs was correlated with expression of the inhibitory receptors ILT5, and not ILT3, on monocytes. Finally, Amish children had increased percentage of CD28null CD8 T cells, and these cells were correlated with increased IFNγ production and a reduction in circulating IgE in Amish children. Interestingly, the percent of CD28null CD8 T cells also correlated with genes within the innate gene-network that was upregulated in Amish blood. Thus, our findings suggest a model in which environmental exposures in the Amish community drive expression of an IRF7/TNF pathway, promote unique T cell phenotypes that produce IFNγ, and inhibit IgE.
We were surprised to find that healthy Amish children between 6–14 years of age already have as much as 80% CD28null CD8 T cells, a phenotype generally associated with aging and chronic diseases, especially chronic viral infections (23–26). In our earlier study of the innate response in Amish children, we reported that IRF7 was a key transcription factor upregulated in peripheral blood leukocytes from Amish compared to Hutterite children (2). IRF7 is well-known as a viral response gene that initiates type 1 interferon production. While we do not know the past or ongoing viral exposures in the Amish children, the positive correlation of CD28null CD8 T cells with IFNγ release suggest that viral exposures could be contributing to this phenotype. Further, our observation that higher IFNγ was associated with lower IgE suggests that this phenotype may be a marker of protection against atopy, although it is unclear if CD28null CD8 T cells directly contribute to this protection. The majority of Amish children also had CD8 T cells expressing CD25, while almost none of the Hutterite children had these cells. While the function of these cells is not known, they express markers consistent with an exhausted type of CD8 T cell (27, 28).
Monocytes from Amish children had low expression of HLA-DR (2), which has been associated with repeated endotoxin exposure (29) and chronic infections (30, 31) in other studies. These HLA-DR-low monocytes expressed high levels of two inhibitory receptors, ILT3 and ILT5 (2) (Figure 3). ILT3 is notable in that it can drive the differentiation of both Tregs and CD8 suppressor cells that can inhibit inflammation (15, 16, 32, 33). Therefore, it was surprising that ILT3 was negatively correlated with CD45RO+ICOS+ Tregs in Amish children. ILT5 is a related ITIM-containing family member that is upregulated in tolerogenic dendritic cells (34). The function of ILT5 and its relationship to T cell differentiation is currently unknown (15), but our data suggest that ILT5 may be important in the induction of Tregs in the Amish environment. Multiple studies have linked increased numbers of Tregs with protection from asthma, and have shown that children exposed to farm environments, and particularly to consumption of raw milk, have increased numbers of Tregs (35, 36). Together, these data support the hypothesis that increased levels of tolerogenic monocytes drive Treg activation in the Amish children.
Our study has a number of limitations. First, differences in cell phenotypes between Amish and Hutterite children may be due to an epiphenomenon. For instance, we have insufficient data to link our findings to either microbial and environmental exposures, or the presence or absence of atopy. Second, while children with high total IgE in our study were more likely to also have additional markers of atopic disease, including increased eosinophils and positive allergen-specific IgE, we cannot rule out the possibility that these findings reflect exposure to unknown parasitic infections in these children. Finally, our study is limited by the overall small sample size and the relatively few atopic children, especially among the Amish children. Future studies, with larger sample sizes, will yield additional insight into the effect of different environments on cell phenotypes associated with allergic sensitization.
Age and sex had little to no effect on our T cell phenotypes, but the level of IFNγ became significantly different in Amish and Hutterite children when these co-variates were removed. This suggests that immune system skewing may change with age or sex and is consistent with known differences in age-dependent allergy and asthma prevalence in males and females (37). While 6 of the 30 Hutterite children were asthmatic, those individuals did not drive the differences in cell phenotypes we observed between the Amish and Hutterite children. Even when asthmatics were excluded, Hutterite children had increased ICOS+ CD4 Tconv cells, decreased ILT receptor expression on monocytes, fewer CD45RO+ICOS+ Tregs, and fewer CD28null CD8 T cells compared to Amish children. We do not know whether these cell phenotypes are associated with asthma-risk or allergic sensitization. Instead, our data suggest that the Hutterite environment is associated with the development of distinct cell phenotypes that are related to higher IgE production.
