Significance
Sunlight exposure has long been associated with the suppression of MS with the reduction in risk credited to vitamin D production. Vitamin D and sunlight have each been reported to protect against EAE, the mouse model of MS. However, little work has focused on conclusively showing whether sunlight is acting via vitamin D. Here, we examine whether UV can suppress EAE either in animals lacking the ability to make vitamin D or in animals lacking the VDR. In each case, UV fully retained its ability to suppress disease, demonstrating that neither vitamin D nor its receptor is required. Future investigation can now focus on uncovering the mechanism whereby UV suppresses EAE and presumably MS.
Keywords: UV light, vitamin D receptor, experimental autoimmune encephalomyelitis
Abstract
Vitamin D and sunlight have each been reported to protect against the development of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS). To date, the contribution of each has been unclear as ultra violet (UV) exposure also causes the generation of vitamin D in the skin. To examine whether the UV based suppression of EAE results, at least, in part from the production of vitamin D, we studied the effect of UV light on EAE in mice unable to produce 7-dehydroxycholesterol (7-DHC), the required precursor of vitamin D. Furthermore, we examined UV suppression of EAE in mice devoid of the vitamin D receptor (VDR). Our results demonstrate that UV light suppression of EAE occurs in the absence of vitamin D production and in the absence of VDR. Future investigations will focus on identifying the pathway responsible for the protective action of UV in EAE and presumably human MS.
There is growing evidence of a connection between sunlight exposure and multiple sclerosis (MS) (1, 2). Owing to the tight link between ultra violet (UV) B (UVB) exposure and vitamin D synthesis (and, subsequently, serum vitamin D status), many researchers have attributed the apparent reduction in MS for individuals with greater sunlight exposure to actions of vitamin D (3). In the skin, synthesis of previtamin D3 occurs when UVB light is absorbed by 7-dehydrocholesterol (7-DHC) resulting in the production of vitamin D (4, 5). While a great deal of scientific effort has been dedicated to understanding whether supplemental vitamin D can actively protect against or treat MS, much less attention has been given to the effects of UVB itself. Narrow band (NB) UV B (UV-NB) light has been shown to prevent experimental autoimmune encephalomyelitis (EAE) in the mouse, a model of MS, without increasing serum 25OH D3 (6, 7).
To elucidate the individual effects of UV-NB and the synthesis of previtamin D3, we utilized mice lacking the enzyme Sc5d, which are unable to produce 7-DHC from lathosterol (and, thus, previtamin D3 upon UVB exposure) (8). Additional studies utilized mice that lack either the enzyme responsible for the generation of the active vitamin D metabolite 1,25-hydroxyvitamin D3 [1,25(OH)D3] (Cypi27B1) or the receptor for that ligand (vitamin D receptor, VDR) to test whether either was required for EAE prevention by UV-NB light. Taken together, these data demonstrate that neither vitamin D metabolites nor the VDR, are required for UV suppression of EAE.
Results
Generation of Previtamin D3 in Skin Is Not Required for UVB Suppression of EAE.
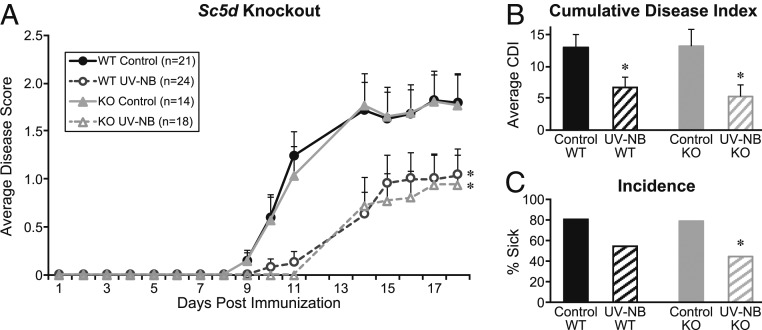
Loss of keratinocyte 7-DHC (and, thus, the ability to generate previtamin D3 upon UVB exposure) in Sc5dfl/fl K14Cre mice had no effect on EAE incidence or disease severity compared to wild-type (WT) controls (Fig. 1A): Both control groups showed onset at Day 8 postimmunization with incidence near 80% (P = 0.79) and similar average cumulative disease scores (P = 0.82). When either the WT or the Sc5d null mice were exposed to daily UV-NB, each showed significant suppression of EAE symptoms compared to their untreated controls (Fig. 1A). Cumulative disease was suppressed with UV-NB exposure in both WT and Sc5dfl/fl K14Cre mice: 13.0 ± 9.2 in WT control versus 6.7 ± 8.0 in WT UV-NB (P = 0.03) and 13.2 ± 9.0 in Sc5dfl/fl K14Cre control versus 5.3 ± 7.1 in Sc5dfl/fl K14Cre UV-NB (P = 0.01, Fig. 1B). Incidence of disease for WT mice exposed to UV-NB was 54%, compared to 81% in WT controls (P = 0.06); in Sc5dfl/fl K14Cre mice, incidence was 44% in the UV-NB-exposed group and 85% in the control group (P = 0.02, Fig. 1C). Overall, the degree of disease abatement was similar between controls and UV-NB exposed regardless of whether mice were able to generate previtamin D3 by UV-NB, demonstrating that the synthesis of previtamin D3 in skin is not required for UV-NB to prevent EAE.
