Significance
How are specific host-symbiont mutualisms stabilized without vertical transmission? This is one of the fundamental questions in evolutionary biology. To ensure specificity, animals and plants have evolved sophisticated sorting mechanisms. Theoretical studies proposed another mechanism, so-called “competition-based selection,” where hosts provide a specific environment to selectively cultivate favorable symbionts and maintain symbiont quality. Although this mechanism is thought to be powerful in open systems, such as gut symbiosis where contaminants regularly invade, until now little experimental evidence has supported its importance in the evolution of symbioses. Through the inoculation of an insect host with a large panel of symbiotic and nonsymbiotic bacteria, we demonstrate that microbial competition is critical to maintain the specificity in an insect gut symbiosis.
Keywords: gut symbiosis, symbiont specificity, stinkbug, Burkholderia, competitiveness
Abstract
Despite the omnipresence of specific host–symbiont associations with acquisition of the microbial symbiont from the environment, little is known about how the specificity of the interaction evolved and is maintained. The bean bug Riptortus pedestris acquires a specific bacterial symbiont of the genus Burkholderia from environmental soil and harbors it in midgut crypts. The genus Burkholderia consists of over 100 species, showing ecologically diverse lifestyles, and including serious human pathogens, plant pathogens, and nodule-forming plant mutualists, as well as insect mutualists. Through infection tests of 34 Burkholderia species and 18 taxonomically diverse bacterial species, we demonstrate here that nonsymbiotic Burkholderia and even its outgroup Pandoraea could stably colonize the gut symbiotic organ and provide beneficial effects to the bean bug when inoculated on aposymbiotic hosts. However, coinoculation revealed that the native symbiont always outcompeted the nonnative bacteria inside the gut symbiotic organ, explaining the predominance of the native Burkholderia symbiont in natural bean bug populations. Hence, the abilities for colonization and cooperation, usually thought of as specific traits of mutualists, are not unique to the native Burkholderia symbiont but, to the contrary, competitiveness inside the gut is a derived trait of the native symbiont lineage only and was thus critical in the evolution of the insect gut symbiont.
A number of animals and plants are associated with beneficial microorganisms, which provide diverse services to the host species, such as enhanced nutrition and protection from antagonists (1, 2). While some symbionts are vertically transmitted from mother to offspring, many animals and plants acquire in every generation the symbionts from the environment (3). Since microorganisms are abundant and diverse in the environment, hosts have evolved elaborate mechanisms for “partner choice” to ensure the specificity of the associations (4, 5). Well-studied model systems, the legume–Rhizobium and squid–Vibrio symbioses, have revealed that the partner specificity is achieved by various host mechanisms, such as signal recognition during initiation and secretion of antimicrobial agents during colonization (6, 7). In addition to such host control mechanisms, theoretical modeling studies have suggested the importance of microbe–microbe competition to ensure the specificity, wherein the host provides a demanding environment to let a favorable microorganism grow dominantly (8, 9). The “competition-based selection” is thought to be more powerful to eliminate contaminants or pathogens and maintain symbiont quality, particularly in open systems, such as the gut, the fungus-farming garden of insects, and the plant rhizosphere, where microbial contamination can regularly occur and potentially disrupt the symbioses. Despite these theoretical studies, empirical support for the impact of microbe–microbe competition on the evolution and stabilization of host–symbiont specificity is very scarce.
The bean bug Riptortus pedestris (Heteroptera: Alydidae) is associated with a Burkholderia symbiont that is confined in symbiosis-specific crypts located in the posterior midgut region M4 (10). The Burkholderia symbiont is not essential but beneficial for growth, reproduction, immunity homeostasis, and pesticide resistance of the insect host (11–13). While most insects vertically transmit their symbionts, bean bugs acquire their microbial partners from the ambient soil in every generation (10, 11). To acquire their specific symbionts from the diverse soil microbiota, stinkbugs appear to utilize a specific organ called the “constricted region,” a narrow passage filled with a mucus-like matrix, located in the midgut immediately upstream of the crypt-bearing region. The constricted region winnows out contaminating microorganisms and the Burkholderia symbiont specifically penetrates into the symbiotic region (14). In addition to this specific apparatus for bacterial sorting, the insect expresses diverse antimicrobial peptides in the symbiotic organ (13, 15), which may also play a role in the partner choice.
To be a bean bug symbiont, therefore, the Burkholderia species should be capable of penetrating the constricted region (initiation), stably colonizing the symbiotic organ (accommodation), and of course, behaving cooperatively with the host (cooperation). Recent studies have revealed part of the symbiont’s mechanisms involved in these processes. The Burkholderia symbiont employs a unique corkscrew flagellar motility to pass through the constricted region (16); it undergoes cytological and metabolic alterations, such as flagella loss and polyhydroxyalkanoate accumulation, to cope with the crypt environment (17–19); and it rapidly proliferates to occupy entirely the available space in the lumen of the symbiotic M4 midgut region, notably by recycling host metabolic wastes into essential amino acids and B vitamins in the M4 crypts, which are thought to be part of the symbiont services provided to the host (17).
The bacterial genus Burkholderia (Betaproteobacteria: Burkholderiaceae), comprises over 100 species with taxonomically validated names and is an ecologically very diverse bacterial group (20). Based on genomic phylogeny, the genus Burkholderia is grouped into at least 3 distinct clades (21) (Fig. 1). The first clade consists of many human-, animal-, and plant-pathogens, including Burkholderia cepacia, Burkholderia pseudomallei and Burkholderia mallei, designated as the “B. cepacia complex and B. pseudomallei” (BCC&P) clade (22). Some BCC&P species are also reported as fungal symbionts (23) and beetle symbionts (24). The second clade includes a number of plant growth-promoting rhizobacteria and nodule symbionts of leguminous plants, and is designated as the “plant-associated beneficial and environmental” (PBE) clade (25). This clade, which also includes farming symbionts of the slime mold Dictyostelium discoideum (26), was recently even nominated as a new genus, Paraburkholderia (27). The third clade mainly consists of environmental species, leaf-nodule symbionts of Rubiaceae plants, and gut symbionts of stinkbugs, and is called the “Burkholderia glathei clade” or the “stinkbug-associated beneficial and environmental” (SBE) clade (10, 28). Also for this clade, a new genus name, Caballeronia, was proposed (29). The outgroup of Burkholderia is the genus Pandoraea (Fig. 1), which are common soil bacteria, although some of them are opportunistic pathogens of humans (30). Almost all of the Burkholderia and Pandoraea species, except the leaf-nodule symbionts in SBE, are easy to culture and genetically manipulable, providing us with an opportunity to clarify how symbiotic traits have evolved in a phylogenetically well-defined bacterial lineage.
Fig. 1.
