Abstract
Schizophrenia is associated with amotivation and reduced goal-directed behavior, which have been linked to poor functional outcomes. Motivational deficits in schizophrenia are often measured using effort-based decision-making (EBDM) paradigms, revealing consistent alterations in effort expenditure relative to controls. While these results have generally been interpreted in terms of decreased motivation, the ability to use trial-by-trial changes in reward magnitude or probability of receipt to guide effort allocation may also be affected by cognitive deficits. To date, it remains unclear whether altered performance in EBDM primarily reflects deficits in motivation, cognitive functioning, or both. We applied a newly developed computational modeling approach to the analysis of EBDM data from two previously collected samples comprising 153 patients and 105 controls to determine the extent to which individuals did or did not use available information about reward and probability to guide effort allocation. Half of the participants with schizophrenia failed to incorporate information about reward and probability when making effort-expenditure decisions. The subset of patients who exhibited difficulties using reward and probability information were characterized by greater impairments across measures of cognitive functioning relative to those patients who did not. Interestingly, even within the subset of patients who successfully used reward and probability information to guide effort expenditure, higher levels of negative symptoms related to motivation and avolition were associated with greater effort aversion during the task. Taken together, these data suggest that prior reports of aberrant EBDM in schizophrenia patients are related to both cognitive function and individual differences in negative symptoms.
Keywords: Motivation, cognition, schizophrenia, effort, computational modeling
General scientific summary:
Individuals with schizophrenia show reductions in willingness to exert effort for rewards relative to healthy controls, but it is unclear whether this difference is related to cognitive functioning or reduced motivation. We use a novel analysis approach to identify individuals who systematically allocate effort based on reward and probability, finding that individuals with schizophrenia are less likely to systematically allocate effort. Our results suggest that observed patterns of reduced effort expenditure in schizophrenia are related to both cognitive functioning and individual differences in motivation.
A cardinal feature of schizophrenia is the reduction of motivation and goal-directed behavior. Importantly, negative symptoms such as amotivation have been linked to poor psychosocial functioning (Fervaha, Foussias, Agid, & Remington, 2013) and are particularly difficult to treat (Sarkar, Hillner, & Velligan, 2015), necessitating a better understanding of the mechanisms that underlie this behavior. While early conceptualizations viewed reduced motivation as a manifestation of anhedonia (Chapman, Chapman, & Raulin, 1976; Meehl, 1975), an emerging body of work suggests that schizophrenia is not associated with blunted hedonic capacity (Abler, Greenhouse, Ongur, Walter, & Heckers, 2008; Aghevli, Blanchard, & Horan, 2003; Barch et al., 2017; Cohen & Minor, 2010; Dowd & Barch, 2012; Gilleen, Shergill, & Kapur, 2015; Llerena, Strauss, & Cohen, 2012). These deficits in motivation may instead be related to altered decision-making (Gold, Waltz, Prentice, Morris, & Heerey, 2008; Heerey, Bell-Warren, & Gold, 2008), particularly regarding cost-benefit decisions of effort expenditure (Barch, Treadway, & Schoen, 2014; Gard et al., 2014).
Consistent with this latter hypothesis, recent studies using effort-based decision-making (EBDM) paradigms have produced remarkably stable results. In 11 published studies testing a combined 499 individuals with schizophrenia or schizoaffective disorder and 245 matched controls (Barch et al., 2014; Fervaha et al., 2015; Fervaha, Graff-Guerrero, et al., 2013; Gold et al., 2013; Hartmann et al., 2014; Huang et al., 2016; McCarthy, Treadway, Bennett, & Blanchard, 2016; Moran, Culbreth, & Barch, 2017; Reddy et al., 2015; Treadway, Peterman, Zald, & Park, 2015; Wang et al., 2015), individuals with schizophrenia were consistently less willing than healthy controls to exert effort for rewards, particularly when reward values and probability of receipt were highest (Barch et al., 2014; Fervaha, Graff-Guerrero, et al., 2013; Gold et al., 2013; Gold, Waltz, & Frank, 2015; McCarthy et al., 2016; Reddy et al., 2015; Treadway et al., 2015). While highly replicable across paradigms and labs, these findings have been somewhat difficult to interpret relative to other research on negative symptoms and reward processing in schizophrenia. Moreover, associations between EBDM behavior and individual differences in negative symptoms have been highly variable (Barch et al., 2014; Fervaha et al., 2015; Fervaha, Graff-Guerrero, et al., 2013; Gold et al., 2013; Strauss et al., 2016; Treadway et al., 2015; Wolf et al., 2014; See Culbreth et al., 2017 for review).
A possible explanation for this pattern of findings is that multiple mechanisms may contribute to alterations in effort expenditure. While they have been traditionally viewed as a measure of motivation, many EBDM tasks may be affected by cognitive performance as well; EBDM tasks typically require participants to make choices regarding effort expenditure across multiple trials in which the magnitude of reward varies (Gold et al., 2013; Pessiglione, Vinckier, Bouret, Daunizeau, & Le Bouc, 2017; Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009). This trial-by-trial change in the expected reward is intended to assess the subjective cost of effort expenditure for each person. Importantly, however, the ability to use trial-by-trial changes in reward magnitude (or probability information, in the case of the EEfRT) to guide effort allocation may be affected by cognitive deficits in schizophrenia. Although motivational impairments and other aspects of negative symptoms have traditionally been viewed as minimally related to cognitive deficits, recent work has suggested possible associations between self-reported measures of motivation and cognitive performance (Fervaha et al., 2014), leading researchers to hypothesize that the observed differences in effort allocation in people with schizophrenia relative to healthy control participants may be driven by disruptions in cognitive functioning (Culbreth, Moran, & Barch, 2017). To date, however, empirical evidence for the role of cognitive deficits in effort-related behavior in schizophrenia is still needed.
In prior EBDM studies, associations between cognitive functioning and effort allocation, assessed as the proportion of high-effort choices, have been highly variable—while some studies have found no significant relationship between willingness to exert effort and cognitive functioning (Hartmann et al., 2014; McCarthy et al., 2016), others have found weak positive associations (Horan et al., 2015), or have only observed relationships at the highest levels of reward (Gold et al., 2013). The current work examines the association between motivation and cognition in schizophrenia by comparing the fit of computational models that vary in terms of their utilization of trial-wise information to guide decisions, allowing us to assess whether trial-by-trial changes in reward magnitude or probability were systematically used to guide choices. By “systematic use” of reward or probability information, we mean that individuals incorporate these values into their choices in a consistent way over the course of the task. Importantly, this question is largely independent from the proportion of high or low effort choices made. For example, someone who is effort averse but who uses reward systematically may choose to complete the high effort option only when the reward magnitude is at the highest levels, while someone else who does not find the high effort option to be particularly burdensome (less effort averse) might have a lower reward threshold for choosing to perform the high effort option. These individuals would have a very different proportion of high effort options (the traditional dependent variable of EBDM tasks), but both make choices that are systematically consistent with regard to the reward information presented. In contrast, an individual could choose a high proportion of hard task choices with little regard for trial-wise changes in reward, selecting the hard option on all trials or in a seemingly random way. For example, they may choose the high effort option for trials with a reward of $2.00 or $2.50, but then choose the low effort option when the reward is $3.00 or $4.00 (See Figure 2). The computational modeling approach presented in this paper allows us to quantify different strategies for incorporating trial-wise reward and probability information when allocating effort, and to test whether the use of particular strategies is associated with cognitive functioning. In essence, our focus here is to determine how individuals made choices (with or without systematic utilization of probability and reward information) as opposed to the more traditional focus on what choices individuals made (proportion of high and low effort options selected). We additionally examine whether controlling for individual differences in the use of trial-by-trial information can clarify the associations between EBDM and motivational impairments.
