Significance
Identifying the critical components of the complex infant experience within the nest that are necessary and sufficient to increase vulnerability to physical and mental disorders is a critical step toward the development of targeted interventions for neurobehavioral deficits observed in maltreated children. While it is generally believed that maternal behavior is correlated with infant outcomes, no assessment of the infant brain during caregiver-related maltreatment has been conducted to uncover causal factors. Here, we use animal models of maltreatment designed to build a bridge between human and animal literature. Our results suggest maltreatment-related social behavior and amygdala dysfunction require both an increase of the stress hormone corticosterone and the context of maternal presence, while hippocampal dysfunction depends only on increased corticosterone.
Keywords: maltreatment, amygdala, hippocampus, social behavior, corticosterone
Abstract
Infant maltreatment increases vulnerability to physical and mental disorders, yet specific mechanisms embedded within this complex infant experience that induce this vulnerability remain elusive. To define critical features of maltreatment-induced vulnerability, rat pups were reared from postnatal day 8 (PN8) with a maltreating mother, which produced amygdala and hippocampal deficits and decreased social behavior at PN13. Next, we deconstructed the maltreatment experience to reveal sufficient and necessary conditions to induce this phenotype. Social behavior and amygdala deficits (volume, neurogenesis, c-Fos, local field potential) required combined chronic high corticosterone and maternal presence (not maternal behavior). Hippocampal deficits were induced by chronic high corticosterone regardless of social context. Causation was shown by blocking corticosterone during maltreatment and suppressing amygdala activity during social behavior testing. These results highlight (1) that early life maltreatment initiates multiple pathways to pathology, each with distinct causal mechanisms and outcomes, and (2) the importance of social presence on brain development.
Early life maltreatment from the caregiver is a risk factor for myriad physical and mental health disorders, most of which emerge in later life in both humans and animal models (1–7). Yet, we still have little understanding of how the infant brain responds to maltreatment and which specific variables in this complex social trauma initiate the aberrant developmental trajectory to induce later life pathology. Two variables have consistently been highlighted as detrimental during early life in both human studies and animal models: increases in stress hormones, particularly glucocorticoids, and trauma associated with the caregiver versus trauma experienced alone (8–13). Moreover, while typical rearing is associated with the caregiver being able to soothe a threatened infant and attenuating stress hormone release (termed social buffering), this process is compromised in maltreatment rearing (14). This suggests that the maltreated infant has pairings of elevated stress hormone while with the mother or other caregiver, which would rarely occur in typically reared children. Here, we manipulate these variables (maternal context of stress, corticosterone levels) and focus on brain regions consistently shown to be targeted by early life trauma in humans and animal models: the amygdala and hippocampus.
The delayed emergence of neurobehavioral vulnerability to pathologies induced by early life trauma challenges our identification of the developmental causes of later dysfunction. However, subtle predictive markers have been identified in young children, such as parental observations of their infant’s heightened anxiety/fear and disrupted infant social behavior within mother–infant interactions (15, 16). For this reason, we assessed social behavior toward the mother as an early life biomarker for abnormal brain development. Understanding the neurobiology of these early life behaviors has also been challenging, although the amygdala and hippocampus have been implicated as loci of dysfunction following trauma in young children and animal models (17–20). Accordingly, we focused on the amygdala and hippocampus to better understand the neural signature of the response to maltreatment.
We used a 2-pronged approach to assess the neurobehavioral response to maltreatment involving (1) a naturalistic paradigm where the mother rat maltreats the pups, providing a natural maltreatment-induced phenotype, and then (2) deconstructing the complex natural experiences associated with maltreatment to identify the necessary and sufficient conditions to mimic the maltreatment-induced phenotype. Specifically, in our deconstructed maltreatment experience, we precisely control and isolate 2 critical features of infant maltreatment: elevation of the stress hormone corticosterone (or control saline) and the social context of stress hormone elevation (with an awake-behaving mother expressing typical caregiving, an anesthetized mother to separate effects of maternal presence from maternal behavior, or a nonsocial tube). We present results suggesting the necessary and sufficient conditions for chronic stress to induce social behavior and amygdala deficits require the social context of the mother, while hippocampal deficits are unconstrained by the social context of stress.
Results
Our naturalistic maltreatment study (experiment 1) and our deconstructed reproduction of some aspects of the maltreatment experience (experiment 2) both begin on postnatal day 8 (PN8) and continuing until testing. In both experiments, pups are removed from the nest on PN13 and given a social behavior test with an anesthetized mother to enable the observation of pups’ neurobehavioral response to the mother without maternal behavioral participation.
Experiment 1.
Maltreatment rearing increases pups’ corticosterone levels and alters social behavior toward the mother.
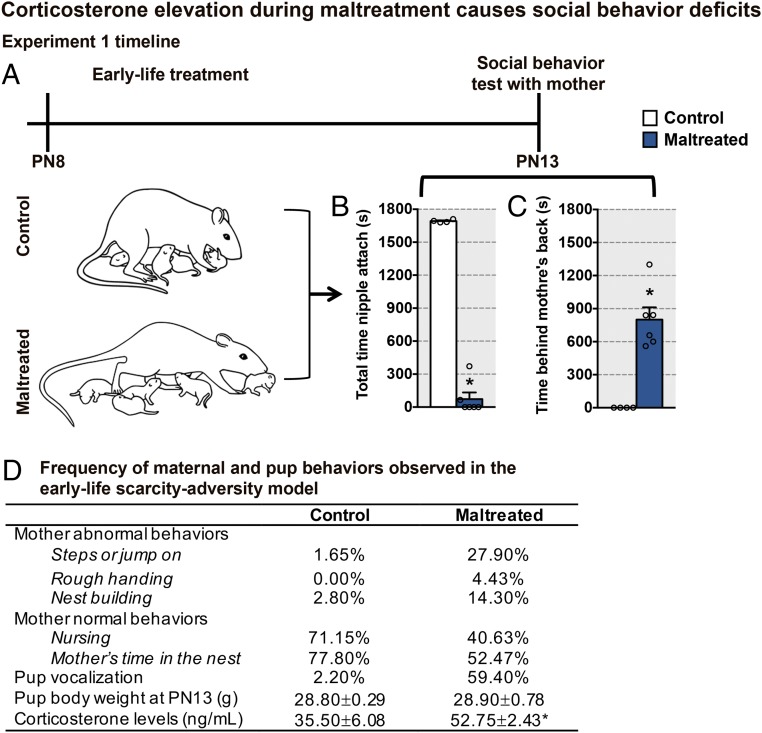
To provide a benchmark for studying the role of corticosterone within a social context and maltreatment-induced deficits, we began by using a well-validated naturalistic maltreatment animal model of early life, scarcity-adversity (Fig. 1A), which is known to produce adult psychopathologies and target the amygdala and hippocampus (16, 21–24). In this model, maltreatment-like maternal behaviors were induced by providing the mother rat with insufficient nest-building materials (Fig. 1D and SI Appendix, Online Materials). This induces rough handling of pups and frequent nest building, although pups gain weight normally. Control mothers were housed with sufficient nest-building materials and did not exhibit maltreatment-like behavior toward pups. Importantly, our results indicate that 5 d of this maltreatment rearing procedure increased pups’ corticosterone levels (Fig. 1D), corroborating previous findings (16).
Fig. 1.
Maltreatment induces social attachment behavior deficits. (A) Experimental design showing that half of the animals were exposed to a scarcity-adversity (maltreated) model of early life in which the mother and her pups were housed in a cage containing low bedding continuously starting from PN8. As a control, the remaining mothers were housed in cages with abundant bedding material for nest building. At PN13, pups received a 30-min pup social behavior test with an anesthetized mother. The use of an anesthetized mother eliminated the contribution of the mother to pup behavior and enabled us to uncover pup neurobehavioral deficits. (B and C) At PN13, pup behavior during the pup social behavior test showed that the maltreated pups showed aberrant social behaviors with the mother [total time nipple attached: t(8) = 21.26, P < 0.0001; time behind the mother’s back: t(8) = 5.75, P = 0.0004]. Over the course of the treatment, approach toward the nonsocial tube stimulus did not differ between corticosterone-treated and saline-treated pups (number of contacts, tube + saline: day 1, 1 ± 0.26; end of treatment, 0.5 ± 0.22; number of contacts, tube + CORT: day 1, 0.83 ± 0.31, end of treatment, 0.83 ± 0.307; number of contacts, mother + saline: day 1, 6 ± 0; end of treatment, 6 ± 0; number of contacts, mother + tube: day 1, 4 ± 0.68; end of treatment, 6 ± 0). (D) Table showing the proportion of maternal behaviors observed during the maltreatment exposure; body weight is not different between rearing conditions [t(8) = 0.10, P = 0.993], and serum corticosterone levels were higher in maltreated pups [t(8) = 3.04, P = 0.016] at PN13. Data are expressed as mean (±SEM) and considered significant when P 0.05. *Maltreated pups were different from control reared pups (n = 4 to 6 for all groups).