Phylogenetic relationship of 113 species of the genus Burkholderia. A multilocus tree based on 6,399 unambiguously aligned amino acid sites of 21 genes is shown. The 21 marker genes were commonly present in the 113 Burkholderia species and 4 betaproteobacteria with well-conserved alignment brocks (>60% of the alignment). Stinkbug-associated SBE, plant-associated PBE, and pathogenic BCC&P groups are highlighted with blue, green, and red, respectively. Recently proposed new genus names of SBE and PBE are also depicted under the group names. Closed circles indicate that the nodes are supported with >70% bootstrap values. Illustrations courtesy of Chiaki Matsuura and Yu Matsuura.
Genetically diverse Burkholderia species or strains are associated with natural R. pedestris populations but nearly all of them belong to the SBE clade (11, 31, 32) (SI Appendix, Fig. S1) and only 1 study reported the infection with BCC&P species in overwintering R. pedestris (33). In addition, in insects reared on soil in the laboratory, we occasionally identified infections with PBE Burkholderia as well as Pandoraea, suggesting that colonization of the symbiotic midgut organ is not strictly limited to SBE species (34). Although we revealed that the bacterial sorting organ in the bean bug gut winnows out symbionts from nonsymbionts, only a limited number of bacterial species (i.e., Eschelichia coli, Pseudomonas putida, and Bacillus subtilis) have been tested as nonsymbionts (14). Here we show by experimental inoculations of diverse bacterial species into R. pedestris that non-SBE Burkholderia and even Pandoraea are capable of passing through the constricted region, stably colonizing the gut symbiotic organ, and behaving cooperatively. We further demonstrate that, through competitive infection assays in conjunction with histological inspections, SBE always outcompetes these nonnative symbionts inside the gut symbiotic organ, highlighting a pivotal role of symbiont competitiveness for stabilizing the insect–microbe gut symbiosis.
Results and Discussion
SBE, PBE, and Pandoraea Species Can Pass through the Constricted Region and Stably Colonize the Symbiotic Organ.
The midgut of R. pedestris consists of 4 distinct sections called M1, M2, M3, and M4. The SBE Burkholderia specifically colonizes the crypt-bearing M4 section. After symbiont infection, the M4 crypts swell and become whitish in color because of the symbiont colonization and proliferation in their lumen (10). In front of M4, a bulbous area called the M4 bulb (M4B) develops, wherein symbiont cells, flowed backward from M4, are entirely digested and the derived nutrients absorbed by the host (15, 17, 35). Probably because of the digested bacteria, the M4B becomes swollen and yellowish (10). The constricted region connects the M3 with the M4B (14). In order to analyze further the infection specificity of the stinkbug–Burkholderia gut symbiosis, we first experimentally inoculated 20 taxonomically diverse bacterial species of 3 major phyla into the bean bug and determined their colonization ability by counting colony forming units (CFU). The tested bacteria (SI Appendix, Table S1) included species of the Burkholderia (SBE [Burkholderia insecticola], PBE [Burkholderia fungorum], and BCC&P [Burkholderia plantarii]), related species of the family Burkholderiaceae (Ralstonia, Chitinimonas, Cupriavidus, and Pandoraea species), as well as more distantly related species of the Alpha- and Gammaproteobacteria. Inoculation was performed in early second instar when the insects are competent for symbiont acquisition (36), and bacterial colonization was investigated at day 2 of the third instar (i.e., 5 d postinoculation), which is 2 to 3 d after completion of the colonization process with B. insecticola (37). Whereas most of the tested species showed little or no infection in the symbiotic organ, the PBE and Pandoraea, as well as the SBE but not the BCC&P, colonized the symbiotic organ to high density, around 107 CFU per organ (Fig. 2A). Microscopic observations with green fluorescent protein (GFP)-labeled strains of E. coli, Burkholderia, and allied Burkholderiaceae species confirmed that while E. coli, Cupriavidus, and BCC&P reached the M3 but did not pass through the constricted region, the SBE, PBE, and Pandoraea species could pass through the constricted region and penetrate into the symbiotic M4 region (Fig. 2B).
Fig. 2.
Infection specificity of bacterial species in the gut symbiotic organ. (A) Infection ability of diverse bacterial species to the gut symbiotic organ. Five days after oral administration, the colonization level in M4 was evaluated by CFU counting. (B) Infection initiation process visualized by confocal microscopy. The infecting bacteria were labeled with GFP. Note that only Pandoraea (P. norimbergensis), PBE (B. fungorum), and SBE (B. insecticola) pass through the sorting organ, the constricted region. Arrows indicate an infection thread invading from M3 to M4B through the constricted region. Abbreviations: CR, constricted region; M3, midgut third section; M4B, M4 bulb.
Infection States of Burkholderia and Pandoraea Species in the Bean Bug.
To further detail the specificity of the interaction and to clarify the infection states of Burkholderia and Pandoraea species in R. pedestris, infection rate (percent of infected insects) and morphology of infected midguts were surveyed for 13 SBE species, 12 PBE species, 7 BCC&P species, 2 species of other Burkholderia clades, and 2 Pandoraea species. Results of the infection assays, morphometric measurements, and microscopic observations, are summarized in Fig. 3 and SI Appendix, Fig. S2 and Table S2, respectively. Details of the infection tests are described in the following sections and shown in SI Appendix, Figs. S3–S10.
Fig. 3.
Morphological alteration of the gut symbiotic organ after colonization of symbiotic and nonsymbiotic bacteria. Images of whole midgut, M4B and M4 region, LSM of M4, and TEM of M4 in an aposymbiotic insect (A), and insects inoculated with SBE (B), PBE (C), and Pandoraea (D). All images are of third-instar nymphs. Abbreviations: H, hindgut; M1, midgut first section; M2, midgut second section; M3, midgut third section; M4B, M4 bulb; M4, crypt-bearing midgut fourth section. In LSM images: green, SYTOX GREEN staining of colonizing bacteria and host nuclei; red, phalloidin staining of cytoskeleton; yellow arrowheads, infected M4 crypts; white arrowheads, uninfected M4 crypts. In TEM images: lm, luminal region of crypt; n, host nucleus.
SBE Burkholderia.
All of the 13 tested SBE Burkholderia species or strains consistently showed high, 80 to 100%, infection rates (SI Appendix, Table S2). In aposymbiotic insects, M4 crypts and M4B were not well developed (Fig. 3A), the luminal space of each crypt was small, and its epithelium cells were thick (Fig. 3A). In contrast, the symbiotic organ infected with SBE (B. insecticola) was well developed (Fig. 3B and SI Appendix, Fig. S2): M4 crypts were bulging by the bacterial proliferation and colonization (SI Appendix, Fig. S2 B and C); the M4B became bulbous in shape (SI Appendix, Fig. S2D) and yellowish in color (SI Appendix, Fig. S2E). Laser-scanning microscopy (LSM) and transmission electron microscopy (TEM) (21) observations confirmed that M4 crypts were entirely filled with SBE cells (Fig. 3B and SI Appendix, Fig. S3), while no or partial colonization occurred in the M4B (SI Appendix, Fig. S4). These morphological features of M4 and M4B were consistently observed in insects infected with the other 12 species of SBE Burkholderia (SI Appendix, Figs. S5 and S9 and Table S2).