Figure 2.
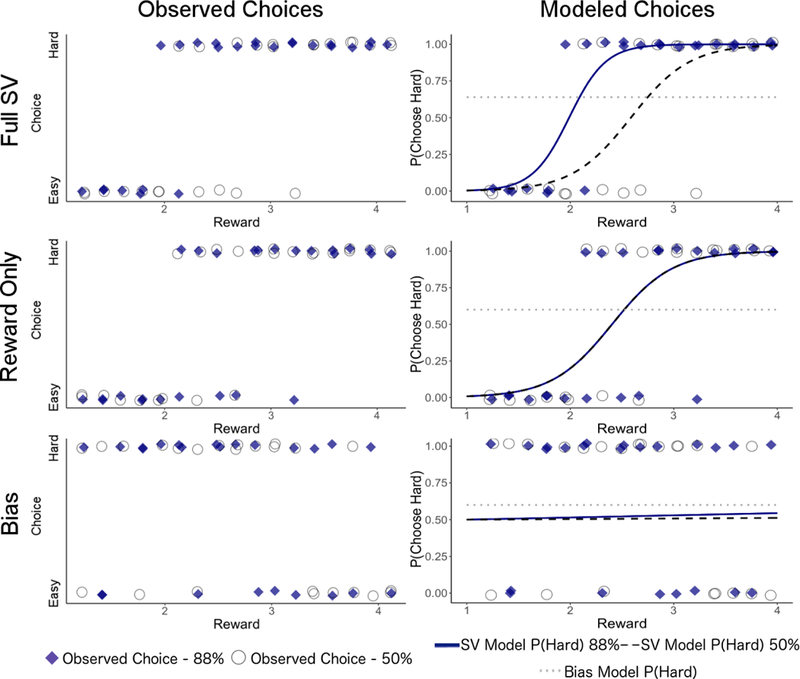
Behavioral data from three sample patients with similar cumulative behavior (each chose high effort on 60–64% of trials). On the left, participant’s observed choices (hard or easy) are plotted by reward value. On the right, observed choices are overlaid with model-predicted probabilities of choosing high effort for 88% probability trials from the SV model, 50% probability trials from the SV model, and bias model (all probabilities). The participant best-fit by the full-SV model (top) chooses high-effort more frequently as reward increases, with different rates for 88% and 50% probability trials. The participant best-fit by the reward-only SV model shows similar effects of reward, but with little effect of probability (note that the lines for 88% and 50% probability conditions exhibit complete overlap). The bottom panel represents data from a patient best-fit by the bias model, where the percentage of high effort choices does not increase as reward increases and choices show little effect of probability.
METHODS
Participants
Participants included 153 individuals with schizophrenia or schizoaffective disorder and 105 healthy controls. Sample 1 (schizophrenia, n = 59, controls n = 39) was collected at Washington University at St. Louis (2008– 2013) and the behavioral findings were previously published (Barch et al., 2014). Three subjects from Sample 1 were excluded for response rates 2.5 standard deviations below the mean (Mean = 44 trials, SD = 9.24; Excluded participants only responded on 2, 3, and 15 trials. After exclusion, Mean = 45.19, SD = 6.41). Sample 2 (schizophrenia, n = 94, controls n = 66) was collected at the Greater Los Angeles VA (GLA) (2013 −2015) and some of the data (n = 134) were previously published (Reddy et al., 2015). No participants were excluded from Sample 2. All study procedures were approved by the Washington University or GLA IRB, respectively, and all subjects provided written informed consent. Analysis was conducted at Emory University (2017–2019). See Supplementary Materials for full inclusion criteria.
Task and Data
Models were fit to data from a variant of the Effort Expenditure for Rewards Task (EEfRT; Treadway et al., 2009; Figure 1A), which has been widely used with schizophrenia patients (Barch et al., 2014; Fervaha, Graff-Guerrero, et al., 2013; McCarthy et al., 2016; Moran et al., 2017; Reddy et al., 2015; Treadway et al., 2015). On each trial, participants were asked to choose between an easy task for $1 or a hard task for a variable amount of reward. In Sample 1, the easy task required participants to make 30 button presses in a period of 7s with their dominant index finder, while the hard task required 100 button presses within 21 seconds with non-dominant pinky finger. In Sample 2, the number of presses required for the hard and easy tasks were individually calibrated for each participant. The number of presses for the hard task was 85% of the participant’s maximum press speed for 30s, while the number of presses for the easy task was one-third of the amount of presses required for the hard task for 7s. Both studies utilized a reward range of $1-$4.30 for the hard task. Participants were told that they would not always win the reward, but were explicitly given the likelihood that they would “win” if they successfully completed the chosen task. The probability levels were 50% and 88%. Each trial started with a fixation cross (1s), followed by a choice period. Participants collected in Sample 1 were given 5 seconds to respond before a task was randomly chosen and made choices over a 15 minute period, while participants in Sample 2 did not have a decision time limit and completed 50 trials. Following the choice, participants were shown the word “Ready” for 1 second, then completed the required number of button presses while a bar on the screen showed their progress. Participants were then told whether they successfully completed the task and whether or not they won money for the trial. As with previous samples, only the first 50 trials were analyzed. Demographic information and measures of symptom expression collected in each sample are in Table 1 and further described in Supplementary Materials.
Figure 1.
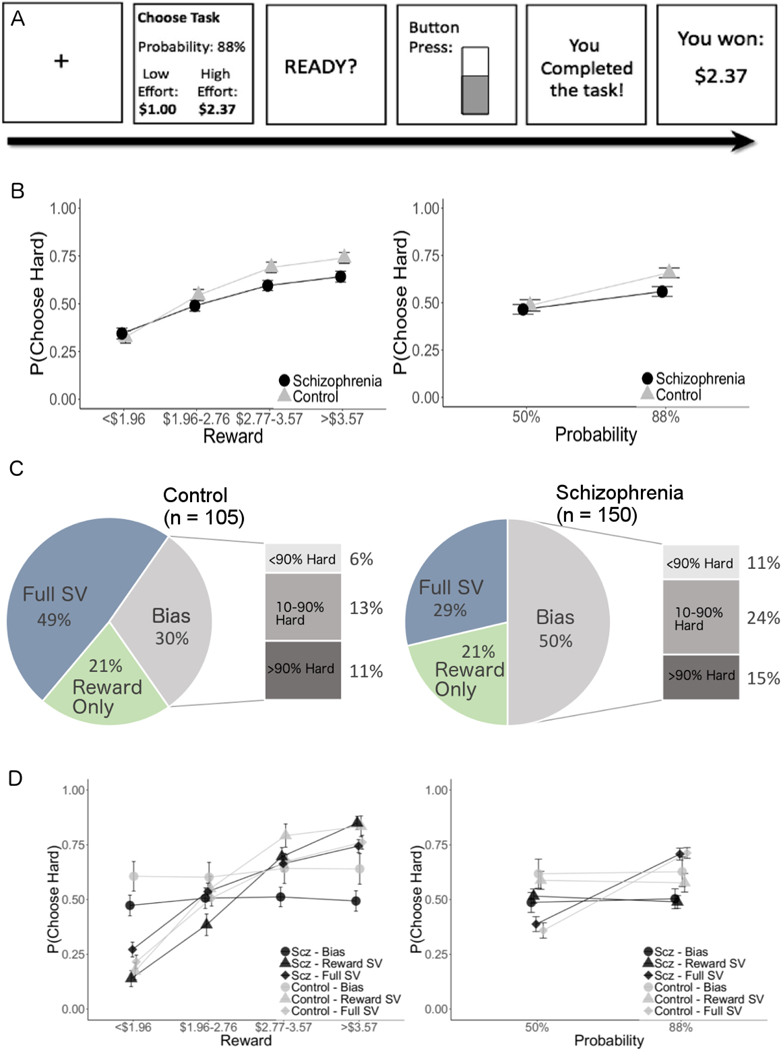
A. Task schematic. B. Proportion of subjects best-fit by the subjective value and bias models, measured using Bayesian Information Criterion. The proportion of individuals (within the bias model) who highly favored one option (i.e. who selected over 90% hard and 90% easy) are also indicated. C. Overall proportion of hard task selection by people with schizophrenia and healthy controls at each reward and probability level. D. Performance of patients with schizophrenia and controls within each subgroup.
