Abstract
Early detection of prenatal alcohol exposure is critical for designing and testing effectiveness of interventional therapeutics. Choline supplementation during and after prenatal alcohol exposure has shown promising benefits in improving outcomes in rodent models and clinical studies. A sheep model of first trimester-equivalent binge alcohol exposure was used in this study to model the dose of maternal choline supplementation used in an ongoing prospective clinical trial involving pregnancies at risk for FASD. Pregnant sheep were randomly assigned to six groups: Saline+Placebo control, Saline+Choline, binge Alcohol+Placebo (light binging), binge Alcohol+Choline, Heavy binge Alcohol+Placebo (heavy binging) and Heavy binge Alcohol+Choline. Ewes received intravenous alcohol or saline on three consecutive days per week from gestational day (GD) 4 to 41 to mimic first trimester-equivalent weekend binge drinking paradigm. Choline (10 mg/kg in the daily food ration) was administered from GD 4 until term. On GD 76, 11 fetal ultrasonographic measurements were collected transabdominally. Heavy binge alcohol exposure reduced fetal Frontothalamic Distance (FTD), Mean Orbital Diameter (MOD) and Mean Lens Diameter (MLD) and increased Interorbital Distance (IOD) and Thalamic Width (TW). Maternal choline supplementation mitigated most of these alcohol-induced effects. Maternal choline supplementation also improved overall fetal femur and humerus bone lengths compared to their respective placebo groups. Taken together these results indicate a potential dose dependent effect that could impact the sensitivity of these ultrasonographic measures in predicting prenatal alcohol exposure. This is the first study in the sheep model to identify biomarkers of prenatal alcohol exposure in-utero with ultrasound and co-administration of maternal choline supplementation.
Keywords: Choline, Ultrasonography, Prenatal alcohol, Diagnosis, FASD
INTRODUCTION
Alcohol consumption during pregnancy can result in fetal alcohol spectrum disorders (FASD), which encompass a range of physical, behavioral, learning, emotional, and social disturbances. Drinking among women of childbearing age remains high despite widespread educational efforts about the dangers of drinking during pregnancy, and the incidence of FASD has failed to decline [1]. Attempts to estimate the prevalence of FASD suggest it may be as high as 2–5% in the United States and many Western European countries, but most FASD prevalence studies have under-identified cases in general populations [2]. FASD is a hidden epidemic and often clinicians are not trained to diagnose these conditions [3]. A number of promising interventional strategies and therapeutics have been devised but optimal implementation in clinical trials of most requires early and reliable identification of alcohol-affected pregnancies.
Ultrasonography, unlike other imaging technologies, is routinely employed during pregnancy. A clinical pilot study [4] and more recently a Ukrainian study through the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) [5] have shown that fetal ultrasonography can identify FASD prenatally. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) strategic plan for research includes the need to “refine and increase knowledge about specific structural alterations in various brain regions for identifying fetal alcohol CNS deficits, and explore the potential for developing low-cost or modest-cost approaches for identifying these structural deficits through prenatal ultrasound and transfontanelle ultrasound of newborns” [6]. It is difficult to accomplish this goal in human studies of prevention or treatment of FASD because of variable consumption patterns of alcohol, dependence on often unreliable self-estimates of alcohol intake by pregnant women, and additive effects of drugs often co-abused with alcohol. Because of the far-reaching effects of alcohol on child growth and development and its prevalence among pregnant women, FASD requires attention from the public health, obstetric, pediatric, and education communities [2]. However, accurate early identification of pregnancies in which the fetus is at risk for FASD has proven difficult. Development of ultrasound measures that are sensitive and specific for prenatal alcohol exposure brain injury would be valuable for the early identification of FASD.
Benefits of early identification of prenatal alcohol exposure by ultrasonography include maternal awareness, with resultant behavior changes that lead to a cessation or reduction in drinking during the rest of pregnancy [7, 8]. Intervention to mitigate harmful effects of prenatal alcohol exposure is beneficial at any point during pregnancy. A decrease in neurologic and neurobehavioral deficits has been observed in offspring of women who abstain from alcohol during the third trimester [9]. The development of therapeutic and preventive interventions has also become a priority. Choline supplementation has shown promising benefits in rodent models as a nutraceutical therapeutic approach to lessen the effects of prenatal alcohol exposure [10–13]. Pregnancy increases the production of phosphatidylcholine in the maternal liver, providing an important source of choline necessary for fetal brain development and function [14]. Maternal alcohol consumption could decrease choline availability to the fetus through complex mechanisms that contribute to the deleterious effects of alcohol on brain development [14, 15].
In a CIFASD clinical trial in Ukraine, the researchers administered a multivitamin and mineral supplement to pregnant women, with a subgroup of participants also receiving choline supplementation (750 mg/day, about 10 mg/kg/day) [16]. In our study, a sheep model was used concurrently to optimize translational comparisons. In sheep, choline supplementation was started on gestation day (GD) 4 at 10 mg/kg/day to maximize the likelihood of benefits from early intervention. A number of recent clinical and preclinical studies have demonstrated that choline supplementation during pregnancy could mitigate adverse effects of prenatal alcohol exposure [12, 13, 17, 18].
