Abstract
We evaluated memory responses and antibody persistence to diphtheria-toxoid, tetanus-toxoid, whole-cell-pertussis (DTwP), and Hepatitis-B vaccines in HIV-unexposed, HIV-exposed-uninfected and HIV-infected children previously randomized to initiate time-limited ART at 6–10 weeks (ART-Immed) or when clinically/immunologically indicated (ART-Def).
All children received DTwP booster at 15–18 months. Antibodies were measured for pertussis-toxoid, filamentous haemagglutinin (FHA), diphtheria-toxoid, tetanus-toxoid and hepatitis-B prior to booster, 1–2 weeks post-booster and at 24-months of age.
Pre-booster antibody GMC were lower in HIV-infected groups than HIV-unexposed children for all epitopes. Post-booster and at 24-months of age, the ART-Def group had lower GMCs, and a lower proportion had antibody concentrations ≥0.1IU/ml for tetanus-toxoid and diphtheria-toxoid compared to HIV-unexposed children. At 24-months of age, the ART-Immed group had higher GMCs, and more likely to maintain antibody titres ≥1.0 IU/ml to tetanus-toxoid and diphtheria-toxoid compared to HIV-unexposed children.
Compared to HIV-unexposed children, at 15 and 24-months of age, persistence of antibody to HBsAg of ≥10mIU/ml was similar in the ART-Immed group, but lower among the ART-Def group. Antibody kinetics indicated more robust memory responses in HIV-exposed-uninfected than HIV-unexposed children to diphtheria-toxoid and wP.
HIV-infected children not on ART at primary vaccination had poorer memory responses, whereas HIV-exposed-uninfected children mounted robust memory responses.
Keywords: diphtheria-toxoid, tetanus-toxoid, pertussis vaccine, hepatitis B vaccine, booster, HIV-infected, HIV-exposed uninfected
1.0. INTRODUCTION
Anamnestic immune response after vaccination is required for long-term protection against the targeted pathogens [1]. HIV-infection induces aberrations in major lymphocyte populations, including B-lymphocytes, the cornerstone of humoral immune responses [2, 3]. T- and B-lymphocyte dysfunction might cause lower rates of sero-conversion post-vaccination, quicker waning of antibody, and impaired generation or accelerated loss of antigen-specific memory B-lymphocytes [4, 5]. Immune response to vaccination, could be optimised in HIV-infected infants by early initiation of antiretroviral therapy (ART) [6], through relatively normal lymphocyte development [2, 3]. In contrast, despite B-lymphocyte function improving following later initiation of ART, memory responses remain attenuated after attaining viral control [7, 8, 9]. We and others previously reported that early ART initiation induced better immune responses to the primary series of childhood vaccines than when ART was deferred until immunological or clinical deterioration [2, 10, 11, 12]. Furthermore, immunological aberrations are also reported in HIV-uninfected children born to HIV-infected women, possibly attributable to in-utero exposure to HIV virions and/or maternal antiretroviral exposure affecting lymphocyte differentiation and function in their offspring [13, 14, 15, 16, 17, 18].
The objective of this study was to evaluate the responses to a booster dose of diphtheria-toxoid (DT)- tetanus-toxoid (TT) -whole-cell pertussis (wP) with or without Haemophilus influenzae type-b conjugate vaccine (DTwP-HibCV) and antibody persistence in HIV-unexposed, HIV-exposed-uninfected (HEU) and HIV-infected children previously randomized to initiate ART within 6–10 weeks of age (ART-Immed) or when clinically or immunologically indicated (ART-Def). We also compared the persistence of antibody to hepatitis-B vaccine (HBV) until 24-months of age across the study groups.
2.0. METHODS
2.1. Study Setting
This study was conducted on archived serum samples from children in whom the effects of HIV-exposure and timing of ART initiation on immune responses to 7-valent pneumococcal conjugate vaccine (PCV7) were evaluated [12]. The initial study assessing immune responses to pneumococcal vaccine was initiated in 2004, before the pneumococcus vaccine was available in the public sector in South Africa. .
Briefly, infants aged 6–12 weeks were enrolled in Soweto (Gauteng) and Tygerberg (Western Cape) in South Africa. Participants included HIV-infected children with CD4+-lymphocytes ≥25% randomized to initiate ART immediately (ART-Immed); or when clinically indicated (CDC Stage C or protocol-defined severe Stage B) and/or when CD4+-lymphocytes decreased below the threshold (<20%) suggested for ART initiation proposed at the time by the WHO ART guidelines (ART-Def) [19, 20, 21]. Children in the ART-Immed group were randomized to interrupt ART at 12- (ART/12m) or 24-months (ART/24m) of age [20, 22]. Study criteria for ART re-initiation were Centers for Disease Control and Prevention (CDC) Stage C or investigator-selected (severe) stage B events, including symptomatic lymphoid interstitial pneumonitis, bronchiectasis, nephropathy, cardiomyopathy, and failure to thrive. Furthermore, children born to HIV-infected mothers who tested negative for HIV (HIV-exposed uninfected [HEU]) or born to HIV-uninfected mothers (HIV-unexposed) were also enrolled [12].