TABLE 1:
Participant Demographic, Clinical, and Self-report Measures
| Sample 1 | |||
|---|---|---|---|
| Schizophrenia | Control | t | |
| n=56 (59% M) | n=39 (49% M) | ||
| Age | 38.92 (8.13) | 37.35 (9.25) | 0.87 |
| Education (Years) | 13.00 (2.12) | 13.85 (2.79) | 1.68 |
| WAIS Matrix Reasoning | 9.70 (3.52) | 11.41 (2.58) | 2.59* |
| SAPS Positive | 3.89 (2.76) | .03 (.16) | 8.72** |
| SANS Negative | 8.05 (1.59) | 1.59 (2.33) | 10.63** |
| SANS Avolition | 2.95 (.94) | .79 (1.22) | 9.70** |
| SANS Anhedonia | 2.55 (.93) | .67 (1.03) | 9.27** |
| SAPS Disorganization | 3.38 (2.85) | 1.41 (1.25) | 4.04** |
| Sample 2 | |||
| Schizophrenia | Control | t | |
| n=94 (69%M) | n=66 (59% M) | ||
| Age | 48.77 (11.56) | 47.00 (7.80) | 1.01 |
| Education | 13.24 (1.82) | 14.79 (1.64) | 5.22** |
| MCCB | 31.59 (12.23) | 46.48 (8.58) | 8.01** |
| PANSS Positive | 18.45 (7.46) | NA | - |
| PANSS Negative | 15.96 (6.96) | NA | - |
| CAINS Motivation | 1.77 (.85) | NA | - |
| CAINS Pleasure | 1.70 (.84) | NA | - |
Note. Standard deviations in parentheses. Demographic table does not include subjects who were excluded from analysis (see Methods). Demographic information was not available for 10 healthy control subjects in Sample 2. SAPS = Scale for the Assessment of Positive Symptoms (Andreasen, 1984); SANS = Scale for the Assessment of Negative Symptoms (Andreasen, 1989); WAIS = Wechsler Adult Intelligence Scale (Wechsler, 2014); MCCB = MATRICS Consensus Cognitive Battery (M.F. Green & Nuechterlein, 2004); PANSS = Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opfer, 1987); CAINS = Clinical Assessment Interview for Negative Symptoms (Kring, Gur, Blanchard, Horan, & Reise, 2013). CAINS motivation is the average of the motivation-related items (motivation for close family/spouse/partner relationships, motivation for close friendships and romantic relationships, motivation for work and school activities; motivation for recreational activities) while CAINS pleasure is the average of pleasure-related items (frequency of expected pleasure social activities-past week; frequency of expected pleasure social activities-next week; frequency of expected pleasurable work & school activities-next week; frequency of pleasurable recreational activities-past week; frequency of expected pleasurable recreational activities-next week).
p < .05,
p < .01.
Modeling Approach
Each trial of the EEfRT provides subjects with two pieces of information to consider when making a selection between the high and low effort options: the reward magnitude for the high effort option and the probability of winning. Prior studies of the EEfRT suggest that healthy participants generally adopt a strategy that incorporates all three pieces of information (reward, probability, effort) when performing the EEfRT (e.g., Treadway, Bossaller, Shelton, & Zald, 2012; Treadway et al., 2009). In the current study, we compared three models that reflected different strategies for allocating effort: a “full subjective value (SV) model” that assumes subjects consistently incorporate both trial-wise reward and probability, a “reward only SV model” that assumes subjects only attend to reward magnitude when allocating effort, and a “bias model” that assumes subjects do not consider reward or probability information.
We note that similar models have long been used to evaluate preferences with varying temporal delay and probability (Frederick, Loewenstein, & O’donoghue, 2002; L. Green & Myerson, 2004) and have recently been applied to EBDM (Hartmann, Hager, Tobler, & Kaiser, 2013; Klein-Flugge, Kennerley, Saraiva, Penny, & Bestmann, 2015; Prévost, Pessiglione, Météreau, Cléry-Melin, & Dreher, 2010). While the value of this approach has been highlighted in recent theoretical work (Chong, Bonnelle, & Husain, 2016; Pessiglione et al., 2017), applications of these approaches to examine EBDM in schizophrenia have been limited (Hartmann et al., 2014; Pessiglione et al., 2017). Detailed descriptions of each model are provided below.
Model 1 – Full Subjective Value Model
The full SV model will fit best for subjects who consistently incorporate trial-wise reward and probability information when allocating effort. For this model, the subjective value of a given trial is calculated by taking the objective reward, R ($1-$4.30), and reducing it by the amount of effort required to obtain it (.3 or 1). Individual differences in the extent to which reward should be discounted by effort are captured by allowing the components to be weighed with free parameters (Eq 1).
| Eq 1 |
Effort perceived as extremely costly is reflected in a higher value of k, while weighting of probability is captured by the value of h. These SVs are transformed into probabilities of selecting each option using the Softmax decision rule (Sutton & Barto, 1998), where t is an inverse temperature parameter that reflects a tendency to favor options with higher SVs:
| Eq 2 |
Thus, the full subjective value (SV) model that we fit to our data has three free parameters: k, h, and t. The k parameter reduces subjective value based on the amount of effort required, the h parameter modifies subjective value according to the probability that the reward will be received, and the t parameter guides choices toward options with higher subjective values.
An additional consideration is some subjects who integrate reward, effort, and probability to guide their decision may be better described by a subjective value model that does not distort probability (i.e. Eq 2 where free parameter h is held constant at 1), and may be over-penalized for the additional free parameter h. To account for this possibility, we fit a variant of the full SV model with h constrained to 1. Participants best-fit by the full SV model with a flexible parameter h or SV model with h = 1 are all included in the “full SV model” group, as being fit by either model supports the integration of reward, effort, and probability to guide choice.