PBE Burkholderia.
Among the 12 tested species of PBE Burkholderia, the infection rate was variable (SI Appendix, Table S2). Five species showed a very high (90 to 100%) infection rate, while 4 species showed moderate (40 to 70%) and 3 species showed low (0 to 10%) infection rates. The morphology of the symbiotic organ infected with the highly infective representative, B. fungorum, was observed in detail. This revealed that the symbiotic organ colonized by PBE Burkholderia (Fig. 3C) was markedly different from the organ colonized by SBE Burkholderia: The M4 crypts were infected but not well bulged (SI Appendix, Fig. S2 B and C); the M4B was significantly larger (SI Appendix, Fig. S2D) but remained whitish and did not adapt the yellowish color characteristic for SBE infection (SI Appendix, Fig. S2E). LSM and TEM observations revealed that this nonnative symbiont occupied strongly the central tract of the M4 but had only partially colonized the crypt lumen (Fig. 3C and SI Appendix, Fig. S2F). When colonized, the luminal space of each crypt was narrower and its epithelial cells were thicker than those of the crypts colonized by SBE (Fig. 3 B and C). Colonizing PBE cells had a typical rod shape and were not notably different from colonizing SBE cells (SI Appendix, Fig. S3). Although no or partial colonization of SBE Burkholderia occurred in the M4B, TEM observations showed that a high number of bacterial cells of B. fungorum colonized this region (SI Appendix, Fig. S4), suggesting that the PBE is digestion-tolerant or that the digesting enzymes are not properly produced and that the colonizing cells cause the swelling and whitish color of M4B. These morphological features (i.e., smaller M4 crypts and swollen and whitish M4B) were consistently observed in insects infected with other PBE species (SI Appendix, Fig. S6 and Table S2). PBE species with low infection rates did not alter morphogenesis of M4B and M4, which resembled the aposymbiotic state. Thus, the infection rate and morphological alterations in M4 and M4B were highly correlated (SI Appendix, Fig. S9 D and E), suggesting that bacterial infection and proliferation in the gut symbiotic organ stimulate the morphological change.
BCC&P Burkholderia and other Burkholderia species.
The 7 tested BCC&P species, including 2 species of biosafety level 3 (BSL3) pathogens, B. mallei and B. pseudomallei, consistently showed a low (0 to 40%) infection rate (SI Appendix, Table S2). In this BCC&P group, even when infection was detected by diagnostic PCR, the M4 and M4B were small and not well-developed, as is the case in aposymbiotic insects (SI Appendix, Figs. S7 and S9). GFP-labeled Burkholderia glumae and Burkholderia pyrrocinia confirmed that the BCC&P species can penetrate the constricted region and reach the M4 region (SI Appendix, Fig. S10), although they did not stably accommodate inside the crypts. Other Burkholderia species of independent clades, Burkholderia andoropogonis and Burkholderia caryophylli, showed no infection (SI Appendix, Figs. S7 and S9 and Table S2).
Pandoraea and other Burkholderiaceae.
Both tested Pandoraea species, Pandoraea norimbergensis and Pandoraea oxalativorans, showed a 100% infection rate (SI Appendix, Table S2). Similar to PBE Burkholderia, M4 crypts were infected but not properly developed and the M4B was strongly swollen in shape but whitish in color (Fig. 3D and SI Appendix, Figs. S2 and S8). LSM and TEM observations revealed that Pandoraea were abundant in the central duct of M4 but only partially colonized the crypt lumen (Fig. 3D and SI Appendix, Fig. S2F). TEM observations revealed that M4 colonized cells were typical rods (SI Appendix, Fig. S3) and Pandoraea cells densely colonized M4B (SI Appendix, Fig. S4), similar to PBE Burkholderia, suggesting that the Pandoraea is also digestion-tolerant and the colonizing cells cause the swelling and whitish color of M4B. Other Burkholderiaceae species, Cupriavidus taiwanensis, Ralstonia solanacearum, and Chitimonas koreensis, did not infect the symbiotic organ (SI Appendix, Figs. S8 and S9 and Table S2).
PBE Burkholderia and Pandoraea Are Beneficial for the Bean Bug.
The inoculation tests revealed that many PBE Burkholderia species and even the outgroup, Pandoraea, passed through the constricted region and stably accommodated in the M4 region, although they did not fully colonize the crypt lumen. To determine if the PBE and Pandoraea species are beneficial for the bean bug, fitness parameters of the insect host (survival rate, growth period, body size, and weight) were measured after infection. B. insecticola (SBE), B. fungorum (PBE), and P. norimbergensis (Pandoraea), all of which show a 100% infection rate, were tested. Compared with aposymbiotic insects, insects infected with either of these strains showed significantly higher survival rates, shorter developmental time before adulthood, larger body size, and heavier body weight (Fig. 4). However, insects infected with the Pandoraea nevertheless showed a slower developmental time and smaller body weight than those infected with the SBE Burkholderia (Fig. 4), while insects infected with the PBE species showed an almost identical body size and weight to those with SBE Burkholderia, although the growth period was significantly delayed (Fig. 4). Taken together, the rearing experiments clearly demonstrated that the nonnative bacteria B. fungorum and P. norimbergensis are not parasitic but enhance survivability, growth, and development of the host insect, although to a slightly lesser extend than the native symbiont B. insecticola.
Fig. 4.
Fitness effect of symbiotic and nonsymbiotic bacteria in the bean bug. Comparison of fitness parameters between aposymbiotic insects (Apo) and insects infected with Pandoraea (Pan: P. norimbergensis), PBE (B. fungorum), and SBE (B. insecticola). (A) Survival rate (= adult emergence rate), (B) time to adulthood, (C) photographs of male and female adults. (D–F) Male insects: (D) maximum thorax width, (E) body length, (F) dry weight. (G–I) Female insects: (G) maximum thorax width, (H) body length, and (I) dry weight. Different letters indicate statistically significant differences (P < 0.05). The fitness parameters were analyzed by a Mann–Whitney U test (B and D), Student t test (E and F), Brunner–Munzel test (G), and Welch t test (H and I), and corrected by the Bonferroni method. Error bars indicate SDs. Number of replicates are depicted on the bars.
SBE Burkholderia Always Outcompete PBE and Pandoraea in the Gut Symbiotic Organ in Coinfection Assays.