Overall, our assessment of T cell subsets in Amish children supports a model in which sustained microbial exposures lead to chronic antigen stimulation. Exposure to this milieu, in turn, results in changes to the innate immune system (2) and is correlated with changes to the adaptive immune system, characterized by low expression of T cell co-stimulatory molecules. Thus, the Amish farm environment broadly regulates both innate and adaptive immunity. Understanding the mechanisms driving the protective effects of the Amish farm environment on both innate and adaptive immunity may generate robust avenues of study for asthma and allergic disease prevention.
Supplementary Material
Key Messages:
Environmental exposures in children can drive unique T cell phenotypes that are associated with circulating IgE.
Regulatory T cells and CD8 T cells from Amish children display a phenotype characteristic of chronic activation, while their conventional CD4 T cells display a less activated phenotype.
Acknowledgments
We would like to thank the Amish and Hutterite families who generously volunteered to participate in our study. We would also like to thank Gorka Alkorta-Aranburu, Maitane Arrubarrena Orbegozo, Kathleen Bailey, Christine Billstrand, Kelly Blaine, Daniel Cook, Donna Decker, Mohammad Jaffery, Courtney Burrows, Katherine Naughton, Raluca Nicolae, Rob Stanaker, Meghan Sullivan, and Emma Thompson for assistance on field trips and sample processing. This work was supported by the National Institutes of Health U19 AI095230; R01 HL085197; T32 HL007605 for CLH and MMS. The University of Chicago Flow Cytometry Core is supported by P30 CA014599.
Abbreviations:
- APC
Antigen presenting cell
- HLA-DR
Human leukocyte antigen –antigen D related
- ICOS
Inducible T-cell costimulator
- IFNγ
Gamma-interferon
- ILT3
Ig-like transcript 3
- ILT5
Ig-like transcript 5
- PBL
Peripheral blood leukocyte
- PD-1
Programmed cell death protein 1
- RBC
Red Blood Cell
- Th2
T helper type-2 CD4+ T cell
- TCR
T cell receptor
- Treg
Regulatory CD4+ T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Von Mutius declares no conflicts of interest with regard to the work under consideration for publication. Relevant financial activities outside the submitted work (within 36 months prior to 11 July 2019): Dr. von Mutius received fees from Springer Medizin Verlag GmbH for editorial work, speaker’s fees from American Thoracic Society, Böhringer Ingelheim International GmbH, HAL Allergie GmbH; consultant fees from OM Pharma SA, PharmaVentures, Peptinnovate Ltd.; fees from Massachusetts Medical Society for serving as NEJM Editorial Board Member; expert fees from Chinese University of Hongkong, European Commission, University of Utrecht, University of Turku/Turun Yliopisto, University of Tampere/Tampereen Yliopisto, University of Helsinki/Helsingin yliopisto; author fees from Springer Verlag, Schattauer Verlag, Georg-Thieme-Verlag, Elsevier and travel costs from Nestlé Deutschland AG. Intellectual Property: Dr. von Mutius is listed as inventor on the following patents: Publication number EP 1411977: Composition containing bacterial antigens used for the prophylaxis and the treatment of allergic diseases. Publication number EP1637147: Stable dust extract for allergy protection. Publication number EP 1964570: Pharmaceutical compound to protect against allergies and inflammatory diseases. Application number LU101064, Barn dust extract for the prevention and treatment of diseases. (Pending). Dr. von Mutius is listed as inventor and has received royalties on the following patent: Publication number EP2361632: Specific environmental bacteria for the protection from and/or the treatment of allergic, chronic inflammatory and/or autoimmune disorders.