Model 2 – Reward Only Subjective Value Model
Although it is common for participants to integrate the information about reward and probability presented on each trial, some participants allocate effort based only on available rewards (See Figure 2). For these subjects, a simpler model that does not incorporate a free parameter for scaling probability information will likely capture their behavior more accurately. The resulting “reward only” SV model is identical to the full model when h assumes a value of zero (Eq 3), and will describe behavior as well as the full SV model for participants who modulate their responses very little based on probability, but are nevertheless systematically guiding effort allocation on the basis of reward magnitude.
| Eq 3 |
Although this model and the full SV model with h=1 both hold h constant and have the same number of free parameters, the interpretation of the two models is very different. Restricting h to be 0 represents choice behavior that does not modulate choice based on probability of receipt, while h = 1 allows for probability to affect subjective value.
Model 3 – Bias Model
In addition to the SV models, we examined a “bias” model. This model is the least complex model that we fit and provides a similar or better fit than the SV models for participants who highly favor one option, respond randomly, or make choices inconsistent with the assumptions of the SV models (i.e. favoring effort allocation for low reward). This model has one free parameter, b, which represents a bias towards the low-effort option. The probability of selecting the high-effort option is simply 1-b. The bias model assumes a consistent probability of choosing low effort across trials, regardless of probability or reward, similar to a consistent subjective value for exerting effort. Critically, comparing the fit of these models can identify participants who systematically allocate effort based on all available information, those who primarily allocate effort based on reward, and those who make decisions that are not strongly or consistently influenced by trial-specific information.
Model Fitting
All models were fit in Matlab using maximum likelihood estimation with the optimization function fminsearch. Models were fit individually to each subjects’ data, and parameters were selected for each participant that optimized the likelihood of the behavioral data. For subjective value models, k and h parameters were constrained to be between 0 and 10, while t was constrained between 0 and 100. All models were fit with 200 random parameter initializations.
The SV models benefit from the flexibility of additional free parameters—while the bias model only has one parameter, the reward-only SV model has two free parameters, and the full SV model has three free parameters (though in the variant with h constrained to 1 the SV model has only two free parameters). To account for this difference in flexibility, we compared model fit using Bayesian Information Criterion (Schwarz, 1978). Importantly, BIC penalizes models that have additional flexibility, favoring more parsimonious models when log-likelihood is the same or similar. BIC incorporates goodness of fit (likelihood, Li), number of free parameters (Vi), and the number of observations (i.e. number of trials, n) using the following equation:
| Eq 4 |
We compared the BIC value for each model to categorize each subject as being better fit by the full SV model (either with 3 free parameters or with h constrained to 1), reward-only SV model, or bias model. The approach of classifying participants based on model fit (BIC) has been referred to as “computational phenotyping” (Lefebvre, Lebreton, Meyniel, Bourgeois-Gironde, & Palminteri, 2017). Figure 2 shows the performance of three patients with similar overall willingness to exert effort (P(hard) ranging from 60 to 64%) best-fit by each of the three models.
While comparing goodness-of-fit allows us to identify participants as being best-fit by one of our candidate models, we are also interested in examining relationships between cognitive functioning and systematic allocation of effort as a continuous measure. We additionally calculated a BIC difference measure (ΔBIC; Dai, Kerestes, Upton, Busemeyer, & Stout, 2015; Lefebvre et al., 2017) to quantify the improvement in goodness-of-fit that the SV model provides over the bias model. Models provide a better fit than baseline models (such as the bias model) only when the more complex model can account for the dependency of choices on trial-wise information (Ahn, Busemeyer, Wagenmakers, & Stout, 2008; Dai et al., 2015). In other words, the ABIC allows us to quantify the fit improvement obtained by including the available information on each trial.
| Eq 5 |
Subjects with a positive ΔBIC are better fit by the SV model, and their choices are better explained by incorporating trial-by-trial variability in reward and probability, while subjects with a negative ABIC exhibit behavior that is better explained by the simpler model.
We conducted simulation analyses to verify that best-fitting parameters were precise enough to be recovered from simulated data. Fitting models to simulated data establishes the ability to identify and recover parameters, and is a necessary step before interpreting parameters (Huys, 2018). For these details and additional assessments of model fit, see Supplementary Materials.
RESULTS
SV and Bias Model Fit Comparison
While the of majority of participants (70%) in the control group were best-fit (lower BIC) by the SV models (full-SV 49%, reward-only 21%, bias 30%), the majority of participants with schizophrenia were best-fit by the bias model (full-SV 29%, reward-only 21%, bias = 50%), 2 (patient/control) x 3 (model fit) chi-square test X2(2, N=255)=12.253, p=.002, Cramer’s V=.219a. These results (Figure 1C) indicate that the behavior of healthy control participants was more frequently captured by models that incorporate trial-by-trial variation, while the behavior of participants with schizophrenia was better explained by the simplest model that does not incorporate trial-by-trial variation in reward and probability. Overall, these results suggest that individuals with schizophrenia were less likely than healthy controls to integrate reward magnitude and probability information to guide effort allocation. However, half of the participants with schizophrenia were best-fit by the SV models, suggesting systematic utilization of reward information to guide effort allocation for these individuals.
As expected, behavioral results (proportion of high-effort choices) across patients and controls were consistent with previous results when aggregated across the two samples, (Figure 1B). Across all subjects, repeated-measures ANOVA on the proportion of high-effort choices with two levels of probability and four levels of reward support main effects of probability F(1, 253)=114.76, p<.001, ηp2=31 90% CI [.24, .38] and reward F(3,759)=199.14, p<.001, ηp2=44 90% CI [.40, .48], non-significant main effect of patient group F(1, 253)=2.51, p=.114, np2=01 90% CI [0, .04], as well as interactions between patient group and probability F(1, 253)=9.12, p=.003, ηp2=04 90% CI [.01, .08], and patient group and reward F(3,759)=5.79,p=.005, ηp2=02 90% CI [.01, .04]. The pattern of results from this combined sample were consistent with prior reports; participants with schizophrenia and healthy controls differed in their willingness to exert effort for rewards at high levels of probability t(253)=2.64,p=.009, d=.33 90% CI [.13,.55] but not at low probability, t(253)=.53, p=.597, d = .067 90% CI [−.14,.28], and only differed in their willingness to exert effort at the highest levels of rewards tlevel3(253)=2.39, p=.018, d=.30 90% CI [.093,.51], tlevel4(253)=2.38,p=018, d=.30 90% CI [.092,.51]. The performance of each model-defined group can be seen in Figure 1D.
Associations between model-based subtype, cognitive function, and negative symptoms
Given that our initial modeling analysis identified sub-groups, or “computational phenotypes”, of individuals in terms of their systematic allocation of effort, we next sought to determine if these groups differed in cognitive function, demographic characteristics or symptom severity including negative symptom subscales. In Sample 1, sub-groups of individuals with schizophrenia based on model fit (full-SV, reward-only, and bias) differed in their scores on WAIS-III Matrix Reasoning, F(2,53)=4.59,p=015, ηp2=148 90% CI [ 018, 27] (Figure 2A), SANS negative symptoms F(2,53)=4.36, p=.018, ηp2=141 90% CI [.015,.27], and years of education F(2. 53) = 7.73, p=.001, ηp2=226 90% CI [.064,.36], and showed marginal differences in SANS avolition F(2,53)=2.47, p=.094, ηp2=085 90% CI [0,.20]. However, the groups did not differ in the anhedonia or disorganization subscales of the SANS, SAPS positive symptoms, age, reaction time, completion rate of chosen effort, or medication type (see Table S2 and Supplementary Materials for additional details).