At PN13, pups in both rearing conditions underwent a 30-min social behavior test with an anesthetized mother. This test eliminates maternal behavior but retains the maternal odor cue to enable pups to identify their mother (25). The mild stress of exposing the pups to this social behavior test uncovered behavioral differences that are not observed within the nest environment (16). Specifically, our results show that maltreated-reared pups displayed aberrant social behavior toward the mother compared with controls (Fig. 1 B and C), as they spend less time nipple-attached and spend more time behind the mother’s back rather than at the ventrum.
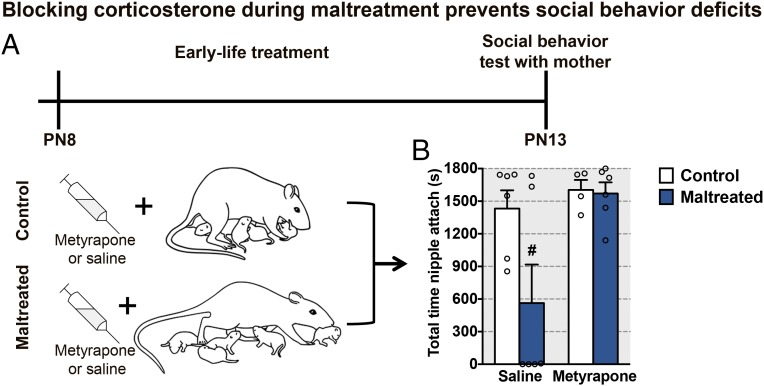
This maltreatment-induced atypical social behavior with the mother was averted by preventing pup corticosterone increases during maltreatment rearing. Specifically, we administered the corticosterone synthesis inhibitor metyrapone (intraperitoneally, 50 mg/kg; Sigma) or saline daily to pups before using the same scarcity-adversity–induced maltreatment described above. However, to limit our blockade of corticosterone to the time of maltreatment, we only depleted the dam’s nesting resources daily for 1 h, again beginning on PN8 and testing on PN13 with an anesthetized mother (Fig. 2). Limiting bedding for 1 h each day reliably increased maltreatment by the mother [replicates (6)] and was associated with pup social behavior deficits toward the mother during the social behavior test, similar to chronic maltreatment.
Fig. 2.
Maltreatment produces corticosterone-dependent alterations in social behavior toward the mother, which are rescued by corticosterone blockade during maltreatment. (A) Schematic of study design in which rat pups received metyrapone (50 mg/kg) or an equal volume of saline before daily bouts of low bedding. (B) Social behavior during the interaction test with the mother shows that attachment deficits associated with maltreatment were prevented in pups that received metyrapone [rearing condition: F(1,18) = 6.64, P = 0.019; metyrapone: F(1,18) = 3.89, P = 0.064; interaction between rearing condition and metyrapone for total time nipple attached: F(1,18) = 3.34, P = 0.084]. #A priori comparison between maltreated saline and maltreated metyrapone pups (P = 0.021). Data are expressed as mean (±SEM) and considered significant when P 0.05 (n = 4 to 6 for all groups).
Data presented in Figs. 1 and 2 indicate that maltreatment increases pup corticosterone levels and disrupts pup social behavior toward the mother, which can be prevented by blocking up-regulation of corticosterone during maltreatment. Taken together, these results suggest that maltreatment impacts social behavior with the mother through up-regulation of stress hormone levels.
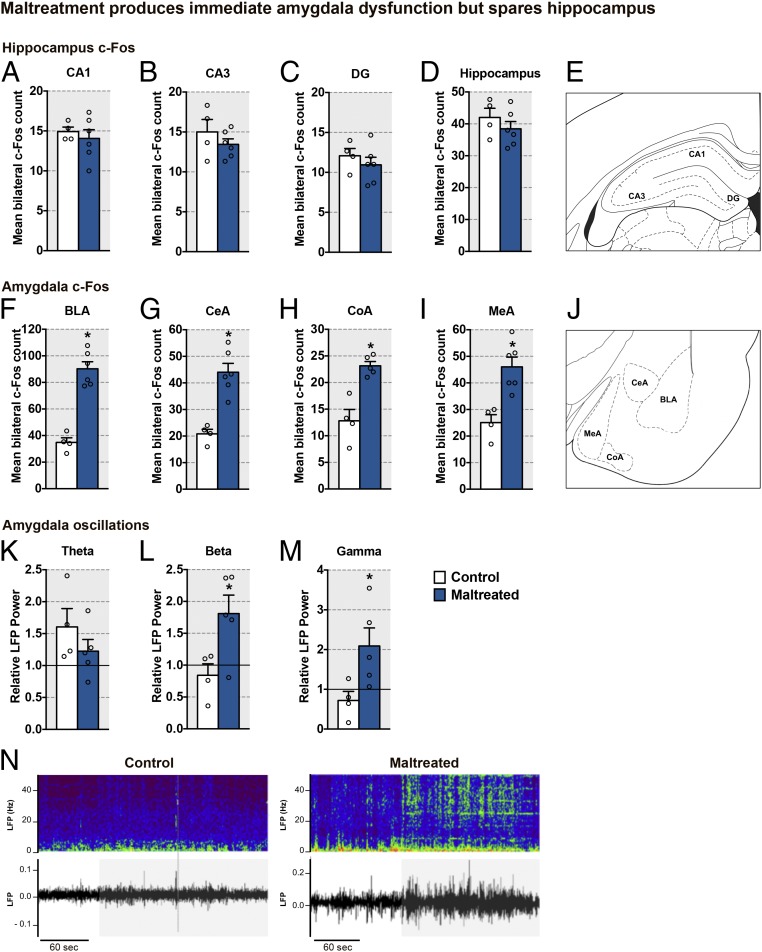
Maltreatment produces immediate amygdala dysfunction but spares the hippocampus.
To explore the neurobiology of maltreatment-induced behavioral deficits, we examined functional and structural changes to the amygdala and hippocampus, 2 brain areas highlighted as targets of stress in the literature (2, 20, 26). As shown in Fig. 3, maltreated pups’ atypical social behavior with the mother at PN13 was associated with amygdala neural hyperactivity but no detectable changes in the hippocampus, as indicated by c-Fos expression 90 min after the mother–pup social behavior test. Specifically, neural activity in amygdala, including the basolateral (BLA), central (CeA), cortical (CoA), and medial (MeA) nuclei, was significantly higher in maltreated pups during social behaviors compared with control pups (Fig. 3 F–J). In contrast, overall hippocampal c-Fos and regional measures in CA1, CA3, and the dentate gyrus were not significantly different between groups (Fig. 3 A–E).
Fig. 3.
Maltreatment alters the neural response to maternal presence. (A–D) c-Fos expression (mean ± SEM) in different subfields of the hippocampus and total hippocampus 90 min following the social behavior test [CA1: t(8) = 0.59, P = 0.570; CA3: t(8) = 1.04, P = 0.330; dentate gyrus (DG): t(8) = 0.82, P = 0.430; total hippocampus: t(8) = 0.97, P = 0.360]. (E) Schematic representation of the hippocampus. (F–I) c-Fos expression (mean ± SEM) in different amygdala nuclei 90 min following the social behavior test [BLA: t(8) = 7.82, P < 0.0001; CeA: t(8) = 5.28, P = 0.0007; CoA: t(7) = 4.97, P = 0.002; MeA: t(8) = 4.04, P = 0.004]. (J) Schematic representation of amygdala nuclei analyzed. (K–M) LFPs (mean ± SEM) in the amygdala [theta: t(7) = 1.16, P = 0.285; beta: t(7) = 2.67, P = 0.032; gamma: t(7) = 2.48, P = 0.042]. (N) Sonogram traces in response to maternal presence (beginning at the time indicated by the gray area) in control (Left) and maltreated (Right) pups. Data are expressed as mean (±SEM) and considered significant when P 0.05. *Maltreated pups were different from control pups (n = 4 to 6 for all groups).
Due to the increased amygdala neural activity in maltreated pups, we measured amygdala local field potentials (LFPs) using telemetry in untethered pups to determine potential dynamic rhythmic neural activity within the amygdala as pups interacted with an anesthetized mother (Fig. 3 K–N). Our previous work indicated that typically reared pups’ LFPs showed dynamic decreases in both the gamma- and beta-frequency bands with maternal presence, which co-occurred in the cortical areas (27) and the somatosensory system (28). Our current results show that the amygdalae of maltreated pups, compared with controls, displayed significantly enhanced power in gamma (35 to 100 Hz) and beta (15 to 35 Hz) frequencies, while the theta-frequency band (5 to 15 Hz) was not altered (Fig. 3 K–N). Overall, pups exposed to maltreatment failed to exhibit the maternal presence-induced decrease in LFP high-frequency oscillations observed in control pups, suggesting diminished ability of maternal sensory cues to influence the maltreatment-reared pups (27).