The two main goals of the current study were to evaluate ultrasonography as a prenatal screening tool for identifying biomarkers of brain and bone development to detect prenatal alcohol exposure in the sheep model and to evaluate the beneficial effect of choline supplementation to mitigate adverse effects of prenatal alcohol exposure. Because binge drinking during pregnancy is a major determinant of the severity of FASD [19], in our study we modeled two levels of binge drinking during the first trimester-equivalent period (designated as “binge alcohol” and “heavy binge alcohol”), while evaluati ng the potential benefits of maternal choline supplementation administered throughout gestation to prevent FASD.
MATERIALS AND METHODS
Animals and Breeding
All animals and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University. Prior to breeding, Suffolk ewes (aged 2–5 years) received multi-species Clostridium bacteria-toxoid (Covexin 8, Intervet/Schering-Plough Animal Health, Summit, NJ) 2 ml intramuscularly, albendazole (Valbazen Suspension 7.5 mg/kg, Pfizer Animal Health, New York, NY) 0.75 ml/25 lb of body weight orally, and ivermectin (Ivomec Drench for Sheep 0.8%, Merial, Inc., Duluth, GA) 3 ml/26 lb body weight orally. Ewes were maintained on a coastal Bermuda grass pasture and a pelleted ewe ration (TAMU Ewe Ration, Nutrena, Cargill, Minneapolis, MN) designed to meet 100% of the National Research Council (NRC) requirements as calculated by ARIES software version 2007, University of California, Davis. Cycling ewes received progesterone impregnated vaginal implants (EAZI-BREED™, CIDR ®, Pharmacia & Upjohn Ltd., Auckland New Zealand); implants were removed 11 days after placement, at which time prostaglandin F2α (Lutalyse 5 mg/mL, Pharmacia & Upjohn, Kalamazoo MI) 4 ml was administered intramuscularly. The next day, ewes were placed with a ram fitted with a marking harness for a period of 24 hours. The day of mating (the day that the ewes were marked by the ram) was designated as GD 0, and ewes entered the experiment the next day. Ewes were penned individually for the experiment but had visual contact at all times with herd mates in adjacent pens in an environmentally regulated facility (22°C and a 12:12 light/dark cycle). Preg nancy was confirmed ultrasonographically on GD 25, and if ewes were not pregnant, they were removed from the experiment. The previously described pelleted ration was fed to ewes based on body weight and stage of gestation, meeting NRC requirements at all times. Ewes were fed twice daily and had free access to drinking water. Maternal food consumption was monitored daily. All ewes consumed all feed offered, and there were no differences between groups in feed consumption.
Choline dose development
Choline dose development and validation were previously described and reported [20]. The high choline doses often used in rodent studies (~250 mg/kg per day) are not likely to be used in clinical studies in pregnant women, in which the recommended dose during human pregnancy is 450 mg/day (~6mg/kg per day). In the CIFASD randomized clinical trial, the choline group was given a daily dose of 750 mg (~10 mg/kg per day), thus helping to guide the decision for the dose of choline used in the sheep model [16]. Therefore, the dosing regimen of 10 mg/kg per day used in this sheep model study is both relevant and highly translational.
Treatment groups
Ewes (n = 49) were randomly assigned to six treatments groups: 1) Saline+Placebo control group that received isotonic saline 0.9% infusions intravenously (n = 8), 2) Saline+Choline group that received isotonic saline 0.9% infusions intravenously along with 10 mg/kg/day choline (n = 8), 3) Binge Alcohol+Placebo group that received 1.75 g/kg treatment of ethanol (n = 9), 4) Binge Alcohol+Choline group that received 1.75 g/kg treatment of ethanol along with 10 mg/kg/day choline (n = 6), 5) Heavy binge Alcohol group that received 2.5 g/kg treatment of ethanol (n = 10), and 6) Heavy binge Alcohol+Choline group that received 2.5 g/kg treatment of ethanol along with 10 mg/kg/day choline (n = 8). The alcohol infusions in ewes modeled a weekend binge drinking pattern [1] over the human first trimester-equivalent period in sheep (GD 4–41) [21], with alcohol administered on three consecutive days per week, followed by four days without treatment (18 treatments in total).
An intravenous catheter (16 ga., 5.25 inch Angiocath™; Becton Dickinson, Sandy, UT) was placed into the jugular vein of each ewe on GD 4. Beginning on this day, alcohol (1.75 g/kg or 2.5 g/kg body weight) or saline was administered intravenously via a pump (VetFlo® 7701B IV Vet Infusion Pump, Grady Medical, Temecula, CA) over a 1-hour period. The alcohol solution was prepared by adding 95% ethanol to sterile 0.9% saline to achieve a 40% w/v alcohol solution. Solutions were prepared under aseptic conditions and passed through a 0.2 µm bacteriostatic filter. The saline control group received an infusion of isotonic saline (0.9%) that was equal in volume to the alcohol infusions. Ewes in the choline supplemented groups received 10 mg/kg oral choline supplement (ReaShure® choline chloride 28.8%; daily dose based on weight of choline, Balchem Corporation, New Hampton, NY) mixed with their daily feed for the entirety of their pregnancy.
