Abstract
Background:
Peanut sublingual immunotherapy (SLIT) for 1 year has been shown to induce modest clinical desensitization in allergic children. Studies of oral immunotherapy, epicutaneous immunotherapy, and SLIT have suggested additional benefit with extended treatment.
Objective:
We sought to investigate the safety, clinical effectiveness, and immunologic changes with long-term SLIT in children with peanut allergy.
Methods:
Children with peanut allergy aged 1 to 11 years underwent extended maintenance SLIT with 2 mg/d peanut protein for up to 5 years. Subjects with peanut skin test wheals of less than 5 mm and peanut-specific IgE levels of less than 15 kU/L were allowed to discontinue therapy early. Desensitization was assessed through a double-blind, placebo-controlled food challenge (DBPCFC) with up to 5000 mg of peanut protein after completion of SLIT dosing. Sustained unresponsiveness was further assessed by using identical DBPCFCs after 2 to 4 weeks without peanut exposure.
Results:
Thirty-seven of 48 subjects completed 3 to 5 years of peanut SLIT, with 67% (32/48) successfully consuming 750 mg or more during DBPCFCs. Furthermore, 25% (12/48) passed the 5000-mg DBPCFC without clinical symptoms, with 10 of these 12 demonstrating sustained unresponsiveness after 2 to 4 weeks. Side effects were reported with 4.8% of doses, with transient oropharyngeal itching reported most commonly. Side effects requiring antihistamine treatment were uncommon (0.21%), and no epinephrine was administered. Peanut skin test wheals, peanut-specific IgE levels, and basophil activation decreased significantly, and peanut-specific IgG4 levels increased significantly after peanut SLIT.
Conclusion:
Extended-therapy peanut SLIT provided clinically meaningful desensitization in the majority of children with peanut allergy that was balanced with ease of administration and a favorable safety profile.
Keywords: Peanut allergy, sublingual immunotherapy, food immunotherapy, food desensitization, sustained unresponsiveness, food allergy treatments
An estimated 6% to 8% of children are affected by food allergy,1,2 and there is evidence the prevalence is increasing globally.1 The most common triggers of severe and fatal food-induced anaphylactic reactions are peanuts and tree nuts.3,4 Unfortunately, peanut allergy is less commonly outgrown than other major food allergies. Currently, there are no approved treatments for food allergy, and the standard of care is strict avoidance of the specific food. Even with extreme vigilance, accidental ingestions are not uncommon,5 and patients remain at risk for potentially life-threatening reactions. As a result, families of children with food allergies report disruptions in daily activities, increased stress and anxiety, and lower quality of life.6,7
Both oral immunotherapy (OIT) and epicutaneous immunotherapy (EPIT) strategies have advanced to late-stage clinical trials and review by the US Food and Drug Administration for use in treating peanut allergy.8,9 However, OIT might have potential limitations in terms of safety and ease of administration, and EPIT might be limited in its ability to generate clinically meaningful immunologic changes. Sublingual immunotherapy (SLIT) could represent a viable alternative for patients because of its simple route of administration and the good overall safety and efficacy seen in smaller trials.10,11
In the first published study directly comparing peanut OITwith peanut SLIT, 21 children aged 7 to 13 years were randomized to receive either 2000 mg of peanut OIT or 3.7 mg of peanut SLIT daily.10 After 12 months of therapy, peanut OIT provided a 141-fold increase in reaction threshold, whereas peanut SLIT provided a lesser but significant 22-fold increase from baseline. Similar changes in peanut skin prick test (SPT) responses, peanut-specific IgE (pn-sIgE) levels, and peanut-specific IgG4 (pn-sIgG4) levels were seen with both therapies. However, 42.8% of peanut OIT doses resulted in symptoms compared with 9% of peanut SLIT doses. Furthermore, peanut OIT was more likely to result in moderate or severe symptoms; symptoms requiring epinephrine, antihistamines, or b-agonists; and treatment withdrawals.
In a multicenter study of adolescents and adults aged 12 to 40 years through the Consortium for Food Allergy Research (CoFAR), peanut SLIT was shown to induce a modest level of clinical desensitization after 44 weeks.12 Extended treatment of these subjects up to 3 years suggested improved desensitization with longer duration of therapy.13 Additional studies of OIT and EPIT have also supported a stronger desensitization effect after longer periods of treatment.14–16
Previously, we demonstrated a modest but significant increase in reaction threshold compared with placebo in young children aged 1 to 11 years with peanut allergy after 12 months of SLIT.17 Here we report on the effectiveness and safety of long-term peanut SLIT in young children after completion of open-label extended maintenance therapy for up to 5 years.