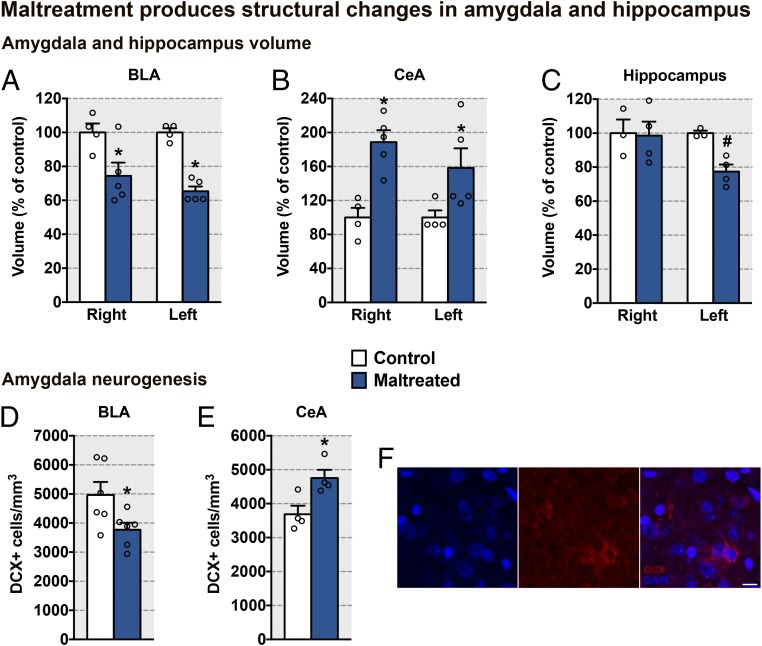
Different from the functional changes, maltreatment-induced structural changes were observed in both the amygdala and hippocampus in PN13 pups. Specifically, we observed volumetric decreases in the left and right BLA nucleus of maltreated pups, compared with control pups (Fig. 4A). Conversely, the volume of the left and right CeA nucleus was increased in maltreated pups, compared with control pups (Fig. 4B). In the hippocampus, however, only the left side was affected, with maltreated pups exhibiting a smaller hippocampal volume compared with controls (Fig. 4C). The volumetric alterations in the BLA and CeA nuclei of maltreated pups were associated with altered neurogenesis [doublecortin expression (DCX), an endogenous protein maximally expressed in neuroblasts and immature neurons at ∼2 wk of age (29, 30)]. As neurogenesis, differentiation, and migration are minimal between PN0 and PN14 in the amygdala (31), the sparse expression patterns observed here may reflect late-emerging embryonic neurogenesis (32) and differences between groups likely represent variation in neuronal survival (neuron density decreases between PN7 and PN14) (31). We observed that, parallel to volumetric alterations, maltreated pups exhibited suppressed DCX in the BLA nucleus and enhanced DCX in the CeA nucleus compared with controls (Fig. 4 D–F). The maltreatment-related increase in CeA volume replicates data from children in whom maltreatment was associated with greater amygdala volume (33). Together, these results provide a clinically relevant template to dissect specific causal features of the highly complex experience of maltreatment that initiates the pathway to pathology.
Fig. 4.
Maltreatment induces amygdala structural alterations. (A–C) Volume (mean ± SEM) of different amygdala nuclei and the hippocampus [BLA, maltreatment: F(1,7) = 23.89, P = 0.002; side: F(1,7) = 1.04, P = 0.342; interaction between maltreatment and side: F(1,7) = 1.09, P = 0.329; CeA, maltreatment: F(1,7) = 11.34, P = 0.01; side F(1,7) = 1.26, P = 0.297; interaction between maltreatment and side: F(1,7) = 0.42, P = 0.536; hippocampus, maltreatment: F(1,5) = 2.66, P = 0.163; side: F(1,5) = 3.41, P = 0.124; interaction between maltreatment and side: F(1,5) = 4.08, P = 0.09]. #A priori comparison between maltreated and control on the left (P = 0.006). (D and E) DCX (mean ± SEM) in different amygdala nuclei [BLA: t(10) = 2.36, P = 0.040; CeA: t(6) = 3.06, P = 0.022]. (F) Representative images of DCX-labeled immature neurons in the amygdala (red, DCX; blue, DAPI). (Scale bar, 10 μm.) Data are expressed as mean (±SEM) and considered significant when P 0.05. *Maltreated pups were different from control pups (n = 3 to 5 for all groups).
Experiment 2.
Daily corticosterone administration paired with maternal presence mimics effects of maltreatment.
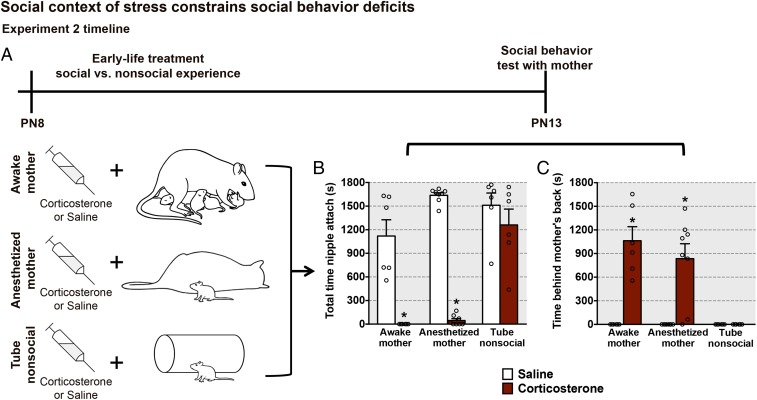
To identify which components of caregiver maltreatment are necessary and sufficient to induce the neurobehavioral deficits observed in experiment 1, we deconstructed pups’ experiences within the maltreating mother–infant dyad. Here, we recapitulated 2 features of maltreatment, chronic high corticosterone and maternal context, using the same treatment age range as in experiment 1. This experiment is illustrated in Fig. 5A and involves daily injections of corticosterone (or saline) to pups while with an awake nurturing mother, an anesthetized mother, or a nonsocial polyethylene tube. In the “awake mother” treatment, pups were reared by a nurturing mother (i.e., typical control mother) and received 1 injection of corticosterone (3 mg/kg; Sigma) or saline once per day for 5 d. This corticosterone injection raised pups’ corticosterone levels for about 60 min (34, 35). This treatment group receiving corticosterone during typical nurturing care from the mother was used to mimic the stress hormone increase observed with abuse (Fig. 1D) but without maltreatment behavior from the mother. In the “anesthetized mother” treatment, pups were also reared by a nurturing mother and removed from the nest for 90 min once per day for 5 d to receive a corticosterone injection to produce an increase in corticosterone limited to the presence of an anesthetized mother. In this condition, pups remained with the anesthetized mother, engaged in social behaviors toward her, and maintained proximity to the mother. Thus, this condition preserved pup behavior while eliminating all maternal behaviors during the period of elevated corticosterone levels. Finally, the maternal context of stress was completely removed in another cohort of pups (“tube nonsocial”) that were removed from the nest, given corticosterone injections, and placed with a nonsocial stimulus (polyethylene tube) for 90 min once per day for 5 d. Similar to experiment 1, all pups were given a social behavior test with an anesthetized mother at PN13, instead of another treatment session. Importantly, no drug treatments occurred during this social behavior test. It should be noted that we did not find differences between our infant treatment groups when they were assessed in the nest with a typically behaving mother at PN13, where maternal behavior can facilitate typical nursing and social behaviors in pups (percentage of time spent nursing: control + saline vs. control + corticosterone [45.4 ± 1.1 vs. 58.54 ± 4.92; t(5) = 1.42, P = 0.214]).
Fig. 5.
High corticosterone levels within a social context mimic effects of maltreatment on social behavior at PN13. (A) Experimental design showing that half of the animals were maintained with high corticosterone levels through daily injections (3 mg/kg), while the other half were injected with saline (control). For the awake mother group, pups were reared by a nurturing mother and received daily injections of corticosterone or saline. For the anesthetized mother group, pups were reared by a nurturing mother, were injected with corticosterone or saline, and were placed in the presence of an anesthetized mother for 90 min daily beginning at PN8, which eliminated all maternal behaviors but limited elevated corticosterone levels to this specific social context. For the tube nonsocial group, pups were reared by a nurturing mother, injected with corticosterone or saline, and placed in the presence of a nonsocial stimulus (odorized tube), which completely removed the maternal influence but maintained elevated corticosterone levels. At PN13, pups received a 30-min social behavior test with an anesthetized mother with no drug treatment. (B and C) Pup behavior during the mother–pup social behavior test [total time nipple attached, social context: F(2,34) = 21.92, P < 0.0001; corticosterone: F(1,34) = 98.53, P < 0.0001; interaction between social context and corticosterone: F(2,34) =15.99, P < 0.0001; time behind the mother’s back, social context: F(2,34) = 11.04, P = 0.0002; corticosterone: F(1,34) = 45.32, P < 0.0001; interaction between social context and corticosterone: F(2,34) = 11.04, P = 0.0002]. Data are expressed as mean (±SEM) and considered significant when P 0.05. *Difference from all other groups (n = 6 to 8 for all groups).