PBE Burkholderia and Pandoraea species are common bacterial groups in soil environments, and detected frequently with SBE Burkholderia (34, 38, 39). Given the high infectivity and benefits of PBE and Pandoraea, why do only SBE Burkholderia prevail in field populations of the bean bug? To clarify this point, colonization competitiveness of the PBE and Pandoraea against the SBE was investigated. For this analysis, B. insecticola, B. fungorum, and P. norimbergensis were used as representatives of SBE, PBE, and Pandoraea, respectively. To investigate in vitro competitiveness, log-phase cultured cells of the SBE (rifampicin resistance) and a competitor (PBE or Pandoraea [chloramphenicol resistance]) were suspended in yeast-glucose (YG) medium, and incubated at 25 °C without shaking for 2 d. The incubated medium was plated on YG agar plates containing either rifampicin or chloramphenicol, and the competitive index (CI) was calculated as (output SBE CFU/input SBE CFU)/(output competitor CFU/input competitor CFU) (12). To determine the in vivo competitiveness, newly molted second-instar nymphs of R. pedestris were individually fed with 1 µL of distilled water containing the same CFUs of the SBE and a competitor (B. fungorum or P. norimbergensis). Two days after rearing, the symbiotic organs were dissected, their homogenates were plated on YG agar plates containing antibiotics, and CI values were calculated based on the CFU counts.
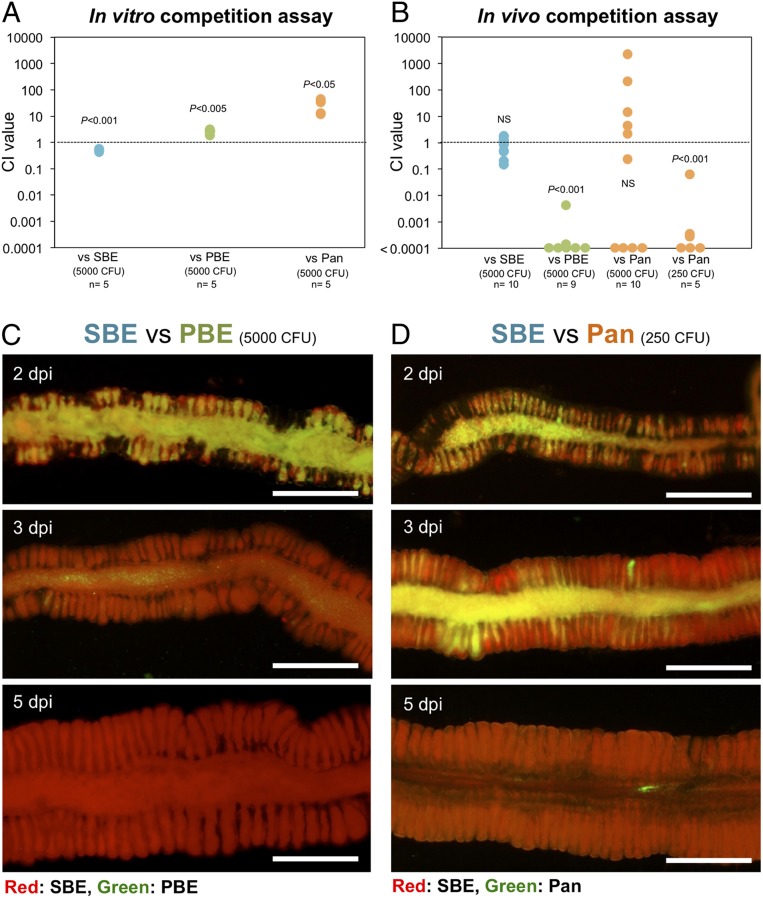
While the SBE Burkholderia did not dominate the 2 other species in vitro (Fig. 5A), the in vivo competitive assay revealed that the SBE Burkholderia significantly outcompeted the PBE species in the gut symbiotic organ, even in a high inoculation dose condition (Fig. 5B). Although the Pandoraea was competitive against the SBE under a high (5,000 cells) infection dose, the SBE became significantly dominant under a lower (250 cells) dose condition (Fig. 5B). Microscopic visualization of the competition process by use of an RFP-labeled B. insecticola (SBE) and GFP-labeled B. fungorum (PBE) or P. norimbergensis (Pandoraea) revealed that, first the SBE and the nonnative bacteria cocolonized the M4 region in a mixed manner, that the SBE then gradually gained the upper hand, and that it eventually solely occupied the symbiotic organ in 5 d after coinoculation (Fig. 5 C and D). Similar results were consistently observed when the SBE species was coinoculated with other PBE Burkholderia species (SI Appendix, Fig. S11 and Table S3) and when another SBE species, Burkholderia cordobensis, was tested against the PBE and Pandoraea species (SI Appendix, Fig. S12 and Table S3). These observations clearly demonstrate that the exclusive prevalence of SBE Burkholderia in natural bean bug populations is supported by the higher competitiveness of SBE species inside the gut symbiotic organ.
Fig. 5.
Infection competitiveness of symbiotic bacteria against nonnative bacteria. Competitiveness of SBE (B. insecticola) against PBE (B. fungorum), and Pandoraea (Pan: P. norimbergensis) in in vitro growth (A) and in vivo (B). Inoculum doses are indicated in CFU. Each dot represents a CI value from a microtube or an insect. The CI values are obtained by (output SBE colony count/input SBE colony count)/(output competitor colony count/input competitor colony count) and statistically evaluated by the 1-sample t test (against CI = 1.0). NS, not significant. (C and D) Visualization of the in vivo competition dynamics. An RFP-labeled SBE strain was coinoculated with a GFP-labeled strain of PBE (C) or Pandoraea (D). dpi, days postinoculation. (Scale bars, 0.2 mm.)
Competitiveness in the Crypt Lumen Is Critical for the Riptortus–Burkholderia Specificity.
Our previous studies have revealed that the bean bug R. pedestris develops a specific sorting organ called the “constricted region” in the midgut and employs in the M4 unique antimicrobial peptides to select bacteria (14, 15). This study confirmed that diverse bacterial species are winnowed out by the constricted region. However, the selective apparatus is not perfect, and bacteria that are closely related to the natural symbiont, including some PBE Burkholderia and Pandoraea species, can penetrate the constricted region and reach the gut symbiotic organ (Fig. 2 and SI Appendix, Table S2). Although the PBE and Pandoraea did not fully colonize the gut crypts (Fig. 3 and SI Appendix, Fig. S2), these nonnative bacteria stably accommodated in the M4 region and, surprisingly, were not harmful but, in contrast, beneficial for host survival, growth rate, and body size (Fig. 4). In contrast, SBE Burkholderia are highly adapted to the internal environment of the gut symbiotic organ and always outcompete PBE and Pandoraea species (Fig. 5 and SI Appendix, Figs. S11 and S12). These results clearly demonstrated that the exclusive prevalence of SBE Burkholderia in natural bean bug populations is underpinned not only by host-mediated partner choice, but also by the higher competitiveness of SBE species inside the gut symbiotic organ.