METHODS
Study population and design
Two studies with peanut SLIT were rolled into a long-term extension protocol. In the initial protocol, which has been previously described,17 subjects underwent peanut or placebo SLIT for 12 months. In this open-label extension study subjects received maintenance doses of peanut SLIT at 2 mg/d for up to a total of 5 years. Subjects receiving active treatment in the original cohort continued directly into this extension protocol. Subjects initially randomized to placebo were crossed over to open-label peanut SLIT and underwent an identical build-up protocol, as previously described,17 before continuing into the extension protocol. An additional cohort of patients with peanut allergy with identical inclusion and exclusion criteria participated in a separate open-label peanut SLIT protocol with identical build-up and maintenance dosing and were rolled into this extension protocol as well.
The extended maintenance protocol was planned for a total of 5 years of peanut SLIT therapy. However, subjects completing at least 3 years of therapy who also demonstrated favorable immune modulation as defined by a peanut SPT response of less than 5 mm and a pn-sIgE level of less than 15 kU/L were allowed to undergo final assessment before age 5 years.
Desensitization was assessed through a double-blind, placebo-controlled food challenge (DBPCFC) with 5000 mg of peanut protein (approximately 16 to 20 peanut kernels) after the final day of SLIT dosing. Subjects passing the desensitization DBPCFC were instructed to discontinue peanut SLIT dosing and avoid peanuts for 2 to 4 weeks. Subjects then underwent another 5000-mg DBPCFC to assess for sustained unresponsiveness (SU).
DBPCFCs
The 5000-mg cumulative dose of each DBPCFC was administered in 6 increasing doses provided 20 minutes apart. Incremental challenge doses were as follows: 250, 500, 1000, 1000, 1000, and 1250 mg. Oat flour was used as a placebo and administered in identical increments. Subjects who consumed 5000 mg of peanut protein without dose-limiting clinical symptoms were considered to have passed the food challenge. Objective or persistent subjective allergic symptoms that resulted in stoppage of the DBPCFC included diffuse hives, severe nasal congestion, lip and tongue swelling, throat pain, coughing, moderate-to-severe abdominal pain, and vomiting. The cumulative ingested amount before the incremental dose causing discontinuation of the food challenge was reported as the successfully consumed dose (SCD).
Safety monitoring
All dose escalations and DBPCFCs were monitored by a study nurse or physician. Parents were instructed to monitor subjects for 2 hours after home dosing and document all dosing and side effects in home diaries. Timing relative to dosing and all treatments were also recorded. Safety assessments during the extended maintenance protocol were reviewed at biannual clinic visits. Eighteen types of dosing side effects were broadly grouped into oropharyngeal, skin, upper respiratory, chest, and abdominal symptoms for reporting purposes.
Peanut SPTs
SPTs were performed with a GREER Pick (Greer, Lenoir, NC) by using a standard 1:20 dilution for peanut extract. Wheal size was calculated as the average of the largest diameter and the perpendicular midpoint diameter. Reaction to peanut was reported as the peanut wheal size minus the wheal size of the saline negative control.
Mechanistic studies
Blood for mechanistic studies was collected at baseline, annually, and at desensitization and SU food challenges. Serum pn-sIgE and pn-sIgG4 levels were assessed by using the ImmunoCAP 100 (Thermo Scientific, Waltham, Mass), as previously described.17,18 Ratios of pn-sIgG4 to pn-sIgE were calculated by first converting both IgG4 and IgE levels into micrograms per liter and then dividing pn-sIgG4 quantities by pn-sIgE quantities. A conversion factor of 2.42 µg/L = 1 kU/L was used for pn-sIgE. Basophil activation was assessed by using whole blood in the presence of IL-3 with several dilutions of crude peanut extract (103, 102, 101, and 100 ng/mL), anti-IgE (103 ng/mL), and media alone. Basophils were identified as CD123+CD203c+Lin− (CD3, CD14, CD19, and CD41) events, with CD63 as the primary marker of activation. Results were analyzed as a ratio of peanut-specific to nonspecific (anti-IgE) activation, as previously described.19
Ethics
The protocol and consent forms were approved by the local institutional review board. The study was conducted under a US Food and Drug Administration investigational new drug application. Written informed consent was obtained from parents/guardians.
Statistical analysis
Statistical analysis was performed with Prism 8.0.1 software (GraphPad Software, San Diego, Calif). Comparison of baseline and end-of-treatment pn-IgE and pn-IgG4 levels and SPT responses was performed by using the paired Wilcoxon signed-rank test. Basophil reactivity was analyzed with the unpaired Wilcoxon rank sum test for each concentration of peanut tested. Pearson correlations between DBPCFC results and peanut-specific immunoglobulin levels and SPT responses were calculated and tested by using the correlation test. A P value of less than .05 was considered statistically significant.
