Abstract
G-protein βγ subunits are key participants in G-protein signaling. These subunits facilitate interactions between receptors and G proteins that are critical for the G protein activation cycle at the plasma membrane. In addition, they play roles in directly transducing signals to an ever expanding range of downstream targets, including integral membrane and cytosolic proteins. Emerging data indicate that Gβγ may play additional roles at intracellular compartments including endosomes, the Golgi apparatus, and the nucleus. Here, we discuss the molecular and structural basis for their ability to coordinate this wide range of cellular activities.
Keywords: G protein, G-protein-coupled receptor, WD-40 repeat proteins, Signal transduction, Membrane interactions, Translocation, Second messengers, Cyclic AMP, Phosphatidylinositol, Adenylate cyclase, G-protein-coupled receptor kinase, Phospholipase C, Phosphatidylinositol 3-kinase
Overview
G-protein-coupled receptors (GPCRs) are a large family of cell-surface receptors that mediate the actions of a wide range of physiological ligands and hormones and serve as major targets of therapeutic drugs. These actions are mediated through the activation of intracellular G-protein heterotrimers consisting of Gα, β, and γ subunits. Upon receptor activation, the G proteins become activated and directly interact with downstream enzymes or ion channels that mediate cell-type-specific changes in cellular physiology.
In the GPCR signal transduction system, G-protein βγ subunits are required for the engagement of the G-protein heterotrimers with the receptor during the nucleotide-exchange reaction [1, 2]. In this reaction, the receptor directly engages the G protein α subunit with tightly bound GDP [3]. Agonist-bound receptors catalyze the release of GDP allowing GTP to bind leading to G protein activation and dissociation of Gα from Gβγ [1, 4, 5]. The Gβ and Gγ subunits remain tightly bound to one another as a constitutive heterodimer. The molecular basis for the requirement for Gβγ in this reaction will be discussed later in this review.
Following subunit dissociation both Gα and Gβγ subunits bind directly to target proteins to regulate their activities [1, 6]. Gα subunits have relatively specific sets of targets, such as adenylate cyclase for Gαs and phospholipase C (PLC) β for Gαq/11, with clearly defined binding determinants. A fascinating feature of Gβγ subunit signaling is the sheer number of targets to which Gβγ can bind and regulate without a clear conservation of binding sequence [7, 8]. This leads to a broad range of physiological functions that are regulated by Gβγ subunits including chemotaxis, cardiac ionotropy, and opioid analgesia. Here, we discuss how Gβγ subunits perform these diverse functions at a molecular and structural level.
Gβγ structure
G-protein β subunits are prototypical members of the WD40 repeat protein family [9], whose structures were determined by the Sprang and Sigler laboratories (PDBs:1GP2 and 1TBG) (Fig. 1) [10–12]. As a family, WD40 repeat proteins have no known catalytic activity, but instead are involved in protein–protein interactions in processes ranging from ubiquitination to histone methylation [9, 13]. Gβ, like most WD40 repeat proteins, folds into a characteristic β propeller structure with seven blades comprised of four antiparallel β sheets. This results in a toroid-like structure with concave top and bottom surfaces. Protein partners bind to either the top, bottom, or clefts on the sides of these WD40 repeat proteins. Binding to Gβγ has unique requirements.
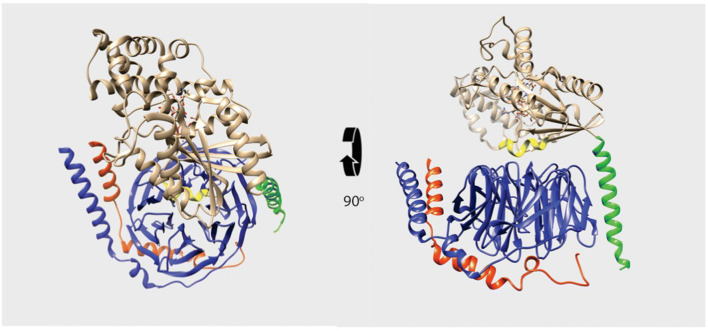
Fig. 1.
G-protein heterotrimer. Ribbon diagram of the structure of the Gαi1β1γ2 heterotrimer (PDB 1GP2). Gβ is in blue and Gγ is in red. The amino-terminal helix of Gαi1 is in green and the switch II helix in Gαi1 is in yellow, with the remainder of Gαi1 shown in beige
Gβ subunits form constitutive dimers with Gγ subunits through an amino-terminal α helix, absent in other WD40 repeat proteins, that forms a coiled-coil interaction with the α-helical amino terminus of the smaller γ subunits [11, 12]. Assembly with Gγ is required for solubility of Gβ, again a unique feature of Gβ subunits, since most WD40 proteins express and fold well in the absence of a binding partner. The C-termini of Gγ subunits are covalently modified with either farnesyl or geranyl lipid moieties, which provides part of the driving force for membrane association of these proteins [14–16]. The well-characterized direct protein–protein interactions of G-protein βγ subunits are primarily mediated by the Gβ subunits, but some examples of interactions with Gγ subunits have also been described.
Subunit diversity
There are numerous genes encoding Gβ and Gγ subunits. There are five distinct β subunit genes encoding the 36 kDa β subunit. Gβ1–4 are 90% identical and are generally interchangeable in terms of defined molecular interactions. Gβ5 is only 50% identical to the other Gβ subunits and does not function as a traditional Gβ subunit, but rather assembles with the R7 family of regulators of G-protein signaling (RGS) containing G protein γ-like (ggl) domains instead of the Gγ subunits [17, 18]. RGS proteins function as G protein GTPase-activating proteins (GAPs) and have not been shown to directly participate in G protein βγ subunit signaling in the traditional sense.
There are 12 distinct Gγ subunit genes encoding the 9–12 kDa subunits [19, 20]. These genes are considerably more diverse than the Gβ subunits with as low as 25% sequence homology. The primary function of this diversity is not clear, but an emergent idea is that, rather than specific regulation of protein–protein interactions, the different γ subunits impart different membrane-binding properties to control subcellular and perhaps submembrane localization of Gβγ subunits.
Activation of Gβγ subunit signaling
Comparison of the X-ray crystal structure of the G-protein heterotrimer with Gβγ alone indicates that Gβγ subunits do not undergo significant conformational changes upon G-protein activation. Thus, the model for the activation of Gβγ signaling relies on G protein α subunit activation, with G protein α-GTP dissociation from Gβγ revealing surfaces on Gβγ competent for Gβγ target interactions. This model is supported by mutagenesis studies as well as structural studies, indicating that most effector-binding surfaces overlap with the Gα-binding surface on Gβγ.
Gβγ protein–protein interactions
Gβγ has multiple surfaces involved in interactions with receptors, effector proteins, and membranes. In this section, we will discuss the evidence supporting roles for multiple protein-binding interactions.
Gα subunit-binding surfaces on Gβγ
The first X-ray crystal structures of any WD40 repeat protein were the transducin β1 subunit solved by the Sigler laboratory [11] and the β1 subunit in complex with Gαi1 solved by the Sprang laboratory [10, 12]. The heterotrimer structure revealed a bivalent interaction between Gα and Gβ (Fig. 1). There is no direct contact between Gα and Gγ. Gα subunits consist of an amino-terminal α helix followed by a Ras-like domain that contains several so-called Switch regions that alter their conformations dependent on nucleotide binding. In addition, Gα contains a unique independent domain consisting primarily of α helices inserted into the Ras-like domain. This domain is involved in forming the binding site for nucleotides and contributes amino acids involved in GTP hydrolysis.