The primary vaccination series of all children included DTwP-HibCV (CombAct-Hib, Sanofi Pasteur, France), monovalent recombinant HBV 10µg dose (HerberBiotec S.A., Cuba) and PCV7 (Prevnar™, Wyeth Vaccines, NJ, USA) at approximately 6, 10 and 14 weeks of age with a 4–6 week interval between doses. The immunogenicity to the primary series has been reported [10]. Participants were then randomized to receive a booster-dose of either HibCV or PCV7 with DTwP at 15–18 months of age. The immune responses to PCV7 and HibCV booster will be reported separately (manuscript in preparation).
2.2. Laboratory Assay
Clotted blood samples were collected prior to the booster dose, 1–2 weeks post-booster and at 24-months of age; serum aliquots were stored at −70°C [10]. Antibodies to DT, TT, hepatitis-B surface antigen (HBsAg), pertussis-toxin (PT) and filamentous hemagglutinin (FHA) were measured by an in-house Luminex-multiplex immunoassay as described previously [10]. All laboratory tests were performed at the Respiratory Meningeal Pathogens Research Unit (RMPRU), Chris Hani Baragwanath Academic Hospital, Johannesburg South Africa. Antibody persistence and booster response were grouped according to putative sero-protective thresholds for long-term protection of ≥0.1IU/ml and ≥1.0IU/ml for DT and TT; ≥10mIU/ml and ≥100mIU/ml for HBsAg-antibody [1, 23]. Since a correlation between serological response and protection against pertussis is not well established, immune responses to pertussis vaccine were evaluated by whether ≥4-fold increase from pre-booster to post-booster antibody levels was achieved [1]. Laboratory staff were blinded to children’s randomization group and other personal information.
2.3. Statistical Analyses
Pre-booster, post-booster and at 24-months of age antibody Geometric Mean Concentrations (GMC) between study-groups were compared using a multiple linear regression model, considering gender, age, race (black African or mixed ancestry), CD4+-lymphocyte percentage, study-centre and pre-booster antibody concentrations (for post-booster and at 24-months of age time-points) as covariates. Percentage of participants with sero-protective antibody levels or sero-conversion was compared between study-groups using multivariate logistic regression, considering the same covariates. Analyses were limited to children who received all 3-doses in the primary series, had the booster-dose at 15–18 months of age and had serum collected within protocol-specified window periods. Children unable/unwilling to adhere to the study protocol were excluded from this analysis. Children with missing data at specific visits were excluded from those time-points (Supplementary Figure 1). The children included in analysis were similar compared to those not included; Supplementary Table 1.
Results from children in the two arms of the ART-Immed group (ART/12m; ART/24m) were similar at all time-points (Supplementary Table 2) and therefore analysed as a single group (ART-Immed). CD4+-lymphocyte percentages pre-booster and at 24-months were compared by paired t-tests. HIV viral load was not assessed at the time of booster. Statistical analyses were performed using GraphPad Prism 5a (GraphPad Software Inc, La Jolla, CA, USA), R (R Foundation for Statistical Computing, Vienna, Austria) and STATA (STATA Corporation, College Station, TX, USA). All tests were two-sided and p-values <0.05 were considered significant. All comparisons were pre-specified.
2.4. Ethics considerations
This sub-study was approved by the Human Research Ethics Committees of the University of the Witwatersrand (M080966) and Stellenbosch University, the Medicine Control Council (South Africa), Division of AIDS of the National Institute for Health (USA) and was registered under Clinical Trials number () for the parent study. The parent/s of participants signed informed, written consent prior to the childs’ participation, including for measuring immune responses to other vaccines.
3.0. RESULTS
Of the 483 children completing the primary vaccination series [10], 465 (96%) returned for booster-vaccination at 15–18 months of age; Table 1 and Supplementary Figure 1. Sixteen percent (11/69) of children in the ART-Def group were started on ART prior to completing their primary series of vaccines, whilst the rest were initiated on ART at the latest by 24-months of age. The median age at ART initiation among the ART-Def group was 4 months; Table 1. Among the ART-Immed group, 70 of 81 and 76 of 90 children who interrupted ART at 12-months (ART/12m) and 24-months of age (ART/24m), respectively, were included in the analysis [22]. The mean CD4+-lymphocytes percentages were similar between the ART-Immed and the ART-Def groups pre-booster and at 24-months of age; Table 1.
Table 1.