In Sample 2, sub-groups of individuals with schizophrenia based on model fit (full-SV, reward-only, and bias) showed differences in cognition as assessed with the MCCB composite score, F(2,91) = 4.27, p=.017, ηp2=09 90% CI [.009,.17]. Participants in Sample 2 did not differ in negative symptoms, positive symptoms, CAINS-MAP, demographic characteristics, completion rate of chosen effort, or medication type (see Table S2 and Supplementary Materials for additional details). Sub-groups in Sample 2 showed significant differences in reaction time, F(2,91) = 10.53,p<.001, ηp2= 19 90% CI [.072,.29], with participants in the full SV group taking longer to respond. In both samples, being best-fit by more complex models was associated with increased cognitive functioning.
In addition to comparing groups based on the best-fitting model, one can also examine individual differences in the magnitude of difference in model fit across two models, thereby permitting a dimensional analysis as a complement to the categorical analyses presented above. To this end, we conducted a stepwise linear regression analysis with ΔBIC as the dependent variable, where ΔBIC indicates the extent to which the full SV model (i.e. the addition of trial-by-trial information) improved goodness of fit relative to the bias model. Here, we compare the fit of the full SV model, as the reward-only SV model (h=0) and full SV model with h = 1 are nested within this model.
For Sample 1, independent variables included group membership (schizophrenia or control), age, sex, years of education, WAIS matrix reasoning, SANS avolition, SANS anhedonia, and SAPS positive symptoms. The model that best predicted ΔBIC only included WAIS matrix reasoning, t(93) = 3.58, p = .001. Demographic characteristics, group membership, positive and negative symptoms and symptom subscales (avolition, anhedonia) were not retained in the regression (See Table S3 for statistics for excluded variables).
For Sample 2, independent variables included demographic characteristics (age/sex/education), MCCB composite score, PANSS positive symptoms, PANSS negative symptoms, and motivation and pleasure subscales derived from the CAINS-MAP. Although motivation and pleasure in the CAINS are combined into a single scale (CAINS-MAP), prior work has demonstrated that individual items from the CAINS-MAP may be combined to assess specific difficulties in motivation (avolition) and hedonic experience (anhedonia) (Strauss & Gold, 2016; See Table 1 note for specific items included in the motivation and pleasure subscales). For Sample 2, only participants with schizophrenia were included in the model as symptom scores were not available for control participants. The model that best predicted ΔBIC only included the MCCB composite score t(91) = 3.55, p = .001. Demographic characteristics, positive and negative symptoms and symptom subscales (motivation, pleasure) were not retained in the regression.
An important consideration is that the relationship between cognitive functioning and ΔBIC in individuals with schizophrenia may reflect an underlying reduction in motivation, as reduced motivation has been found to relate to cognitive test performance (Fervaha et al., 2014). To assess this possibility, we examined the partial correlations between cognitive functioning and ABIC controlling for individual differences in SANS avolition and anhedonia in Sample 1, r(52) = .277, p = .043, and both CAINS motivation and pleasure in Sample 2, r(90) = .320, p = .002 (Figure 3b). Overall, these results indicate that the extent to which effort was systematically allocated was related to cognitive functioning in both samples of individuals will schizophrenia independent of negative symptom severity.
Figure 3.
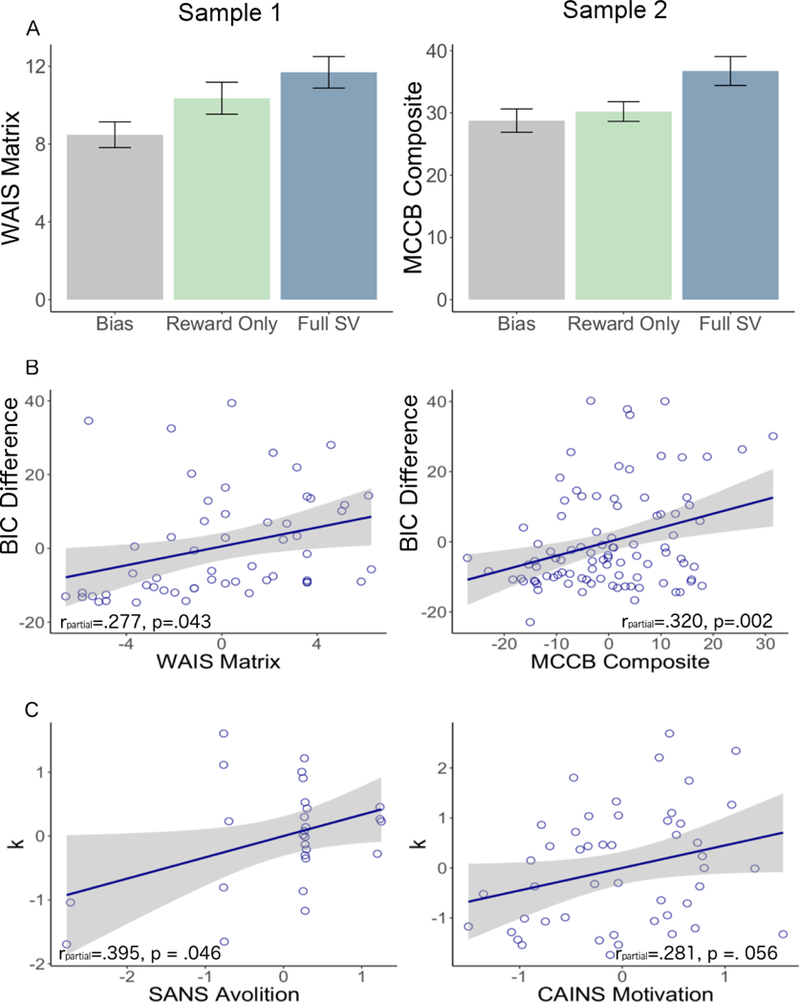
Relationship between cognitive measures and model fit in participants with schizophrenia. A. Differences in cognitive functioning between individuals with schizophrenia in each subgroup. B. Correlation between ΔBIC and measures of cognition in both samples of individuals with schizophrenia (all patients), controlling for negative symptoms. WAIS: Wechsler Adult Intelligence Scale, MCCB: MATRICS Consensus Cognitive Battery, BIC: Bayesian Information Criterion. C. Effort-sensitivity parameter (k) association with negative symptoms in people with schizophrenia best-fit by the subjective value models (full or reward only), controlling for cognitive functioning. CAINS: Clinical Assessment Interview for Negative Symptoms, SANS: Scale for the Assessment of Negative Symptoms. Higher scores indicate greater impairment.
Associations between model-parameters, cognitive function, and negative symptoms
While model-fitting yielded evidence for subgroups of individuals in terms of systematic allocation of effort and cognitive function, we also wanted to examine whether our model-based approach would help uncover associations between effort discounting and symptom severity. We conducted a stepwise linear regression analysis with free parameter k from the subjective value model as the dependent variable, where the k parameter represents the extent to which available rewards are discounted based on the effort required to obtain them. For Sample 1, independent variables included group membership (schizophrenia or control), age, sex, years of education, WAIS matrix reasoning, SANS avolition, SANS anhedonia, and SAPS positive symptoms. The model that best predicted k only included SANS avolition, t(93) = 2.98, p = .004. For Sample 2, independent variables included demographic characteristics (age/sex/education), MCCB composite score, PANSS positive symptoms, PANSS negative symptoms, and motivation and pleasure subscales derived from the CAINS. For this sample, no variables were retained in the stepwise regression.