Fig. 1.
Mice lacking expression of Sc5d and, thus, the ability to produce 7-DHC from lathosterol in skin show suppression of EAE by UV-NB. (A) The inability to generate 7-DHC (gray solid line) has no impact on the development of EAE in control mice (versus WT, black solid line). Sc5d knockout mice that lack the ability to generate previtamin D3 upon UV exposure show similar suppression of disease with UV-NB (dashed gray line) versus WT (dashed black line). (B) Cumulative disease index (CDI) does not differ between WT and knockout control animals. The degree of overall disease suppression is similar between WT (black hatched bars) and knockout mice (gray hatched bars) exposed to UV-NB compared to their respective controls. (C) Disease incidence is reduced with UV-NB, regardless of the ability to produce 7-DHC upon UV-NB exposure. Data shown represent cumulative data from 2 independent studies. *P < 0.05 versus respective controls.
Neither Cyp27B1 nor VDR Are Required for EAE Suppression by UVB.
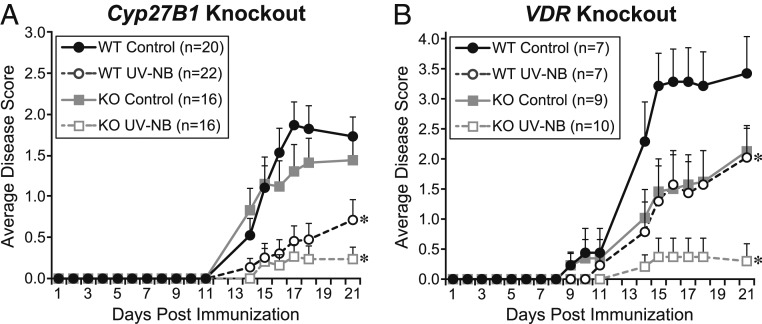
As reported previously, WT mice showed significant suppression of EAE when exposed to UV-NB beginning at immunization (Fig. 2). At the study termination, the average EAE score for WT mice exposed to daily UV-NB was significantly suppressed compared to controls (Experiment A: 0.7 ± 0.2 versus 1.7 ± 0.2, P = 0.002; Experiment B: 2.0 ± 0.5 versus 3.4 ± 0.6, P < 0.05). The degree of suppression by UV-NB was not altered in mice lacking Cyp27B1, the enzyme responsible for conversion to the hormone 1,25(OH)D3, compared to controls (0.2 ± 0.1 versus 1.4 ± 0.3, P = 0.009, Fig. 2A). UV-NB was also equally suppressive in mice lacking VDR, indicating that the VDR is not involved in the UV suppression of EAE (0.3 ± 0.3 versus controls 2.1 ± 0.4, P = 0.005, Fig. 2B).
Fig. 2.
Mice lacking either the 1⍺-hydroxylase enzyme or the VDR show similar suppression of EAE with UV-NB compared to WT mice. WT mice show significant disease suppression when exposed to UV-NB (dashed black lines). (A) Cyp27B1 knockout mice, which are unable to produce the active metabolite 1,25(OH)D3, show similar suppression of disease with UV-NB (dashed gray line) compared to WT controls (solid gray line). (B) VDR knockout mice, which lack the receptor for 1,25 dihydroxy vitamin D, show a similar degree of EAE suppression with UV-NB (dashed gray line) compared to WT controls (solid gray line). *P < 0.05 versus respective controls.