In the Gαβγ heterotrimer, the Ras-like domain of Gα interacts with top surface of the WD-40 repeat toroid of Gβ, while the Gα subunit amino-terminal α helix contacts the side of the Gβ torus on blade 1 of the β propeller [10, 12]. Biochemical analyses show that the deletion of the amino terminus of the Gα subunit prevents the formation of a stable complexes with Gβ and the Gα amino terminus alone cannot bind stably to Gβ [21, 22]. This indicates that these individual Gα subunit domains alone have low affinity for Gβγ, but together, these domains cooperate in a two-site interaction that results in an apparent dissociation constant of Gα for Gβ subunits in the low nM range [23].
Exchange of GDP for GTP in the Gα subunit causes a conformational change at the switch two region in the Ras-like domain that contacts the top of the β subunit toroid [24]. It is likely that these changes lead to a loss of affinity of the Ras-like domain for the Gβ surface reducing the overall affinity of the complex. This leads to a situation, where the amino terminus retains contact with the side of the Gβ torus through relatively weak interactions ultimately allowing for subunit dissociation. There is some evidence, however, that some G protein heterotrimers do not fully dissociate upon Gα subunit activation [25, 26]. It is possible that some heterotrimers remain associated through Gα subunit amino-terminal interactions with Gβ during G protein activation.
Receptor interactions
The data are clear demonstrating that Gβγ subunits are required for receptor-stimulated nucleotide exchange on Gα, but in the X-ray crystal structure of the β2AR-Gs, heterotrimer complex multiple contacts with the G-protein α subunit are evident, particularly at the Gαs C terminus, but no contacts are seen between the βAR and the Gβ or γ subunits [3]. Thus, this structure does not reveal a mechanistic basis for the Gβγ requirement for receptor stimulation of nucleotide exchange other than to suggest that the requirement is indirect. Binding of Gβγ to Gα orders the amino terminus of Gα involved in interactions with intracellular loop 2 in the β-adrenergic receptor. Thus, a possible role of the β subunit may be to present Gα in the correct conformation for receptor engagement. An additional role may be to stabilize Gα interactions with membranes, such that they can be accessed by receptors [27]. An interesting recent discovery is that small fragments of the Gα subunits called mini G’s are capable of binding activated receptors both in vitro for crystallization and in intact cells [28–30]. Thus, these fragments of Gα subunits are capable of binding to GPCRs in the absence of Gβγ, but these proteins are not competent for nucleotide exchange.
Interestingly, several recent cryo-EM structures of class B GPCRs have revealed contacts between regions of the C-terminal tail of the GPCR and Gβ subunits. In the case of the GLP1 (PDB: 6B3J), calcitonin (PDB: 5UZ7), CGRP (PDB: 6E3Y), and PTH (PDB: 6NBF) receptors structures the 8th helix of the receptor that sits parallel to the inner surface of the plasma membrane contacts the side of the Gβ torus at blade 7 (amino acids 307–312) (Fig. 3) [31–34]. Truncation of the 8th helix to remove contacts with Gβ strongly reduces calcitonin-dependent cAMP production, but it is unclear if this is a direct consequence of the loss of Gβ interactions. In a recent structure of Rhodopsin bound to Gαiβ1γ2 a region of the C-tail beyond the helix eight contacts the Gβ subunit at a crevasse between the Gα subunit and Gβ subunits (PDB:6QNO) [35]. No mutagenic studies were done to evaluate the significance of these interactions.
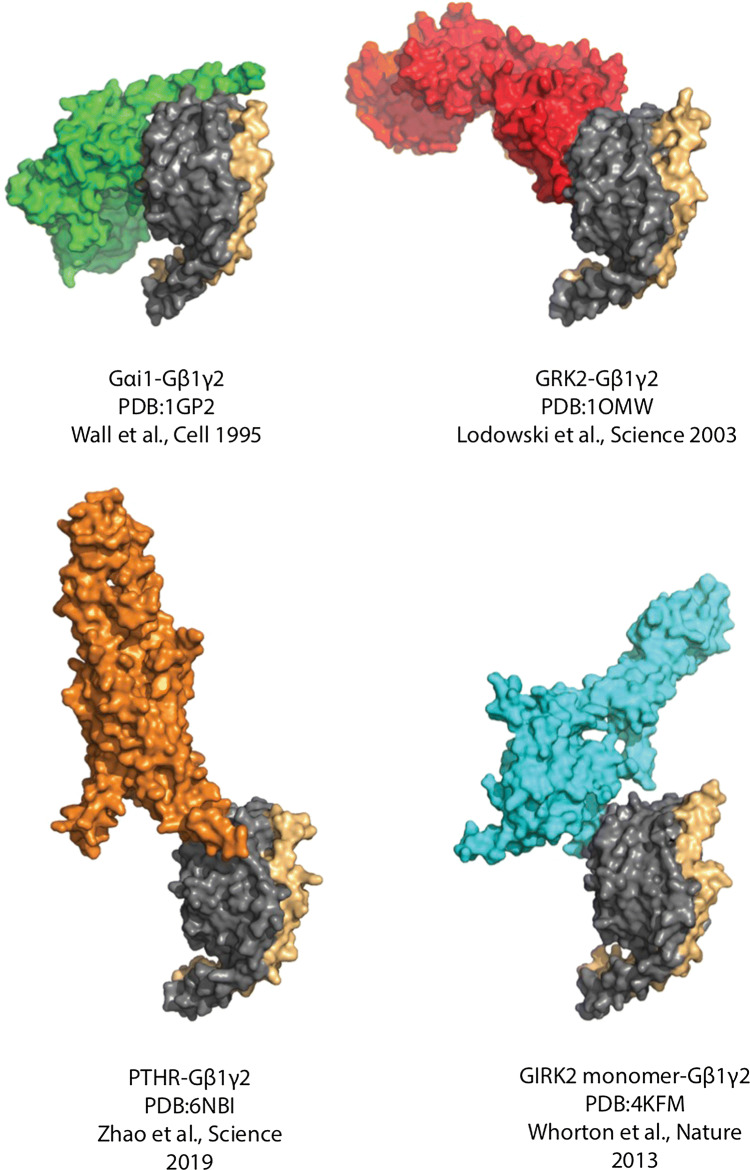
Fig. 3.
Gβγ subunit interactions with effector proteins. Gβ1 is shown in gray and Gγ2 in beige, with various effectors shown in different colors: Gαi1 is shown in green, GRK2 is shown in red, GIRK2 is shown in cyan, and the PTH receptor is shown in orange. These complexes show the various ways that Gβγ can engage with effectors. While Gα has a bivalent interaction with Gβγ, the other effectors illustrated here interact with Gβ at a single surface. These interaction interfaces differ between the binding partners. It is important to note that most of these effectors share interaction surfaces with Gα and, therefore, interact only with free Gβγ, apart from the PTH receptor C-terminal helix contacting the side of the Gβ toroid
These contacts between the receptor and the Gβ subunits are interesting, but they do not reveal a mechanistic role for these interactions leaving us to speculate on the functions of these interactions. These interactions are in concordance with the previous work with fragments of receptors demonstrating binding to Gβ, and thus, these speculations have arisen before [36–38]. Several possibilities can be considered. One concept supported by biochemical and cell biological data is of pre-coupling or pre-binding of G proteins to receptors to increase specificity and improve coupling efficiency [25]. In this model, the heterotrimer would be tethered to the receptor prior to agonist-dependent activation, but would not be engaged productively with the activating core of the receptor. Therefore, it is possible that Gβ interactions with the C terminus of the receptor in the GDP bound trimer could mediate this scaffolding interaction. Another possibility is that Gβ remains transiently associated with the C terminus of the receptor to facilitate the binding of downstream effectors, such that they are in close proximity to the receptor. As an example, just distal to the Gβ-binding site on the receptor are amino acids that are phosphorylated by G-protein-coupled receptor kinases (GRKs) to promote the binding of arrestins. Receptor-bound Gβγ could “recruit” GRK2/3 subfamily the C-tail of receptor to facilitate receptor phosphorylation at this location [35, 36]. Alternatively, this binding could facilitate scaffolding of downstream signal transduction targets to increase the efficiency and specificity of signaling. Finally, it has been suggested that Gβγ subunits promote the assembly of receptor-signaling complexes in the Golgi apparatus prior to plasma membrane (PM) delivery [39].