Social context of stress constrains social behavior deficits.
As illustrated in Fig. 5, behavioral effects of maltreatment could be mimicked by elevating corticosterone levels in the presence of the mother (both awake and anesthetized), but not by elevating corticosterone in the presence of a nonsocial stimulus. Specifically, during the PN13 social behavior test, pups that received daily treatment with corticosterone in the context of a mother, regardless of whether she was awake or anesthetized, resulted in aberrant social behavior with the mother, as pups showed reduced time nipple-attached and spent more time behind the mother’s back compared with controls (Fig. 5 B and C). Remarkably, exposure to high corticosterone levels within a nonsocial context (tube) did not induce any infant social behavior deficits with the mother during the PN13 test. Together, these results suggest that the association between high corticosterone levels and the mother’s presence, but not the mother’s behavior, results in social behavior deficits in infancy.
Social context of stress constrains amygdala dysfunction but not the hippocampus.
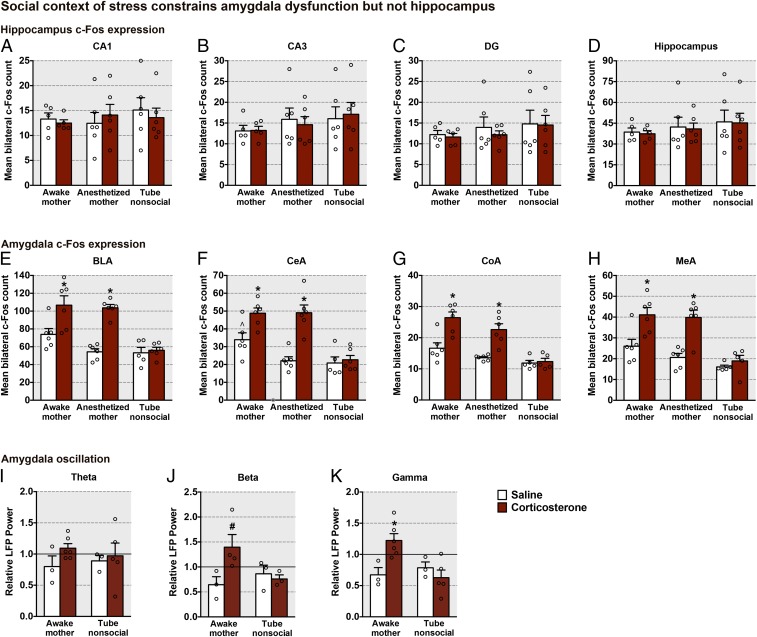
The aberrant social behavior with the mother observed in pups previously exposed to high corticosterone levels in the presence of a social context (awake or anesthetized mother) was associated with amygdala hyperactivity (Fig. 6 E–H). Indeed, evaluation of c-Fos expression after the mother–pup social behavior test indicated that amygdala (BLA, CeA, MeA, and CoA nuclei) neural activity was significantly higher in all PN13 pups exposed to corticosterone paired with an awake or anesthetized mother, compared with pups that received saline injections. Importantly, no alteration in amygdala neural activity was observed in pups that were exposed to daily high corticosterone levels within a nonsocial context (tube). Hippocampal neural activity was not altered by any of the treatments (Fig. 6 A–D).
Fig. 6.
High corticosterone levels within a social context daily over 5 d mimic the effects of maltreatment on amygdala function at PN13. (A–D) c-Fos expression (mean ± SEM) in the hippocampus 90 min following the social behavior test [CA1, social context: F(2,28) = 0.29, P = 0.749; corticosterone: F(1,28) = 0.02, P = 0.892; interaction between social context and corticosterone: F(2,28) = 0.38, P = 0.687; CA3, social context: F(2,28) = 1.02, P = 0.373; corticosterone: F(1,28) = 0.002, P = 0.987; interaction between social context and corticosterone: F(2,28) = 0.13, P = 0.876; dentate gyrus (DG), social context: F(2,28) = 0.79, P = 0.463; corticosterone: F(1,28) = 0.23, P = 0.632; interaction between social context and corticosterone: F(2,28) = 0.07, P = 0.932; total hippocampus, social context: F(2,28) = 0.75, P = 0.478; corticosterone: F(1,28) = 0.05, P = 0.826; interaction between social context and corticosterone: F(2,28) = 0.001, P = 0.998]. (E–H) c-Fos expression (mean ± SEM) in different amygdala nuclei 90 min following the social behavior test [BLA, social context: F(2,29) = 16.04, P < 0.0001; corticosterone: F(1,29) = 31.07, P < 0.0001; interaction between social context and corticosterone: F(2,29) = 7.01, P = 0.003; CeA, social context: F(2,29) = 18.25, P < 0.0001; corticosterone: F(1,29) = 29.17, P < 0.0001; interaction between social context and corticosterone: F(2,29) = 7.18, P = 0.003; MeA, social context: F(2,28) = 16.04, P < 0.0001; corticosterone, F(1,28) = 27.23, P < 0.0001; interaction between social context and corticosterone: F(2,28) = 4.05, P = 0.028; CoA, social context: F(2,28) = 20.57, P < 0.0001; corticosterone: F(1,28) = 29.30, P < 0.0001; interaction between social context and corticosterone: F(2,28) = 5.97, P = 0.007]. (I–K) LFP (mean ± SEM) in the amygdala [theta, social context: F(1,13) = 0.10, P = 0.921; corticosterone: F(1,13) = 1.47, P = 0.247; interaction between social context and corticosterone: F(1,13) = 0.46, P = 0.507; beta, social context: F(1,9) = 1.08, P = 0.324; corticosterone: F(1,9) = 2.62, P = 0.140; interaction between social context and corticosterone: F(1,9) = 4.54, P = 0.06]. #A priori comparison between awake mother paired with saline and awake mother paired with corticosterone (P = 0.036) for beta-frequency [gamma, social context: F(1,13) = 3.71, P = 0.08; corticosterone: F(1,13) = 2.46, P = 0.140; interaction between social context and corticosterone: F(1,13) = 7.98, P = 0.01]. Data are expressed as mean (±SEM) and considered significant when P 0.05. *Difference from all other groups (n = 3 to 6 for all groups).
The elevated LFP beta- and gamma-band activity found in PN13 pups exposed to continuous maltreatment rearing in their nest was also mimicked by daily 90-min treatments of corticosterone injections within a social context (Fig. 6 I–K). Similar to control-reared pups in experiment 1, daily saline-treated pups showed a decrease in high-frequency oscillations when the mother was placed in the testing area, consistent with previous work (27). In contrast, pups that had simply received corticosterone injections with the maternal presence for 5 d before testing exhibited enhanced amygdala beta- and gamma-frequency oscillations when the mother was placed in the testing area (Fig. 6 I–K). In contrast, pups injected daily with corticosterone or saline in the presence of a tube failed to show these beta- and gamma-band elevations when exposed to a mother on PN13.
Social context of stress constrains amygdala structural alterations but not the hippocampus.