Maternal blood alcohol concentration
To measure peak blood alcohol concentration, blood was drawn from the jugular vein of each ewe one hour after alcohol infusions began on GDs 6, 27, and 41, as previously described [22, 23]. A 20 µl aliquot of blood was collected in a microcapillary tube and transferred into a vial containing 0.6 N perchloric acid and 4 mM n-propyl alcohol (internal standard) in distilled water. The vial was tightly capped with a septum-sealed lid and stored at room temperature until analysis within 24 hours of collection by headspace gas chromatography (Varian Associates model 3900, Palo Alto, CA).
Ultrasonographic examinations
Transabdominal ultrasonographic examinations were performed by an ultrasonographer, blinded to the alcohol exposure status of the ewes, using a MyLab 30 Gold machine (Esaote North America, Indianapolis, Indiana) with a convex microarray transducer (8–1 MHz) and a microconvex array transducer (9–3 MHz). We identified GD 76 as the optimum time for ultrasonography of the brain in fetal sheep because the fetal brain is well developed but ossification of the calvarium is incomplete. After complete ossification, structure identification and image quality are poor. Ultrasonographic norms have been established for fetal sheep, and their potential for use in assessing congenital abnormalities in sheep models has been validated [24] Fetal brain measurements in the current study were modeled after an ultrasound pilot study in humans [4] and more recent second trimester human ultrasound study [5].
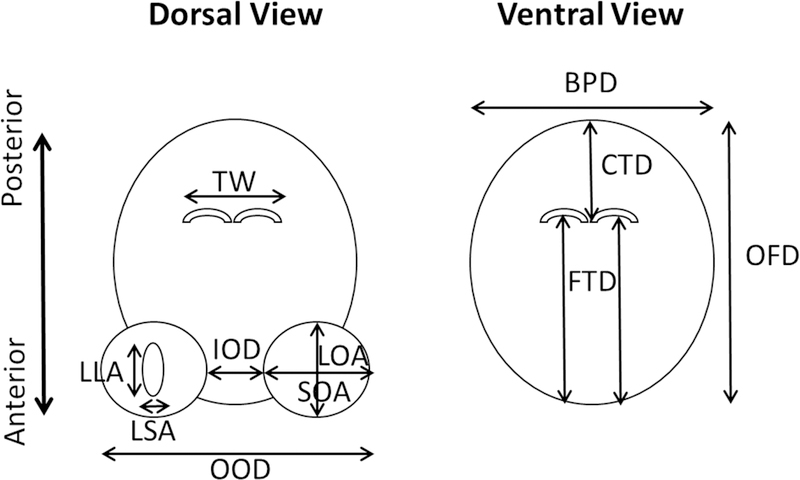
On GD 76 (during the second trimester human equivalent), 11 measurements of the fetal skull, lens, brain, and legs were collected from each ewe in the six treatment groups. The measurements included the following parameters: 1) Mean lens diameter (MLD) calculated by averaging the long and short lens axis diameters, 2) Interorbital distance (IOD) measured as the distance between the orbits, 3) Mean orbital diameter (MOD) calculated by averaging the long and short orbital axis diameters, 4) Outer orbital distance (OOD) measured as the distance between the outer edges of the orbits, 5) Biparietal distance (BPD) measured as the distance between the inner surfaces of the lateral calvaria, 6) Caudothalamic distance (CTD) measured as the distance between the posterior margin of the thalami and the inner surface of the posterior calvarium, 7) Frontothalamic distance (FTD) measured as the distance between the inner surface of the anterior calvarium and the posterior margin of the thalami, 8) Occipitofrontal distance (OFD) measured as the distance between the inner surface of the anterior calvarium and the inner surface of the posterior calvarium, 9) Thalamic width (TW) measured as the width of the developing thalamus, 10) Femur length (FL) measured as the length of the femur from proximal to distal, and 11) Humerus length (HL) measured as the length of the humerus from proximal to distal. Diagrams depicting these ultrasonography measures and representative actual images are shown in Figure 1 and Figure 2, respectively.
Figure 1:
Diagram depicting cranio-facial measurements of fetal lamb on GD 76. LLA, Long Lens Axis diameter; SLA, Short Lens Axis diameter; IOD, Interorbital Distance; LOA, Long Orbital Axis diameter; SOA, Short Orbital Axis diameter; OOD, Outer Orbital Distance; BPD, Biparietal Distance; CTD, Caudothalamic Distance; FTD, Frontothalamic Distance; OFD, Occipitofrontal Distance; TW, Thalamic Width.