Effector interaction surfaces
Regulation of Gβγ-signaling downstream to target-binding molecules is regulated by the activation state of the Gα subunit. In all assays of effector regulation by Gβγ, it is strongly inhibited by GαGDP. Pertussis toxin, which inhibits Gi protein heterotrimer activation by receptors, inhibits the activation of Gβγ downstream signaling in many cell-based assays [1, 8]. The model that arose from these observations is that GαGDP sterically occludes effector-binding sites on Gβ and that subunit dissociation uncovers these surfaces, making them available for effector interactions. This has been supported by both mutagenesis studies and by crystallographic structures of complexes between Gβγ and a limited series of effector targets [40–42].
As discussed above, the Gα interface is a bipartite interface defined by a region at blade1 of the β propeller that interacts with the N-terminal α helix of Gα on the side of the torus, and a switch interface on the top of the torus formed by loops from all of the blades that interact with switch 2 of the Gα subunit (see Fig. 1). Thus, either or both these two interfaces are potential sites for binding to Gβγ effector targets (Fig. 2).
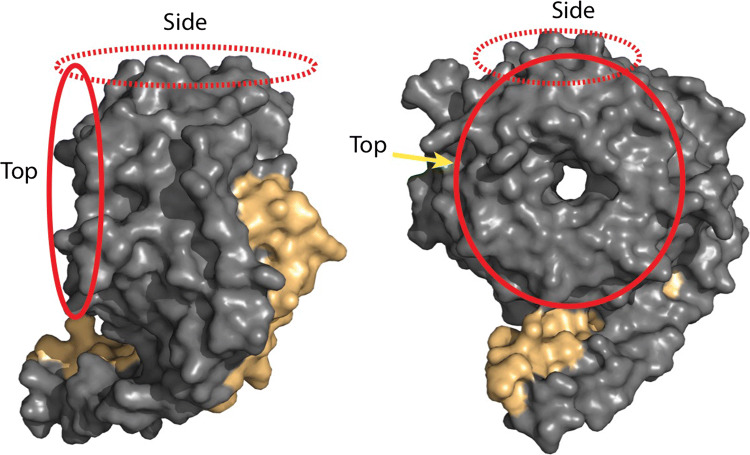
Fig. 2.
Protein-binding interfaces on Gβγ subunits. Space-filling model of Gβ1γ2 with Gβ1 in gray and Gγ2 in beige. The model has been rotated to better show the subunit interfaces. Circled in red, are the two effector interaction surfaces of G-β that have been shown to bind effectors. Interface I, circled by a solid line, is considered the primary interaction interface that is occluded by Gα Ras-like domain binding and revealed upon nucleotide exchange, while Interface II, circled by a dotted line, contacts the Gα subunit amino-terminal α-helix
The first structure of Gβγ with a bona fide effector was the GRK2-Gβγ co-crystal structure (PDB:1OMW) (Fig. 3) [41]. In this structure, the pleckstrin homology domain of GRK2 makes contact primarily with the switch interface on top of the Gβ torus and has limited contacts with the N-terminal interface. In contrast, in the complex between Gβ and the G protein-regulated inwardly rectifying potassium (GIRK) channel, GIRK makes contacts almost exclusively with the interface on the edge of blades 1 and 7 of the β propeller of the Gβ subunit that interacts with the N terminus of Gα (PDB:4KFM) (Fig. 3) [42]. These represent two extremes of the effector footprint on Gβ. Mutagenesis studies indicate that other effectors interact with either or both these surfaces, but the modes of interactions with these surfaces are generally distinct. Given that Gβγ effectors do not contain a general consensus-binding sequence this is not entirely surprising but leads to the question as to what features of Gβγ structure drive effector specificity.
Hotspot on Gβγ
In an attempt to understand the basis for recognition of multiple targets by Gβγ, we conducted a random peptide phage display screen to identify linear peptide epitopes that could mediate binding to Gβγ subunits [7]. The rationale was based on established data, showing that linear peptides representing sequences from Gβγ targets involved in Gβγ binding could, on their own, bind to Gβγ and inhibit interactions between Gβγ and effectors [43–45]. To conduct this screen, Gβγ was purified as the target and immobilized. This unbiased approach could potentially identify peptides that bind to any surface on Gβγ, not just those involved in biologically relevant protein–protein interactions. To our surprise, not only did the screen identify peptides that bound to biologically relevant sites on Gβγ, but competition analysis indicated that all of the diverse peptides that were selected bound to a common surface on Gβ. One of the selected peptides, SIGK, was co-crystallized with Gβγ and found to bind to the top of the Gβ torus in a similar position to the switch 2 region of Gα (PDB: 1XHM) [46]. It was quite surprising that in this unbiased screen, where the entire surface of Gβγ was available for binding, only one biologically relevant site was bound by phage displayed peptides. This type of result has been seen before for other protein–protein interactions, where a single-binding site has the physico-chemical properties ideal for protein–protein interactions. These types of protein–protein interaction site have been termed “hotspots” and are commonly selected by phage display [47–49]. Thus, we have proposed that the top of the Gβ torus is a hotspot, consistent with the idea that this surface can bind to multiple target proteins without a single consensus-binding sequence.
Gβγ amino-terminal coiled-coil
Some studies have shown that the amino-terminal coiled-coil region of Gβγ interacts with targets. The first evidence for Gβγ regulation of signaling downstream of a GPCR was in the Ste2 mating pathway in yeast [50]. In this pathway, the GPCR Ste2, when activated by α-factor pheromone, leads to the activation of a MAP kinase cascade in part through Gβγ-dependent recruitment of the MAP kinase scaffolding protein Ste5 and activating Ste20, the yeast analog of p21-activated protein kinase (PAK). To identify binding sites on Gβ required for this downstream signaling cascade, a yeast-based Gβ saturation mutagenesis screen identified dominant negative mutants in Gβ that disrupt the mating signaling cascade [51]. This screen identified two interaction sites, one corresponding to the N-terminal coiled-coil region. Subsequent mutagenesis studies indicated that this region is involved in direct binding of Ste20 [52]. This is far outside the well-defined effector-binding regions discussed above for binding mammalian effectors. Our laboratory identified the same region as a site for binding of PLCβ using a peptide crosslinking approach [53]. Mutation of this site in Gβ increases the activation of PLCβ2 by Gβ suggesting that, in this case, the binding of PLC to this amino-terminal site may be inhibitory. Another study showed that this amino-terminal site is important for the Gβγ-dependent regulation of type 5 adenylyl cyclase [54]. A number of effectors have been shown to bind to the inactive G-protein heterotrimer. Since this N-terminal region of Gβ is outside the Gα subunit-binding site, we proposed that this may be a docking site that allows pre-scaffolding of Gβγ with effectors.
Additional evidence for the N terminus of Gγ subunits affecting signaling via changing membrane and/or protein–protein interactions arises from work in yeast. Ste18, the yeast homolog of Gγ subunits, has phosphorylation site at the N terminus. Using phosphomimetic and phospho-dead mutants, it was shown that this phosphorylation affects the efficacy and kinetics of downstream MAP kinase activation in the yeast pheromone pathway stimulated by α factor [55]. It remains to be determined whether these changes in signaling arise from altered protein–protein interactions or via alteration of membrane interactions of Ste18. With respect to mammalian systems, the amino terminus of Gγ12 can be phosphorylated at Ser1 by protein kinase C [56, 57], which alters the apparent affinity of β1γ12 dimers for adenylate cyclase II but not PLCβ [56]. A recent phospho-proteomic screen found multiple mammalian Gγ subunits to be enriched in phosphorylation sites at their amino terminus [58]. The extreme amino termini of Gγ subunits are not resolved in X-ray crystal structures, likely because they are disordered making it difficult to speculate on the exact functional roles for these phosphorylation events. These studies are provocative, and if these phosphorylation events are clearly shown to have functional effects it would suggest that the Gγ amino terminus is involved in protein–protein or protein–membrane interactions that have yet to be defined.