The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Genuneit J, Strachan DP, Buchele G, Weber J, Loss G, Sozanska B, et al. The combined effects of family size and farm exposure on childhood hay fever and atopy. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2013;24(3):293–8. [DOI] [PubMed] [Google Scholar]
- 2.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbreich M, Genuneit J, Weber J, Braun-Fahrlaender C, Waser M, von Mutius E. Amish children living in Northern Indiana have a very low prevalence of allergic sensitization. J Allergy Clin Immunol. 2012;129:1671–3. [DOI] [PubMed] [Google Scholar]
- 4.Motika CA, Papachristou C, Abney M, Lester LA, Ober C. Rising prevalence of asthma is sex-specific in a US farming population. J Allergy Clin Immunol. 2011;128(4):774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapko N, Zawadka M, Polosak J, Worthen GS, Danet-Desnoyers G, Puzianowska-Kuznicka M, et al. Long-term Monocyte Dysfunction after Sepsis in Humanized Mice Is Related to Persisted Activation of Macrophage-Colony Stimulation Factor (M-CSF) and Demethylation of PU.1, and It Can Be Reversed by Blocking M-CSF In Vitro or by Transplanting Naive Autologous Stem Cells In Vivo . Frontiers in immunology. 2017;8:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfortmueller CA, Meisel C, Fux M, Schefold JC. Assessment of immune organ dysfunction in critical illness: utility of innate immune response markers. Intensive care medicine experimental. 2017;5(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin CY, Tsai IF, Ho YP, Huang CT, Lin YC, Lin CJ, et al. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. Journal of hepatology. 2007;46(5):816–26. [DOI] [PubMed] [Google Scholar]
- 8.Galbraith NJ, Manek S, Walker S, Bishop C, Carter JV, Cahill M, et al. The effect of IkappaK-16 on lipopolysaccharide-induced impaired monocytes. Immunobiology. 2018;223(4–5):365–73. [DOI] [PubMed] [Google Scholar]
- 9.Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PloS one. 2012;7(10):e47209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124(1):99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vocanson M, Rozieres A, Hennino A, Poyet G, Gaillard V, Renaudineau S, et al. Inducible costimulator (ICOS) is a marker for highly suppressive antigen-specific T cells sharing features of TH17/TH1 and regulatory T cells. The Journal of allergy and clinical immunology. 2010;126(2):280–9, 9.e1–7. [DOI] [PubMed] [Google Scholar]
- 12.Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358(9288):1129–33. [DOI] [PubMed] [Google Scholar]
- 13.Busse M, Krech M, Meyer-Bahlburg A, Hennig C, Hansen G. ICOS mediates the generation and function of CD4+CD25+Foxp3+ regulatory T cells conveying respiratory tolerance. Journal of immunology (Baltimore, Md : 1950). 2012;189(4):1975–82. [DOI] [PubMed] [Google Scholar]
- 14.Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. Journal of immunology (Baltimore, Md : 1950). 2012;188(3):1064–74. [DOI] [PubMed] [Google Scholar]
- 15.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transplant immunology. 2003;11(3–4):245–58. [DOI] [PubMed] [Google Scholar]
- 16.Brenk M, Scheler M, Koch S, Neumann J, Takikawa O, Hacker G, et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. Journal of immunology (Baltimore, Md : 1950). 2009;183(1):145–54. [DOI] [PubMed] [Google Scholar]
- 17.Shilling RA, Pinto JM, Decker DC, Schneider DH, Bandukwala HS, Schneider JR, et al. Cutting edge: polymorphisms in the ICOS promoter region are associated with allergic sensitization and th2 cytokine production. J Immunol. 2005;175(4):2061–5. [DOI] [PubMed] [Google Scholar]
- 18.Clay BS, Shilling RA, Bandukwala HS, Moore TV, Cannon JL, Welcher AA, et al. Inducible costimulator expression regulates the magnitude of Th2-mediated airway inflammation by regulating the number of Th2 cells. PloS one. 2009;4(11):e7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelfand EW, Dakhama A. CD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthma. The Journal of allergy and clinical immunology. 2006;117(3):577–82. [DOI] [PubMed] [Google Scholar]