RESULTS
Study population
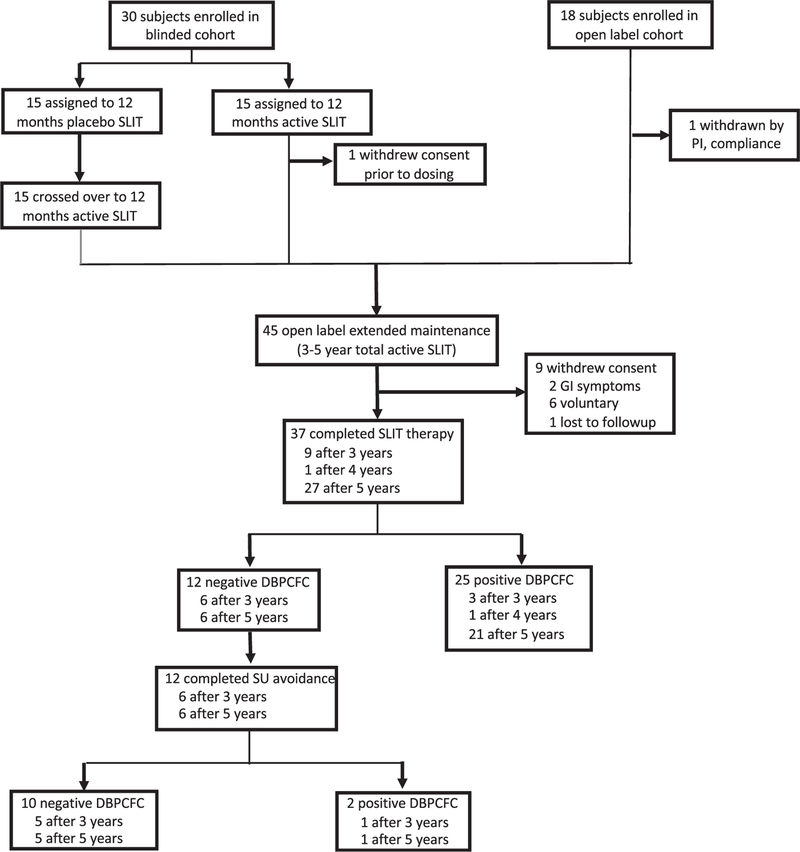
Forty-eight participants included those who were previously reported after the 12-month double-blind phase (19 subjects)17 and additional subjects (11 subjects) who were subsequently enrolled plus subjects (18 subjects) who were rolled over from an open-label cohort receiving peanut SLIT under the identical dosing protocol (Fig 1). Key inclusion criteria included age of 1 to 11 years at the start of SLIT therapy, clinical history of reaction after peanut ingestion, and pn-sIgE levels of 7 kU/L or greater. The median age of the cohort at enrollment was 6.5 years, and 67% were male. The cohort represented a highly allergic population with median peanut SPT wheals of 11.8 mm and median pn-sIgE levels of 83.9 kU/L and 56.3%, 68.8%, 62.5%, and 50% with concomitant asthma, atopic dermatitis, allergic rhinitis, and additional food allergies, respectively (Table I).
FIG 1.
Participant disposition throughout the trial.
TABLE I.
Subjects’ baseline characteristics
| Peanut SLIT (n = 48) | |
|---|---|
| Male sex, no. (%) | 32 (67) |
| Median age (y [range]) | 6.5 (1.6–11.9) |
| Race, no. (%) | |
| White | 46 (96) |
| African American | 0 |
| Asian | 2 (4) |
| Median peanut SPT response (mm [range]) | 11.8 (2.5–28) |
| Median peanut IgE level (kUA/L [range]) | 83.9 (7.7–1636) |
| Other food allergy, no. (%) | 24 (50) |
| Allergic rhinitis, no. (%) | 30 (63) |
| Asthma, no. (%) | 27 (56) |
| Atopic dermatitis, no. (%) | 33 (69) |
Eleven (22.9%) subjects withdrew from the study, with 1 subject discontinuing after consenting but before study drug dosing. During build-up, 1 subject was withdrawn by the principal investigator because of poor compliance. During maintenance dosing, 2 subjects discontinued because of recurrent abdominal pain with dosing, 6 subjects voluntarily withdrew citing difficulty with compliance, and 1 subject was lost to follow-up.