Membrane binding
The binding of Gβγ to membranes is driven in large part by C-terminal modification of Gγ subunits with either farnesyl or geranyl–geranyl isoprenoid moieties via a thioether linkage to a C-terminal cysteine [14]. The replacement of this cysteine with a serine, eliminating post-translational isoprenylation, results a protein that does not associate with membranes and can be purified from the soluble fraction of insect cells [59]. Eliminating post-translation isoprenylation also eliminates the ability of Gβγ complexes to activate effectors. Reasons for this are not entirely clear, since the lipids do not mediate direct protein–protein interactions with effectors. Since many Gβγ targets are activated at membrane surfaces, the modification is likely involved in orienting the Gβγ subunits at the membrane to productively engage these targets.
Whether Gγ subunits are modified with a farnesyl or geranyl–geranyl moiety is encoded by the C-terminal four amino acids cysteine–aliphatic–aliphatic-X (CaaX) motif. The identity of the X amino acid determines whether the Gγ is modified with either with a 15 carbon farnesyl or 20 carbon geranyl–geranyl moiety. After isoprenylation, the C-terminal aaX is cleaved followed by the carboxy methylation of the C-terminal carboxylate. The nature of the modification (15 or 20 carbon) has significant effects on both membrane association as discussed below, but also on interaction with effectors [60, 61]. In general, Gβγ complexes containing Gγ subunits modified with 15 carbon farnesyl have lower affinities for both membranes and effectors than do geranyl–geranyl modified Gβγ subunits.
Recent work has emerged indicating that sequence elements in Gγ subunits beyond lipid modification are important for determining the strength of Gβγ subunit membrane association. In particular, amino acid sequences at the C terminus of Gγ subunits immediately proximal to the isoprenylated cysteine significantly influence membrane binding [62]. These sequences are either enriched in basic amino acids or hydrophobic amino acids contributing additional driving force for PM binding through, either ionic interaction with negatively charged PM lipids, or through hydrophobic interactions with the membrane, respectively. Gγ subunits lacking these types of amino acids have reduced affinity for the membrane and have been shown to dissociate from the membrane in response to receptor activation. Using fluorescence recovery after photo bleaching (FRAP) and/or live cell fluorescence imaging, the Gautam group showed that Gβγ subunits with different Gγ isoforms have different rates of translocation from the plasma membrane to internal compartments. As alluded to above, this rate was dictated by hydrophobic and positively charged residues of the C-terminal tail of Gγ subunits, as well as whether the Gγ subunit was modified with farnesyl or geranyl–geranyl [62–64]. A proposed model is that tight membrane association requires a cooperative interaction between isoprenylation and proximal Gγ sequences to provide the driving force necessary for stable Gβγ membrane association. In addition, in the Gβγ–Gα heterotrimer, determinants for membrane association arise from both Gγ-dependent mechanisms described above, as well as myristoylation and/or palmitoylation of the Gα subunit to provide multivalent contacts with the PM. Upon receptor activation, the activated Gα and Gβγ dissociate resulting in Gβγ membrane association being dependent solely on Gγ membrane-binding determinants [62].
The consequences of membrane tethering on Gβγ-dependent signal transduction have been addressed by the Karunarathne laboratory demonstrating that in an RAW macrophage cell line, Gβγ-dependent PI3Kγ activation was dependent on the γ subunit subtype expressed. Gγ3, a high plasma membrane affinity γ subunit, allowed for robust activation of PI3K, whereas Gγ9, a low PM affinity Gγ subunit, was unable to cause PI3Kγ activation. In addition, a Gγ9 chimera with the C-terminal eight residues of γ3 including the CAAX motif was able to activate PI3Kγ, where Gγ3 with C-terminal eight residues of Gγ9 was not. This suggests that signaling efficacy of the Gβγ subunits for some effectors is at least partially dependent on the affinity of the γ subunit for the plasma membrane [65].
Update on Gβγ-binding partners
Gβγ interacts with multiple protein targets. We compiled a list of these in a previous review in 2008 in this Journal [8]. Others have also reviewed canonical [20] and non-canonical effectors [66]. Since that time, a number of other Gβγ targets have been identified and a subset of those will be discussed here.
Dopamine reuptake transporter (DAT)
The Torres group has identified DAT as being regulated by Gβγ. They showed that Gβγ coprecipitates with DAT immunoprecipitated from rat striatum and binds directly to a C-terminal fragment of DAT [67]. Initial work indicated that Gβγ inhibits DAT dopamine reuptake activity, but later, it was shown that Gβγ stimulated dopamine efflux through binding to a specific sequence in the C terminus of DAT [67, 68]. This is very interesting, because reverse transport by DAT is thought to underly the excitatory effects of amphetamines and suggests that Gβγ may be involved in this process. Indeed, blockade of Gβγ activity in rats with gallein inhibited both amphetamine-dependent dopamine release in the nucleus accumbens and amphetamine-dependent stimulation of locomotor activity [69]. Collectively these data strongly support Gβγ-dependent regulation of this transporter. How Gβγ is regulated to produce these effects remains to be determined.
Elmo/Dock
The Elmo/Dock complex is a guanine nucleotide-exchange factor for Rac required for Gi-coupled chemoattractant receptors to mediate directional chemotaxis. The interaction between DOCK and Gβγ was originally discovered in Dictyostelium discoideum and was shown to be important for stimulating actin polymerization and chemotaxis [70]. Later, studies in mammalian systems identified DOCK1 as a Gβγ-binding partner in Hela cells that regulates migration in response to CXCR4 activation [71].
Transient receptor potential M3 (TrpM3)
Transient receptor potential M3 (TrpM3) is an ion channel found in high abundance in sensory neurons involved in promoting pain stimuli. Recently, three independent laboratories showed that Gi-coupled receptors inhibit TrpM3 through Gβγ signaling [72–74]. The Rohacs laboratory used purified Gβγ subunits to inhibit TrpM3 currents in inside-out patches, and coimmunoprecipitation experiments to identify TrpM3 as a likely direct Gβγ-binding target [72]. In addition, the inhibition of Gβγ function with the Gβγ inhibitors GRK2ct or gallein blocked μ-opioid receptor or GABA receptor-dependent inhibition of TrpM3 currents. Finally, all three groups showed that the inhibition of TRM3 currents by μ-opioid or GABA receptors inhibits nociceptive responses in mouse models of peripheral pain.
Radil
Radil was identified in a proteomics screen as protein that binds to Gβγ and is involved in regulating cell migration and adhesion downstream of Rap [75]. In collaboration with Rap1, Gβγ binding to Radil promotes integrin activation in both neutrophils and in HT1080 fibrosarcoma cells [76]. Gβγ binding promotes Radil translocation to the plasma membrane to sites of adhesion, but how exactly this leads to integrin activation is unclear.
