Abstract
We have explored the opportunities for enhanced ratiometric pH sensing using the well-known carboxy seminaphthofluorescein (SNAFL-2) and silver island films (SiFs). Our results show that the metallic surfaces can provide up to a 40-fold increase in probe fluorescence intensity as compared to nonmetallic surfaces with the same probe coverage. However, while the S/N is significantly better for pH sensing, the emission wavelength ratiometric values are similar to that obtained in solution, due to the fact that the emission of both the acidic and basic forms of the probe are enhanced to similar extents. To the best of our knowledge this is the first report of enhanced ratiometric fluorescence sensing on metallic surfaces.
Keywords: Radiative decay engineering, metal-enhanced fluorescence, ratiometric pH sensing, enhanced sensitivity
INTRODUCTION
In recent years, our laboratories have fabricated numerous noble-metal surfaces for metal-enhanced fluorescence (MEF) detection, whereby fluorophores can undergo modifications in their radiative decay rate, Γ, producing favorable effects such as enhanced fluorescence emission and reduced fluorophore lifetimes (enhanced photostabilities) [1–8].
Silver island films (SiFs), formed an 3-(aminopropyl) triethoxysilane (APS)-coated glass slides by the reduction of silver nitrate by glucose, have proved a versatile surface, providing up-to 10 fold increases in fluorescence signal of appropriate fluorophores [1–5]. Silver colloid coated surfaces have yielded up-to 50-fold enhancements [6], while silver-fractal-like coatings have been shown to increase fluorescein emission, several thousand fold [7,8]. These findings have been most encouraging and suggest the future use of metallic nanostructures in surface assays [4] and high-throughput screening plate-well formats [9].
In this short paper, we further extend our work on MEF phenomena to MEF sensing, and show how SiFs can be used to enhance the emission and therefore signal-to-noise ratio, of a surface immobilized pH sensitive probe, while still retaining the pH sensing characteristics of the probe. In this regard, we have employed a dextran/carboxy seminaphthofluorescein (SNAFL-2) conjugate, which was selected due to the well known response of SNAFL probes to pH, coupled with the well-defined surface chemistry for immobilization of dextran onto solid substrates [10]. This immobilization procedure is based on the surface chemistry that is currently being used for the preparation of surface plasmon resonance chips by Biocore, thus demonstrating the potential widespread applicability of our approach.
MATERIALS AND METHODS
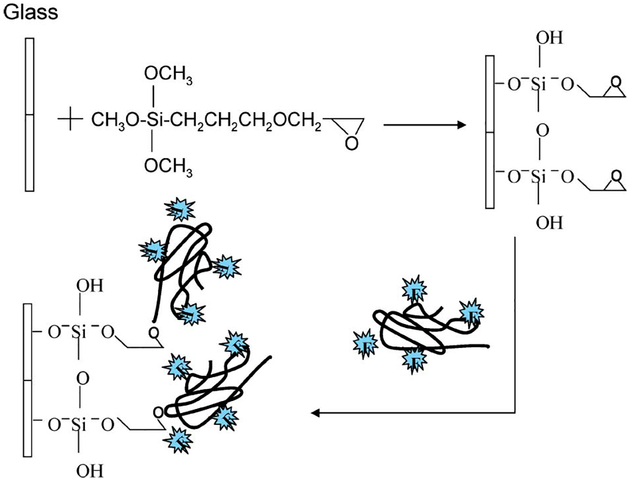
The pH probe, dextran/SNAFL-2 conjugate (obtained from Molecular Probes, Inc., Eugene, OR), was immobilized onto both glass substrates and SiFs using two different methods. For the immobilization of the dextran/SNAFL-2 conjugate onto the glass substrate, precleaned glass substrates (with a mixture of H2SO4 and H2O2) were soaked in a 2% v/v solution of 3-(glycidoxypropyl) trimethoxysilane (GOPTES) in ethanol for 2 hr (Fig. 1). The GOPTES coated glass substrates were then removed from the solution and rinsed several times with ethanol. In order to remove unbound deposited materials, the substrates were additionally placed in ethanol in the ultrasonic bath for 5 min. This procedure was repeated at least four more times. After drying the substrates in a stream of dry nitrogen, they were placed in a basic solution of dextran/SNAFL-2 conjugate and incubated for 20 hr. The glass substrates were then removed from the dextran solution, rinsed with deionized water, and were then finally dried in a stream of dry nitrogen.