Discussion
Elimination of the ability of skin to produce vitamin D upon exposure to UV-NB did not diminish the capacity of UV-NB to suppress EAE. Furthermore, UV-NB was equally suppressive of disease whether or not the VDR or the ability to make 1,25(OH)D3 was present. Taken together, these data demonstrate that UV light prevents the development of EAE independent of vitamin D.
Previous reports from our laboratory supported the idea that disease suppression by UV light is independent of vitamin D. Historically, 1 wk of pretreatment with broadband (BB) UV (280–360 nm) exposure was utilized prior to immunization for EAE. These studies demonstrated that mice exposed to this UV pretreatment had significantly elevated 25-hydroxyvitamin D3 [25(OH)D3] levels on the day of immunization, but these levels did not remain elevated during the study even with continuing UV exposure that suppressed disease (6). These BB bulbs clearly raise serum vitamin D levels even if only transiently. Additional work showed that 300–315 nm narrow band wavelengths were responsible for disease suppression while longer wavelengths were not (7). These UV-NB wavelengths are generally beyond those required for robust vitamin D production, and indeed, Wang et al. showed that, following 37 d of 10 kJ/day UV-NB treatment, serum 25(OH)D3 levels were not different from controls (7). In our previously reported studies using UV-NB, the mice were vitamin D sufficient. In the present study, vitamin D deficient mice were used having 25(OH)D3 levels <4 ng/mL (undetectable by current methods). Following UV-NB, the level of 25(OH)D3 rose to 9 ng/mL showing that UV-NB will produce small amounts of vitamin D that would not have been detected in our previous study utilizing normal mice having serum 25(OH)D3 levels >50 ng/mL. In the current report, we have carried these experiments an additional step using mice unable to produce vitamin D via UV light, yet the UV-NB remained fully effective against EAE. Extending this even further, we have also shown that the absence of the VDR had no effect on the UV suppression of EAE. In addition, as previously demonstrated, 1,25(OH)D3 production is not required for UV-NB suppression and, in fact, may potentiate suppression even further (9).
Accumulating evidence in humans suggests that the link between increased UV exposure and decreased MS risk is independent of vitamin D levels. Lucas et al. found that higher levels of leisure-time sun exposure were associated with a reduced risk of first demyelinating events and was independent of 25(OH)D3 measures (10). Baarnhielm et al. arrived at the same conclusion finding that the association with UV exposure remained even after accounting for vitamin D status (11). Increased lifetime UV exposure is associated with decreased MS risk independent of 25(OH)D3 measurements in both blacks and whites, even though blacks consistently have lower circulating 25(OH)D3 levels compared to whites (12). Even when various polymorphisms related to vitamin D metabolism were accounted for, the independence of UV from vitamin D in MS risk reduction was evident (13). Additional studies are nicely summarized by Breuer et al. (14).
The experimental evidence presented here demonstrates that the suppression of EAE by UV light is independent of vitamin D. Our laboratory has previously demonstrated that spinal cord inflammation and demyelination are both reduced by UV exposure (15). However, the mechanism at the UV-skin interface whereby this downstream protection is offered has yet to be uncovered. There have been many proposals of mechanisms by which UV may exert its beneficial effects against autoimmune disease. UV exposure is known to induce nitric oxide synthesis and affect melanin, serotonin, and endorphin secretion. The induction of urocanic acid (UCA) isomerization by UV exposure is another well-known mechanism of immunosuppression. Although, UCA likely does not play a role in EAE suppression by UV (16).
Despite the evidence for a strong positive effect of UVB on both the SJL/myelin basic protein and the C57BL/6/MOG mouse models of MS, we are aware of only 1 study that has been conducted to examine whether UV-NB could alleviate human MS. Of 20 total participants with Clinically Isolated Syndrome, half of which received 24 UV-NB exposures over 8 wk (starting at a dose of 20 mJ/cm2 and increasing by 20–40% increments through the study), 30% fewer patients in the phototherapy arm converted to MS during the 12-mo follow-up, compared to controls (17). In this small trial, UV exposure occurred only 3 times per week and lasted for only 8 wk. In our studies on mice, optimal suppression of EAE is reached with daily UVB exposure. Although we have found that as little as 2 kJ UV-NB—equivalent to roughly 20 min in the sun on a cloudless day—given each week day can dampen the development and progression of EAE in the mouse (SI Appendix, Fig. S1).