Safety
Overall, SLIT was well tolerated. Of 75,366 total doses, 3,599 (4.78%) were associated with symptoms affecting 45 of 48 subjects. The majority of symptoms self-resolved, with only 158 (0.21%) symptoms requiring antihistamines and none requiring epinephrine. Three episodes of wheezing or cough after SLIT dosing were treated with albuterol in addition to antihistamines. No dosing reactions were treated with oral steroids. As expected, oropharyngeal itching was the most common symptom, representing 75% of reported symptoms and affecting 3.6% of all doses taken. Oropharyngeal itching appeared to decrease with continued dosing, with 89% of episodes reported within the first 24 months of SLIT dosing. Local lip swelling was reported with 0.15% of doses. Gastrointestinal symptoms, including belly pain, vomiting, and diarrhea, were reported with 0.3% of doses (Table II). Both subjects who withdrew because of recurrent abdominal pain with dosing had immediate and full resolution of symptoms after discontinuation of dosing, and no further work-up was pursued. Compliance was strong, with 95.5% of doses successfully administered.
TABLE II.
Peanut SLIT dosing safety and compliance
| Peanut SLIT (n = 48) | |
|---|---|
| Total dosing days | 78,915 |
| Missed doses | 3,549 (4.5%) |
| Total doses taken | 75,366 (95.5%) |
| Dosing symptoms | 3,599 (4.8%) |
| Local | |
| Oropharyngeal pruritus | 2699 (3.6%) |
| Lip swelling | 115 (0.2%) |
| Skin | 387 (0.5%) |
| Upper respiratory tract | 75 (0.1%) |
| Lower respiratory tract | 69 (0.1%) |
| Gastrointestinal | |
| Belly pain | 225 (0.3%) |
| Vomiting | 20 (0.03%) |
| Diarrhea | 5 (0.01%) |
| Treatment administered | |
| Antihistamine | 158 (0.2%) |
| Epinephrine | 0 |
Upper respiratory tract symptoms included runny nose, sneeze, and nasal congestion. Lower respiratory tract symptoms included cough and wheeze.
DBPCFCs
Thirty-seven subjects completed the protocol (n = 9 for 3 years, n = 1 for 4 years, and n = 27 for 5 years) and were considered in the per-protocol (PP) analysis. During the DBPCFC, 32 subjects (intention to treat [ITT], 67%; PP, 86.5%) successfully consumed 750 mg or more, 23 subjects (ITT, 48%; PP, 62.2%) successfully consumed 1750 mg or more, and 17 subjects (ITT, 35%; PP, 45.9%) successfully consumed 2750 mg or more of peanut protein. Twelve subjects (ITT, 25%; PP, 32.4%) passed the 5000-mg DBPCFC without clinical symptoms (Fig 2). The median and mean SCDs of peanut for completers were 1750 and 2561 mg, respectively. Of the 12 subjects who passed the DBPCFC, 10 discontinued SLIT for 4 weeks, 1 discontinued for 3 weeks, and 1 discontinued for 2 weeks. Ten subjects again passed the DBPCFC without clinical symptoms, demonstrating SU. One subject avoiding for 4 weeks and the other avoiding for 2 weeks had negative DBPCFC results; however, both tolerated 3750 mg of peanut protein. The 10 subjects meeting the criteria to complete SLIT therapy before 5 years (SPT < 5 mm, pn-sIgE < 15 kU/L) performed well at the DBPCFC, with 6 subjects tolerating 5000 mg, 3 tolerating 3750 mg, and 1 tolerating 1750 mg of peanut protein. Of the 6 subjects passing the DBPCFC, 5 demonstrated SU by passing the DBPCFC again after peanut SLIT avoidance. Twelve subjects were treated with epinephrine during the end-of-treatment DBPCFC, the majority of which was for moderate-to-severe abdominal pain or vomiting. One subject required 2 doses of epinephrine for diffuse rash, wheeze, and abdominal pain and subsequent development of vomiting, nasal congestion, and decreased perfusion. No subjects were treated with epinephrine during the SU avoidance DBPCFC.
FIG 2.
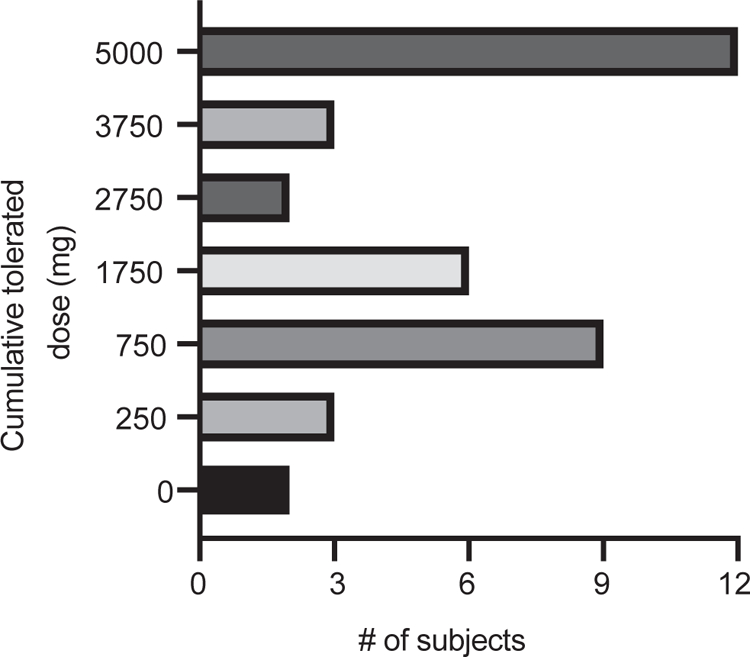
Desensitization thresholds during DBPCFC post-SLIT therapy: Maximum cumulative tolerated dose achieved for each subject during post-SLIT therapy 5000 mg DBPCFC.