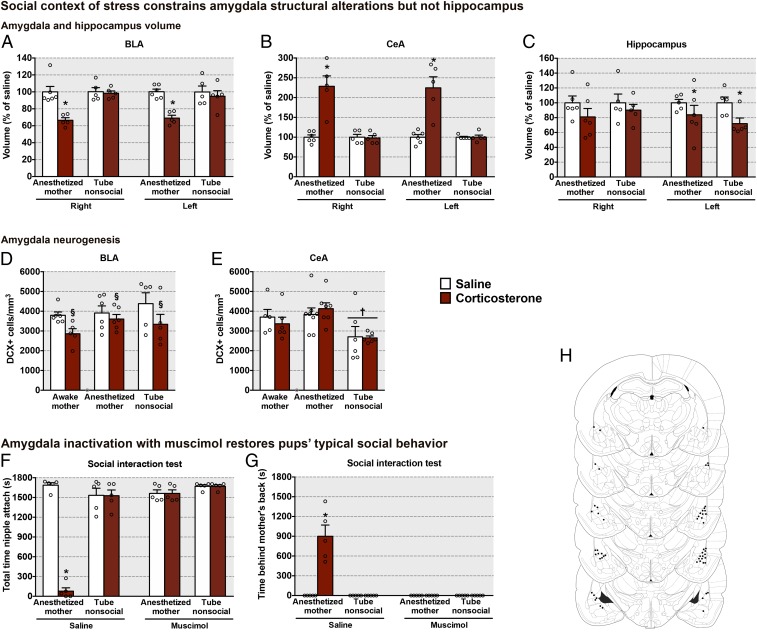
The functional alterations in amygdala responsivity to the mother observed in pups exposed to high corticosterone levels in the presence of the mother were also accompanied by structural alterations. Indeed, the volume of the left and right BLA nucleus was smaller in pups exposed to high corticosterone levels while in the presence of an anesthetized mother compared with pups exposed to high corticosterone levels within a nonsocial context (Fig. 7A). Conversely, the volume of the left and right CeA nucleus was larger in pups exposed to high corticosterone levels while in the presence of an anesthetized mother compared with pups exposed to high corticosterone levels within a nonsocial context (Fig. 7B).
Fig. 7.
High corticosterone levels within a social context induce amygdala structural alterations, and amygdala infusions of muscimol (GABA agonist) restored pups’ typical social attachment behavior. (A–C) Volume (mean ± SEM) of different amygdala nuclei and hippocampus [BLA, social context: F(1,17) = 29.13, P < 0.0001; corticosterone: F(1,17) = 15.93, P = 0.009; side: F(1,17) = 1.83, P = 0.193; interaction between social context and corticosterone: F(1,17) = 9.96, P = 0.006; CeA, social context: F(1,17) = 19.06, P = 0.0004; corticosterone: F(1,17) = 21.15, P = 0.0002; side: F(1,17) = 0.99, P = 0.333; interaction between social context and corticosterone: F(1,17) = 21.91, P = 0.0002; hippocampus, social context: F(1,18) = 0.18, P = 0.678; corticosterone: F(1,18) = 4.22, P = 0.05; side: F(1,17) = 14.07, P = 0.001; interaction between side and corticosterone: F(1,18) = 5.54, P = 0.03]. (D and E) DCX (mean ± SEM) in different amygdala nuclei [BLA, social context: F(2,27) = 1.25, P = 0.303; corticosterone: F(1,27) = 6.84, P = 0.014; interaction between social context and corticosterone: F(2,27) = 0.65, P = 0.527; CeA, social context: F(2,32) = 7.48, P = 0.002; corticosterone: F(1,32) = 0.01, P = 0.912; interaction between social context and corticosterone: F(2,32) = 0.42, P = 0.662]. †Significant main effect of social context, where all tube nonsocial animals are different from awake and anesthetized mother animals independent of corticosterone. §Significant main effect of corticosterone exposure, where all animals exposed to corticosterone are different from animals exposed to saline independent of social context. (F and G) Pup behavior during the social behavior test [total time nipple attached, interaction between social context, corticosterone, and muscimol: F(1,32) = 85.65, P < 0.0001; time behind the mother’s back, interaction between social context, corticosterone, and muscimol: F(1,32) = 28.04, P < 0.0001]. (H) Representative cannula placements in the amygdala for animals receiving either a muscimol or saline infusion at PN13. Schematic brain section images are displayed from the most rostral to most caudal orientation. Adapted from ref. 90. Data are expressed as mean (±SEM) and considered significant when P 0.05. *Difference from all other groups (n = 5 to 8 for all groups).
Corticosterone pairings with social context was not required for all neural deficits associated with maltreatment. The volume of the left hippocampus in all pups exposed to high corticosterone levels, independent of context, was smaller when compared with the hippocampal volume of pups that received saline (Fig. 7C). The volume of the right hippocampus was not affected by exposure to high corticosterone. The volumetric alterations observed in the BLA and CeA nuclei of pups exposed to high corticosterone levels while in the presence of an anesthetized mother did not necessary align with changes in immature neurons. Specifically, pups exposed to high corticosterone levels showed decreased DCX in the BLA nucleus when compared with pups that received saline (Fig. 7D). Additionally, all pups exposed to the tube nonsocial context showed decreased DCX in the CeA nucleus when compared with pups exposed to a social context, regardless of the corticosterone treatment (Fig. 7E). Together, these social constrained amygdala and nonsocial constrained hippocampal results recapitulate the results induced by the scarcity-adversity maltreatment of experiment 1.
Amygdala engagement is causal in disrupted social behavior following chronic stress within a social context.
To directly assess whether amygdala engagement was causal in the behavior deficits observed in the pups exposed to chronic stress in a social context, we suppressed amygdala neural hyperactivity of pups during the PN13 social behavior test. Specifically, pups were implanted with bilateral amygdala cannulae at PN12 following the corticosterone–mother treatment. At PN13, we temporarily silenced the amygdala during the mother–pup social behavior test by intraamygdala infusions of the gamma-aminobutyric acid (GABA) agonist muscimol (0.4 nmol; Sigma). Suppression of amygdala hyperactivity by muscimol infusion reestablished pups’ typical social behavior with the mother (Fig. 7 F–H), while saline infusions did not prevent expression of deficits. Muscimol and vehicle infusions did not affect the typical social behavior of pups that had been exposed to daily saline injections with maternal presence or pups exposed to daily corticosterone/saline injections in the nonsocial tube condition. These results suggest that (1) the amygdala does not normally participate in infant rat social behavior, and (2) experience with chronic high corticosterone levels within a social context prematurely engages the amygdala to disrupt social behavior. This is consistent with research in infant nonhuman primates, where amygdala engagement has been suggested to put a “brake” on infant social behavior (36).
Discussion
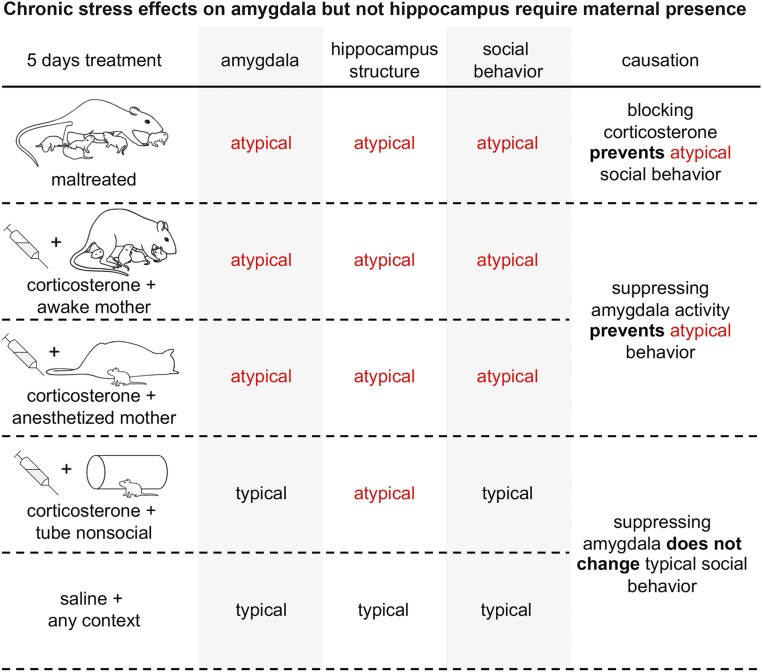
The link between infant maltreatment and later life vulnerability to psychopathologies is well documented. Here, we present specific mechanisms underlying abnormal amygdala and hippocampus development, with only the amygdala requiring social context for initiation of abnormal development. In the present series of experiments, rat pups were reared for 5 d with a maltreating mother beginning on PN8, which increased PN13 pup corticosterone levels, impacted social behaviors with the mother, and altered hippocampal structure (volume, neurogenesis) and amygdala function (c-Fos, LFP) and structure (volume, neurogenesis) (Fig. 8). Next, we deconstructed the infant maltreatment experience to reveal sufficient and necessary conditions to induce these outcomes using the same treatment ages. While hippocampal damage could be phenocopied by merely elevating corticosterone levels under any experimental condition, unexpectedly, pairing corticosterone with the mother, even when anesthetized, was required to recapitulate maltreatment effects on social behavior and the amygdala. Causation was shown by blocking corticosterone during maltreatment or suppressing amygdala activity during social behavior testing. These results significantly extend our current understanding of maternal behavior and late-life outcomes by defining immediate infant mechanisms initiating the neurobehavioral developmental trajectory, ultimately increasing vulnerability to psychopathologies.
Fig. 8.