Demographic and clinical information of participants who had received three doses of DTwP-HibCV and hepatitis-B vaccines and where available for follow-up
| Variable | Total | HIV-unexposeda | HEUb | ART-Immedc | ART-Defd |
|---|---|---|---|---|---|
| Number vaccinated | 465 | 110 | 115 | 171 | 69 |
| Male, n (%) | 218 (47) | 59 (54) | 63 (55) | 71 (42) | 25 (36) |
| Black race, n (%) | 420 (90) | 81 (74) | 108 (94) | 163 (95) | 68 (99) |
| Coloured race, n (%) | 45 (10) | 29 (26) | 7 (6) | 8 (5) | 1 (2) |
| Soweto study center, n (%) | 312 (67) | 71 (65) | 80 (70) | 116 (68) | 45 (65) |
| Stellenbosch study center, n (%) | 153 (33) | 39 (36) | 35 (30) | 55 (32) | 24 (35) |
| Mean time in months between last dose in primary series and booster dose (±SD) | 12.0 (0.6) | 11.9 (0.4) | 11.8 (0.6) | 12.0 (0.4) | 12.2 (0.8) |
| Mean age in months (±SD) Pre-boostere | 15.5 (0.5) | 15.3 (0.3) | 15.4 (0.5)f | 15.6 (0.4)gh | 15.7 (0.6)gi |
| Post-boosterj | 15.9 (0.5) | 15.7 (0.3)k | 15.9 (0.5)l | 15.9 (0.4) | 16.1 (0.6)m |
| 24 months of age | 25.3 (0.6) | 25.2 (0.5)n | 25.4 (0.6)o | 25.4 (0.7) | 25.2 (0.9) |
| Total initiated ART between first and last dose of primary series n/N (%) | NDp | ND | ND | 171/171 (100) | 11/69 (16) |
| Total on ART between last dose of primary series and booster dose n/N (%) | ND | ND | ND | 171/171 (100) | 59/69 (86) |
| Time to ART initiation relative to dose 1 of primary series, median months (range) | ND | ND | ND | ND | 4q (3 to 8); n=66 |
| Booster median CD4+ T-lymphocyte count (IQR)r | ND | ND | ND | 1592 (1138 to 2166) | 1611 (1357 to 2272) |
| Booster mean CD4+ T-lymphocyte % (±SD) | ND | ND | ND | 31 (8.7) | 32 (7.1) |
| 24 months of age median CD4+ T-lymphocyte count (IQR) | ND | ND | ND | 1404 (982 to 1765); n=107 | 1826 (1272 to 2092); n=43 |
| 24 months of age mean CD4+ T-lymphocyte % (±SD) | ND | ND | ND | 34 (7.5); n=107 | 36 (7.1); n=43 |
Footnote:
HIV-unexposed: HIV-uninfected children born to HIV-uninfected mothers;
HEU: HIV-uninfected children born to HIV-infected mothers;
ART-Immed: HIV-infected children with CD4+ ≥25% at enrolment who were initiated on antiretroviral therapy (ART) at enrolment;
ART-Def: HIV-infected children with CD4+ ≥25% at enrolment randomized to deferred ART;
Blood draw was undertaken just prior to and on the same day of DTP booster vaccination;
p-value=0.04 HIV-unexposed compared to HEU;
p-value<0.001 HIV-unexposed compared to ART-Immed and ART-Def;
p-value=0.001 HEU compared to ART-Immed;
p-value=0.002 HEU compared to ART-Def;
Children who received booster dose within protocol-defined window periods and available immunogenicity data within 1–2 weeks after booster;
p-value<0.001 ART-Immed and ART-Def compared to HIV-unexposed;
p-value=0.001 HIV-unexposed compared to HEU;
p-value=0.02 ART-Immed compared to ART-Def;
p-value=0.001 HEU compared to HIV-unexposed;
p-value=0.03 w HIV-unexposed compared to ART-Immed;
ND: not done;
Median time in months for ART initiation of the 66 children in the ART-Def group who had started ART;
IQR: interquartile range.
3.1. Pre- and post-booster antibody kinetics to tetanus-toxoid vaccine
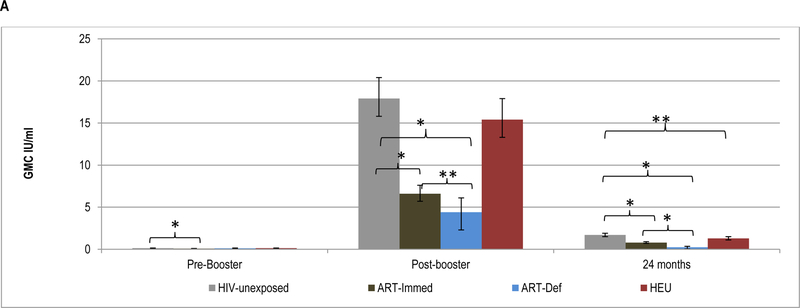
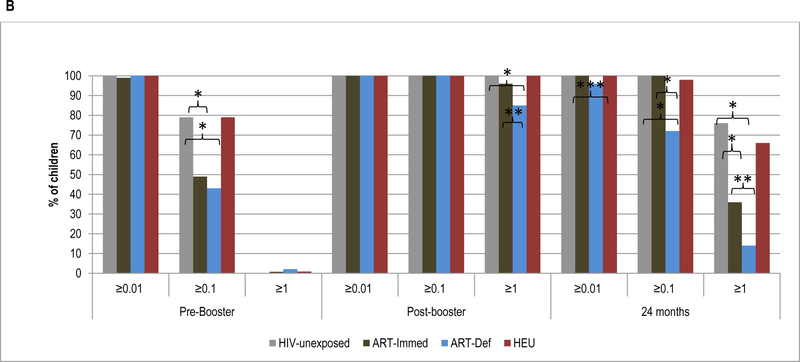
Pre-booster, TT-antibody GMC were higher in HIV-unexposed (0.13IU/ml) compared to ART-Immed children (0.08IU/ml, p<0.001), but similar to the ART-Def group (0.11IU/ml, p=0.09); Figure 1 and Supplementary Table 3. Nevertheless, ≥99% of all children had TT-antibody levels ≥0.01IU/ml; however, compared to HIV-unexposed children (79%), ART-Immed (49%, p<0.001) and ART-Def (43%, p<0.001) groups were less likely to have TT-antibody levels ≥0.1IU/ml; Figure 1 and Supplementary Table 4.