Fig. 1.
Experimental procedure for immobilization of the pH probe onto glass microscope slides.
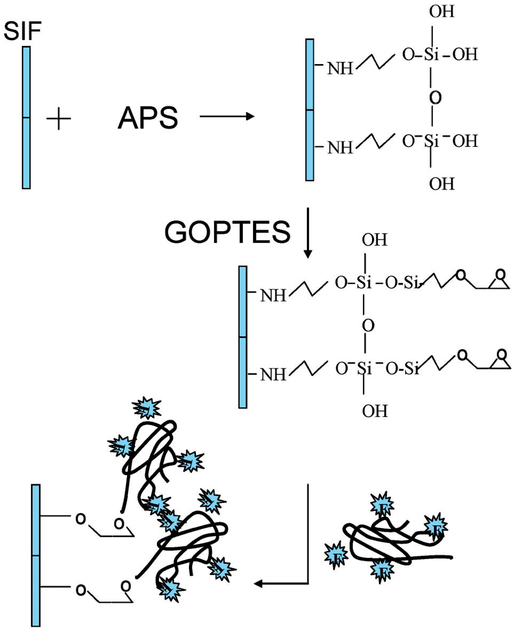
Immobilization of Dextran-SNAFL-2 onto SiFs was performed as follows (Fig. 2): SiFs were prepared according to the literature procedure [3]. The SiFs were then soaked in a 2% v/v solution of APS in ethanol for 2 hr. The APS coated SiFs were removed from the solution and rinsed several times with ethanol to remove the unbound APS. The APS-coated SiFs were then soaked in a 2% v/v solution of GOPTES in ethanol for 2 hr. The unbound GOPTES was removed by rinsing the substrates with ethanol several times. After drying the substrates in a stream of dry nitrogen, they were then placed in a basic solution of dextran/SNAFL-2 conjugate and subsequently incubated for 20 hr. The glass substrates were then removed from the dextran solution, rinsed with deionized water, and then dried in a stream of dry nitrogen.
Fig. 2.
Experimental procedure for the immobilization of the pH probe onto SiFs (silver island films).
For the ratiometric intensity plots and data shown in Fig. 4 and Table I, respectively, fluorescence intensity ratio’s on either glass or SiFs was determined using a laser-scanning microscope (LSM 410, Zeiss), a 488 nm argon laser line (0.5 mW), a CCD camera and the range of filters shown in Table I.
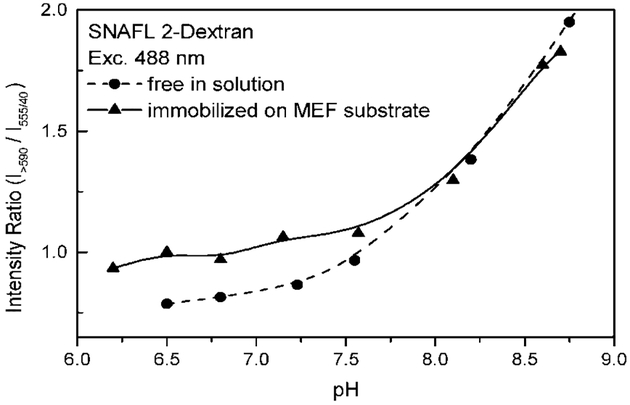
Fig. 4.
The pH sensitivity of SNAFL-2 is retained in the presence of silver nanoparticles. The intensity on the MEF substrate is about 30–40 times brighter than the intensity of probe immobilized on glass slides. The ratios however are similar.
Table I.