An important consideration is that the effort sensitivity obtained from the subjective value model (parameter k) may relate to negative symptoms only in individuals who systematically allocate effort (i.e. those best-fit by one of the SV models). Among individuals with schizophrenia who were best fit by a subjective value model, k was positively associated with SANS avolition in Sample 1, r(25) = .395, p = .041 and with CAINS motivation in Sample 2, r(46) = .297, p =. 040. In both samples, greater impairments in functioning (i.e. less motivation/more avolition) were associated with increased effort discounting. When controlling for differences in cognitive functioning (WAIS matrix in Sample 1, MCCB composite in Sample 2) the partial correlation between k and SANS avolition remained significant in Sample 1, r(24) = .395, p = .046, and the relationship between k and MCCB composite remained marginally significant in Sample 2, r(45) = .281, p =. 056 (Figure 3c).
Overall, this pattern of results suggests that within the subgroup of people with schizophrenia who were better fit by the SV model (and therefore systematically allocated effort) computationally-derived measures of effort sensitivity may be associated with clinically-rated measures of motivational impairment, although this should be further examined in larger samples.
DISCUSSION
In this paper, we examined whether well-established patterns of aberrant effort-expenditure in participants with schizophrenia relative to healthy controls reflected a single deficit in motivation, or was partially driven by cognitive impairments. By adopting a novel computational modeling approach to the analysis of an EBDM task, we examined three computational subtypes for effort allocation. As a whole, individuals with schizophrenia were less likely to favor strategies that incorporated trial-by-trial reward and probability information in a consistent way over the course of the task, suggesting a core deficit in “systematic” effort allocation. Further analysis found that failure to utilize trial-wise information was characterized by deficits in cognitive functioning. Importantly, however, our modeling approach also identified that half of the participants with schizophrenia did not exhibit impairment in the systematic allocation of effort based on reward information (including 29% who also incorporated probability information). Within these individuals, model-estimated parameters were associated with severity of motivational impairment. Taken together, our findings highlight the importance of cognitive variables in determining behavior in EBDM tasks and suggest that only a subset of individuals with schizophrenia-those best fit by the bias model-fail to employ a strategy that considered trial-wise reward and probability information, consistent with dysfunctional effort allocation.
Unlike those participants best fit by the SV or reward only models, participants who were better characterized by the bias model-the simplest model that we fit-exhibited a pattern of responding that showed very little modulation of choice behavior as a function of available reward or probability information (Figure 1D). Critically, participants with schizophrenia were more likely to be better fit by this simple model than healthy controls. While prior work found that individuals with schizophrenia modulated their choices as a function of reward information, the subset of individuals best-fit by the bias model showed a general insensitivity to reward and probability information that was not localized to high reward values. Consequently, this analysis suggests that for half of participants with schizophrenia (those fit by the bias model) the magnitude of impairment in effort allocation may be more severe than previously understood.
Participants with schizophrenia who were best fit by the bias model also showed reductions in measures of cognitive functioning. This finding is consistent with the idea that systematically incorporating reward, probability, and effort information to guide choices is cognitively demanding, suggesting an important role for cognitive processing in effortful goal-directed behavior and its assessment. An emerging body of research has examined the interaction between motivation and cognition in shaping decision-making and goal-directed behavior (see (Braver et al., 2014, for a review), highlighting the idea that allocation of working memory and cognitive resources is a motivated process. Thus, the observed reliance on simple strategies for making choices in a subset of people with schizophrenia may be a manifestation of reduced motivation. Consistent with this hypothesis, a recent study of reinforcement learning with variable working memory demands in patients with schizophrenia found that working memory capacity and reliability could account entirely for putative learning deficits (Collins, Brown, Gold, Waltz, & Frank, 2014). As with the current results, this finding highlights the extent to which apparent performance differences in a reward-related symptom domain may be driven by symptoms related to cognition for some individuals. Thus, some individuals who were best fit by the bias model may have simply lacked the motivation to put forth the mental effort required to develop a systematic allocation strategy with regard to reward and probability information. While this possibility does diminish the interpretability of participants’ choices, it still suggests that when placed in a context where they were able to exchange effort for reward, these individuals were uninterested in or unable to exert the requisite mental effort to develop a systematic strategy.
Interestingly, among the subsets of participants who were better fit by the SV models, individuals with schizophrenia allocated effort at rates very similar to healthy controls (Figure 1D). Variability in effort sensitivity across individuals-as reflected by model parameters-was associated with levels of amotivation. Thus, even within participants showing systematic effort allocation at the group level, the application of computational models was useful for characterizing individual differences in effortful behavior related to negative symptoms. Moreover, the association with negative symptoms was specific to avolition and items of the MAP scale assessing low motivation. This dissociation is consistent with a large amount of prior preclinical and theoretical work suggesting that impairments in effort expenditure should be distinct from deficits associated with hedonic capacity (Barch & Dowd, 2010; Berridge, 2007; Kring, Gur, Blanchard, Horan, & Reise, 2013; Salamone & Correa, 2012; Treadway & Zald, 2011; Treadway & Zald, 2013).
While the associations between our model-derived effort aversion parameter and negative symptom severity are intriguing, the appropriate interpretation of this result is unclear. Effort aversion in Sample 1 was associated with avolition across all participants, while motivation in Sample 2 was only associated with effort aversion in patients who were best-fit by a subjective value model. While one possibility is that SANS avolition measures a construct closer to behavioral effort aversion, the possibility that we consider to be more likely is that the value of effort discounting parameter k is more meaningful in participants who are better fit by the model (and who systematically integrate effort). The isolation of participants who were well fit by the SV models may also reduce a key source of “noise” in the data: these individuals likely met the cognitive and motivational requirements to understand and comply with the task, and the relatively higher degree of cognitive function in these participants may have resulted in more accurate reporting of negative symptoms, as retrospective reporting of negative symptoms requires intact executive function (Strauss & Gold, 2012). Consequently, the construct validity and measurement sensitivity of the task is likely enhanced for these participants, thereby resulting in clearer associations with negative symptoms. It should be reiterated that this association between negative symptom severity and model-derived effort aversion will require replication. A sizeable number of prior studies have reported inconsistent relationships between EBDM and negative symptom assessments (Barch et al., 2014; Fervaha et al., 2015; Fervaha, Graff-Guerrero, et al., 2013; Gold et al., 2013; Strauss et al., 2016; Treadway et al., 2015; Wolf et al., 2014), lowering the degree to which a single result can be interpreted with confidence.