Phosphoinositide 3 kinase β (PI3Kβ)
GPCRs are important regulators of PI3kinase activities. Initial studies identified of PI3Kγ consisting of the P110γ catalytic subunit and the P100 regulatory subunit as major targets of direct activation by Gβγ subunits’ downstream of chemoattractant receptors responsible for directional migration in neutrophils [77–80]. The activation of this isoform is highly restricted to monocytes largely due to the restricted expression of the p100 subunit which renders the complex more sensitive to Gβγ. PI3Kβ, on the other hand, is ubiquitously expressed and is also a target for Gβγ activation [81, 82]. PI3Kβ consists of a p110β catalytic subunit and p85, p55, or p50 regulatory subunits. Purified PI3Kβ is activated in vitro by purified Gβγ subunits, demonstrating direct activation [81, 83]. The enzyme is also regulated by tyrosine phosphorylation and is synergistically activated by phosphorylation and Gβγ subunits and is thus poised as a coincidence detector for activation by receptor tyrosine kinase (RTK) and GPCR-regulated pathways [81, 83]. Direct binding sites for Gβγ on the PI3K p110β subunit were identified by hydrogen/deuterium exchange mass spectrometry (DXMS) and mutagenic analysis [82, 84]. PI3Kβ has a number of unique signaling functions, including vesicle trafficking, mitosis, and cancer that have been described in detail elsewhere [85].
P-Rex exchange factor
P-Rex is a guanine nucleotide-exchange factor for the small GTPase Rac and is coordinately activated by binding to both PIP3 and Gβγ. It was originally identified in migrating immune cells as a critical factor regulating immune cell migration in response to chemotactic stimuli [86], and was subsequently found to be involved in cancer cell metastasis [87]. Very recently, the cryo-EM reconstruction of P-Rex in complex with Gβγ was determined adding to the relatively few atomic structures for Gβγ complexed to a downstream effector (J. Tesmer and J. Cash, personal communication). In this case P-Rex1 has contacts with the entire upper surface of the βγ toroid thus overlapping with the binding of other effector targets and the α subunit switch 2 binding interface, but the binding mode is completely different from that seen with GRK2, the GIRK channel, and Gα subunits. This supports the idea that each effector may have its own binding mode on the Gβ surface. Another unique feature is an interaction with the amino terminus of the Gβ and Gγ subunits. This has not been observed in any structures of other Gβγ subunit complexes with other binding partners. The significance of this interaction remains to be determined, although it seems to be a minor contributor as compared to the interaction of P-Rex with the core of Gβ.
Mechanisms of Gβγ-dependent regulation
In addition to having multiple binding surfaces that can coordinate binding of multiple target molecules or membrane surfaces, either separately or together, there are several potential modes of target regulation. These include translocation, allosteric regulation, scaffolding, or combinations of these (Fig. 4).
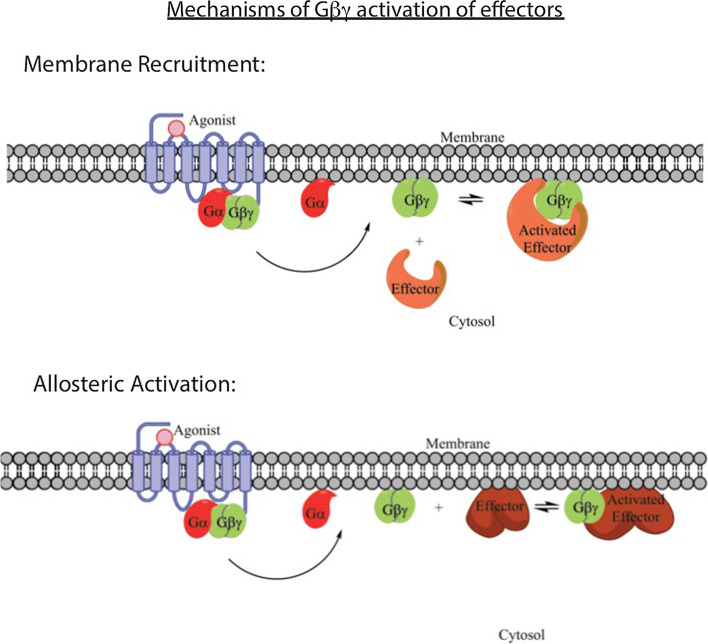
Fig. 4.
Mechanisms of effector activation by Gβγ subunits. There are two general mechanisms for effector regulation by Gβγ the mechanism for each effector is dependent on whether the target is cytosolic, or membrane-bound. In the case of cytosolic proteins such GRK2, whose substrates are localized to the plasma membrane, a potential mechanism for activation is recruitment to the plasma membrane by membrane-bound Gβγ, where when Gβγ dissociates from Gα, the binding site for the cytosolic effector is revealed allowing for the effector to bind, recruiting it to the plasma membrane, where it can act on its substrate. For other transmembrane effectors, such as or GIRK channel, Gβγ binding must cause conformational rearrangement of the target protein to a more active state, since the effector is already localized to the plasma membrane. These two mechanisms of activation are not mutually exclusive, and some effectors use a combination of both
Translocation
As discussed above, Gβγ subunits are tethered to the plasma membrane via lipid modification at the C terminus of Gγ subunits with either a farnesyl 15 carbon or a geranyl–geranyl 20 carbon isoprenoid, as well as other amino acid determinants at the C terminus of Gγ. Thus, Gβγ subunits are tethered to membranes and activate targets at membrane surfaces. We will focus on plasma membrane signaling here, but roles for Gβγ at other intracellular membrane surfaces have been described [66, 88, 89]. A number of Gβγ-regulated proteins are found in the cytosol, including PI3Kγ, PLCβ, and GRK2/3. For all of these proteins, the primary substrates, phosphoinositides for PI3K and PLCβ, and GPCRs for GRK2/3 are at the plasma membrane. Thus, a potential mechanism for the activation of these proteins is through Gβγ-dependent “recruitment” of these effectors from the cytosol to the membrane, such that the proteins are enriched near their substrates. GRK2 is the classic example of this, where there is no evidence for an increase in enzymatic activity of GRK2 upon binding to Gβγ. Thus, upon G-protein activation, the GRK2-binding surface on Gβγ is revealed at the plasma membrane leading to GRK2 binding to Gβγ at the PM and subsequent phosphorylation of nearby receptors. As discussed above, the C terminus of some GPCRs bind to Gβγ and may provide an additional layer of recruitment directly to the relevant GPCRs.
For PI3Kγ and PI3Kβ, the situation is likely more complex, since PI3K is found both at the PM and in the cytoplasm. Thus, translocation could be part of its activation mechanism, but since some PI3K is membrane-localized, this pool could be sufficient for activation by an allosteric mechanism. Indeed, PI3Kβ and PI3Kγ have been suggested to be activated by both translocation and allosteric activation [82, 90].
Allosteric activation
Another class of effectors are the membrane-associated targets of Gβγ. These include ion channels and adenylyl cyclases. Since these effectors are integral membrane proteins, their activation mechanisms must involve allosteric regulation. This has been most carefully studied for the GIRKs. PIP2 and Gβγ binding to the channel are required for activation [91]. The X-ray crystal structure of the GIRK2 has been solved both alone and in complex with Gβγ and PIP2 [42]. Gβγ and PIP2 bind to the C-terminal cytoplasmic domains of the tetrameric structure at a 1:1 stoichiometry per channel subunit. This binding leads to a rotational reorientation of the cytoplasmic domains and consequent opening of an inner helix gate at the inner pore that allows flow of potassium ions. Binding of both Gβγ and PIP2 is required for this activation.
PLCβ may also be subject to allosteric regulation, because Gβγ has been shown to have no effect on the membrane localization of PLC during activation [92]. A mechanism for PLCβ activation by G proteins that has been proposed involves reorientation on the PM to promote membrane-induced movement of a cap on the catalytic site. Here, a negatively charged linker region in the TIM barrel catalytic domain occludes the active site preventing access to the membrane substrate. It has been proposed that reorientation on the PM upon G-protein-binding causes the negatively charged membrane to position the linker region out of the active site [93]. This is attractive, because it could suggest a universal mechanism for PLC activation by various stimuli including Gβγ activation. Deletion of the linker region causes the dramatic activation of the enzyme, but these deletions do not prevent G-protein activation, suggesting that other mechanisms are also involved. In addition, there is no direct biophysical evidence for membrane-induced reorientation of this linker region.