Peanut SPTs
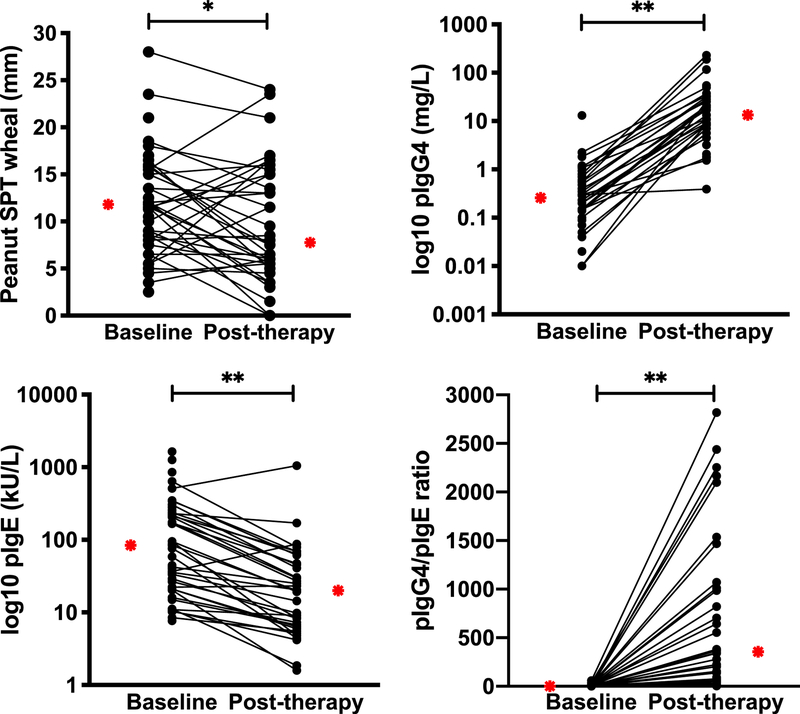
The median baseline wheal size to peanut SPT was 11.8 mm (range, 2.5–28 mm). Peanut SPTs at study completion were significantly decreased, with a median wheal size of 7.8 mm (range, 0–24 mm; P = .049; Fig 3, A). For the 10 subjects demonstrating SU, the baseline peanut SPT response was 11.5 mm (range, 3.5–15 mm) and the end-of-study peanut SPT response was 5.8 mm (range, 0–16 mm).
FIG 3.
Change in peanut-specific immunoglobulins and SPT: Significant changes from baseline to posttherapy for peanut SPT (*P = .05), pn-sIgE (**P < .001), pn-sIgG4 (**P < .001), and pn-sIgG4/pn-sIgE ratio (**P < .001). Median levels depicted by red stars.
Peanut-specific immunoglobulins
The median baseline pn-sIgE level was 83.9 kU/L (range, 7.7–1636 kU/L), and the median pn-sIgE level decreased significantly at study completion to 20.0 kU/L (range, 1.6–1051.8 kU/L; P < .0001; Fig 3, B). For the 10 subjects demonstrating SU, the baseline pn-sIgE level was 28.0 kU/L (range, 10.3–219 kU/L) and the end-of-study pn-sIgE level was 7.8 kU/L (range, 4.2–51.4 kU/L). The median baseline pn-sIgG4 level was 0.3 mg/L (range, 0–13.1 mg/L). The median pn-sIgG4 level significantly increased at study completion to 10.9 mg/L (range, 0–231.0 mg/L; P < .0001; Fig 3, C). For the 10 subjects demonstrating SU, the baseline pn-sIgG4 level was 0.4 mg/L (range, 0.1–2.3 mg/L), and the end-of-study pn-sIgG4 level was 10.9 mg/L (range, 0–231.0 mg/L). The median baseline pn-sIgG4/pn-sIgE ratio was 1.45 (range, 0–58.4), which increased significantly to 356.3 (range, 3.4–2818.2; P < .0001; Fig 3, D).