Intensity Enhancement Factors for the pH Probe SNAFL 2-dextran, for Several Emission Filter Settings and an Excitation Wavelength of 488 nm. The Enhancement Ratio is Determined Relative to that Obtained on a Glass Substrate (i.e. No Silver)
| Sample | 555 ± 40 nm | 615 ± 80 nm | >550 nm | >590 nm |
|---|---|---|---|---|
| pH 6.80 | 36.7 | 42.9 | 48.8 | 45.2 |
| pH 8.90 | 37.0 | 31.1 | 25.4 | 30.9 |
RESULTS AND DISCUSSION
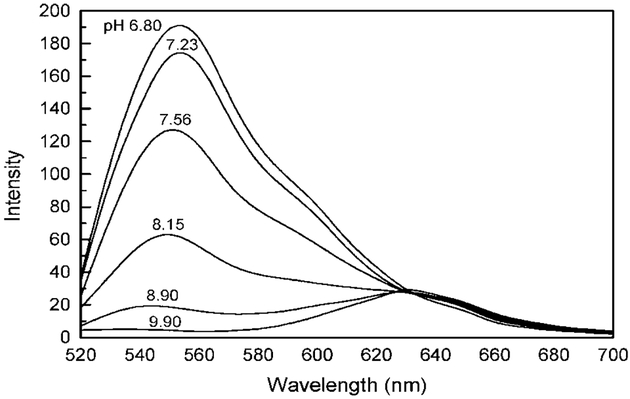
Seminaphthofluoresceins are class of pH probes readily used for both excitation and emission wavelength ratiometric pH sensing [11]. The acid form of the probe displays a spectrum with an emission peak at ≈552 nm, the base form at ≈635 nm (Fig. 3). Large spectral shifts between both forms of the probe readily allow for convenient ratiometric pH sensing using the intensity ratio at two emission wavelengths.
Fig. 3.
Emission spectra of SNAFL-2-Dextran in solution in various pH buffers (PBS buffer), with 130 mM KCl. The excitation wavelength was 488 nm and temperature 23°C.
Several filter settings were used to determine the intensity enhancement (intensity on silver/glass) when the probe was immobilized on SiFs. The average enhancement factor was determined for the acid form (pH 6.8) and the base form (pH 8.9) at four spectral ranges, noting that the pKa of these probes is in the range 7.6–7.9, which accounts for their use in physiological sensing [11]. The results are summarized in Table I. The acidic form of SNAFL-2 typically shows a higher MEF (average of 43.4) than that of the basic form (average of 31.1), i.e. 30–40 fold more fluorescence signal was obtained from the silver as compared to the glass substrate at all the filter settings. This may be due to the acidic form of SNAFL-2 (protonated) interacting more strongly with the silver nanoparticles due to a similar resonance energy, noting that the absorption peak of the SiFs is centered around 440 nm. In this regard, one typically observes a greater enhancement for the lowest quantum yield species, i.e. MEF enhancement = l/Q0, where Q0 is the quantum yield in the absence of metal [1,2].
The pH sensitivity of SNAFL-2 is also retained on SiFs (Fig. 4). The magnitude of the intensity ratio (intensity of base form, >590 nm versus intensity of acid form 35–575 nm) is decreased as compared to the free probe in aqueous solution, which is probably due to a greater enhancement of the acidic form of SNAFL-2 as compared to the basic form, also noting that this becomes more pronounced at lower pH values, Fig. 4. This suggests the use of silver particles to increase the S/N ratio for SNAFL-2 in low pH sensing measurements.
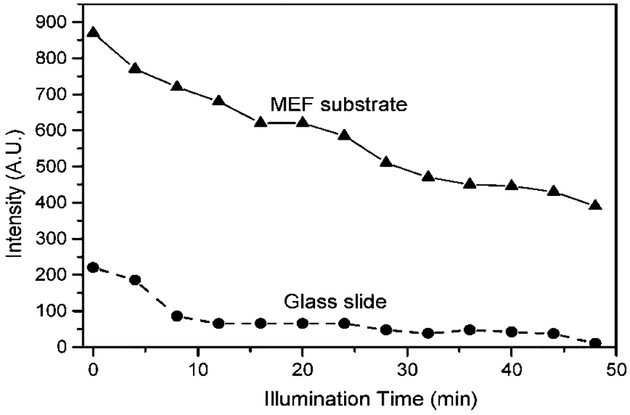
Another particular advantage of using SiFs is shown in Fig. 5. When SNAFL-2 is immobilized onto SiFs it photo-bleaches at a similar rate as compared to when it is immobilized onto a glass surface, but with the added attraction of a greater total fluorescence (photon) flux from the MEF substrate. Enhanced fluorescence intensities and improved probe photostabilities are some of the most desired features for probes for chemical sensing and cellular imaging, allowing the use of lower probe concentrations (less toxicity, less problems associated with probe loading), the use of a lower power excitation source (less damage to cells), and relatively shorter data acquisition times (less exposure damage to cells). Moreover, enhanced intensities from pH probes on SiFs may also allow one to use low power excitation sources such as LED’s (Light Emitting Diodes) and laser diodes for field deployable sensing devices, areas of active research.