Multiple contact sites for a single effector
For Gβγ targets for which the structures have been solved, there is a clear 1:1 stoichiometry of binding of Gβγ to each protein unit. The K+ channel has four subunits and four bound Gβγ subunits. In case of other effectors, there are reports of multiple binding sites that may involve different Gβγ surfaces. These include PI3Kγ, adenylate cyclase, and PLCβ. Structures of these complexes have not been solved. For adenylate cyclase type 5, the N terminus and the C2 domains seem to have distinct binding sites for Gβγ that involve multiple regions of the Gβγ subunit [54]. Mutagenic disruption of either the hotspot or the amino-terminal coiled coil on Gβ partially inhibited AC5 but not AC6 binding, but the disruption of both eliminated AC5 binding. Interestingly, the activation of AC5 or AC6 was entirely dependent on the amino-terminal coiled-coil region, while for other ACs, the “hotspot” was more important.
A similar situation exists for PLCβ2/3. Multiple binding sites for Gβγ on PLCβ have been identified including the N-terminal pleckstrin homology (PH) domain and the catalytic Y domain [45, 94, 95]. In addition, two sites on Gβγ have been found to be involved in PLCβ activation [53]. The mutation of sites in the hotspot of Gβγ strongly inhibits PLCβ2 activation, but surprisingly, the mutation of sites in the amino-terminal coiled–coiled region of Gβ enhanced the efficacy of PLCβ2 activation. From these data, we proposed that the amino-terminal coiled-coil region of Gβγ serves as a docking site for PLCβ2 in the inactive G-protein heterotrimer.
Summary
Gβγ subunits play versatile multifactorial roles in signaling downstream of GPCRs with unique surfaces involved in various interactions, including Gα subunits, receptors, membranes, and effectors. These functions could operate independently or cooperatively to scaffold membranes, receptors, and effectors in signal transduction complexes. To date, there are few atomic level structures available for Gβγ complexes leaving an incomplete understanding of the molecular events that drive effector activation. The advent of cryo-EM methods has the potential to lead to and emergence of multiple new structures of Gβγ complexes with targets and receptors. The role of direct interaction of receptors with Gβγ revealed by Cryo-EM has identified surprising new interactions, but their significance remains to be determined. An emergent area of GPCR signaling is internalized receptor signaling from endosomes and other compartments, including the nucleus. Such signaling may have the same requirements as has been discussed above for classical PM processes, but how Gβγ reaches these compartments and what these subunits interact with in these compartments is not well defined. Overall, Gβγ subunits are very interesting multi-functional proteins that likely have many novel functions that have yet to be explored.
Acknowledgements
National Institutes of Health R35GM127303.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 2.Oldham WM, Hamm E. Structural basis of function in heterotrimeric G proteins. Q Rev Biophys. 2006;39(02):117–166. doi: 10.1017/S0033583506004306. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen SGF, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dror RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, Arlow DH, et al. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science. 2015;348(6241):1361–1365. doi: 10.1126/science.aaa5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9(1):60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 6.Clapham DE, Neer EJ. G protein βγ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 7.Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA, et al. Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein βγ subunits is used for recognition of a subclass of effectors. EMBO J. 2001;20(4):767–776. doi: 10.1093/emboj/20.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smrcka AV. G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65(14):2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- 10.Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379(6563):311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 11.Sondek J, Bohm A, Lambright DG, Hamm HE, Sigler PB. Crystal structure of a G-protein βγ dimer at 2.1Å resolution. Nature. 1996;379(6563):369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 12.Wall MA, Coleman DE, Lee E, Iniguez-Lluhi JA, Posner BA, Gilman AG, et al. The structure of the G protein heterotrimer Giα1β1γ2. Cell. 1995;83(6):1047–1058. doi: 10.1016/0092-8674(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 13.Smith TF, Gaitatzes C, Saxena K, Neer EJ. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci. 1999;24(5):181–185. doi: 10.1016/S0968-0004(99)01384-5. [DOI] [PubMed] [Google Scholar]
- 14.Mumby SM, Casey PJ, Gilman AG, Gutowski S, Sternweis PC. G protein γ subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci USA. 1990;87:5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akgoz M, Kalyanaraman V, Gautam N. G protein βγ complex translocation from plasma membrane to Golgi complex is influenced by receptor γ subunit interaction. Cell Signal. 2006;18(10):1758–1768. doi: 10.1016/j.cellsig.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedegaertner PB, Wilson PT, Bourne HR. Lipid modifications of trimeric G proteins. J Biol Chem. 1995;270(2):503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 17.Snow BE, Krumins AM, Brothers GM, Lee SF, Wall MA, Chung S, et al. A G protein γ subunit-like domain shared between RGS11 and other RGS proteins specifies binding to Gβ5 subunits. Proc Natl Acad Sci USA. 1998;95(22):13307–13312. doi: 10.1073/pnas.95.22.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witherow DS, Wang Q, Levay K, Cabrera JL, Chen J, Willars GB, et al. Complexes of the G protein subunit gβ5 with the regulators of G protein signaling RGS7 and RGS9. Characterization in native tissues and in transfected cells. J Biol Chem. 2000;275(32):24872–24880. doi: 10.1074/jbc.M001535200. [DOI] [PubMed] [Google Scholar]
- 19.Gautam N, Northup J, Tamir H, Simon MI. G protein diversity is increased by associations with a variety of gamma subunits. Proc Natl Acad Sci USA. 1990;87(20):7973–7977. doi: 10.1073/pnas.87.20.7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, et al. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev. 2013;65(2):545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 21.Neer EJ, Pulsifer L, Wolf LG. The amino terminus of G protein α subunits is required for interaction with βγ. J Biol Chem. 1988;263(18):8970–8996. [PubMed] [Google Scholar]
- 22.Denker BM, Neer EJ, Schmidt CJ. Mutagenesis of the amino terminus of the α subunit of the G protein Go. In vitro characterization of αo-βγ interactions. J Biol Chem. 1992;267(9):6272–6277. [PubMed] [Google Scholar]
- 23.Sarvazyan NA, Remmers AE, Neubig RR. Determinants of Giα and βγ binding: measuring high affinity interactions in a lipid environment using flow cytometry. J Biol Chem. 1998;273(14):7934–7940. doi: 10.1074/jbc.273.14.7934. [DOI] [PubMed] [Google Scholar]
- 24.Sprang SR. G protein mechanisms: insights from structural analysis. Ann Rev Biochem. 1997;66:639–678. doi: 10.1146/annurev.biochem.66.1.639. [DOI] [PubMed] [Google Scholar]
- 25.Gales C, Van Durm JJ, Schaak S, Pontier S, Percherancier Y, Audet M, et al. Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol. 2006;13(9):778–786. doi: 10.1038/nsmb1134. [DOI] [PubMed] [Google Scholar]
- 26.Klein S, Reuveni H, Levitzki A. Signal transduction by a nondissociable heterotrimeric yeast G protein. Proc Natl Acad Sci USA. 2000;97(7):3219–3223. doi: 10.1073/pnas.97.7.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Florio VA, Sternweis PC. Mechanisms of muscarinic receptor action on Go in reconstituted phospholipid vesicles. J Biol Chem. 1989;264:3909–3915. [PubMed] [Google Scholar]