Basophil activation
The ratio of peanut-specific basophil activation to nonspecific (anti-IgE) activation decreased significantly from baseline to the end of treatment. This decrease was seen at all 4 concentrations of crude peanut extract evaluated (103 ng/mL, P < .004; 102 ng/mL, P <.0006; 101 ng/mL, P <.0001; and 100 ng/mL, P <.0001; Fig 4).
FIG 4.
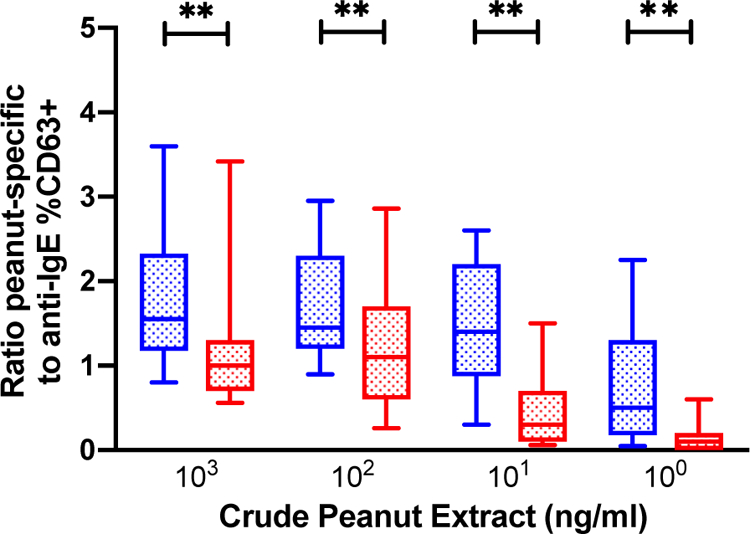
Decrease in peanut-specific to nonspecific (anti-IgE) basophil activation: Significant decreases from baseline (blue) to post-therapy (red) across 4 concentrations of crude peanut extract in %CD63+ basophils as a ratio of peanut-specific to nonspecific (anti-IgE) activation (**P < .001). Box and whiskers represent 10–90 percentiles.
DISCUSSION
As therapies for peanut allergy approach US Food and Drug Administration consideration, there has been increasing attention on patient preferences for a therapy. A recent report investigated caregiver goals of therapy while considering peanut OIT or EPIT therapy for their children.20 The primary themes that were noted were a desire for a buffer against unintentional peanut exposure and a low tolerance for risk from the treatment itself. In a recent study investigating reaction thresholds during oral food challenges, the median eliciting dose across 347 peanut oral food challenges with positive results was 75 mg of peanut protein.21 Based on these data, the dose predicted to elicit a reaction in 50% of pediatric patients with peanut allergy was 29.9 mg of peanut protein. In our study of extended peanut SLIT therapy, 67% (PP, 86%) of subjects tolerated at least 750 mg of peanut protein, with a median SCD of 1750 mg, suggesting a clinically significant buffer for the majority of subjects. In comparison, peanut EPIT in a recently completed multinational phase 3 study demonstrated a median cumulative reactive dose of 444 mg.9 Furthermore, in the current study 48% (PP, 62%) and 35% (PP, 46%) of subjects tolerated 1750 and 2750 mg, respectively. Comparing these data with those of a recent phase 3, multinational study of peanut OIT that demonstrated response rates of 76.6% to 443 mg, 67.2% to 1043 mg, and 50.3% to 2043 mg of peanut protein,8 peanut SLIT provides near-comparable desensitization in children with peanut allergy.
In an effort to translate food immunotherapy outcomes to real-world clinical benefits, a quantitative risk assessment model has been developed.22 Repeated simulations were conducted by using various clinical thresholds in patients with peanut allergy combined with national consumption data and estimates of peanut residue in common snack foods. Increasing the clinical threshold to 300 mg of peanut protein was estimated to provide a greater than 95% reduction of risk for allergic reactions to common foods, such as chips, cookies, snack cakes, and ice cream. Achieving a clinical threshold of 1000 mg increased this risk reduction to nearly 99%. These data further support a clinically meaningful level of desensitization for the majority of our subjects treated with peanut SLIT.
Dosing safety was anticipated as a significant advantage of SLIT therapy. In our study of peanut SLIT, symptoms were reported after less than 5% of doses, and the majority of these symptoms were transient oropharyngeal itching. Despite the majority of side effects occurring at home without medical supervision, antihistamines were rarely administered, and epinephrine was not used for any dosing symptoms. Only 2 subjects discontinued therapy because of recurrent abdominal pain without vomiting compared with generally greater than 10% of patients who receive OIT. Abdominal symptoms resolved immediately on stopping the study drug, and no eosinophilic esophagitis was observed in the study.