In symbiotic associations with environmental transmission, host organisms have evolved elaborate mechanisms for partner choice and sanctions of cheaters (4). Thus, to establish a specific association with their hosts, symbiotic microorganisms have to be capable of passing a sorting mechanism (initiation), colonizing the symbiotic organ (accommodation), and behaving cooperatively in the hosts (cooperation). Mutualists are generally characterized by these features. Strikingly, the inoculation of a broad range of Burkholderia and allied species revealed that these fundamental features of symbiotic microorganisms are not restricted to SBE but shared by PBE, and even the outgroup Pandoraea, indicating that these symbiotic traits are ancestral (Fig. 6). In contrast, the advanced adaptation to and strong competitiveness in the gut symbiotic organ evolved exclusively in the SBE lineage (Fig. 6). These features enable the SBE Burkholderia to dominantly and stably occupy the symbiotic gut portion of the bean bug and underpin the host–symbiont specificity. Previous studies on the SBE species B. insecticola have revealed some of the genetic bases for the initiation, accommodation, and cooperation, all of which are conserved in PBE and Pandoraea species: Flagellar motility for initiation (14), polyhydroxyalkanoate/purine/cell wall/LPS biosynthesis for accommodation (18, 35, 40, 41), and nitrogen and sulfur recycling for cooperation (17). The extent to which differential activity of these functions in competitive and noncompetitive strains contributes to the colonization competitiveness will deserve attention in future studies.
Fig. 6.
Evolutionary hypothesis of the symbiotic traits in the Burkholderiaceae. Abilities for initiation, accommodation, and cooperation in the bean bug gut have evolved in the common ancestor of the genera Pandoraea and Burkholderia (black box in the dendrogram), and only the competitiveness in the gut symbiotic organ has evolved in the SBE lineage (blue box in the dendrogram). These traits, except the initiation ability, are lost in BCC&P (white box in the dendrogram).
At this stage, it also remains unclear where and how the competitive traits have evolved in the SBE lineage. It is plausible that the competitive traits have been selected in the insect gut. This scenario requires that the SBE Burkholderia gain fitness through the symbiotic association, for example by an efficient recolonization of the soil environment from the insect midgut crypts. Although this aspect of the symbiosis is currently poorly investigated (10), recolonization of the soil does not seem impossible because in all stages of the insect life cycle, a high number of viable bacteria are present in the posterior midgut. Alternatively, the competitive traits may have evolved due to selection on SBE Burkholderia lifestyles in a different environment, such as soil and rhizosphere, and the symbiont could outcompete other bacteria in the M4 incidentally. To clarify this point, the symbiont’s benefit gained from the association should be investigated in forthcoming work.
Possible Mechanisms Underpinning the Competitiveness.
The molecular mechanisms supporting the competitiveness of SBE in the gut symbiotic organ are at present unclear. Possible mechanisms for the competitiveness could be categorized into 2 groups: Direct and indirect mechanisms. In the former mechanism, symbionts directly interfere with competitors, while in the latter mechanism, symbionts are selected by host-served specific conditions, such as nutrients and antimicrobial agents. As for the former mechanism, the T6SS is known as a competition factor in the squid–Vibrio system and honey bee gut microbiota (42, 43). However, the T6SS of SBE Burkholderia is strongly repressed in vivo (17) and T6SS mutants outcompeted PBE and Pandoraea species, similarly to the wild-type (SI Appendix, Fig. S13), strongly suggesting that the cT6SS is not involved in the in vivo competitiveness of SBE Burkholderia. In addition, SBE species lack the contact-dependent growth inhibition system, which is also known as a microbe–microbe toxin delivery system in some Burkholderia (44).
Taking into account the histological observations, the latter, indirect competition mechanism seems more plausible to the bean bug system. The microscopic observations revealed that PBE and Pandoraea colonize and proliferate well in M4B and the central tract of M4 but show a colonization defect inside crypts (Fig. 3), suggesting that the environment of the crypt lumen is different from the central tract of the M4 and is highly specialized for housing SBE Burkholderia. This could contribute to the in vivo competitiveness of the symbiont. Although speculative, SBE Burkholderia may efficiently utilize nutrients served by the host in the crypt lumen, and also adapt to specific chemical conditions (e.g., pH, osmolarity, and oxygen concentration) of the luminal environment. Since diverse crypt-specific antimicrobial peptides are highly expressed (15), it is also plausible that SBE Burkholderia are more tolerant to the host gut immunity than the less-competitive species. Furthermore, theoretical studies have pointed out that the adhesion ability is an additional pivotal character to determine the competitiveness and stability of a bacterium in complex microbiota developing on a surface substrate, such as biofilms and intestinal flora (45). As an alternative mechanism, therefore, SBE Burkholderia may have an adhesin-mediated higher affinity to the crypt epithelia. Future genome comparisons in conjunction with gene-deletion or -transfer experiments could lead to the identification of key genes responsible for the SBE-specific colonization and competitiveness in the midgut crypts.
Diversity of the Symbiont Specificity in Stinkbugs.
In addition to the bean bug, other heteropteran species of the superfamilies Coreoidea and Lygaeoidea and the family Largidae are commonly associated with Burkholderia in the midgut crypts. Although the bean bug and most members of the Coreoidea and Lygaeoidea are exclusively associated with SBE Burkholderia (32), some species are associated mainly with SBE but to a lesser extend also with PBE and even with BCC&P (46–50). In addition, members of the Largidae harbor a specific group of PBE Burkholderia (51, 52). These previous surveys support the hypothesis that the symbiotic traits were ancestral in the genus Burkholderia, and also suggest that the competition-based selection favors different Burkholderia clades in different stinkbug taxa.
Competition-Based Selection of an Optimal Symbiont.