Summary of behavioral and neural effects of maltreatment and corticosterone injection paired with a social context (awake or anesthetized mother) or a nonsocial context (tube). Maltreatment impacts both the hippocampus and amygdala. The effects of maltreatment on the hippocampus can be mimicked simply by repeatedly injecting pups with corticosterone, regardless of context, ranging from experience with a nurturing mother to placement with the nonsocial context of a tube. On the other hand, the effects of maltreatment on the amygdala required a social context that was independent of maternal behavior since stress hormone increased within the context of a maltreating mother, a nurturing mother, and an anesthetized mother all produced similar amygdala outcomes.
Maltreatment-Induced Changes to the Hippocampus Could Be Recapitulated with or without a Social Context.
During maltreatment or any of our deconstructed stress treatment paradigms, the hippocampus showed no activity changes (c-Fos), but pups exhibited a smaller left hippocampal volume compared with controls. The late onset of hippocampal engagement in social behavior likely contributes to our failure to show functional hippocampal changes during social behaviors (37–40). However, the reduced left hippocampal volume observed here replicates extensive work in humans showing enhanced effects of maltreatment on the left hippocampus (41–45). Although mechanisms remain unknown, indirect mediation of glucocorticoid effects via glutamate may play a role in these effects (46). Some have speculated that asymmetry in the distribution of N-methyl-D-aspartate (NMDA) receptors between the left and right hippocampus (47) translates to hemispheric differences in NMDA receptor function and synaptic plasticity in hippocampal subfields to produce these outcomes. Moreover, the ability of stress, regardless of social context and regardless of maternal behavior, to disrupt hippocampal development is consistent with the literature showing this region to be a target of a wide range of early life trauma (48). While stress hormones are well known to impact brain development (2, 49, 50), our data also suggest that some effects are dependent upon maternal presence during stress and illustrate the importance of the social figure in guiding some features of brain development (20, 51).
The Amygdala Is Not Typically Incorporated into the Infant Social Behavior Circuit but Is Prematurely Engaged by Chronic Stress Hormone Elevation.
The present results suggest the amygdala is not part of the social behavior network of typically developing infants and its precocious engagement disrupts social behavior with the mother. This is consistent with the nonhuman primate literature, where precocious activation of the amygdala puts a brake on social behavior (36, 52, 53). Leveraging the advantages of animal research, we silenced the amygdala in pups following treatment with high corticosterone levels in the presence of a social context; removing the amygdala from the infant social behavior network was sufficient to return social behavior to control levels, while leaving control social behavior undisturbed. Together, these data suggest that chronic stress in a social context precociously engages the amygdala, disrupting social behavior toward the mother.
The literature also suggests that stress hormone elevation may be required to engage the amygdala in the social behavior circuit (16). Indeed, it is well documented that behavioral deficits are difficult to detect in children but can be uncovered by challenges and stress, such as occurs in the classic Strange Situation Procedure developed by Ainsworth and Bell (15). Only after repeated challenges of separation and reunion with the mother and a stranger can one observe attachment disorders (aberrant social behaviors with the attachment figure), as can be induced by caregiver maltreatment and the resultant disordered attachment (15, 54). In the present study, where maltreatment is ongoing, pups still have high stress hormone levels and readily express social behavior deficits. This is significant, as stress levels return to baseline following termination of maltreatment and pup behavior becomes indistinguishable from controls until around weaning, when neurobehavioral deficits again emerge (16). It should be noted that we also did not find differences between groups when they were assessed in the nest with a typically behaving mother, where maternal behavior can facilitate typical nursing and social behaviors in pups. Thus, our design permitted us to characterize immediate neurobehavioral deficits when stress hormone differences are detectable, before maltreatment effects become latent, reemerging at weaning (21).
It is important to note that during testing, pups do not appear to respond to the mother as an aversive stimulus. Indeed, regardless of infant treatment within our naturalistic and experimentally controlled rearing conditions, all pups continue to respond to the mother as an attachment figure, as evidenced by continued approach and contact with the mother (attached or behind) and expression of the nipple attachment that is well documented to occur only to a mother rat that expresses the maternal odor learned by the pup (55). What we find statistically significant between groups is pups’ behavior once contact is made with the mother (i.e., pups’ social partner): Pups reared with a maltreating mother or that received corticosterone in the presence of a mother (awake or anesthetized) showed reduced prosocial behaviors toward the mother.
As with any ecologically relevant naturalistic experimental paradigm using social stimuli, it is impossible to completely separate the social dimension from confounds, such as stimulus complexity (56–59). It is for this reason that we experimentally deconstructed the pup’s complex social experience with the mother and progressively eliminated some of the complexity. The data presented here complement and expand work from our laboratory and others showing that specific sensory components of the mother and/or her behavior can be experimentally broken down to uncover myriad “hidden” causal relationships between very specific maternal behaviors or sensory stimuli and very specific outcomes [i.e., Hofer’s “hidden regulators” (60)]. For example, the mere odor of the mother, a relatively simple social stimulus, is sufficient to drive neurobehavioral responses in the pup that mimic the effects of the complex social stimulus of the mother’s presence (61–63). Our work also shows that pups’ experience with a maltreating mother degrades the value of this maternal odor, which greatly attenuates the ability of this social stimulus to alter the neurobehavioral impact of this odor throughout the lifespan (62, 64, 65). On a broader scale, literature from across the lifespan has shown that social stimuli can engage specific neurobehavioral responses, including within the amygdala (66). It is also noteworthy that there is a large literature suggesting that a social context guides the brain’s response to stress, with stress within a social context compared with a nonsocial context having a distinct neurobehavioral signature (67–70). Here, we have highlighted a specific “hidden” infant experience within the complex mother–infant social relationship that is causally related to neurobehavioral changes in the developmental trajectory, one of which is socially bound, while the other is not. Our research and others’ clearly highlight that other specific “hidden” relationships coexist within the mother–infant relationship (71, 72).
The Amygdala Structural and Functional Alterations Did Not Require Maternal Behavior but Did Require the Presence of the Mother.
Elevating stress hormone levels with an anesthetized, nonbehaving mother was sufficient to phenocopy the neurobehavioral effects of maltreatment. These data complement research highlighting the impact of specific features of maternal behavior associated with infant maltreatment (73, 74). Moreover, the current findings demonstrating that corticosterone has unique amygdala effects depending on the social context open a previously under-explored avenue to investigate why some but not all adverse childhood experiences lead to neurobehavioral deficits. However, the downstream effectors of corticosterone that may mediate these effects remain elusive. We have previously shown that systemic corticosterone levels and maternal presence drive amygdala plasticity at the level of signaling molecular cascades (62, 75), and mitogen-activated protein kinase has been identified as a downstream mediator of corticosterone at the synapse (76). Future work will be necessary to determine the specific effectors of corticosterone on amygdala plasticity (77, 78).
How Do We Interpret These Results within the Context of Abundant Evidence Suggesting the Importance of Maternal Behavior for Brain and Behavioral Development?
Abundant evidence has accumulated to show that maternal sensory stimuli, such as licking or time spent nursing, can impact brain development within a typical range, while experiencing maternal behavior inducing trauma and pain during maltreatment can program the brain for later maladaptive behavior (20, 77, 79, 80). The current findings do not challenge the data, some of which were generated by our laboratory, supporting this notion. Instead, we suggest that the value of the caregiver be expanded to include more than simple overt maternal behavior, including the learned maternal odor in rodents and the learned sight, smell, and sound of the mother in children. Indeed, as our manipulations progressively eliminated maternal behavior, they did not eliminate the maternal olfactory and somatosensory stimuli received by pups. These stimuli gain hedonic value as the infant interacts with the mother or other caregiver, including adoptive or foster parents for children, rodents, and nonhuman primates (14, 65, 81). Our results highlight the importance of these sensory cues in patterning infant brain function identified in the child and animal developmental literatures.
The maternal odor is a powerful stimulus for children and rodents, where the odor helps guide the infant’s social behaviors with the mother, decreases trauma-induced stress elevation (social buffering), and decreases pain. It is important to note that altricial infants should rarely have a stress hormone increase while with the mother. When threatened or stressed, altricial infants have a stress hormone increase but use the caregiver as a “safe haven” and rapidly approach the caregiver for protection (82, 83). This contact with the attachment figure lowers stress hormone levels, a process termed social buffering (14, 84, 85). This process of social buffering is greatly reduced in compromised caregiver–infant dyads involving maltreatment in children, nonhuman primates, and rodents (48, 54, 86). Therefore, we suggest that our deconstructed modeling of the stress hormone elevation in maltreatment provides clues about the specific neurobehavioral pathologies that are induced by a maltreating caregiver who cannot socially buffer the offspring. In other words, the combined effect of stress hormones and social pairings may reflect one pathway by which a compromised attachment with the caregiver may initiate a specific developmental perturbation.