Figure 1.
Geometric mean concentration and percentage of children with sero-protective antibody levels to tetanus-toxoid at pre-booster, post-booster and at 24 months of age.
Footnote: HIV-unexposed: HIV-uninfected children born to HIV-uninfected mothers; ART-Immed: HIV-infected children with CD4+% ≥25% at enrolment who were initiated on antiretroviral therapy (ART) at enrolment; ART-Def: HIV-infected children with CD4+ ≥25% at enrolment randomized to deferred ART; HEU: HIV-uninfected children born to HIV-infected mothers. A) TT geometric mean concentration at pre-booster, post-booster and 24 months of age. Vertical bars represent 95% CI. B) Percentage of children sero-protected (≥0.01; ≥0.1 and ≥1.0 IU/ml) at pre-booster, post-booster and 24 months of age.
* p-value <0.001; ** p-value =0.01; *** p-value =0.03.
Following the booster dose, the fold-increase in TT-antibody was lower among the ART-Def (39.8, p<0.001) and ART-Immed (87.9, p=0.001) than HIV-unexposed (135.6) children; Supplementary Table 3. This was also associated with higher TT-antibody GMC among HIV-unexposed (17.9IU/ml) than the ART-Immed (6.6 IU/ml, p<0.001) or ART-Def (4.4IU/ml, p<0.001) groups. Within the HIV-infected groups, the fold-increase in TT-antibody post-booster in ART-Immed was higher than in ART-Def children. Following the booster dose, all HIV-unexposed and HIV-infected children had TT-antibody levels ≥0.1IU/ml. The majority also had titers ≥1.0IU/ml, although less so among ART-Def (85%) than HIV-unexposed (100%, p<0.001) and ART-Immed (96%, p=0.01) children; Figure 1 and Supplementary Table 4.
At 24-months of age, TT GMC were higher in HIV-unexposed (1.7IU/ml) compared to ART-Immed (0.79IU/ml, p<0.001) and ART-Def (0.23IU/ml, p<0.001) children; and the fold-decline was greater among ART-Def (−21.3) than either HIV-unexposed (−10.1, p<0.001) or ART-Immed (−9.7, p=0.002) groups; Supplementary Table 3. Nevertheless, >96% of all children maintained TT-antibody ≥0.01IU/ml; whilst a lower percentage of ART-Def (72%) had levels ≥0.1IU/ml compared to HIV-unexposed and ART-Immed groups (100% each, p<0.001). Further, 76% of HIV-unexposed had TT-antibody ≥1.0IU/ml compared to 36% of ART-Immed (p<0.001) and 14% of ART-Def (p<0.001); Figure 1 and Supplementary Table 4.
TT-antibody kinetics were similar pre- and post-booster in HIV-unexposed compared to HEU children. TT-antibody GMC were, however, lower in HEU at 24-months of age (1.3 vs. 1.7IU/ml, p=0.01) and 66% of HEU compared to 76% of HIV-unexposed children (p=0.05) had TT-antibody ≥1.0IU/ml; Figure 1.