Theoretical studies predicted the importance of host control (i.e., partner choice and partner fidelity) for the evolution and maintenance of mutualistic associations (4, 53). Furthermore, more recent modeling studies proposed competition-based mechanisms as an alternative to assure symbiont quality (8, 9, 45, 54), where the host serves as a selective environment to let a favorable microorganism grow preferentially. Herein, we experimentally point out the importance of this mechanism in the Riptortus–Burkholderia symbiotic system. Recent transcriptomic analyses revealed that SBE Burkholderia recycle the host’s metabolic wastes into utilizable nutrients, such as essential amino acids and B vitamins (17). Combined with histological data, the gene-expression patterns in the symbiotic organ further suggest that the Burkholderia symbiont proliferates in the M4 crypts and that excess symbiont cells move into the M4B region, where they are entirely digested and all of the derived nutrients absorbed by the host (15, 17). In other words, the bean bug cultivates the symbiont in the M4 and digests them in the M4B. In such a “cultivation symbiosis,” growth rate and regeneration efficiency of host metabolites in the symbiotic organ seem to be directly linked to the host fitness, whereby the host could gain a significant benefit from the better grower. If so, we could speculate that the host insect facilitates microbial competition inside the symbiotic organ not only for winnowing out potential parasites but also for selecting a better grower. In this context, it is notable that SBE showed greater benefits to the host than PBE and Pandoraea species in terms of growth rate and body weight (Fig. 4). Moreover, the apparent absence of bacterial degradation in the M4B in PBE or Pandoraea infected insects (SI Appendix, Fig. S3) could be a further reason for the reduced benefits provided by these bacteria.
A competition-based symbiont selection also plays a pivotal role in the fungus-cultivating ambrosia beetles (55), suggesting that in cultivation symbiosis, where hosts harvest growing symbionts, the competition-based mechanism is commonly important not only for keeping symbiont quality but also maybe for selecting the better-growing symbionts. More generally, broader surveys could clarify the general impact of microbe–microbe competition in the evolution and stabilization of symbiotic associations maintained via environmental symbiont transmission, as suggested by recent examples (42, 43).
The genus Burkholderia (Burkholderia/Paraburkholderia/Caballeronia) includes a variety of symbiotic lifestyles, such as the nodule symbionts in leguminous plants and farming symbionts in the social amoeba, as well as the gut symbionts in stinkbugs, in which the molecular mechanisms underpinning the specificity (and probably importance of symbiont’s competitiveness) seem different between each system (56, 57). The amazing diversity of symbiotic lifestyles in this monophyletic group would provide a great opportunity to find out the different paths a microorganism can take to become a symbiont in the competitive microbial world.
Materials and Methods
Insects and Bacterial Strains.
The R. pedestris strain used in this study was originally collected from a soybean field in Tsukuba, Ibaraki, Japan, and maintained in the laboratory for over 10 y. The insects were reared in Petri dishes (90-mm diameter, 20-mm high) at 25 °C under a long-day regimen (16-h light, 8-h dark) and fed with soybean seeds and distilled water containing 0.05% ascorbic acid. Bacterial species/strains used in this study and their culture conditions are listed in SI Appendix, Tables S1 and S2.
Oral Administration of Cultured Bacteria.
Taxonomically diverse bacteria.
Bacterial species were grown in specific optimal growth condition (SI Appendix, Table S1) to an early log phase on a gyratory shaker at 150 rpm. Exponentially growing cells (approximately OD600 = 0.5) were harvested by centrifugation and suspended in distilled water so that the final concentration was 104 cells/µL. Newly molted second-instar nymphs were deprived of water overnight to make the insects thirsty and willing to ingest the bacteria-containing suspensions. The nymphs were fed with symbiont-containing water for 48 h, after which it was replaced by sterile water. Two days after the third-instar molt (approximately 5 d after inoculation), the symbiotic organs were dissected under a binocular microscope (S8APO, Leica), rinsed twice in sterilized water, and homogenized in sterilized water. Dilution series of the solution were then plated on agar plates to examine the infection status of the insects. If any colonies were detected, the number of CFUs was calculated.
Burkholderia and other Burkholderiaceae species.
Burkholderia and other Burkholderiaceae species were grown at 30 °C to an early log phase in YG medium (0.5% yeast extract, 0.4% glucose, 0.1% NaCl) for Burkholderia, Pandoraea, Cupriavidus, and Ralstonia, or R2A broth (Nippon Pharmaceuticals) for Chitinimonas on a gyratory shaker at 150 rpm. Inoculations of the bacterial species were performed as described above. Two days after the third-instar molt, the symbiotic organs were dissected and examined for infection with the Burkholderia or other Burkholderiaceae species by diagnostic PCR with specific primer sets for the Burkholderia 16S rRNA gene (BF and BR) (58) or universal ones for the bacterial 16S rRNA gene (515F and 806R) (59), respectively, as previously described (32).
B. mallei and B. pseudomallei.
Inoculation tests of 2 BSL3 pathogens, B. mallei and B. pseudomallei, were performed at the BSL3 facility of the Hokkaido University Research Center for Zoonosis Control. The 2 species were also grown at 30 °C to an early log phase in YG medium on a gyratory shaker at 150 rpm, as well as other Burkholderia species, as described above. Oral administrations of the 2 species were performed as described above. Two days after the third-instar molt, insects were preserved in acetone during 1 wk so that eventual bacterial cells were completely killed. Total DNA was extracted from the abdominal part (without dissection) and subjected to diagnostic PCR to determine if the BCC&P species had colonized the gut symbiotic organ of R. pedestris.
GFP- or RFP-Labeling of Bacterial Species.
PBE species (B. fungorum, Burkholderia phytofirmans, Burkholderia graminis, Burkholderia kururiensis), BCC&P species (B. plantarii, B. glumae, B. pyrrocinia), P. norimbergensis, E. coli, and C. taiwanensis were labeled with GFP by the Tn7 minitransposon system, as previously described (14). For labeling SBE Burkholderia (B. insecticola and B. cordobensis) with red fluorescent protein (RFP), pMini-Tn7-kan-rfp (60) was used under the same experimental conditions.
Histological Observations.
Insects were inoculated with Burkholderia or Pandoraea species (listed in SI Appendix, Table S2) at the second instar, and dissected 2 d after the third-instar molt (approximately 5 d after inoculation). The whole midgut was dissected from each insect in PBS, and photographed by a digital camera (EC3, Leica) connected to a dissection microscope (S8APO, Leica). To investigate colonization properties in more detail, GFP-labeled B. insecticola (SBE), B. fungorum (PBE), and P. norimbergensis (Pandoraea) were inoculated, and infected crypts were observed under a confocal LSM (TCS SP8, Leica). The dissected tissues were fixed with 4% paraformaldehyde for 10 min at room temperature, washed in PBS twice, incubated in PBS containing 0.1% Triton X-100 for 5 min, stained with 0.5 µM of SYTOX Green, and 5 units/mL of Alexa Fluor555 phalloidin (Thermofisher) in PBS for 20 min, washed in PBS twice, and mounted on silane-coated glass slides for observations.
Competitive Infection Assays.
In vitro competition assay.