In summary, the current results begin to unravel the complexity of natural mother–infant interactions and identify specific causal mechanisms for neurobehavioral deficits found within the ubiquitous effects of being reared by an abusive caregiver. The major significance of our results is that social context paired with stress hormones is required to produce amygdala-dependent social behavior deficits, while stress hormones in any context produce corticosterone-induced hippocampal deficits. Importantly, within a strong attachment relationship, the caregiver should protect the infant from corticosterone elevation and socially buffer the infant. The developmental psychology literature identifies social buffering importance during both transient, infrequent caregiver-induced stressors and external stressors that can be socially buffered once the infant approaches the caregiver for comfort and protection. Maltreating caregivers typical fail to socially buffer the infant under either context, potentially exposing the infant to the repeated, chronic caregiver social context while stress hormone levels are elevated. This may represent one way in which maltreatment initiates an aberrant developmental trajectory associated with social behavior and amygdala deficits, although many others are likely to coexist.
Materials and Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the Nathan Kline Institute and New York University, in accordance with guidelines from the NIH. More details are provided in SI Appendix, Online Materials.
Subjects.
Male and female Long–Evans rats were born and bred on-site. Unless otherwise indicated, an equal number of males and females were used, with 1 male and 1 female per condition from a given litter. Based on previous work from our laboratory, where we did not observe sex differences in animals at this young age following maltreatment (21, 87, 88), the current study did not include sex as a variable. The rearing environment was altered at PN8 using either continuous or 90-min daily manipulations. At PN13, behavioral, amygdala, and hippocampus data were collected.
Maltreatment.
Infant maltreatment was induced by the scarcity-adversity model of low bedding (89), which disrupts maternal care by reducing the mother’s resources for nest building (Fig. 1 A and E). This procedure is validated to produce maternal maltreatment of pups (i.e., rough treatment, such as stepping on pups) and results in the later life amygdala disruption, depressive-like and anxiety-like behavior, and dysregulation of fear expression (21–24).
Corticosterone Manipulations.
In experiment 1, pups received daily administration of metyrapone (50 mg/kg; Sigma) or an equal volume of saline 90 min before exposure to a dam with low bedding to reduce pups’ stress hormone release during the 1-h maltreating (or control) treatment. In experiment 2, pups received daily (5 d) administration of corticosterone 2-hydroxypropyl-β-cyclodextrin complex (3 mg/kg; Sigma) or an equal volume of saline 30 min before being placed with an awake nurturing mother, with an anesthetized mother, or in a chamber with a polyethylene tube for 90 min per day. A radioimmunoassay was used to assess pup serum corticosterone.
Social Behavior Testing, c-Fos, Neurogenesis, and Structural Measurements.
At PN13, all pups received a 30-min social behavior test with an anesthetized mother (milk letdown blocked) placed on her side to give pups access to nipples (16). Pups were tested individually, with free interaction with the mother. Tests were recorded and scored blinded to experimental condition. Sixty minutes after the test, brains were harvested and processed for amygdala and hippocampus c-Fos expression, DCX, and volume.
Electrophysiology.
PN12 pups were anesthetized and implanted with a wireless telemetry transmitter (DSI) with a recording electrode targeting the BLA nucleus and a reference electrode targeting the right posterior cortex. LFPs were recorded during the PN13 social behavior test. The pup was placed in a small arena in a sound-attenuated recording booth, and amygdala LFP activity was recorded continuously (10 min of habituation, followed by the addition of an anesthetized dam for 20 min). Neural signals were amplified, filtered (0.5 to 300 Hz), digitized at 2 kHz with Spike2 software (CED, Inc.), and analyzed offline. Recordings were all from the left amygdala. Fast Fourier transform power analyses were performed on the raw LFP data to quantify oscillatory power in 2.9-Hz frequency bins from 0 to 100 Hz (Hanning). Power in the theta-frequency (5 to 15 Hz), beta-frequency (15 to 35 Hz), and gamma-frequency (35 to 80 Hz) bands was calculated for each specified behavioral window bin (1 min). The change in LFP oscillatory power as a function of the mother’s presence was calculated as the ratio of LFP power during maternal presence versus alone. Electrode placement was verified histologically.
Cannulation and Muscimol Administration.
PN12 pups were anesthetized by isoflurane inhalation, and cannulae were implanted bilaterally into the amygdaloid complex targeting the BLA nucleus (caudal: −0.90 mm; lateral: ±4.50 mm from bregma). At PN13, vehicle or muscimol (0.4 nmol) was infused bilaterally via a Harvard syringe pump, and pups were given the social behavior test. Cannula placement was verified histologically.
Statistical Analysis.
Data were analyzed with Student’s t tests or 2-way or repeated measures ANOVA, followed by Newman–Keuls post hoc tests. Further analyses utilized planned comparisons to test the a priori hypothesis that (1) maltreatment or corticosterone injection will alter outcomes compared with controls and (2) blocking corticosterone will prevent the behavioral effects of high corticosterone.
Supplementary Material
Acknowledgments
We thank Katarzyna Stepien for her illustrations and Tom Cooper for generously providing access to his laboratory equipment. This research was funded by NIH Grants F32MH112232 (to M.O.), Brain & Behavior Research Foundation (BBRF) National Alliance for Research on Schizophrenia & Depression (NARSAD) Young Investigator (to M.O.), T32MH019524 (to M.O.), and R37HD083217 (to R.M.S.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907170116/-/DCSupplemental.
References
- 1.de Kloet E. R., Sibug R. M., Helmerhorst F. M., Schmidt M. V., Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev. 29, 271–281 (2005). Correction in: Neurosci. Biobehav. Rev. 30, 576 (2006). [DOI] [PubMed] [Google Scholar]
- 2.McEwen B. S., Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 9, 149–154 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Andersen S. L., et al. , Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 20, 292–301 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan A., et al. , Childhood maltreatment, depression, and suicidal ideation: Critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front. Psychiatry 6, 42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher M. H., Childhood trauma and the enduring consequences of forcibly separating children from parents at the United States border. BMC Med. 16, 146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty T. S., Blaze J., Keller S. M., Roth T. L., Phenotypic outcomes in adolescence and adulthood in the scarcity-adversity model of low nesting resources outside the home cage. Dev. Psychobiol. 59, 703–714 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tost H., Champagne F. A., Meyer-Lindenberg A., Environmental influence in the brain, human welfare and mental health. Nat. Neurosci. 18, 1421–1431 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Graham A. M., et al. , Maternal cortisol concentrations during pregnancy and sex-specific associations with neonatal amygdala connectivity and emerging internalizing behaviors. Biol. Psychiatry 85, 172–181 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron J. L., Eagleson K. L., Fox N. A., Hensch T. K., Levitt P., Social origins of developmental risk for mental and physical illness. J. Neurosci. 37, 10783–10791 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suomi S. J., Eisele C. D., Grady S. A., Harlow H. F., Depressive behavior in adult monkeys following separation from family environment. J. Abnorm. Psychol. 84, 576–578 (1975). [DOI] [PubMed] [Google Scholar]
- 11.Gould E., Woolley C. S., McEwen B. S., Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J. Comp. Neurol. 313, 479–485 (1991). [DOI] [PubMed] [Google Scholar]
- 12.Demers L. A., et al. , Separable effects of childhood maltreatment and adult adaptive functioning on amygdala connectivity during emotion processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 116–124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan R. M., Holman P. J., Transitions in sensitive period attachment learning in infancy: The role of corticosterone. Neurosci. Biobehav. Rev. 34, 835–844 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunnar M. R., Hostinar C. E., Sanchez M. M., Tottenham N., Sullivan R. M., Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc. Neurosci. 10, 474–478 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ainsworth M. D., Bell S. M., Attachment, exploration, and separation: Illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 41, 49–67 (1970). [PubMed] [Google Scholar]
- 16.Raineki C., Moriceau S., Sullivan R. M., Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol. Psychiatry 67, 1137–1145 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin K. A., et al. , Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology 41, 1956–1964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham A. M., et al. , Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev. Cogn. Neurosci. 18, 12–25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tottenham N., Human amygdala development in the absence of species-expected caregiving. Dev. Psychobiol. 54, 598–611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldji C., et al. , Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U.S.A. 95, 5335–5340 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raineki C., Cortés M. R., Belnoue L., Sullivan R. M., Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 32, 7758–7765 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raineki C., et al. , Paradoxical neurobehavioral rescue by memories of early-life abuse: The safety signal value of odors learned during abusive attachment. Neuropsychopharmacology 40, 906–914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth T. L., Sullivan R. M., Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry 57, 823–831 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Walker C. D., et al. , Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: Critical considerations of methodology, outcomes and translational potential. Stress 20, 421–448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perry R. E., Blair C., Sullivan R. M., Neurobiology of infant attachment: Attachment despite adversity and parental programming of emotionality. Curr. Opin. Psychol. 17, 1–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meaney M. J., Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Sarro E. C., Wilson D. A., Sullivan R. M., Maternal regulation of infant brain state. Curr. Biol. 24, 1664–1669 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courtiol E., Wilson D. A., Shah R., Sullivan R. M., Teixeira C. M., Maternal regulation of pups’ cortical activity: Role of serotonergic signaling. eNeuro 5, ENEURO.0093-18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempermann G., Song H., Gage F. H., Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 7, a018812 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown J. P., et al. , Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Chareyron L. J., Lavenex P. B., Lavenex P., Postnatal development of the amygdala: A stereological study in rats. J. Comp. Neurol. 520, 3745–3763 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Bayer S. A., Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J. Comp. Neurol. 194, 845–875 (1980). [DOI] [PubMed] [Google Scholar]