An additional advantage of SLIT was thought to be its simple administration. Overall, 23% of subjects withdrew from the study, with only 2 of these 11 subjects citing adverse events. In comparison, 25 (11%) of 238 subjects receiving active EPIT in a recent phase 3 study withdrew, with 4 citing adverse events,9 and 80 (21%) of 374 subjects receiving active OIT withdrew from a recent phase 3 study, with 43 citing adverse events.8 It is possible that recurrent oropharyngeal pruritus or the medicinal taste led to oral aversion and ultimately to discontinuation. Increasing scheduling conflicts as the children grew older might have also played a role. It is noteworthy that in the 3-year CoFAR study of peanut SLIT in adolescents and adults, 62% of subjects withdrew from the study, many because of difficulty with compliance.13 The significantly greater subject retention in our study, despite its longer duration, might suggest an advantage to starting therapy when children are younger.
In recent studies of OIT looking at up to 1 month off therapy, variable levels of SU have been reported.14,23 In this current cohort we demonstrate 2-to 4-week SU in 21% (PP, 27%) of subjects enrolled in the protocol. With a median age at study completion of 8.3 years, this group would appear to be beyond the age expected for natural resolution of their peanut allergy. SU was reported in only 11% of subjects in the older CoFAR peanut SLIT cohort,13 possibly suggesting an advantage with younger age; however, subjects in this study underwent 8 weeks of avoidance, complicating any direct comparisons.
Similar to other forms of food immunotherapy, there was a significant modulatory effect on the allergic immune response after peanut SLIT. We previously reported that pn-sIgE levels increased over the initial months of SLIT therapy only to return to baseline by 12 months.17 After up to 5 years of peanut SLIT therapy, pn-sIgE levels decreased significantly to less than baseline levels from a median of 83.9 to 20 kU/L. Simultaneously, pn-sIgG4 levels increased from a median of 0.3 to 13.4 mg/L, and concurrently, the pn-sIgG4/pn-sIgE ratio increased significantly. Suppression of immediate effector mast cells and basophils were also observed, with peanut SPT wheal size decreasing from a median of 11.8 to 7.8 mm, and basophil activation decreasing across all 4 concentrations of crude peanut extract tested. With regard to potential biomarkers of clinical response, there was a strong correlation between the desensitization DBCPFC and the end-of-study SPT response that was not seen with the pn-sIgE level, the pn-sIgG4 level, or the pn-sIgG4/IgE ratio (see Fig E1 in this article’s Online Repository at www.jacionline.org).
There are several weaknesses of the study, the most prominent being the lack of a placebo group beyond the 12-month time point. For this proof-of-concept study, the potential for placebo therapy for up to 5 years was not considered ethically appropriate. The older age of the cohort at DBPCFC was thought to address some of the concern for natural resolution. In addition, the median tolerated dose of 1750 mg was at least 20 times greater than what has been described for placebo groups in other food immunotherapy trials.12,17,24
This study also did not include a baseline DBPCFC before SLIT therapy. At the beginning of the study, a clinical history of reaction to peanut and pn-sIgE cutoff of 7 kU/L was estimated to provide at least 80% positive predictive value. Further evidence supporting that subjects do in fact have peanut allergy in this cohort is that they are nearly identical to the subjects who had entry DBPCFC’s in recently published multicenter studies of peanut OIT and peanut EPIT,8,9 with a median pn-sIgE level of 83.9 kU/L and peanut SPT response of 11.8 mm and 56.3%, 68.8%, and 50% with concomitant asthma, atopic dermatitis, and additional food allergies. SU was assessed over a relatively short interval of 2 to 4 weeks compared with other studies, which might not be long enough to differentiate a gradual decrease in desensitization versus a truly sustained level of protection after SLIT therapy. Finally, like many other food immunotherapy studies, the population was very homogeneous and represented by mostly white male subjects, limiting the generalizability of the results.
In this long-term open-label study of peanut SLIT in children with peanut allergy, we have demonstrated a substantial and clinically meaningful desensitization effect coupled with strong compliance and dosing safety. Extending therapy from 1 to 5 years also resulted in more prominent immunologic changes demonstrating modulation of the allergic response. Further study is needed to determine whether greater doses of peanut SLIT might provide additional benefit. In addition, better understanding of the durability of the desensitization effect after peanut SLIT could have clinically meaningful implications. Finally, identification of biomarkers that can reliably predict response to treatment without the need for a food challenge remains a critical need. In summary, the balance of safety, efficacy, and simple dosing administration support peanut SLIT as a viable alternative for the treatment of peanut allergy.
Supplementary Material
Clinical implications:
Extended therapy with peanut SLIT in children for up to 5 years provides clinically significant desensitization with evidence of immune modulation. Peanut SLIT is safe and well tolerated.