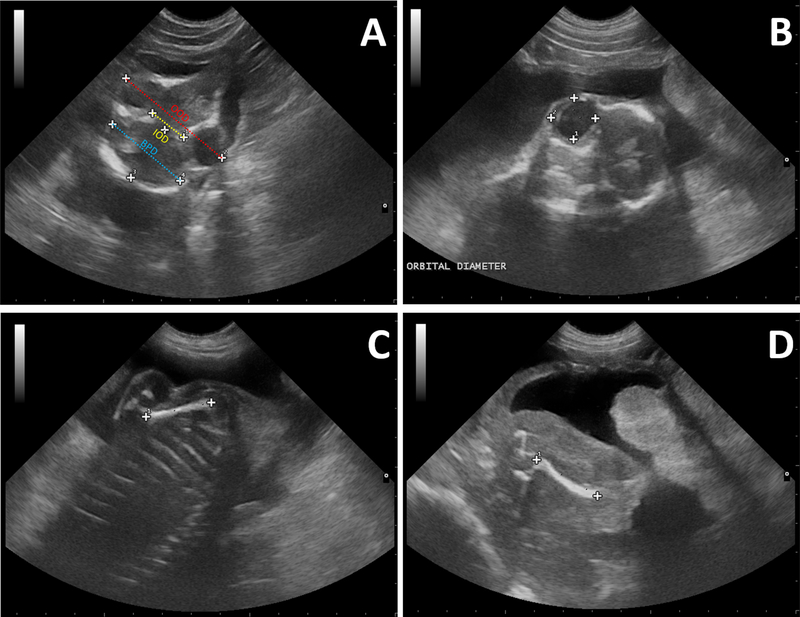
Figure 2:
(A) Axial ultrasonographic image at GD 76 illustrating measurements of Interorbital Distance (IOD) (yellow dotted line), Outer Orbital Distance (OOD) (red dotted line) and Biparietal diameter (BPD) (blue dotted line). (B) Ultrasonographic image depicting measurement of fetal Orbital Diameter. (C-D) Lateral ultrasonographic image at GD 76 illustrating measurements of the humeral length (C) and femoral length (D).
Statistical Analysis
Statistical analysis was performed with SigmaStat® (Version 3.5 Systat Software, Inc). Data are presented as mean ± standard error of the mean (SEM). Two-way analysis of variance (ANOVA) was performed with alcohol exposure (Saline v/s Low Ethanol v/s High Ethanol) and choline supplementation (Placebo v/s Choline) as two independent factors. Significant effects in these ANOVAs were followed by pairwise comparisons using Fisher’s protected least significant difference method. Level of significance was established a priori at P<0.05.
RESULTS
Maternal blood alcohol concentration
The mean ± SEM maternal blood alcohol concentrations at the end of alcohol infusion (1 hour; point in time at which blood alcohol concentrations are known to peak) for the 4 alcohol receiving groups are tabulated in Table 1. Two-way ANOVA showed main effect of alcohol dose (F(1,18)=21.20, p<0.001). Pairwise comparisons showed overall significantly higher blood alcohol concentrations in the heavy binge alcohol group compared to the binge alcohol group (P<0.001). Heavy binge alcohol+placebo group had significantly higher BAC than the binge alcohol+placebo group (P=0.024). Heavy binge alcohol+choline group had significantly higher BAC than the binge alcohol+choline group (P<0.001). There were no statistically significant differences in BACs between binge alcohol+placebo and binge alcohol+choline groups or heavy binge alcohol+placebo and heavy binge alcohol+choline groups indicating that choline supplementation did not alter alcohol bioavailability.
Table 1:
Blood Alcohol Concentrations (BACs). Numbers are Mean ± SEM
| Binge Alcohol+Placebo | Binge Alcohol+Choline | Binge Heavy Alcohol+Placebo | Binge Heavy Alcohol+Choline | |
|---|---|---|---|---|
| BAC (mg/dL) | 197.69 ± 13.44 | 190.09 ± 16.18 | 274.83 ± 34.72 | 297.51 ± 17.51 |
Ultrasonographic examinations
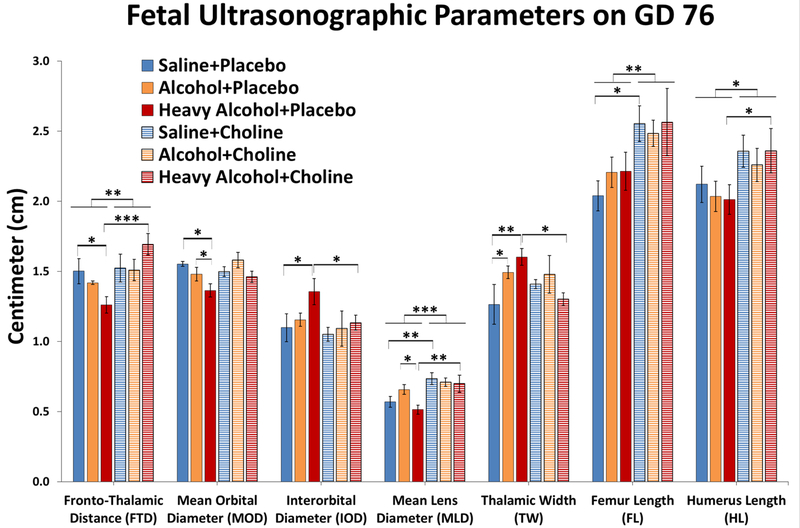
The mean ± SEM values for all 11 fetal parameters measured ultrasonographically are listed in Table 2 and selected parameters that most directly inform the effects of alcohol dosing or choline supplementation are presented in Figure 3. Maternal alcohol consumption significantly decreased fetal frontothalamic distance (FTD) and choline supplementation was able to rescue this phenotype. Two-way ANOVA revealed the significant main effect of choline supplementation (F(1,41)=9.07, P=0.004) and a significant interaction between alcohol dosing and choline supplementation (F(2,41)=4.79, P=0.013). Follow-up comparisons confirmed that fetal FTD was significantly reduced in the heavy binge alcohol+placebo group compared to the saline+placebo control group (P=0.019), but the lower fetal FTD in the binge alcohol+placebo group compared to the saline+placebo group did not reach significance. Choline supplementation showed a protective effect on fetal FTD parameter, as shown by the significant increase in the heavy binge alcohol+choline group compared to the heavy binge alcohol+placebo group (P<0.001).