- 33.Lyons-Ruth K., Pechtel P., Yoon S. A., Anderson C. M., Teicher M. H., Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav. Brain Res. 308, 83–93 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leeper L. L., Schroeder R., Henning S. J., Kinetics of circulating corticosterone in infant rats. Pediatr. Res. 24, 595–599 (1988). [DOI] [PubMed] [Google Scholar]
- 35.Moriceau S., Wilson D. A., Levine S., Sullivan R. M., Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J. Neurosci. 26, 6737–6748 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amaral D. G., The amygdala, social behavior, and danger detection. Ann. N. Y. Acad. Sci. 1000, 337–347 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Gómez R. L., Edgin J. O., The extended trajectory of hippocampal development: Implications for early memory development and disorder. Dev. Cogn. Neurosci. 18, 57–69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavenex P., Banta Lavenex P., Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behav. Brain Res. 254, 8–21 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Qin S., et al. , Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat. Neurosci. 17, 1263–1269 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raineki C., et al. , Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus 20, 1037–1046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teicher M. H., Anderson C. M., Polcari A., Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. U.S.A. 109, E563–E572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bremner J. D., et al. , Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse–A preliminary report. Biol. Psychiatry 41, 23–32 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frodl T., Reinhold E., Koutsouleris N., Reiser M., Meisenzahl E. M., Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J. Psychiatr. Res. 44, 799–807 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Stein M. B., Koverola C., Hanna C., Torchia M. G., McClarty B., Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 27, 951–959 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Vermetten E., Schmahl C., Lindner S., Loewenstein R. J., Bremner J. D., Hippocampal and amygdalar volumes in dissociative identity disorder. Am. J. Psychiatry 163, 630–636 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armanini M. P., Hutchins C., Stein B. A., Sapolsky R. M., Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 532, 7–12 (1990). [DOI] [PubMed] [Google Scholar]
- 47.Kawakami R., et al. , Asymmetrical allocation of NMDA receptor ε2 subunits in hippocampal circuitry. Science 300, 990–994 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Tottenham N., Sheridan M. A., A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 3, 68 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupien S. J., McEwen B. S., Gunnar M. R., Heim C., Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Provencal N., et al. , Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proc. Natl. Acad. Sci. U.S.A., 201820842 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callaghan B. L., Richardson R., Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav. Neurosci. 125, 20–28 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Emery N. J., et al. , The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 515–544 (2001). [PubMed] [Google Scholar]
- 53.Bachevalier J., Málková L., Mishkin M., Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 545–559 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Nachmias M., Gunnar M., Mangelsdorf S., Parritz R. H., Buss K., Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Dev. 67, 508–522 (1996). [PubMed] [Google Scholar]
- 55.Landers M. S., Sullivan R. M., The development and neurobiology of infant attachment and fear. Dev. Neurosci. 34, 101–114 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Opendak M., Gould E., Adult neurogenesis: A substrate for experience-dependent change. Trends Cogn. Sci. (Regul. Ed.) 19, 151–161 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Lagace D. C., et al. , Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. U.S.A. 107, 4436–4441 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peña C. J., et al. , Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 356, 1185–1188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel D., Kas M. J., Chattarji S., Buwalda B., Rodent models of social stress and neuronal plasticity: Relevance to depressive-like disorders. Behav. Brain Res. 369, 111900 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Hofer M. A., Early relationships as regulators of infant physiology and behavior. Acta Paediatr. Suppl. 397, 9–18 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Ifrán M. C., Suárez A. B., Pautassi R. M., Kamenetzky G. V., Maternal odor exposure modulates acceptance of a bitter taste in newborn and infant rats. Front. Psychol. 9, 1327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Opendak M., Zanca R. M., Anane E., Serrano P. A., Sullivan R. M., Developmental transitions in amygdala PKC isoforms and AMPA receptor expression associated with threat memory in infant rats. Sci. Rep. 8, 14679 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Debiec J., Sullivan R. M., Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc. Natl. Acad. Sci. U.S.A. 111, 12222–12227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al Aïn S., et al. , Neurobehavioral assessment of maternal odor in developing rat pups: Implications for social buffering. Soc. Neurosci. 12, 32–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry R. E., Al Aïn S., Raineki C., Sullivan R. M., Wilson D. A., Development of odor hedonics: Experience-dependent ontogeny of circuits supporting maternal and predator odor responses in rats. J. Neurosci. 36, 6634–6650 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y., et al. , Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171, 1176–1190.e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skuse D., Morris J., Lawrence K., The amygdala and development of the social brain. Ann. N. Y. Acad. Sci. 1008, 91–101 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Felix-Ortiz A. C., Tye K. M., Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 34, 586–595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rasia-Filho A. A., Londero R. G., Achaval M., Functional activities of the amygdala: An overview. J. Psychiatry Neurosci. 25, 14–23 (2000). [PMC free article] [PubMed] [Google Scholar]
- 70.Modi M. E., Sahin M., A unified circuit for social behavior. Neurobiol. Learn. Mem., S1074-7427(18)30195-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hofer M. A., Hidden regulators in attachment, separation, and loss. Monogr. Soc. Res. Child Dev. 59, 192–207 (1994). [PubMed] [Google Scholar]
- 72.Sarro E. C., Sullivan R. M., Barr G., Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience 258, 147–161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Claessens S. E., et al. , Development of individual differences in stress responsiveness: An overview of factors mediating the outcome of early life experiences. Psychopharmacology (Berl.) 214, 141–154 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rincón-Cortés M., et al. , Enduring good memories of infant trauma: Rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proc. Natl. Acad. Sci. U.S.A. 112, 881–886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Opendak M., et al. , Neurobiology of maternal regulation of infant fear: The role of mesolimbic dopamine and its disruption by maltreatment. Neuropsychopharmacology 44, 1247–1257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Revest J. M., et al. , BDNF-TrkB signaling through Erk1/2 MAPK phosphorylation mediates the enhancement of fear memory induced by glucocorticoids. Mol. Psychiatry 19, 1001–1009 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bagot R. C., et al. , Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 92, 292–300 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Xiong H., et al. , mTOR is essential for corticosteroid effects on hippocampal AMPA receptor function and fear memory. Learn. Mem. 22, 577–583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Curley J. P., Champagne F. A., Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 40, 52–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Opendak M., Gould E., Sullivan R., Early life adversity during the infant sensitive period for attachment: Programming of behavioral neurobiology of threat processing and social behavior. Dev. Cogn. Neurosci. 25, 145–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Logan D. W., et al. , Learned recognition of maternal signature odors mediates the first suckling episode in mice. Curr. Biol. 22, 1998–2007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kerns K. A., Mathews B. L., Koehn A. J., Williams C. T., Siener-Ciesla S., Assessing both safe haven and secure base support in parent-child relationships. Attach. Hum. Dev. 17, 337–353 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Hornstein E. A., Fanselow M. S., Eisenberger N. I., A safe haven: Investigating social-support figures as prepared safety stimuli. Psychol. Sci. 27, 1051–1060 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Sanchez M. M., McCormack K. M., Howell B. R., Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci. 10, 512–526 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sullivan R. M., Perry R. E., Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Soc. Neurosci. 10, 500–511 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Callaghan B. L., Tottenham N., The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology 41, 163–176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Junod A., Opendak M., LeDoux J. E., Sullivan R. M., Development of threat expression following infant maltreatment: Infant and adult enhancement but adolescent attenuation. Front. Behav. Neurosci. 13, 130 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robinson-Drummer P. A., et al. , Infant trauma alters social buffering of threat learning: Emerging role of prefrontal cortex in preadolescence. Front. Behav. Neurosci. 13, 132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perry R. E., et al. ; Family Life Project Key Investigators , Developing a neurobehavioral animal model of poverty: Drawing cross-species connections between environments of scarcity-adversity, parenting quality, and infant outcome. Dev. Psychopathol. 31, 399–418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paxinos G., Watson C., The Rat Brain in Stereotaxic Coordinates (Academic Press, Boston, MA, 2003). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.