Fig. 5.
Photo stability of the SNAFL-2-Dextran (pH 6.3) immobilized on the MEF substrate (SiFs) and glass (control sample). The illumination conditions were identical for the both samples, green LED, 535 nm, and 0.5 mW.
It is well-known that both forms of SNAFL-2 are fluorescent, affording for ratiometric lifetime sensing [11]. However, in all our reports of MEF [1–8] we have shown that the lifetime of fluorophores in close proximity to silver nanostructures is dramatically reduced, <100 ps. Given that this is most likely to occur here, and coupled with the complexities associated with lifetime measurements on silver surfaces, we therefore chose not to additionally measure the lifetimes of the substrates reported here.
CONCLUSIONS
We have shown that immobilized silver nanoparticles on glass substrates are indeed useful sensing platforms producing a significantly brighter immobilized pH probe, which retains its pH sensing capability. We have found that up to a 40-fold greater fluorescence intensity can be obtained using silver nanostructures as compared to glass substrates. However, the silver nanoparticles are efficient at enhancing both the acid and base from of SNAFL-2 to a similar extent, which indicates that any ratiometric measurement will have a dynamic range similar to the solution based measurements, but operating at a 40-fold higher S/N. In addition, it appears that the acidic form of SNAFL-2 is enhanced more than the basic form, suggesting sensing advantages at lower pH values.
ACKNOWLEDGMENT
This project was supported by the National Institutes of Health, 1 R43-CA97569–01.
ABBERIVIATIONS:
- MEF
Metal-enhanced fluorescence
- RDE
Radiative decay engineering
- GOPTES
3-(glycidoxypropyl) trimethoxysilane
- APS
3-(aminopropyl) triethoxysilane
REFERENCES
- 1.Lakowicz JR (2001). Radiative decay engineering: Biophysical and biomedical applications. Appl. Biochem 298, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geddes CD and Lakowicz JR (2002). Metal-enhanced fluorescence. J. Fluoresc 12(2), 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, and Gryczynski I (2002). Radiative decay engineering 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal. Biochem 301, 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geddes CD, Aslan K, Gryczynski I, Malicka J, and Lakowicz JR (2004). Noble metal nanostructure for metal-enhanced fluorescence In Geddes CD and Lakowicz JR (Eds.), Review Chapter for Annual Reviews in Fluorescence, Kluwer Academic/Plenum Publishers, New York, USA, pp. 365–01. [Google Scholar]
- 5.Gryczynski I, Malicka J, Geddes CD, and Lakowicz JR (2003). The CFS engineers the intrinsic radiative decay rate of low quantum yield fluorophores. J. Fluoresc 12(1), 11–13. [Google Scholar]
- 6.Geddes CD, Cao H, Gryczynski I, Gryczynski Z, Fang J, and Lakowicz JR (2003). Metal-enhanced fluorescence due to silver colloids on a planar surface: Potential applications of Indocyanine green to in vivo imaging. J. Phys. Chem. A 107, 3443–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parfenov A, Gryczynski I, Malicka J, Geddes CD, and Lakowicz JR (2003). Enhanced fluorescence from fluorophores on fractal silver surfaces. J. Phys. Chem. B 107(34), 8829–8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geddes CD, Parfenov A, Roll D, Gryczynski I, Malicka J, and Lakowicz JR (2003). Silver fractal-like structures for metal-enhanced fluorescence: Enhanced fluorescence intensities and increased probe photostabilities. J. Fluoresc 13(3), 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aslan K, Gryczynski I, Malicka J, Lakowicz JR, and D Geddes C (2005). Metal enhanced fluorescence: application to high-throughput screening and drug discovery In Gad Shayne (Ed.), Drug Discovery Handbook, Wiley &Sons, New Jersey: In press. [Google Scholar]
- 10.Lofas S and Johnson B (1990). A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J. Chem. Soc. Comm 21, 1526–1528. [Google Scholar]
- 11.Lakowicz JR (1999). Principles of Fluorescence Spectroscopy, Kluwer, New York. [Google Scholar]