For competition assays, a spontaneous rifampicin-mutant of B. insecticola (SBE) and spontaneous chloramphenicol-mutants of B. fungorum (PBE) and P. norimbergensis (Pandoraea) were used. Log-phase cultured cells of the SBE and a competitor (PBE or Pandoraea) were suspended in YG medium, adjusted to the same concentration at 5,000 CFU and mixed. The adjusted cells were diluted to 1 mL with YG medium in a 1.5-mL microtube, and incubated at 25 °C without shaking. Two days after the inoculation, the medium was serially diluted and plated on YG agar plates containing either rifampicin or chloramphenicol. After 3-d incubation at 25 °C, colonies of the SBE and competitor were counted on the plates. The CI was calculated by (output SBE CFU/input SBE CFU)/(output competitor CFU/input competitor CFU) (12).
In vivo competition assay.
Log-phase cultured cells of the SBE and a competitor (B. fungorum or P. norimbergensis) were suspended in distilled water, mixed, and adjusted to the same concentration at either 250 CFU/µL or 5,000 CFU/µL infection dose. In each assay, newly molted second-instar nymphs of R. pedestris were individually fed with 1 µL of the bacteria-containing suspensions, as previously described (12). Insects were reared at 25 °C under a long-day regimen and fed with soybean seeds and distilled water. Two days after the inoculation, the symbiotic organ was dissected from each of the insects and homogenized with sterile distilled water. The output homogenate was serially diluted and plated on YG agar plates containing antibiotics. After 3-d incubation at 25 °C, colonies of the SBE and competitor were counted on the plates and CI values were calculated.
To visualize the in vivo competition, an RFP-labeled SBE Burkholderia (B. insecticola or B. cordobensis) and a GFP-labeled competitor (B. fungorum, B. phytofirmans, B. graminis, B. kururiensis, or P. norimbergensis) were coinoculated, and the colonization process was investigated by fluorescence microscopy (DMI4000B, Leica). Based on the microscope images, red and green fluorescent areas were calculated by ImageJ (61) and used to evaluate the competitiveness of the SBE Burkholderia species.
To clarify whether T6SS is involved in the competition ability, 2 T6SS genes, vgrG and clpV (SI Appendix, Fig. S13A), were deleted in B. insecticola (SBE) by the homologous recombination technique, as previously described (18). vgrG, a tip protein playing a role as spike as well as an effector, is an essential gene for functional T6SS (62). clpV, encoding an AAA+ ATPase, is also 1 of the pivotal genes for the T6SS (62). The ΔvgrG and ΔclpV mutants were labeled with RFP and subjected to the in vivo competition assay, as described above.
Supplementary Material
Acknowledgments
We thank I. Nishi and N. Nakamura for technical assistance; C. Matsuura and Y. Matsuura for beautiful artwork; H. Shimoji for significant comments on statistics; and P. Mergaert for helpful comments on this manuscript. This work has benefited from the BSL3 center of the Hokkaido University Research Center for Zoonosis Control. This work was supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research 19K15724 (to H.I.) and Ministry of Education, Culture, Sports, Science, and Technology Grants-in-Aid for Scientific Research 15H05638 (to Y.K.), the Canon foundation (H.I.), the Japan Society for the Promotion of Science (JSPS) Bilateral Joined Research Project (Y.K.), and JSPS Research Fellowship for Young Scientists 201911493 (to S.J.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912397116/-/DCSupplemental.
References
- 1.Margulis L., Fester R., Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis (MIT Press, Cambridge, MA, 1991). [PubMed] [Google Scholar]
- 2.Ruby E., Henderson B., McFall-Ngai M., Microbiology. We get by with a little help from our (little) friends. Science 303, 1305–1307 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Bright M., Bulgheresi S., A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 8, 218–230 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs J. L., Mueller U. G., Wilcox T. P., Bull J. J., The evolution of cooperation. Q. Rev. Biol. 79, 135–160 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Simms E. L., et al. , An empirical test of partner choice mechanisms in a wild legume-rhizobium interaction. Proc. Biol. Sci. 273, 77–81 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondorosi E., Mergaert P., Kereszt A., A paradigm for endosymbiotic life: Cell differentiation of Rhizobium bacteria provoked by host plant factors. Annu. Rev. Microbiol. 67, 611–628 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Nyholm S. V., McFall-Ngai M. J., The winnowing: Establishing the squid-vibrio symbiosis. Nat. Rev. Microbiol. 2, 632–642 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Archetti M., et al. , Economic game theory for mutualism and cooperation. Ecol. Lett. 14, 1300–1312 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Scheuring I., Yu D. W., How to assemble a beneficial microbiome in three easy steps. Ecol. Lett. 15, 1300–1307 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeshita K., Kikuchi Y., Riptortus pedestris and Burkholderia symbiont: An ideal model system for insect-microbe symbiotic associations. Res. Microbiol. 168, 175–187 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi Y., Hosokawa T., Fukatsu T., Insect-microbe mutualism without vertical transmission: A stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 73, 4308–4316 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi Y., et al. , Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. U.S.A. 109, 8618–8622 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park K. E., et al. , The roles of antimicrobial peptide, rip-thanatin, in the midgut of Riptortus pedestris. Dev. Comp. Immunol. 78, 83–90 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Ohbayashi T., et al. , Insect’s intestinal organ for symbiont sorting. Proc. Natl. Acad. Sci. U.S.A. 112, E5179–E5188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futahashi R., et al. , Gene expression in gut symbiotic organ of stinkbug affected by extracellular bacterial symbiont. PLoS One 8, e64557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinosita Y., Kikuchi Y., Mikami N., Nakane D., Nishizaka T., Unforeseen swimming and gliding mode of an insect gut symbiont, Burkholderia sp. RPE64, with wrapping of the flagella around its cell body. ISME J. 12, 838–848 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohbayashi T., et al. , Comparative cytology, physiology and transcriptomics of Burkholderia insecticola in symbiosis with the bean bug Riptortus pedestris and in culture. ISME J. 13, 1469–1483 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J. K., et al. , Polyester synthesis genes associated with stress resistance are involved in an insect-bacterium symbiosis. Proc. Natl. Acad. Sci. U.S.A. 110, E2381–E2389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S. H., et al. , PhaR, a negative regulator of PhaP, modulates the colonization of a Burkholderia gut symbiont in the midgut of the host insect, Riptortus pedestris. Appl. Environ. Microbiol. 83, e00459-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eberl L., Vandamme P., Members of the genus Burkholderia: Good and bad guys. F1000Res. 5, 1007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beukes C. W., et al. , Genome data provides high support for generic boundaries in Burkholderia sensu lato. Front. Microbiol. 8, 1154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahenthiralingam E., Urban T. A., Goldberg J. B., The multifarious, multireplicon Burkholderia cepacia complex. Nat. Rev. Microbiol. 3, 144–156 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Levy A., et al. , Invasion of spores of the arbuscular mycorrhizal fungus Gigaspora decipiens by Burkholderia spp. Appl. Environ. Microbiol. 69, 6250–6256 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flórez L. V., et al. , Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect-defensive mutualism. Nat. Commun. 8, 15172 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suárez-Moreno Z. R., et al. , Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb. Ecol. 63, 249–266 (2012). [DOI] [PubMed] [Google Scholar]