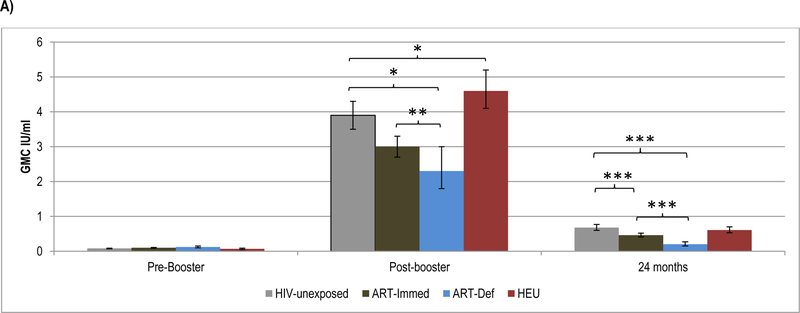
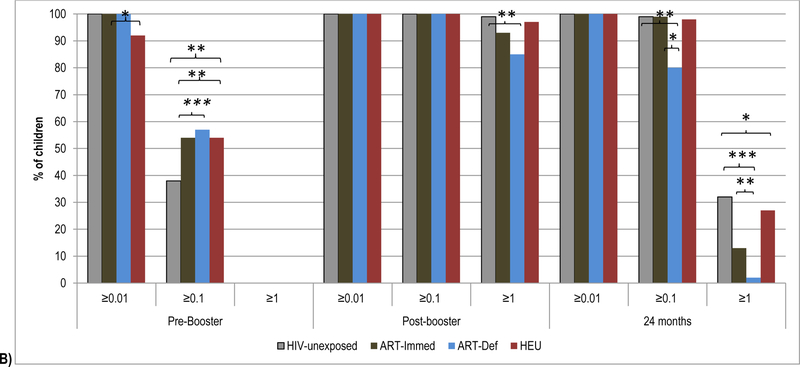
3.2. Pre- and post-booster antibody kinetics to diphtheria-toxoid vaccine
Pre-booster, DT-antibody GMC were similar in HIV-unexposed (0.08IU/ml) compared to ART-Immed (0.10IU/ml; p=0.22) or ART-Def (0.12IU/ml, p=0.05) children; Figure 2 and Supplementary Table 3. A higher percentage of HIV-infected children (ART-Immed: 54%, p=0.001 and ART-Def: 57%, p=0.02) had pre-booster levels ≥0.1IU/ml than HIV-unexposed (38%) children; Figure 2 and Supplementary Table 4.
Figure 2.
Geometric mean concentration and percentage of children with sero-protective antibody levels to diphtheria-toxoid at pre-booster, post-booster and at 24 months of age.
Footnote: HIV-unexposed: HIV-uninfected children born to HIV-uninfected mothers; ART-Immed: HIV-infected children with CD4+% ≥25% at enrolment who were initiated on antiretroviral therapy (ART) at enrolment; ART-Def: HIV-infected children with CD4+ ≥25% at enrolment randomized to deferred ART; HEU: HIV-uninfected children born to HIV-infected mothers. A) DT geometric mean concentration at pre-booster, post-booster and 24 months of age. Vertical bars represent 95% CI. B) Percentage of children sero-protected (>0.01; >0.1 and >1.0 IU/ml) at pre-booster, post-booster and 24 months of age.
* p-value <0.01; ** p-value <0.05; *** p-value <0.001.
The post-booster DT-antibody fold-increase was, however, higher among the HIV-unexposed group (49.6) than ART-Immed (30.4; p=0.08) and ART-Def (17.9; p=0.001) groups. Also post-booster GMC were higher among HIV-unexposed children (3.9IU/ml) than ART-Immed (3.0IU/ml, p<0.001) and ART-Def (2.3IU/m; p=0.003) groups. All HIV-unexposed and HIV-infected children, however, had post-booster DT-antibody ≥0.1IU/ml. Further, ≥93% of all groups had DT-antibody levels ≥1.0IU/ml, except among the ART-Def group (85% vs. 99% in HIV-unexposed children; p=0.02); Figure 2.
At 24-months of age, compared to HIV-unexposed children (0.68IU/ml), DT-antibody GMC were lower in ART-Immed (0.46IU/ml, p<0.001) and ART-Def (0.20IU/ml, p<0.001) groups. The fold-decline in titers was greater among the ART-Def (−11.4; p=0.001) and ART-Immed (−6.8; p=0.008) groups than HIV-unexposed (−5.5) children. All HIV-unexposed and HIV-infected children maintained DT-antibody ≥0.01IU/ml, although a lower percentage of ART-Def (80%) had levels ≥0.1IU/ml than HIV-unexposed (99%, p=0.02) or ART-Immed (99%, p=0.004) children. Further, a higher percentage of HIV-unexposed (32%) children had antibody levels ≥1.0IU/ml than ART-Immed (13%, p=0.001) and ART-Def (2%, p=0.003), Figure 2.
Comparing HEU to HIV-unexposed children, pre-booster DT-antibody GMC were similar; although HEU children were more likely to have levels ≥0.1IU/ml (54%, p=0.03). HEU children demonstrated higher fold-increase post-booster (68.4, p=0.004), resulting in higher GMC (4.6IU/ml) than HIV-unexposed children (3.9IU/ml, p=0.002). The percentage with titers ≥1.0 IU/ml was, however, similar between these two groups (>97%). Similarly, at 24-months of age GMC and percentage of children with sero-protective levels (for both ≥0.1 and ≥1IU/ml) were similar; however, antibody decay was greater in HEU compared to HIV-unexposed children (fold change −7.7 vs. −5.5; p=0.004, respectively), Supplementary Table 4.