Limitations and Future Directions
While we believe this work to be informative, several limitations and directions of improvement should be noted. First, while the EEfRT task has several levels of reward, the inclusion of only two levels of effort obscures our ability to confidently disentangle increased sensitivity to effort from decreased sensitivity to reward. As such, the application of similar modeling methods to other tasks (e.g. Arulpragasam, Cooper, Nuutinen, & Treadway, 2018; Le Bouc et al., 2016), or the addition of a wider range of effort levels to the EEfRT would be useful in future studies to dissociate these constructs. The application of paradigms that have been adapted for neuroimaging would also be useful in dissociating whether those fit by the bias model show differences in neural representation of subjective value, or whether subjective values are similar represented but ultimately not used to guide choice, possibly suggesting difficulties in the association of these values with motor responses. Future work may also benefit from a simplified design, specifically equated temporal requirements for hard and easy tasks and consistent probability, to measure effort discounting in the absence of possible temporal discounting or risk sensitivity.
It should also be highlighted that approximately one third of healthy controls were best-fit by the bias model. While individuals within this group are characterized by a failure to modulate choice based on this available information, there are several different behavioral profiles within this group that should be parsed in future work. Better fit of the bias model may include individuals who respond randomly, who allocate effort for reward in suboptimal ways (i.e. being more willing to exert effort for lower rewards or lower probabilities), or who select all one option. While a participant who exclusively selects the easy option may genuinely have a high reward threshold for exerting effort, it is also possible that they failed to understand the task. The inclusion of debriefing questions regarding self-reported strategy implemented by the participant would be critical in future studies to allow for the identification of participants who show a full understanding of the task. Debriefing questionnaires may also be useful in identifying other valuation strategies implemented by participants in this task that could aid in the development of new models.
This work advances previous methods of analysis of EBDM tasks to address how individuals make choices rather than focusing exclusively on what choices are made. Our results suggest that the failure to systematically allocate effort occurs more frequently in individuals with schizophrenia than healthy controls, that many participants with schizophrenia exhibit systematic effort allocation, and that disrupted effort allocation is related to cognitive functioning. These data underscore the importance of considering patient heterogeneity when evaluating task performance on experimental paradigms, and highlight the advantages of computational models to help identify potential sub-groups that may be characterized by distinct underlying pathophysiology. Moreover, only after controlling for differences in systematic allocation were we able to detect clear relationships between effort aversion and negative symptoms across both samples. Future work in several domains is needed to fully understand the implications of these results, their extension to daily life, and their specificity to schizophrenia relative to other diagnoses that are associated with aberrant effort expenditure.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health: MH102355 and MH108605 to MTT, MH066031 to DMB, and F32 MH115692 to JAC, and by an investigator-initiated research collaboration funded by Amgen Inc. to MFG. Original behavioral data were published in Journal of Abnormal Psychology (Barch et al., 2014) and Schizophrenia Bulletin (Reddy et al., 2015). Preliminary results were presented at Society of Biological Psychiatry 2018.
Footnotes
DISCLOSURES
The authors report no conflicts of interest. MFG has consulted for AiCure and Lundbeck and is on the Scientific Advisory Board of Cadent. He has received research funds from Amgen and Forum. Sample 2 was supported by an investigator-initiated research collaboration funded by Amgen Inc. to MFG. In the past 3 years, MTT has served as a paid consultant to NeuroCog Trials, Avanir Pharmaceuticals, and Blackthorn Therapeutics. MTT is a co-inventor of the EEfRT, which was used in this study. Emory University and Vanderbilt University licensed this software to BlackThorn Therapeutics. Under the IP Policies of both universities, Dr. Treadway receives licensing fees and royalties from BlackThorn Therapeutics. Additionally, Dr. Treadway has a paid consulting relationship with BlackThorn. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies, and no funding from these entities was used to support the current work. All views expressed are solely those of the authors.
These results were consistent when using AIC, see Supplementary Materials.
References
- Abler B, Greenhouse I, Ongur D, Walter H, & Heckers S (2008). Abnormal reward system activation in mania. Neuropsychopharmacology, 33(9), 2217–2227. doi: 10.1038/sj.npp.1301620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghevli MA, Blanchard JJ, & Horan WP (2003). The expression and experience of emotion in schizophrenia: a study of social interactions. Psychiatry Res, 119(3), 261–270. [DOI] [PubMed] [Google Scholar]
- Ahn WY, Busemeyer JR, Wagenmakers EJ, & Stout JC (2008). Comparison of decision learning models using the generalization criterion method. Cognitive science, 32(8), 1376–1402. [DOI] [PubMed] [Google Scholar]
- Andreasen N (1984). The Scale for the Assessment of Positive Symptoms in Schizophrenia (SAPS). Iowa City, Ia: University of Iowa. [Google Scholar]
- Andreasen NC (1989). The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. The British Journal of Psychiatry. [PubMed] [Google Scholar]
- Arulpragasam AR, Cooper JA, Nuutinen MR, & Treadway MT (2018). Corticoinsular circuits encode subjective value expectation and violation for effortful goal-directed behavior. Proceedings of the National Academy of Sciences, 201800444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Gold JM, Johnson SL, Kring AM, MacDonald AW, … Strauss ME. (2017). Explicit and implicit reinforcement learning across the psychosis spectrum. J Abnorm Psychol, 126(5), 694–711. doi: 10.1037/abn0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, & Dowd EC (2010). Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36(5), 919–934. doi:sbq068 [pii] 10.1093/schbul/sbq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, & Schoen N (2014). Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. JAbnorm Psychol, 123(2), 387–397. doi: 10.1037/a0036299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl), 191(3), 391–431. doi: 10.1007/s00213-006-0578-x [DOI] [PubMed] [Google Scholar]
- Braver TS, Krug MK, Chiew KS, Kool W, Westbrook JA, Clement NJ, … group M. (2014). Mechanisms of motivation-cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci, 14(2), 443–472. doi: 10.3758/s13415-014-0300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, & Raulin ML (1976). Scales for physical and social anhedonia. J Abnorm Psychol, 85(4), 374–382. [DOI] [PubMed] [Google Scholar]
- Chong TT, Bonnelle V, & Husain M (2016). Quantifying motivation with effort-based decision-making paradigms in health and disease. Prog Brain Res, 229, 71–100. doi: 10.1016/bs.pbr.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Cohen AS, & Minor KS (2010). Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull, 36(1), 143–150. doi : 10.1093/schbul/sbn061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AG, Brown JK, Gold JM, Waltz JA, & Frank MJ (2014). Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci, 34(41), 13747–13756. doi: 10.1523/JNEUR0SCI.0989-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Moran EK, & Barch DM (2017). Effort-cost decision-making in psychosis and depression: could a similar behavioral deficit arise from disparate psychological and neural mechanisms? Psychol Med, 1–16. doi: 10.1017/S0033291717002525 [DOI] [PubMed] [Google Scholar]
- Dai J, Kerestes R, Upton DJ, Busemeyer JR, & Stout JC (2015). An improved cognitive model of the Iowa and Soochow Gambling Tasks with regard to model fitting performance and tests of parameter consistency. Frontiers in psychology, 6, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd EC, & Barch DM (2012). Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One, 7(5), e35622. doi: 10.1371/journal.pone.0035622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Duncan M, Foussias G, Agid O, Faulkner GE, & Remington G (2015). Effort-based decision making as an objective paradigm for the assessment of motivational deficits in schizophrenia. Schizophr Res, 168(1–2), 483–490. doi: 10.1016/j.schres.2015.07.023 [DOI] [PubMed] [Google Scholar]