Table 2:
Fetal parameters measured ultrasonographically on GD 76.
| Saline +Placebo (n=8) | Binge Alcohol +Placebo (n=9) | Binge Heavy Alcohol +Placebo (n=10) | Saline +Choline (n=8) | Binge Alcohol +Choline (n=6) | Binge Heavy Alcohol +Choline (n=8) | |
|---|---|---|---|---|---|---|
| Fetal Parameters Measured | ||||||
| Mean Lens Diameter (MLD)* | 0.57 ± 0.04 | 0.66 ± 0.04 | 0.51 ± 0.03 | 0.74 ± 0.04 | 0.71 ± 0.03 | 0.70 ± 0.06 |
| Interorbital Distance (IOD)* | 1.10 ± 0.10 | 1.15 ± 0.05 | 1.36 ± 0.09 | 1.05 ± 0.05 | 0.99 ± 0.10 | 1.13 ± 0.05 |
| Mean Orbital Diameter (MOD)* | 1.55 ± 0.02 | 1.48 ± 0.05 | 1.36 ± 0.05 | 1.50 ± 0.04 | 1.58 ± 0.05 | 1.46 ± 0.04 |
| Outer Orbital Distance (OOD) | 4.22 ± 0.06 | 4.13 ± 0.11 | 4.04 ± 0.10 | 4.20 ± 0.12 | 4.27 ± 0.19 | 3.96 ± 0.15 |
| Biparietal Distance (BPD) | 2.77 ± 0.06 | 2.87 ± 0.09 | 2.73 ± 0.06 | 2.83 ± 0.14 | 2.97 ± 0.12 | 2.84 ± 0.04 |
| Caudothalamic Distance (CTD) | 0.61 ± 0.05 | 0.58 ± 0.05 | 0.52 ± 0.05 | 0.60 ± 0.04 | 0.59 ± 0.03 | 0.59 ± 0.04 |
| Frontothalamic Distance (FTD)* | 1.50 ± 0.09 | 1.42 ± 0.01 | 1.26 ± 0.06 | 1.52 ± 0.10 | 1.51 ± 0.08 | 1.69 ± 0.08 |
| Occipitofrontal Distance (OFD) | 2.10 ± 0.05 | 2.06 ± 0.07 | 2.09 ± 0.17 | 2.18 ± 0.09 | 2.20 ± 0.09 | 2.24 ± 0.06 |
| Thalamic Width (TW)* | 1.39 ± 0.08 | 1.50 ± 0.05 | 1.60 ± 0.06 | 1.41 ± 0.03 | 1.48 ± 0.13 | 1.30 ± 0.04 |
| Femur Length (FL)* | 2.04 ± 0.11 | 2.21 ± 0.11 | 2.21 ± 0.14 | 2.55 ± 0.13 | 2.49 ± 0.09 | 2.56 ± 0.24 |
| Humerus Length (HL)* | 2.12 ± 0.13 | 2.03 ± 0.11 | 2.01 ± 0.11 | 2.37 ± 0.12 | 2.26 ± 0.12 | 2.36 ± 0.16 |
indicate parameters with statistically significant (P<0.05) effects of alcohol dosing or choline supplementation and these selected parameters are also presented in Figure 3. Values are Mean ± SEM in Centimeters.
Figure 3:
Fetal ultrasonographic parameters on GD 76. Values are mean ± SEM *, ** and *** indicate P<0.05, 0.01 and 0.001, respectively.
Maternal alcohol consumption significantly decreased fetal mean orbital diameter (MOD), confirmed by a significant main effect of alcohol dosing (F(2,43)=5.09, P=0.010). Follow-up comparisons indicated the orbital diameter was significantly reduced in the heavy binge alochol+placebo group compared to the saline+placebo (P=0.002) group and to the binge alcohol+placebo (P=0.043) group. For interorbital distance (IOD) the two-way ANOVA yielded no significant main or interactive effects of alcohol treatment (F(2,42)=2.57, P=0.089) or choline supplementation (F(1,42)=2.81, P=0.101) despite the trend for increased IOD only in the heavy binge alcohol+placebo group (see Table 2), reflecting the limited power to detect a two-way interaction (F(2,42)=0.77, P=0.468). Recent second-trimester ultrasound studies have reported increased IOD in alcohol-exposed pregnancies in [5]. Therefore, despite of non-significant main effect, we relied on post hoc pairwise comparison analysis to test effects on IOD in the heavy binge alcohol groups, and these indicated the heavy binge alcohol+placebo group had significantly greater IOD than the saline+placebo group (P=0.02) and the heave binge alcohol+choline was reduced relative to heavy binge alcohol+placebo, P=0.043). It is important to note that an increased IOD in the heavy binge alcohol group could be a manifestation of a decreased MOD, rather than a true increase in inter-orbital spacing.
Fetal mean lens diameter (MLD) was significantly increased after choline supplementation, as confirmed by the significant main effect of choline supplementation (F(1,43)=15.42, P<0.001). MLD was significantly increased in the heavy binge alcohol+choline group compared to its respective placebo group (P=0.002) as well as in the saline+choline group compared its respective placebo group (P=0.008). MLD was significantly decreased in the heavy binge alcohol+placebo group compared to binge alcohol+placebo group (P=0.012).