While current practice dictates that an individual can wholly replace time spent in the sun with a vitamin D supplement, this is likely not an adequate replacement for the benefits of UV exposure. The current results are promising for the use of UV-NB to suppress the development of MS and pave the way to determine the biochemical impact of UV-NB and isolation of the resulting active substance that suppresses EAE.
Methods
Animals and Diet.
All procedures were approved by the Research Animal Resources Committee of the College of Agricultural and Life Sciences at the University of Wisconsin-Madison. All animals were maintained in the Department of Biochemistry vivarium with a 12 h:12 h light:dark cycle.
7-DHC null mice.
For the experiment utilizing the mouse deleted for Sc5d, mice carrying a floxed allele of the Sc5d gene were generously donated by Dr. Ervin Epstein (8). Mice were fed Lab Diet 5015 (Purina Mills, Richmond, IN). Floxed Sc5d mice (Sc5dfl/fl) were crossed with keratinocyte-specific Cre mice (K14Cre, Stock 018964, Jackson Laboratory) to generate constitutive keratinocyte-specific deletion of Sc5d. Pairings occurred such that control mice containing only either the Cre enzyme or the floxed allele of Sc5d as well as complete WT mice were also produced. At 10–14 wk of age, mice were immunized for EAE; half of the study mice also received UV-NB exposure beginning at immunization. Pilot studies showed no difference in EAE incidence or disease severity among WT mice and Sc5dfl/fl or K14Cre mice; therefore, these genotypes have been combined and jointly labeled as WT for subsequent analysis.
VDR and Cyp27B1 null mice.
VDR null mice (Stock 006133, Jackson Laboratory) were bred with C57BL/6J mice (Stock 000664, Jackson Laboratory) to obtain heterozygous mice for breeding. Female and male VDR+/− mice were then paired to generate VDR−/− and VDR+/+ offspring for experiments. Cyp27B1 null mice (18) were bred in a similar fashion to yield experimental offspring. All mice were maintained on a rescue diet containing 2% calcium, 1.2% phosphorous, and 20% lactose (TD.96348, Envigo) to maintain mineral balance in the absence of proper vitamin D signaling; WT mice were maintained on the same diet for consistency. At 10–14 wk of age, mice were bled by retro-orbital sinus for blood chemistries and immunized for EAE; half of the study mice also received UV-NB exposure beginning at immunization.
Calcium rescue.
Serum calcium elevation is a sensitive indicator of the presence of vitamin D (19). Mice from our vitamin D deficient breeding colony were placed on a 0.02% calcium diet for 2 wk to deplete serum calcium. Blood was collected and serum analyzed to confirm calcium depletion and the absence of 25(OH)D3. Mice were then exposed to 5 kJ UV-NB each weekday. Serum calcium was again measured at 5 and 10 d of UV exposure.
EAE Induction and Scoring.
Induction of disease was carried out as reported previously (16). Briefly, mice were immunized with myelin oligodendrocyte glycoprotein peptide ([MOG], Hooke Laboratories, Lawrence, MA), a model of primary progressive MS. MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) was emulsified in complete Freund’s adjuvant and contained inactivated Mycobacterium tuberculosis H37Ra. On day zero, 20 μL of MOG emulsion was injected s.c., followed by 200 ng of pertussis toxin diluted in sterile PBS given intraperitoneally 2–4 h later (PTX, List Biological Laboratories, Campbell, CA); 24 h later, a second 200 ng PTX booster injection was given. Mice were scored each weekday for clinical signs of EAE using the following scale: 0, no clinical disease; 1, loss of tail tone; 2, unsteady gait; 3, hind limb paralysis; 4, forelimb paralysis; 5, death.
UVB Radiation Treatment.
Radiation treatment was carried out as reported previously (16). Briefly, prior to treatment, an electric razor was used to shave the dorsum of each animal. Beginning at immunization, animals were irradiated daily with a bank of UV-NB lamps with an emission spectrum of 300–325 nm (Solarc Systems, Minesing, ON). The radiation output was measured by placing a UV radiometer equipped with a UVX-31 sensor with a calibration point of 310 nm and bandpass 280–340 nm (UVP LLC, Upland, CA) at 5 locations within the cage, representing the positions occupied by the animals. An average output was calculated, and the time adjusted to expose mice to 8.3 kJ/m2 per treatment. This UV dose results in significant EAE suppression with minimal reddening of the skin. These readings were confirmed using a wide band spectroradiometer RPS900 (International Light, Peabody, MA).