- Fervaha G, Foussias G, Agid O, & Remington G (2013). Amotivation and functional outcomes in early schizophrenia. Psychiatry Res, 210(2), 665–668. doi: 10.1016/j.psychres.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, & Remington G (2013). Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res, 47(11), 1590–1596. doi: 10.1016/j.jpsychires.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, & Remington G (2014). Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry, 71(9), 1058–1065. doi: 10.1001/jamapsychiatry.2014.1105 [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, & O’donoghue T (2002). Time discounting and time preference: A critical review. Journal of economic literature, 40(2), 351–401. [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, & Vinogradov S (2014). Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? JAbnorm Psychol, 123(4), 771–782. doi: 10.1037/abn0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleen J, Shergill SS, & Kapur S (2015). Impaired subjective well-being in schizophrenia is associated with reduced anterior cingulate activity during reward processing. Psychol Med, 45(3), 589–600. doi: 10.1017/S0033291714001718 [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, & Frank MJ (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry, 74(2), 130–136. doi: 10.1016/j.biopsych.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, & Frank MJ (2015). Effort cost computation in schizophrenia: a commentary on the recent literature. Biol Psychiatry, 78(11), 747–753. doi: 10.1016/j.biopsych.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, & Heerey EA (2008). Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull, 34(5), 835–847. doi: 10.1093/schbul/sbn068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, & Myerson J (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull, 130(5), 769–792. doi: 10.1037/0033-2909.130.5.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, & Nuechterlein KH (2004). The MATRICS initiative: developing a consensus cognitive battery for clinical trials. Schizophrenia research, 72(1), 1–3. [DOI] [PubMed] [Google Scholar]
- Hartmann MN, Hager OM, Reimann AV, Chumbley JR, Kirschner M, Seifritz E, … Kaiser S. (2014). Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophrenia bulletin, 41(2), 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann MN, Hager OM, Tobler PN, & Kaiser S (2013). Parabolic discounting of monetary rewards by physical effort. Behav Processes, 100, 192–196. doi: 10.1016/j.beproc.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, & Gold JM (2008). Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry, 64(1), 62–69. doi: 10.1016/j.biopsych.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Reddy LF, Barch DM, Buchanan RW, Dunayevich E, Gold JM, … Green MF. (2015). Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 2-External Validity and Correlates. Schizophr Bull, 41(5), 1055–1065. doi: 10.1093/schbul/sbv090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yang XH, Lan Y, Zhu CY, Liu XQ, Wang YF, … Chan RC. (2016). Neural substrates of the impaired effort expenditure decision making in schizophrenia. Neuropsychology, 30(6), 685–696. doi: 10.1037/neu0000284 [DOI] [PubMed] [Google Scholar]
- Huys QJ (2018). Bayesian approaches to learning and decision-making In Computational Psychiatry (pp. 247–271): Elsevier. [Google Scholar]
- Kay SR, Fiszbein A, & Opfer LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin, 13(2), 261. [DOI] [PubMed] [Google Scholar]
- Klein-Flugge MC, Kennerley SW, Saraiva AC, Penny WD, & Bestmann S (2015). Behavioral modeling of human choices reveals dissociable effects of physical effort and temporal delay on reward devaluation. PLoS Comput Biol, 11(3), e1004116. doi: 10.1371/journal.pcbi.1004116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The clinical assessment interview for negative symptoms (CAINS): final development and validation. American journal of psychiatry, 170(2), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouc R, Rigoux L, Schmidt L, Degos B, Welter ML, Vidailhet M, … Pessiglione M. (2016). Computational Dissection of Dopamine Motor and Motivational Functions in Humans. JNeurosci, 36(25), 6623–6633. doi: 10.1523/JNEUROSCI.3078-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre G, Lebreton M, Meyniel F, Bourgeois-Gironde S, & Palminteri S (2017). Behavioural and neural characterization of optimistic reinforcement learning. Nature Human Behaviour, 1(4), 0067. [Google Scholar]
- Llerena K, Strauss GP, & Cohen AS (2012). Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr Res, 142(1–3), 65–70. doi: 10.1016/j.schres.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, & Blanchard JJ (2016). Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res, 170(2–3), 278–284. doi: 10.1016/j.schres.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl PE (1975). Hedonic capacity: some conjectures. BullMenninger Clin, 39(4), 295–307. [PubMed] [Google Scholar]
- Moran EK, Culbreth AJ, & Barch DM (2017). Ecological momentary assessment of negative symptoms in schizophrenia: Relationships to effort-based decision making and reinforcement learning. JAbnorm Psychol, 126(1), 96–105. doi: 10.1037/abn0000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessiglione M, Vinckier F, Bouret S, Daunizeau J, & Le Bouc R (2017). Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain. doi: 10.1093/brain/awx278 [DOI] [PubMed] [Google Scholar]
- Prévost C, Pessiglione M, Météreau E, Cléry-Melin M-L, & Dreher J-C (2010). Separate valuation subsystems for delay and effort decision costs. Journal of Neuroscience, 30(42), 14080–14090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, … Green MF. (2015). Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 1-Psychometric Characteristics of 5 Paradigms. Schizophr Bull, 41(5), 1045–1054. doi: 10.1093/schbul/sbv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, & Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76(3), 470–485. doi:S0896–6273(12)00941–5 [pii] 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Hillner K, & Velligan DI (2015). Conceptualization and treatment of negative symptoms in schizophrenia. World J Psychiatry, 5(4), 352–361. doi: 10.5498/wjp.v5.i4.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G (1978). Estimating the dimension of a model. The annals of statistics, 6(2), 461–464. [Google Scholar]
- Strauss GP, & Gold JM (2012). A New Perspective on Anhedonia in Schizophrenia. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2011.11030447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, & Gold JM (2016). A psychometric comparison of the clinical assessment interview for negative symptoms and the brief negative symptom scale. Schizophrenia bulletin, 42(6), 1384–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, & Frost KH (2016). Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a Progressive Ratio task. Schizophr Res, 170(1), 198–204. doi: 10.1016/j.schres.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, & Barto AG (1998). Reinforcement learning: An introduction (Vol. 1): MIT press; Cambridge. [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, & Zald DH (2012). Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol, 121(3), 553–558. doi: 10.1037/a0028813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, & Zald DH (2009). Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE, 4(8), e6598. doi: 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, & Park S (2015). Impaired effort allocation in patients with schizophrenia. Schizophr Res, 161(2–3), 382–385. doi: 10.1016/j.schres.2014.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35(3), 537–555. doi:S0149–7634(10)00112–0 [pii] 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2013). Parsing Anhedonia Translational Models of Reward-Processing Deficits in Psychopathology. Current Directions in Psychological Science, 22(3), 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Huang J, Yang X. h., Lui SS, Cheung EF, & Chan RC (2015). Anhedonia in schizophrenia: Deficits in both motivation and hedonic capacity. Schizophrenia research, 168(1), 465–474. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2014). Wechsler adult intelligence scale-Fourth Edition (WAIS-IV). San Antonio, Texas: Psychological Corporation. [Google Scholar]
- Wolf DH, Satterthwaite TD, Kantrowitz JJ, Katchmar N, Vandekar L, Elliott MA, & Ruparel K (2014). Amotivation in schizophrenia: integrated assessment with behavioral, clinical, and imaging measures. Schizophr Bull, 40(6), 1328–1337. doi: 10.1093/schbul/sbu026 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