- 20.Dakhama A, Collins ML, Ohnishi H, Goleva E, Leung DY, Alam R, et al. IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy. 2013;68(5):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenk AD, Gorbacheva V, Rabant M, Fairchild RL, Valujskikh A. Effector functions of donor-reactive CD8 memory T cells are dependent on ICOS induced during division in cardiac grafts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godlove J, Chiu WK, Weng NP. Gene expression and generation of CD28-CD8 T cells mediated by interleukin 15. Experimental gerontology. 2007;42(5):412–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodge G, Mukaro V, Reynolds PN, Hodge S. Role of increased CD8/CD28(null) T cells and alternative co-stimulatory molecules in chronic obstructive pulmonary disease. Clinical and experimental immunology. 2011;166(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pita-Lopez ML, Ortiz-Lazareno PC, Navarro-Meza M, Santoyo-Telles F, Peralta-Zaragoza O. CD28-, CD45RA(null/dim) and natural killer-like CD8+ T cells are increased in peripheral blood of women with low-grade cervical lesions. Cancer cell international. 2014;14(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almanzar G, Schmalzing M, Trippen R, Hofner K, Weissbrich B, Geissinger E, et al. Significant IFNgamma responses of CD8+ T cells in CMV-seropositive individuals with autoimmune arthritis. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2016;77:77–84. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W, et al. Co-inhibitory Molecule B7 Superfamily Member 1 Expressed by Tumor-Infiltrating Myeloid Cells Induces Dysfunction of Anti-tumor CD8(+) T Cells. Immunity. 2018;48(4):773–86.e5. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Zhang S, Hu Y, Yang Z, Li J, Liu X, et al. Targeting PD-1 and Tim-3 Pathways to Reverse CD8 T-Cell Exhaustion and Enhance Ex Vivo T-Cell Responses to Autologous Dendritic/Tumor Vaccines. Journal of immunotherapy (Hagerstown, Md : 1997). 2016;39(4):171–80. [DOI] [PubMed] [Google Scholar]
- 29.Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, et al. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. Journal of leukocyte biology. 2009;86(4):863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer CD, Romero-Tejeda M, Sirignano M, Sharma S, Allen TM, Altfeld M, et al. Naturally Occurring Subclinical Endotoxemia in Humans Alters Adaptive and Innate Immune Functions through Reduced MAPK and Increased STAT1 Phosphorylation. Journal of immunology (Baltimore, Md : 1950). 2016;196(2):668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucin P, Mahmutefendic H, Blagojevic Zagorac G, Ilic Tomas M. Cytomegalovirus immune evasion by perturbation of endosomal trafficking. Cellular & molecular immunology. 2015;12(2):154–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen MA, Yanowitch RN, Reder AT, White DM, Arnason BG. Immunoglobulin-like transcript 3, an inhibitor of T cell activation, is reduced on blood monocytes during multiple sclerosis relapses and is induced by interferon beta-1b. Multiple sclerosis (Houndmills, Basingstoke, England). 2010;16(1):30–8. [DOI] [PubMed] [Google Scholar]
- 33.Chui CS, Li D. Role of immunolglobulin-like transcript family receptors and their ligands in suppressor T-cell-induced dendritic cell tolerization. Human immunology. 2009;70(9):686–91. [DOI] [PubMed] [Google Scholar]
- 34.Velten FW, Duperrier K, Bohlender J, Metharom P, Goerdt S. A gene signature of inhibitory MHC receptors identifies a BDCA3(+) subset of IL-10-induced dendritic cells with reduced allostimulatory capacity in vitro. Eur J Immunol. 2004;34(10):2800–11. [DOI] [PubMed] [Google Scholar]
- 35.Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, et al. Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. The Journal of allergy and clinical immunology. 2014;133(2):551–9. [DOI] [PubMed] [Google Scholar]
- 36.Schroder PC, Illi S, Casaca VI, Lluis A, Bock A, Roduit C, et al. A switch in regulatory T cells through farm exposure during immune maturation in childhood. Allergy. 2016. [DOI] [PubMed] [Google Scholar]
- 37.Fuseini H, Newcomb DC. Mechanisms Driving Gender Differences in Asthma. Current allergy and asthma reports. 2017;17(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein MM, Hrusch CL, Sperling AI, Ober C. Effects of an FcgammaRIIA polymorphism on leukocyte gene expression and cytokine responses to anti-CD3 and anti-CD28 antibodies. Genes and immunity. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.