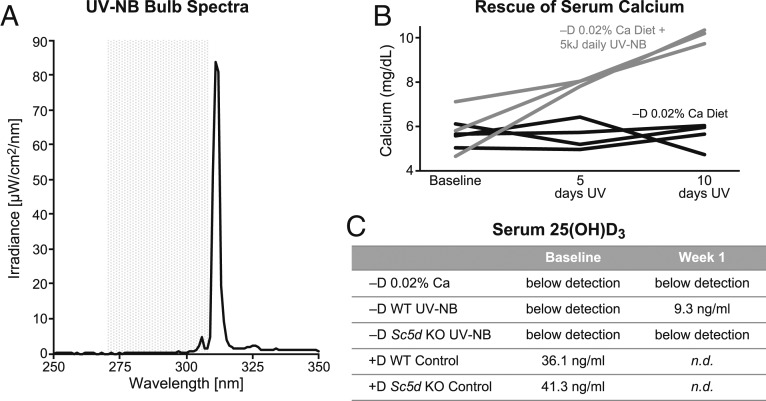
While UV-NB is generally beyond the wavelength required for the generation of previtamin D3 in skin (Fig. 3A), we have shown that a moderate daily dose of UV-NB can rapidly restore serum calcium levels in calcium depleted vitamin D deficient mice (Fig. 3B). Owing to the relatively high limit of detection (4 ng/mL), serum 25(OH)D3 levels are a less sensitive indicator of vitamin D production by UV than the serum calcium response. Nevertheless, 25(OH)D3 levels in mice maintained on a vitamin D deficient diet and exposed to UV-NB rise above the detection threshold after only 5 daily doses of UV (Fig. 3C). Although the increase in serum 25(OH)D3 upon UV-NB exposure is modest, the robust ability to restore serum calcium in depleted mice indicates that the exposure is enough to elicit a strong biological response.
Fig. 3.
UV-NB is able to restore serum calcium in depleted mice through the generation of vitamin D. (A) The spectral output from UV-NB bulbs (300–325 nm, peak output 311–312 nm, black line) is generally beyond the wavelength required for robust generation of previtamin D3 in skin (shaded gray area) (20). (B) However, a moderate daily dose of UV-NB (gray lines) can rapidly restore serum calcium levels in calcium depleted vitamin D deficient mice (black lines) to normal levels. Mice bred under vitamin D deficient conditions were placed on a vitamin D deficient diet containing 0.02% calcium for 2 wk to achieve hypocalcemia. A subset of the mice was then exposed to UV-NB (black lines) for 5 consecutive days and their serum calcium measured; this was repeated the following week. UV-NB was able to partially rescue serum calcium by 5 d of exposure with full rescue occurring by 10 d of exposure. Each line represents the time course from 1 animal. (C) WT mice maintained on a vitamin D deficient diet and exposed to UV-NB show a rise above the detection threshold for serum 25(OH)D3 after only 5 daily doses of UV. By contrast, mice lacking the Sc5d enzyme are unable to raise their serum 25(OH)D3 by UV-NB. Each value represents a single sample pooled from 4 to 8 mice. n.d., not determined.
Blood Chemistries.
Blood was obtained at various time points from the retro-orbital sinus to assess serum calcium and 25(OH)D3. Serum calcium was measured by atomic absorption spectrometry on the PerkinElmer 900H instrument. Serum 25(OH)D3 concentrations were measured by the DiaSorin Liaison 25 OH Vitamin D Total Assay (Stillwater, MN).
Statistical Analysis.
Data are expressed as mean ± SEM. Average clinical scores were calculated at termination (typically day 21). Average disease score was determined by averaging all clinical scores within a treatment group for a particular day. CDI was determined by summing all daily scores for each individual animal across the study; these values were then averaged across each group. Statistical analysis was performed using the 2-tailed Fisher exact probability test for incidence and the Wilcoxon rank sum test for clinical scores. A value of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Ervin Epstein for generously sharing the Sc5d null mice, Hae Rin Lee for her technical skills, the Animal Care Staff for the care of the animals, Mindy Kendrick for conducting serum calcium measurements, Laura Vanderploeg (Biochemistry Department Media Laboratory) for figure preparation, and Debra Noltner for her assistance in the preparation of the paper. This work was supported by a fund from the Wisconsin Alumni Research Foundation.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913294116/-/DCSupplemental.