Acknowledgments
Supported by the National Institutes of Health/National Center for Complementary and Integrative Health (R01-AT-004435) and the Wallace Research Foundation.
Study coordination and support were provided by Pamela Steele, Jan Kamilaris, Sarah Bennick, Emily English, Deanna Hamilton, and Lauren Herlihy. Editorial support was provided by Jennifer King, with funding provided by the author.
Disclosure of potential conflict of interest: E. H. Kim reports consultancy with Aimmune therapeutics, DBV Technologies, AllerGenis, and Allakos; clinical medical advisory board membership with DBV Technologies; and receipt of grant funding from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID), the NIH/National Center for Complementary and Integrative Health (NCCIH), Food Allergy Research & Education (FARE), and the Wallace Research Foundation. A. W. Burks reports minority stock holder in Allertein and Mast Cell Pharmaceuticals; advisory board membership with Aimmune Therapeutics, Consortia TX, and Prota Therapeutics; consultancy fees from DBV Technologies and N-fold; royalties from UpToDate; speaking fees from Gordon Research Conferences for pediatric allergy and asthma meetings; and sponsored research from FARE and the NIH. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- CoFAR
Consortium for Food Allergy Research
- DBPCFC
Double-blind, placebo-controlled food challenge
- EPIT
Epicutaneous immunotherapy
- ITT
Intention to treat
- OIT
Oral immunotherapy
- pn-sIgE
Peanut-specific IgE
- pn-sIgG4
Peanut-specific IgG4
- PP
Per-protocol
- SCD
Successfully consumed dose
- SLIT
Sublingual immunotherapy
- SPT
Skin prick test
- SU
Sustained unresponsiveness
REFERENCES
- 1.Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol 2014;133:291–308. [DOI] [PubMed] [Google Scholar]
- 2.Iweala OI, Choudhary SK, Commins SP. Food allergy. Curr Gastroenterol Rep 2018;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol 2007;119:1016–8. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol 2001;107:191–3. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer DM, Perry TT, Atkins D, Wood RA, Burks AW, Jones SM, et al. Allergic reactions to foods in preschool-aged children in a prospective observational food allergy study. Pediatrics 2012;130:e25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol 2006;96:415–21. [DOI] [PubMed] [Google Scholar]
- 7.Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010;65:933–45. [DOI] [PubMed] [Google Scholar]
- 8.Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, Hourihane JO, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med 2018;379:1991–2001. [DOI] [PubMed] [Google Scholar]
- 9.Fleischer DM, Greenhawt M, Sussman G, Begin P, Nowak-Wegrzyn A, Petroni D, et al. Effect of epicutaneous immunotherapy vs placebo on reaction to peanut protein ingestion among children with peanut allergy: the PEPITES randomized clinical trial. JAMA 2019;321:946–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol 2015;135:1275–82, e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol 2012;129:448–55, e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol 2013;131:119–27, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burks AW, Wood RA, Jones SM, Sicherer SH, Fleischer DM, Scurlock AM, et al. Sublingual immunotherapy for peanut allergy: long-term follow-up of a randomized multicenter trial. J Allergy Clin Immunol 2015;135:1240–8, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol 2014;133:468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol 2016;137:1117–27.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson HA, Shreffler WG, Yang WH, Sussman GL, Brown-Whitehorn TF, Nadeau KC, et al. Effect of varying doses of epicutaneous immunotherapy vs placebo on reaction to peanut protein exposure among patients with peanut sensitivity: a randomized clinical trial. JAMA 2017;318: 1798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 2011;127:640–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burk CM, Kulis M, Leung N, Kim EH, Burks AW, Vickery BP. Utility of component analyses in subjects undergoing sublingual immunotherapy for peanut allergy. Clin Exp Allergy 2016;46:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow’s milk tolerance. J Allergy Clin Immunol 2013;131:180–6, e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhawt M, Marsh R, Gilbert H, Sicherer S, DunnGalvin A, Matlock D. Understanding caregiver goals, benefits, and acceptable risks of peanut allergy therapies. Ann Allergy Asthma Immunol 2018;121:575–9. [DOI] [PubMed] [Google Scholar]
- 21.Purington N, Chinthrajah RS, Long A, Sindher S, Andorf S, O’Laughlin K, et al. Eliciting dose and safety outcomes from a large dataset of standardized multiple food challenges. Front Immunol 2018;9:2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumert JL, Taylor SL, Koppelman SJ. Quantitative assessment of the safety benefits associated with increasing clinical peanut thresholds through immunotherapy. J Allergy Clin Immunol Pract 2018;6:457–65.e4. [DOI] [PubMed] [Google Scholar]
- 23.Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol 2017;139:173–81.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol 2011;127: 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.