- 28.Carpenter B, Tate CG. Engineering a minimal G protein to facilitate crystallisation of G protein-coupled receptors in their active conformation. Protein Eng Des Sel. 2016;29(12):583–594. doi: 10.1093/protein/gzw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nehme R, Carpenter B, Singhal A, Strege A, Edwards PC, White CF, et al. Mini-G proteins: novel tools for studying GPCRs in their active conformation. PLoS One. 2017;12(4):e0175642. doi: 10.1371/journal.pone.0175642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wan Q, Okashah N, Inoue A, Nehme R, Carpenter B, Tate CG, et al. Mini G protein probes for active G protein-coupled receptors (GPCRs) in live cells. J Biol Chem. 2018;293(19):7466–7473. doi: 10.1074/jbc.RA118.001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang YL, Khoshouei M, Radjainia M, Zhang Y, Glukhova A, Tarrasch J, et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature. 2017;546(7656):118–123. doi: 10.1038/nature22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang YL, Khoshouei M, Glukhova A, Furness SGB, Zhao P, Clydesdale L, et al. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature. 2018;555(7694):121–125. doi: 10.1038/nature25773. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Sun B, Feng D, Hu H, Chu M, Qu Q, et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature. 2017;546(7657):248–253. doi: 10.1038/nature22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao L-H, Ma S, Sutkeviciute I, Shen D-D, Zhou XE, de Waal PW, et al. Structure and dynamics of the active human parathyroid hormone receptor-1. Science. 2019;364:148–153. doi: 10.1126/science.aav7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai C-J, Marino J, Adaixo RJ, Pamula F, Muehle J, Maeda S, et al. (2019) Cryo-EM structure of the rhodopsin-Gαi-βγ complex reveals binding of the rhodopsin C-terminal tail to the Gβ subunit. Elife. 10.7554/eLife.46041 [DOI] [PMC free article] [PubMed]
- 36.Wu GY, Bogatkevich GS, Mukhin YV, Benovic JL, Hildebrandt JD, Lanier SM. Identification of Gβγ binding sites in the third intracellular loop of the M-3-muscarinic receptor and their role in receptor regulation. J Biol Chem. 2000;275(12):9026–9034. doi: 10.1074/jbc.275.12.9026. [DOI] [PubMed] [Google Scholar]
- 37.Taylor JM, Jacob-Mosier GG, Lawton RG, VanDort M, Neubig RR. Receptor and membrane interaction sites on Gβ. A receptor- derived peptide binds to the carboxyl terminus. J Biol Chem. 1996;271(7):3336–3339. doi: 10.1074/jbc.271.7.3336. [DOI] [PubMed] [Google Scholar]
- 38.Kan W, Adjobo-Hermans M, Burroughs M, Faibis G, Malik S, Tall GG, et al. M3 muscarinic receptor interaction with phospholipase C β3 determines its signaling efficiency. J Biol Chem. 2014;289(16):11206–11218. doi: 10.1074/jbc.M113.538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupre DJ, Robitaille M, Rebois RV, Hebert TE. The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu Rev Pharmacol Toxicol. 2009;49(1):31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, Shektar LR, et al. Molecular basis for interactions of G protein βγ subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 41.Lodowski DT, Pitcher JA, Capel WD, Lefkowitz RJ, Tesmer JJG. Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science. 2003;300(5623):1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- 42.Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature. 2013;498(7453):190–197. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, DeVivo M, Dingus J, Harry A, Li J, Sui J, et al. A region of adenylyl cyclase 2 critical for regulation by G protein βγ subunits. Science. 1995;268(5214):1166–1169. doi: 10.1126/science.7761832. [DOI] [PubMed] [Google Scholar]
- 44.Krapivinsky G, Kennedy ME, Nemec J, Medina I, Krapivinsky L, Clapham DE. Gβ binding to GIRK4 subunit is critical for G protein-gated K + channel activation. J Biol Chem. 1998;273(27):16946–16952. doi: 10.1074/jbc.273.27.16946. [DOI] [PubMed] [Google Scholar]
- 45.Sankaran B, Osterhout J, Wu D, Smrcka AV. Identification of a structural element in phospholipase C β2 that interacts with G protein βγ subunits. J Biol Chem. 1998;273(12):7148–7154. doi: 10.1074/jbc.273.12.7148. [DOI] [PubMed] [Google Scholar]
- 46.Davis TL, Bonacci TM, Sprang SR, Smrcka AV. Structural and molecular characterization of a preferred protein interaction surface on G protein βγ subunits. Biochemistry. 2005;44(31):10593–10604. doi: 10.1021/bi050655i. [DOI] [PubMed] [Google Scholar]
- 47.Clackson T, Ultsch MH, Wells JA, de Vos AM. Structural and functional analysis of the 1: 1 growth hormone: receptor complex reveals the molecular basis for receptor affinity. J Mol Biol. 1998;277(5):1111–1128. doi: 10.1006/jmbi.1998.1669. [DOI] [PubMed] [Google Scholar]
- 48.Fairbrother WJ, Christinger HW, Cochran AG, Fuh G, Keenan CJ, Quan C, et al. Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry. 1998;37(51):17754–17764. doi: 10.1021/bi981931e. [DOI] [PubMed] [Google Scholar]
- 49.Ma B, Wolfson HJ, Nussinov R. Protein functional epitopes: hot spots, dynamics and combinatorial libraries. Curr Opin Struct Biol. 2001;11(3):364–369. doi: 10.1016/S0959-440X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 50.Whiteway M, Hougan L, Dignard D, Thomas DY, Bell L, Saari GC, et al. The STE4 and STE18 genes of yeast encode potential b and g subunits of the mating factor receptor-coupled G protein. Cell. 1989;56:467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- 51.Leberer E, Dignard D, Hougan L, Thomas DY, Whiteway M. Dominant-negative mutants of a yeast G-protein β subunit identify two functional regions involved in pheromone signalling. EMBO J. 1992;11:4805–4813. doi: 10.1002/j.1460-2075.1992.tb05586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leeuw T, Wu C, Schrag JD, Whiteway M, Thomas DY, Leberer E. Interaction of a G-protein βγ subunit with a conserved sequence in Ste20/PAK family protein kinases. Nature. 1998;391(6663):191–195. doi: 10.1038/34448. [DOI] [PubMed] [Google Scholar]
- 53.Bonacci TM, Ghosh M, Malik S, Smrcka AV. Regulatory interactions between the amino terminus of G-protein βγ subunits and the catalytic domain of PLC β2. J Biol Chem. 2005;280:10174–10181. doi: 10.1074/jbc.M412514200. [DOI] [PubMed] [Google Scholar]
- 54.Brand CS, Sadana R, Malik S, Smrcka AV, Dessauer CW. Adenylyl cyclase 5 regulation by Gβγ involves isoform-specific use of multiple interaction sites. Mol Pharmacol. 2015;88:758–767. doi: 10.1124/mol.115.099556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choudhury S, Baradaran-Mashinchi P, Torres MP. Negative feedback phosphorylation of Gγ subunit Ste18 and the Ste5 scaffold synergistically regulates MAPK activation in yeast. Cell Rep. 2018;23(5):1504–1515. doi: 10.1016/j.celrep.2018.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasuda H, Lindorfer MA, Myung C-S, Garrison JC. Phosphorylation of the G protein γ12 subunit regulates effector specificity. J Biol Chem. 1998;273(34):21958–21965. doi: 10.1074/jbc.273.34.21958. [DOI] [PubMed] [Google Scholar]
- 57.Morishita R, Nakayama H, Isobe T, Matsuda T, Hashimoto Y, Okano T, et al. Primary structure of a γ subunit of G protein, γ12, and its phosphorylation by protein kinase C. J Biol Chem. 1995;270(49):29469–29475. doi: 10.1074/jbc.270.49.29469. [DOI] [PubMed] [Google Scholar]
- 58.Dewhurst HM, Choudhury S, Torres MP. Structural analysis of PTM hotspots (SAPH-ire)—a quantitative informatics method enabling the discovery of novel regulatory elements in protein families. Mol Cell Proteom. 2015;14:2285–2297. doi: 10.1074/mcp.M115.051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iniguez-Lluhi JA, Simon MI, Robishaw JD, Gilman AG. G protein βγ subunits synthesized in Sf9 cells. J Biol Chem. 1992;267:23409–23417. [PubMed] [Google Scholar]
- 60.Ueda N, Iniguez-Lluhi JA, Lee E, Smrcka AV, Robishaw JD, Gilman AG. G protein βγ subunits. Simplified purification and properties of novel isoforms. J Biol Chem. 1994;269(6):4388–4395. [PubMed] [Google Scholar]