Binge alcohol exposure significantly increased fetal thalamic width (TW) and choline supplementation was able to rescue this phenotype, yielding a significant alcohol treatment X choline supplementation interaction (F(2,38)=3.44, P=0.043). Significant increases in fetal TW was evident in the binge alcohol+placebo (P=0.048) and heavy binge alcohol+placebo (P=0.004) groups compared to the saline+placebo group. Choline supplementation significantly decreased fetal TW in the heavy binge alcohol+choline (P=0.016) group compared to the heavy binge alcohol+placebo group.
Previously we reported that third trimester-equivalent alcohol exposure reduces fetal bone length, diameter and strength [25, 26]. Surprisingly, in the current study we did not observe any significant alterations among alcohol exposed groups for fetal femoral and humerus length. This lack of effect could be attributed to early measures (GD 76) in this study rather than end of third trimester measures in our previous studies. Nonetheless, choline supplementation increased fetal femoral and humerus length in all groups compared to their respective placebo groups, confirmed by significant main effects of choline for femur (F(1,42)=10.71, P=0.002) and humerus (F(1,42)=7.12, P=0.011), respectively. Fetal femoral length was significantly higher in the saline+choline group compared to the saline+placebo group (P=0.013). Fetal femur and humerus lengths were higher in the heavy binge alcohol+choline group compared to the heavy binge alcohol+placebo group (P=0.081 and 0.047, respectively).
DISCUSSION AND CONCLUSION
Three major findings can be gleaned from this study. First, prenatal heavy binge alcohol exposure during the first-trimester equivalent period in a sheep model results in an increase in thalamic width (TW) and decreases in frontothalamic distance (FTD), mean orbital diameter (MOD), and mean lens diameter (MLD) of the fetus observed during second trimester-equivalent (GD 76) ultrasonographic examination. This supports the hypothesis that ultrasonographic measures in the sheep model of binge alcohol drinking in the first trimester can predict prenatal exposure. Second, maternal choline supplementation mitigates the adverse effect of prenatal alcohol exposure and significantly rescued fetal FTD and TW parameters, along with non-significant improvements in MOD and IOD. Choline also increased the MLD in the control and the heavy binge alcohol exposure groups. Third, maternal choline supplementation increased fetal appendicular bone (femur and humerus) length in all groups. Taken together, these findings support the hypothesis that choline supplementation can mitigate adverse effects of prenatal alcohol exposure and promote fetal growth in the first trimester-equivalent sheep model of binge alcohol drinking.
Ultrasonographic examinations are routinely performed in pregnant women and because of this, fetal screening using this technique has attracted attention as a possible way to detect FASD during pregnancy. In a pilot study, ultrasound examinations were performed on pregnant women in the Ukraine, where prenatal alcohol exposure was common [4]. Based on self-reporting, the women were divided into alcohol exposed and unexposed groups; based on these groupings, several fetal brain measures were found to be predictive of alcohol exposure. The strongest predictor, caval-calvarial distance, was significantly reduced by 38% in the second trimester, but this measurement did not differ significantly between groups in the third trimester. The frontothalamic distance (FTD) was also smaller and found to be predictive during the second and third trimesters. Our study identified a reduced FTD in the heavy binge alcohol group during the second trimester-equivalent period in the sheep model. Similarly, during the third trimester, smaller orbital diameter was also predictive in the Kfir et al., 2009 study [4]. Our study confirmed these findings and showed that the MOD was smaller in the heavy binge alcohol group. In the Kfir et al., 2009 study, exposed fetuses also had a significantly shorter femur length in the second trimester. Similarly, using a sheep model, we have also reported alterations in fetal bone dimensions and strength after prenatal alcohol exposure [25, 26]. However, in this current study we didn’t identify any length changes in the humerus or femur between groups on GD 76 after in-utero ultrasonographic measurements. On contrary, ex-utero bone analysis in our previous studies was performed towards the end of third trimester-equivalent period. Effect of alcohol exposure on fetal bones could be culmination of changes that occur throughout the pregnancy and early second trimester-equivalent detection may not be able to detect those alterations.
In a more recent study of second trimester ultrasounds by Montag et al., 2016 it was found that interorbital distance (IOD) was significantly larger in alcohol exposed infants [5], and our study found a significant increase in IOD in a post hoc comparison between the heavy binge alcohol+placebo group and the saline+placebo group. Increased IOD is a characteristic dysmorphic feature of alcohol exposure and is consistent with hypertelorism. The current study also found significantly decreased mean orbital diameter (MOD) in the heavy binge alcohol group. Developmental research done using rat, mouse and zebrafish models have shown that alcohol exposure results in visual defects including generalized small eye size and reduced electroretinograms responses [27–29].