- 26.DiSalvo S., et al. , Burkholderia bacteria infectiously induce the proto-farming symbiosis of Dictyostelium amoebae and food bacteria. Proc. Natl. Acad. Sci. U.S.A. 112, E5029–E5037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawana A., Adeolu M., Gupta R. S., Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front. Genet. 5, 429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeters C., et al. , Phylogenomic study of Burkholderia glathei-like organisms, proposal of 13 novel Burkholderia species and emended descriptions of Burkholderia sordidicola, Burkholderia zhejiangensis, and Burkholderia grimmiae. Front. Microbiol. 7, 877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobritsa A. P., Samadpour M., Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int. J. Syst. Evol. Microbiol. 66, 2836–2846 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Jeong S. E., Lee H. J., Jia B., Jeon C. O., Pandoraea terrae sp. nov., isolated from forest soil, and emended description of the genus Pandoraea Coenye et al. 2000. Int. J. Syst. Evol. Microbiol. 66, 3524–3530 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi Y., Meng X. Y., Fukatsu T., Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 71, 4035–4043 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikuchi Y., Hosokawa T., Fukatsu T., An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 5, 446–460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung M., Lee D. H., Abundance and diversity of gut-symbiotic bacteria, the genus Burkholderia in overwintering Riptortus pedestris (Hemiptera: Alydidae) populations and soil in South Korea. PLoS One 14, e0218240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh H., et al. , Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J. 12, 909–920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J. K., et al. , Bacterial cell wall synthesis gene uppP is required for Burkholderia colonization of the stinkbug gut. Appl. Environ. Microbiol. 79, 4879–4886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuchi Y., Hosokawa T., Fukatsu T., Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl. Environ. Microbiol. 77, 4075–4081 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kikuchi Y., Fukatsu T., Live imaging of symbiosis: Spatiotemporal infection dynamics of a GFP-labelled Burkholderia symbiont in the bean bug Riptortus pedestris. Mol. Ecol. 23, 1445–1456 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stopnisek N., et al. , Genus-wide acid tolerance accounts for the biogeographical distribution of soil Burkholderia populations. Environ. Microbiol. 16, 1503–1512 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Itoh H., et al. , Bacterial population succession and adaptation affected by insecticide application and soil spraying history. Front. Microbiol. 5, 457 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J. K., et al. , Purine biosynthesis-deficient Burkholderia mutants are incapable of symbiotic accommodation in the stinkbug. ISME J. 8, 552–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J. K., et al. , The lipopolysaccharide core oligosaccharide of Burkholderia plays a critical role in maintaining a proper gut symbiosis with the bean bug Riptortus pedestris. J. Biol. Chem. 292, 19226–19237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speare L., et al. , Bacterial symbionts use a type VI secretion system to eliminate competitors in their natural host. Proc. Natl. Acad. Sci. U.S.A. 115, E8528–E8537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steele M. I., Kwong W. K., Whiteley M., Moran N. A., Diversification of type VI secretion system toxins reveals ancient antagonism among bee gut microbes. MBio 8, e01630-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia E. C., Perault A. I., Marlatt S. A., Cotter P. A., Interbacterial signaling via Burkholderia contact-dependent growth inhibition system proteins. Proc. Natl. Acad. Sci. U.S.A. 113, 8296–8301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLoughlin K., Schluter J., Rakoff-Nahoum S., Smith A. L., Foster K. R., Host selection of microbiota via differential adhesion. Cell Host Microbe 19, 550–559 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Itoh H., et al. , Evidence of environmental and vertical transmission of Burkholderia symbionts in the oriental chinch bug, Cavelerius saccharivorus (Heteroptera: Blissidae). Appl. Environ. Microbiol. 80, 5974–5983 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boucias D. G., et al. , Detection and characterization of bacterial symbionts in the Heteropteran, Blissus insularis. FEMS Microbiol. Ecol. 82, 629–641 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Garcia J. R., et al. , Partner associations across sympatric broad-headed bug species and their environmentally acquired bacterial symbionts. Mol. Ecol. 23, 1333–1347 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Kuechler S. M., Matsuura Y., Dettner K., Kikuchi Y., Phylogenetically diverse Burkholderia associated with midgut crypts of spurge bugs, Dicranocephalus spp.(Heteroptera: Stenocephalidae). Microbes Environ. 31, 145–153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohbayashi T., Itoh H., Lachat J., Kikuchi Y., Mergaert P., Burkholderia gut symbionts associated with European and Japanese populations of the dock bug Coreus marginatus (Coreoidea: Coreidae). Microbes Environ. 34, 219–222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeshita K., et al. , Burkholderia of plant-beneficial group are symbiotically associated with bordered plant bugs (Heteroptera: Pyrrhocoroidea: Largidae). Microbes Environ. 30, 321–329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon E. R. L., McFrederick Q., Weirauch C., Phylogenetic evidence for ancient and persistent environmental symbiont reacquisition in Largidae (Hemiptera: Heteroptera). Appl. Environ. Microbiol. 82, 7123–7133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bull J. J., Rice W. R., Distinguishing mechanisms for the evolution of co-operation. J. Theor. Biol. 149, 63–74 (1991). [DOI] [PubMed] [Google Scholar]
- 54.Schluter J., Foster K. R., The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 10, e1001424 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranger C. M., et al. , Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl. Acad. Sci. U.S.A. 115, 4447–4452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lardi M., de Campos S. B., Purtschert G., Eberl L., Pessi G., Competition experiments for legume infection identify Burkholderia phymatum as a highly competitive β-rhizobium. Front. Microbiol. 8, 1527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haselkorn T. S., et al. , The specificity of Burkholderia symbionts in the social amoeba farming symbiosis: Prevalence, species, genetic and phenotypic diversity. Mol. Ecol. 28, 847–862 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Tago K., et al. , A fine-scale phylogenetic analysis of free-living Burkholderia species in sugarcane field soil. Microbes Environ. 29, 434–437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caporaso J. G., et al. , Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Norris M. H., Kang Y., Wilcox B., Hoang T. T., Stable, site-specific fluorescent tagging constructs optimized for burkholderia species. Appl. Environ. Microbiol. 76, 7635–7640 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abràmoff M. D., Magalhães P. J., Ram S. J., Image processing with ImageJ. Biophoton. Int. 11, 36–42 (2004). [Google Scholar]
- 62.Cianfanelli F. R., Monlezun L., Coulthurst S. J., Aim, load, fire: The type VI secretion system, a bacterial nanoweapon. Trends Microbiol. 24, 51–62 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.