- 61.Myung C-S, Yasuda H, Liu WW, Harden TK, Garrison JC. Role of isoprenoid lipids on the heterotrimeric G protein γ subunit in determining effector activation. J Biol Chem. 1999;274(23):16595–16603. doi: 10.1074/jbc.274.23.16595. [DOI] [PubMed] [Google Scholar]
- 62.O’Neill PR, Karunarathne WKA, Kalyanaraman V, Silvius JR, Gautam N. G-protein signaling leverages subunit-dependent membrane affinity to differentially control βγ translocation to intracellular membranes. Proc Natl Acad Sci. 2012;109(51):E3568–E3577. doi: 10.1073/pnas.1205345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ajith Karunarathne WK, O’Neill PR, Martinez-Espinosa PL, Kalyanaraman V, Gautam N. All G protein βγ complexes are capable of translocation on receptor activation. Biochem Biophys Res Commun. 2012;421(3):605–611. doi: 10.1016/j.bbrc.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saini DK, Kalyanaraman V, Chisari M, Gautam N. A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation. J Biol Chem. 2007;282(33):24099–24108. doi: 10.1074/jbc.M701191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Senarath K, Payton JL, Kankanamge D, Siripurapu P, Tennakoon M, Karunarathne A. Gγ identity dictates efficacy of Gβγ signaling and macrophage migration. J Biol Chem. 2018;293(8):2974–2989. doi: 10.1074/jbc.RA117.000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan SM, Sung JY, Hébert TE. Gβγ subunits—different spaces, different faces. Pharmacol Res. 2016;111:434–441. doi: 10.1016/j.phrs.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Olivares J, Torres-Salazar D, Owens WA, Baust T, Siderovski DP, Amara SG, et al. Inhibition of dopamine transporter activity by G protein βγ subunits. PLoS One. 2013;8(3):e59788. doi: 10.1371/journal.pone.0059788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Olivares J, Baust T, Harris S, Hamilton P, Galli A, Amara SG, et al. Gβγ subunit activation promotes dopamine efflux through the dopamine transporter. Mol Psychiatry. 2017;22(12):1673–1679. doi: 10.1038/mp.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mauna JC, Harris SS, Pino JA, Edwards CM, DeChellis-Marks MR, Bassi CD, et al. G protein βγ subunits play a critical role in the actions of amphetamine. Transl Psychiatry. 2019;9(1):81. doi: 10.1038/s41398-019-0387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L, et al. A Gβγ effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis. Dev Cell. 2012;22(1):92–103. doi: 10.1016/j.devcel.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Xu X, Pan M, Jin T. ELMO1 directly interacts with Gβγ subunit to transduce GPCR signaling to Rac1 activation in chemotaxis. J Cancer. 2016;7(8):973–983. doi: 10.7150/jca.15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Badheka D, Yudin Y, Borbiro I, Hartle CM, Yazici A, Mirshahi T, et al. Inhibition of transient receptor potential melastatin 3 ion channels by G-protein βγ subunits. Elife. 2017;6:e26147. doi: 10.7554/eLife.26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quallo T, Alkhatib O, Gentry C, Andersson DA, Bevan S. G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. Elife. 2017;6:e26138. doi: 10.7554/eLife.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dembla S, Behrendt M, Mohr F, Goecke C, Sondermann J, Schneider FM, et al. Anti-nociceptive action of peripheral mu-opioid receptors by G-βγ protein-mediated inhibition of TRPM3 channels. Elife. 2017;6:e26280. doi: 10.7554/eLife.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed SM, Daulat AM, Meunier A, Angers S. G protein βγ subunits regulate cell adhesion through Rap1a and its effector Radil. J Biol Chem. 2010;285(9):6538–6551. doi: 10.1074/jbc.M109.069948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L, Aerbajinai W, Ahmed SM, Rodgers GP, Angers S, Parent CA. Radil controls neutrophil adhesion and motility through β2-integrin activation. Mol Biol Cell. 2012;23(24):4751–4765. doi: 10.1091/mbc.e12-05-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits. Cell. 1994;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 78.Stephens LR, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka AV, et al. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89(1):105–114. doi: 10.1016/S0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLCβ2 and β3 and PI3K in chemoattractant-mediated signal transduction. Science. 2000;287(5455):1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 80.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science. 2000;287(5455):1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 81.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S, Fukui Y, et al. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272(39):24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 82.Dbouk HA, Vadas O, Shymanets A, Burke JE, Salamon RS, Khalil BD, et al. G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness. Sci Signal. 2012;5(253):ra89. doi: 10.1126/scisignal.2003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maier U, Babich A, Nürnberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J Biol Chem. 1999;274(41):29311–29317. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- 84.Vadas O, Dbouk HA, Shymanets A, Perisic O, Burke JE, Abi Saab WF, et al. Molecular determinants of PI3Kγ-mediated activation downstream of G-protein-coupled receptors (GPCRs) Proc Natl Acad Sci USA. 2013;110(47):18862–18867. doi: 10.1073/pnas.1304801110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bresnick AR, Backer JM. PI3Kb-A versatile transducer for GPCR, RTK, and small GTPase signaling. Endocrinology. 2019;160(3):536–555. doi: 10.1210/en.2018-00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, et al. P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108(6):809–821. doi: 10.1016/S0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 87.Lindsay CR, Lawn S, Campbell AD, Faller WJ, Rambow F, Mort RL, et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun. 2011;2:555. doi: 10.1038/ncomms1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jamora C, Yamanouye N, Van Lint J, Laudenslager J, Vandenheede JR, Faulkner DJ, et al. Gbg-mediated regulation of Golgi organization is through the direct activation of protein kinase D. Cell. 1999;98(1):59–68. doi: 10.1016/S0092-8674(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 89.Saini DK, Karunarathne WK, Angaswamy N, Saini D, Cho JH, Kalyanaraman V, et al. Regulation of Golgi structure and secretion by receptor-induced G protein bg complex translocation. Proc Natl Acad Sci USA. 2010;107(25):11417–11422. doi: 10.1073/pnas.1003042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, et al. Roles of Gβγ in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the βγ subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA. 1998;95(3):1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romoser V, Ball R, Smrcka AV. Phospholipase C β2 association with phospholipid interfaces assessed by fluorescence resonance energy transfer. G protein βγ subunit-mediated translocation is not required for enzyme activation. J Biol Chem. 1996;271(41):25071–25078. doi: 10.1074/jbc.271.41.25071. [DOI] [PubMed] [Google Scholar]
- 93.Hicks SN, Jezyk MR, Gershburg S, Seifert JP, Harden TK, Sondek J. General and versatile autoinhibition of PLC isozymes. Mol Cell. 2008;31(3):383–394. doi: 10.1016/j.molcel.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han DS, Golebiewska U, Stolzenberg S, Scarlata SF, Weinstein H. A dynamic model of membrane-bound phospholipase Cβ2 activation by Gβγ subunits. Mol Pharmacol. 2011;80(3):434–445. doi: 10.1124/mol.111.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang T, Dowal L, El-Maghrabi MR, Rebecchi M, Scarlata S. The pleckstrin homology domain of phospholipase C-beta(2) links the binding of gbetagamma to activation of the catalytic core. J Biol Chem. 2000;275(11):7466–7469. doi: 10.1074/jbc.275.11.7466. [DOI] [PubMed] [Google Scholar]