Prenatal choline supplementation has been shown to mitigate neurodevelopmental damage caused by prenatal alcohol exposure in rats [10, 11], suggesting its potential use as an intervention for FASD. The recommended human choline intake during pregnancy is 450 mg/day, and 3500 mg/day is the tolerable upper limit or roughly a dose of 60 mg/kg in a 60 kg woman. Dosages used in rodent studies have exceeded this range, with no apparent side effects at 200 mg/kg in pregnant mice and 250 mg/kg in pregnant rats [30, 31]. However, in baboons, choline administration at a dose roughly equivalent to 20 mg/kg together with alcohol resulted in hepatotoxicity [32], likely due to lower choline oxidase activity in this species. Sheep, like primates, have lower choline oxidase activity than rodents and therefore probably have a lower maximum tolerable intake level of choline than rats; choline dietary requirements and tolerable upper limits have not been determined in sheep. While the dietary dose of 10 mg choline per kg of body weight compares closely between the dose used in this study and the study conducted in women [16] (both are roughly 10 mg/kg), we cannot compare how it relates to choline requirements in other species. We consider the ovine model appropriate for investigating the safety and efficacy of choline supplementation, and the current study supports the conclusion that concurrent prenatal choline supplementation can improve measures of second-trimester craniofacial development in alcohol-exposed pregnancies.
The sheep model of FASD has numerous translational benefits in preclinical research in which dose, pattern, and timing of alcohol exposure are experimentally controlled [33]. Sheep have body weights and head/brain sizes that are more comparable to humans and they have long gestational periods (147 days) closely resembling human gestation (280 days). Also, all three trimester-equivalents of brain development occur in-utero in the sheep model and can be matched more directly to prenatal brain development in humans [33, 34]. The ovine model is slightly limited because beyond GD 76, the fetal skull becomes ossified and ultrasound of the developing brain is no longer possible thus limiting examination in the human third trimester-equivalent period. Findings in this study illustrate the translational value of the sheep model, confirming that the effects of alcohol on brain and cranio-facial measurements are replicable in multiple species. In summary, our study provides a tool for detecting alcohol-induced intrauterine growth deficits. Intrauterine brain growth deficits are known to predict infant neuronal growth trajectory, infant behavior and adult behavioral health [35–38]. A number of studies have shown protective effects of various nutritional intervention strategies to rescue alcohol-induced fetal deficits [23, 39–42]. This study also provides novel evidence that maternal choline supplementation during pregnancy can mitigate some of the detrimental effects of binge alcohol drinking in the first trimester. Select ultrasonographic measures may provide an optimal structural biomarker of the effects of first trimester binge drinking associated with FASD.
HIGHLIGHTS.
In-utero ultrasonography has diagnostic potential for identifying fetal alcohol exposure
Amount and timing of alcohol exposure impacts fetal cranio-facial anomalies
Choline supplementation mitigates alcohol-induced fetal cranio-facial anomalies
ACKNOWLEDGEMENTS
We would like to thank Dr. Bunita Eichelberger and Dr. Keith Chaffin for their assistance with determining and optimizing ultrasonographic methods. We would like to thank Ms. Raine Lunde for her assistance with procedures. This work was supported by the National Institutes of Health, NIAAA Grants AA17120, AA18166 (S.E.W.) and the Knights Templar Eye Foundation Pediatric Ophthalmology Career Starter Research Grant KTEF1806 (O.B.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
References
- 1.Caetano R, et al. , The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res, 2006. 30(6): p. 1023–30. [DOI] [PubMed] [Google Scholar]
- 2.May PA, et al. , Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev, 2009. 15(3): p. 176–92. [DOI] [PubMed] [Google Scholar]
- 3.Clarren SK and Lutke J, Building clinical capacity for fetal alcohol spectrum disorder diagnoses in western and northern Canada. Can J Clin Pharmacol, 2008. 15(2): p. e223–37. [PubMed] [Google Scholar]
- 4.Kfir M, et al. , Can prenatal ultrasound detect the effects of in-utero alcohol exposure? A pilot study. Ultrasound in Obstetrics and Gynecology, 2009. 33(6): p. 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montag AC, et al. , Second-Trimester Ultrasound as a Tool for Early Detection of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res, 2016. 40(11): p. 2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services, N.I.o.H., Alcohol across the lifespan, N.I.o.A.A.a.A.F.Y.S.P.F. 2009–14, Editor. 2008. [Google Scholar]
- 7.Handmaker NS, et al. , Impact of alcohol exposure after pregnancy recognition on ultrasonographic fetal growth measures. Alcohol Clin Exp Res, 2006. 30(5): p. 892–8. [DOI] [PubMed] [Google Scholar]
- 8.Sedgmen B, et al. , The impact of two-dimensional versus three-dimensional ultrasound exposure on maternal-fetal attachment and maternal health behavior in pregnancy. Ultrasound Obstet Gynecol, 2006. 27(3): p. 245–51. [DOI] [PubMed] [Google Scholar]
- 9.Coles CD, et al. , Neonatal neurobehavioral characteristics as correlates of maternal alcohol use during gestation. Alcohol Clin Exp Res, 1985. 9(5): p. 454–60. [DOI] [PubMed] [Google Scholar]
- 10.Idrus NM and Thomas JD, Fetal Alcohol Spectrum Disorders: Experimental Treatments and Strategies for Intervention. Alcohol Research & Health, 2011. 34(1): p. 76–85. [PMC free article] [PubMed] [Google Scholar]