3.3. Pre- and post-booster antibody kinetics to pertussis antigens
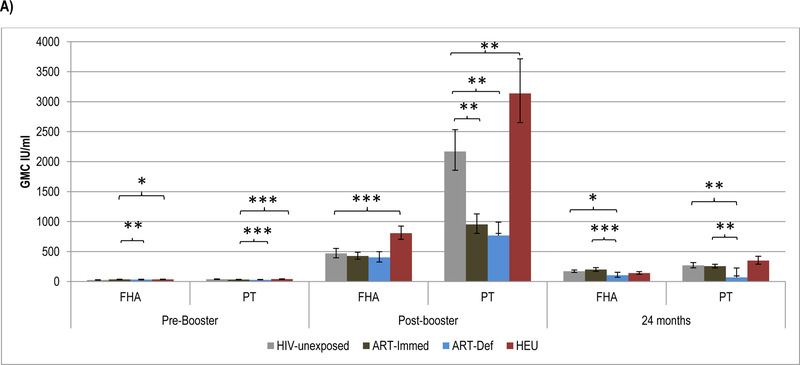
Prior to wP booster, FHA-antibody GMC were lower among HIV-unexposed (24.6IU/ml) than ART-Immed (34.0 IU/ml, p=0.001) children, and similar to ART-Def children (31.9; p=0.09). Pertussis-toxoid antibody GMC were, however, higher in HIV-unexposed (38.3IU/ml) than ART-Immed (31.8IU/ml, p=0.004) and ART-Def (27.2IU/ml, p=0.004) groups; Figure 3 and Supplementary Table 3.
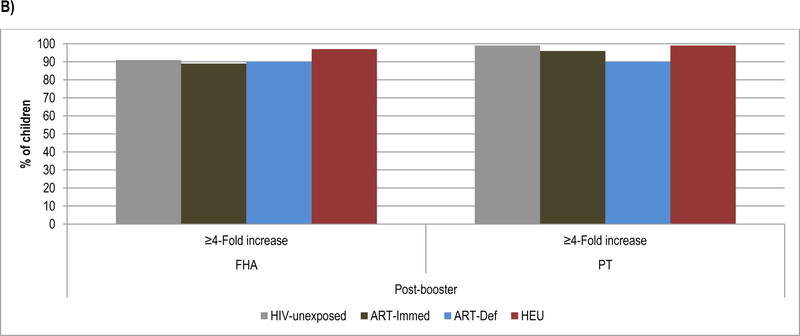
Figure 3.
Geometric mean concentration and percentage of children with ≥4-Fold increase in antibody levels to Pertussis antigens at pre-booster, post-booster and at 24 months of age.
Footnote: HIV-unexposed: HIV-uninfected children born to HIV-uninfected mothers; ART-Immed: HIV-infected children with CD4+% ≥25% at enrolment who were initiated on antiretroviral therapy (ART) at enrolment; ART-Def: HIV-infected children with CD4+ ≥25% at enrolment randomized to deferred ART; HEU: HIV-uninfected children born to HIV-infected mothers. A) FHA and PT geometric mean concentration at pre-booster, post-booster and 24 months of age. Vertical bars represent 95% CI. B) Percentage of children who sero-converted to pertussis antigens (≥4-Fold increase in antibody level) at post-booster.
* p-value <0.05; ** p-value <0.001; *** p-value <0.01.
The fold-increase in FHA-antibody post-booster was similar between HIV-unexposed (18.7) and HIV-infected children (12.3–13.0); all groups achieved similar GMC. The PT-antibody fold-increase was, however, higher in HIV-unexposed (56.1) than ART-Immed (30.1, p=0.01) and ART-Def (27.9, p=0.04) children. Consequently, PT-antibody post-boost GMC were higher in HIV-unexposed (2168.8 IU/ml) than ART-Immed (951.5IU/ml, p<0.001) and ART-Def (767.6IU/ml, p<0.001) groups. There were, however, no differences in the sero-conversion rates (i.e. % with ≥4-fold increase) between HIV-unexposed and HIV-infected children for either FHA (>88%) or PT (≥90%); Figure 3.
At 24-months of age, the ART-Def group showed a larger fold-decline for FHA-antibody (−3.6, p=0.04) and PT-antibody (−12, p=0.01) compared to HIV-unexposed (−2.6 and −7.7, respectively). The ART-Immed group had similar antibody decay for FHA compared to HIV-unexposed (−2.1; p=0.40) but had a lower decline in PT-antibody (−4.0; p<0.001); Supplementary Table 3. Further, ART-Def group had lower FHA- and PT-antibody GMC than HIV-unexposed and ART-Immed groups (p≤0.02 for all observations); Supplementary Table 3 and Figure 3.
HEU compared to HIV-unexposed children had higher pre-booster FHA-antibody (34.5 vs. 24.6IU/ml, p=0.01), but similar PT-antibody GMC. Post-booster fold-increases were also greater in HEU than HIV-unexposed to both FHA (23.1 vs. 18.7, p=0.01) and PT (56.1 vs. 79.7, p=0.001); and consequently HEU had higher GMC than HIV-unexposed children to both FHA (806.2IU/ml vs. 467.6IU/ml, p<0.001) and PT (2168.8 vs. 3138.2IU/ml, p<0.001); Figure 3. There was, however, no difference in the percentage showing sero-conversion to PT (99%) or FHA (>93%) between HEU and HIV-unexposed children. Between post-booster and 24-months of age, HEU children showed a greater fold-decrease than HIV-unexposed for both FHA-antibody (−5.8, p<0.001) and PT-antibody (−9.6, p=0.06), but there were no differences in GMC at 24-months of age, Supplementary Table 3.