References
- 1.Simpson S., Jr, et al. ; Ausimmune/AusLong Investigators Group , Sun exposure across the life course significantly modulates early multiple sclerosis clinical course. Front. Neurol. 9, 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallagher L. G., et al. , Lifetime exposure to ultraviolet radiation and the risk of multiple sclerosis in the US radiologic technologists cohort study. Mult. Scler. 25, 1162–1169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg P., Multiple sclerosis: Vitamin D and calcium as environmental determinants of prevalence (a viewpoint). Part 1: Sunlight, dietary factors, and epidemiology. Int. J. Environ. Stud. 6, 19–27 (1974). [Google Scholar]
- 4.Okano T., Yasumura M., Mizuno K., Kobayashi T., Photochemical conversion of 7-dehydrocholesterol into vitamin D3 in rat skins. J. Nutr. Sci. Vitaminol. (Tokyo) 23, 165–168 (1977). [DOI] [PubMed] [Google Scholar]
- 5.Holick M. F., et al. , Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem. Biophys. Res. Commun. 76, 107–114 (1977). [DOI] [PubMed] [Google Scholar]
- 6.Becklund B. R., Severson K. S., Vang S. V., DeLuca H. F., UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc. Natl. Acad. Sci. U.S.A. 107, 6418–6423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., et al. , Suppression of experimental autoimmune encephalomyelitis by 300-315 nm ultraviolet light. Arch. Biochem. Biophys. 536, 81–86 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Makarova A. M., Pasta S., Watson G., Shackleton C., Epstein E. H. Jr, Attenuation of UVR-induced vitamin D3 synthesis in a mouse model deleted for keratinocyte lathosterol 5-desaturase. J. Steroid Biochem. Mol. Biol. 171, 187–194 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Marling S. J., Martino V. M., Prahl J. M., Deluca H. F., The absence of 25-hydroxyvitamin D3-1α-hydroxylase potentiates the suppression of EAE in mice by ultraviolet light. J. Steroid Biochem. Mol. Biol. 163, 98–102 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Lucas R. M., et al. , Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 76, 540–548 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Bäärnhielm M., et al. , Sunlight is associated with decreased multiple sclerosis risk: No interaction with human leukocyte antigen-DRB1*15. Eur. J. Neurol. 19, 955–962 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Langer-Gould A., et al. , MS sunshine study: Sun exposure but not vitamin D is associated with multiple sclerosis risk in blacks and hispanics. Nutrients 10, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langer-Gould A., et al. , Vitamin d-binding protein polymorphisms, 25-hydroxyvitamin d, sunshine and multiple sclerosis. Nutrients 10, 4–6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breuer J., Loser K., Mykicki N., Wiendl H., Schwab N., Does the environment influence multiple sclerosis pathogenesis via UVB light and/or induction of vitamin D? J. Neuroimmunol. 329, 1–8 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Marling S. J., Beaver E. F., Severson K. S., Deluca H. F., UV light selectively inhibits spinal cord inflammation and demyelination in experimental autoimmune encephalomyelitis. Arch. Biochem. Biophys. 567, 75–82 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Irving A. A., Marling S. J., Plum L. A., DeLuca H. F., Suppression of experimental autoimmune encephalomyelitis by ultraviolet light is not mediated by isomerization of urocanic acid. BMC Neurosci. 18, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart P. H., et al. , A randomised, controlled clinical trial of narrowband UVB phototherapy for clinically isolated syndrome: The PhoCIS study. Mult. Scler. J. Exp. Transl. Clin. 4, 2055217318773112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanhooke J. L., et al. , CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proc. Natl. Acad. Sci. U.S.A. 103, 75–80 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLuca H. F., Vitamin D: Historical overview. Vitam. Horm. 100, 1–20 (2016). [DOI] [PubMed] [Google Scholar]
- 20.MacLaughlin J., Anderson R., Holick M., Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 216, 1001–1003 (1982). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.