- 11.Idrus NM, Breit KR, and Thomas JD, Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol, 2017. 59: p. 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas JD, Abou EJ, and Dominguez HD, Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol, 2009. 31(5): p. 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas JD, et al. , Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol, 2010. 88(10): p. 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeisel SH, What Choline Metabolism Can Tell Us About the Underlying Mechanisms of Fetal Alcohol Spectrum Disorders. Molecular neurobiology, 2011. 44(2): p. 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw GM, et al. , Choline and risk of neural tube defects in a folate-fortified population. Epidemiology, 2009. 20(5): p. 714–9. [DOI] [PubMed] [Google Scholar]
- 16.Coles CD, et al. , Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Matern Child Health J, 2015. 19(12): p. 2605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson SW, et al. , Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol Clin Exp Res, 2018. 42(7): p. 1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson SW, et al. , Feasibility and Acceptability of Maternal Choline Supplementation in Heavy Drinking Pregnant Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol Clin Exp Res, 2018. 42(7): p. 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May PA and Gossage JP, Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Res Health, 2011. 34(1): p. 15–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Birch SM, et al. , Maternal choline supplementation in a sheep model of first trimester binge alcohol fails to protect against brain volume reductions in peripubertal lambs. Alcohol, 2016. 55: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryden MM, Evans HE, and Binns W, Embryology of the sheep. I. Extraembryonic membranes and the development of body form. Journal of Morphology, 1972. 138(2): p. 169–185. [DOI] [PubMed] [Google Scholar]
- 22.Conover EA and Jones KL, Safety concerns regarding binge drinking in pregnancy: a review. Birth Defects Res A Clin Mol Teratol, 2012. 94(8): p. 570–5. [DOI] [PubMed] [Google Scholar]
- 23.Washburn SE, et al. , Acute alcohol exposure, acidemia or glutamine administration impacts amino acid homeostasis in ovine maternal and fetal plasma. Amino Acids, 2013. 45(3): p. 543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward VL, et al. , Fetal sheep development on ultrasound and magnetic resonance imaging: a standard for the in utero assessment of models of congenital abnormalities. Fetal Diagn Ther, 2006. 21(5): p. 444–57. [DOI] [PubMed] [Google Scholar]
- 25.Sawant OB, et al. , The role of acidemia in maternal binge alcohol-induced alterations in fetal bone functional properties. Alcohol Clin Exp Res, 2013. 37(9): p. 1476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramadoss J, et al. , Binge alcohol exposure during all three trimesters alters bone strength and growth in fetal sheep. Alcohol, 2006. 38(3): p. 185–92. [DOI] [PubMed] [Google Scholar]
- 27.Lantz CL, et al. , Visual defects in a mouse model of fetal alcohol spectrum disorder. Front Pediatr, 2014. 2: p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz LM and Fox DA, Prenatal ethanol exposure alters scotopic and photopic components of adult rat electroretinograms. Invest Ophthalmol Vis Sci, 1991. 32(11): p. 2861–72. [PubMed] [Google Scholar]
- 29.Bilotta J, et al. , Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol, 2004. 26(6): p. 737–43. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JD, et al. , Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth defects research. Part A, Clinical and molecular teratology, 2010. 88(10): p. 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young JK, et al. , Nutrition Implications for Fetal Alcohol Spectrum Disorder. Advances in Nutrition, 2014. 5(6): p. 675–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieber CS, et al. , Choline fails to prevent liver fibrosis in ethanol-fed baboons but causes toxicity. Hepatology, 1985. 5(4): p. 561–72. [DOI] [PubMed] [Google Scholar]
- 33.Cudd TA, Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood), 2005. 230(6): p. 389–93. [DOI] [PubMed] [Google Scholar]
- 34.Sawant OB, et al. , Different patterns of regional Purkinje cell loss in the cerebellar vermis as a function of the timing of prenatal ethanol exposure in an ovine model. Neurotoxicol Teratol, 2013. 35: p. 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu G, et al. , Maternal nutrition and fetal development. J Nutr, 2004. 134(9): p. 2169–72. [DOI] [PubMed] [Google Scholar]
- 36.Pryor J, The identification and long term effects of fetal growth restriction. Br J Obstet Gynaecol, 1997. 104(10): p. 1116–22. [DOI] [PubMed] [Google Scholar]
- 37.Riley EP and McGee CL, Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood), 2005. 230(6): p. 357–65. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, et al. , Cognitive and Behavioral Outcomes of Intrauterine Growth Restriction School-Age Children. Pediatrics, 2016. 137(4). [DOI] [PubMed] [Google Scholar]
- 39.Schneider RD and Thomas JD, Adolescent Choline Supplementation Attenuates Working Memory Deficits in Rats Exposed to Alcohol During the Third Trimester Equivalent. Alcohol Clin Exp Res, 2016. 40(4): p. 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballard MS, Sun M, and Ko J, Vitamin A, folate, and choline as a possible preventive intervention to fetal alcohol syndrome. Med Hypotheses, 2012. 78(4): p. 489–93. [DOI] [PubMed] [Google Scholar]
- 41.Sawant OB, Wu G, and Washburn SE, Maternal L-glutamine supplementation prevents prenatal alcohol exposure-induced fetal growth restriction in an ovine model. Amino Acids, 2015. 47(6): p. 1183–92. [DOI] [PubMed] [Google Scholar]
- 42.Sawant OB, et al. , Effects of L-glutamine supplementation on maternal and fetal hemodynamics in gestating ewes exposed to alcohol. Amino Acids, 2014. 46(8): p. 1981–96. [DOI] [PMC free article] [PubMed] [Google Scholar]