3.4. Persistence and antibody kinetics to hepatitis-B vaccine
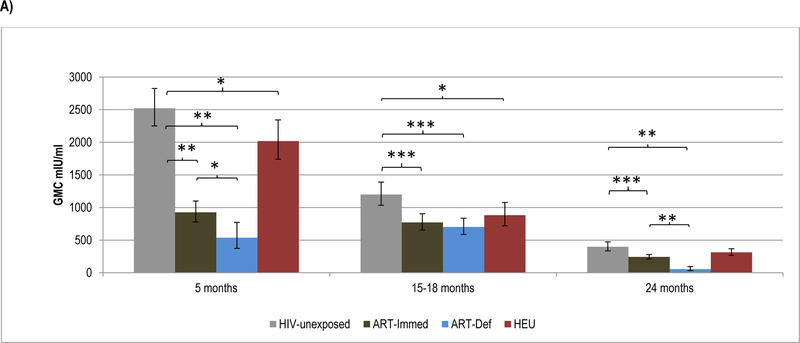
No booster dose of HBV was scheduled in this study; hence, we only measured antibody persistence to the primary series. At 15-months of age, all children maintained sero-protective HBsAg antibody levels ≥10mIU/ml and 94%−100% had levels ≥100mIU/ml. HBsAg antibody GMC were, however, lower in ART-Immed (769.4mIU/ml, p=0.001) and ART-Def (700.2mIU/ml, p=0.002) than HIV-unexposed (1198.5mIU/ml) children. HEU (880.1mIU/ml, p=0.02) also had lower HBsAg antibody GMC than HIV-unexposed children; Figure 4. All groups showed a decrease in HBsAg antibody GMC between post-primary series (5-months of age) and 15-months of age, except for the ART-Def group in whom there was a 0.8-fold increase. The fold-decrease in GMC was lower in ART-Immed (−1.2, p<0.001) than HIV-unexposed children (−2.1); Supplementary Table 3.
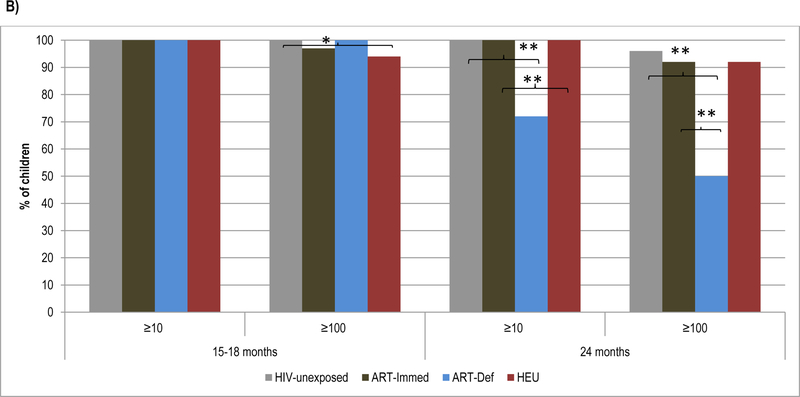
Figure 4.
Geometric mean concentration and percentage of children with sero-protective antibody titers to HBsAg at 5 months, 15–18 months and at 24 months of age.
Footnote: HIV-unexposed: HIV-uninfected children born to HIV-uninfected mothers; ART-Immed: HIV-infected children with CD4+% ≥25% at enrolment who were initiated on antiretroviral therapy (ART) at enrolment; ART-Def: HIV-infected children with CD4+ ≥25% at enrolment randomized to deferred ART; HEU: HIV-uninfected children born to HIV-infected mothers. A) HBsAg-antibody geometric mean concentration at 5 months, 15–18 months and 24 months of age. Vertical bars represent 95% CI. B) Percentage of children sero-protected (≥10 and ≥100 mIU/ml) at 5 months, 15–18 months and 24 months of age.
* p-value <0.05; ** p-value <0.001; *** p-value <0.01.
At 24-months of age, GMC were lower in ART-Immed (242.0mIU/ml, p=0.001) and ART-Def (54.8mIU/ml, p<0.001) groups compared to HIV-unexposed children (396.1mIU/ml). ART-Def children were also less likely to maintain levels ≥10mIU/ml (72%) and ≥100mIU/ml (50%) compared to HIV-unexposed or ART-Immed (92%−100%, p<0.001 for all comparisons); Figure 4. Also, between 15 to 24-months of age, HBsAg antibody decay was greater among ART-Def (−13.3) than HIV-unexposed and ART-Immed groups (−3, p<0.001 for all comparisons).
Comparing HIV-unexposed to HEU children, despite similar decline in HBsAg antibody between 5 and 15-months of age, HEU had lower GMC (880.1, p=0.02) and were less likely to have titres ≥100mIU/ml (94% vs. 100%, p=0.01). These differences were, however, no longer evident by 24-months of age; Figure 4.
4.0. DISCUSSION
Timing of ART initiation during early infancy had an on-going effect on the immune responses to booster-dose of some vaccines. Notably, even though all children in the ART-Def group began ART during their first year of life and had similar CD4+-lymphocytes counts compared to the ART-Immed group at the time of booster, they had an attenuated booster response to most vaccine epitopes compared to HIV-unexposed (for TT, DT and PT) and ART-Immed children (for TT and DT). Memory responses and antibody persistence were generally similar between the ART-Immed and the HIV-unexposed children. Furthermore, ART interruption at 12-months of age did not negatively impact the booster responses or persistence of antibodies to the evaluated vaccine epitopes (Supplementary material Table 2). ART-Def children were unable to maintain HBsAg antibody concentrations ≥10IU/ml at 24-months of age in contrast to the other study groups. It remains unclear, however, whether HBV booster is required for children initiating ART later, as memory responses might be present even in the absence of detectable HBsAg antibody [24]. Another finding from our study, is that the memory responses to vaccine epitopes were similar and occasionally more robust (for DT, PT and FHA) in HEU children.
Previous studies reported that a high percentage of HIV-infected children on ART (±90%) maintained serologic response to TT one year post-vaccination [25, 26, 27], corroborated by a recent meta-analysis in which 75% of HIV-infected children maintained TT-antibody titres ≥0.1IU/ml 2 to 5-years post TT-vaccination [28]. In a small study of pre- and post-booster responses to TT-vaccine, stratified by incomplete or full viral suppression, modest humoral and cellular responses were identified and antibody decayed rapidly within one year [29]. Despite reconstitution of the CD4+-lymphocyte count over the first year of ART in our ART-Def group, additional booster doses might be necessary to maintain sero-protective immunity, as only 72% of these children maintained TT-antibody concentrations ≥0.1IU/ml at 24-months of age.
Diphtheria-toxoid is a weaker antigen than TT, with steeper antibody decay in healthy children [30]. In our study, in addition to an inferior anamnestic response to DT in the ART-Def group, these children were less likely to maintain sero-protective levels of ≥0.1IU/ml (80%) than all other groups (>98%) at 24-months of age. Nevertheless, responses to DT in the ART-Def group were higher than previously reported in children on ART (40–65% of children with viral suppression achieved sero-protective antibody levels), however, immunological responses were only assessed 4–6 years post-booster dose in the study by Zaccarelli-Filho et al. [25].
Interpretation of immune responses to pertussis vaccination is challenging, as correlates of protection are not established [1]. Immune responses to PT and FHA were, however, high in our study, with ≥4-fold increase from pre-booster to post-booster vaccination observed for each pertussis antigen in >87% of all groups. The high sero-conversion rates seen in our HIV-infected groups were similar to that observed in healthy HIV-uninfected children after DTP boosting [31]. Although the HIV-infected children had significant increases in antibody concentration to PT and FHA post-booster, GMC were lower than in HIV-uninfected children especially for PT, which is considered key virulent factor of Bordetella pertussis [32]. A USA cohort study of children on stable ART given acellular-pertussis vaccine reported low antibody responses to a booster-dose and shorter duration of protective immunity than in HIV-uninfected children, despite receiving at least a four dose primary series [33].
In our study, persistence of HBsAg antibody 12-months post-primary series was high in all groups, although GMC were lower in HIV-infected than HIV-unexposed children. Furthermore, by 24-months of age the percentage of ART-Def children with levels ≥10mIU/ml decreased from 100% at 15-months age to 72%, and only 50% had levels ≥100mIU/ml, compared to 92% among ART-Immed children. A study on antibody persistence following HepB vaccination in HIV-infected children on ART, reported that those with titers ≥10mIU/ml following three doses of HepB vaccine, were 71%, 61%, 38%, and 25% at 48 weeks, 2 years, 4 years and 6 years of age, respectively [34]. The results from our study support further booster doses of HBV in children not on ART at the time of receipt of their primary series of vaccines considering the high prevalence of chronic hepatitis-B infection in HIV-infected individuals [35].
In our study a similar proportion of HEU and HIV-unexposed children maintained sero-protective levels to TT, DT and HBsAg; and had comparable PT and FHA GMC 12-months post-primary vaccination. This corroborated results from an earlier South African study, where HEU children had similar antibody levels compared to HIV-unexposed children at 12-months of age to TT, DT and HBsAg [36]. Anamnestic responses were also similar between HEU and HIV-unexposed, although HEU had higher FHA and PT GMC than HIV-unexposed children, the clinical significance of which is unclear in the absence of correlate of protection against pertussis. Although we did not examine the immunological basis for the higher post-boost GMCs in HEU compared to HIV-unexposed children; it could be that the memory responses induced with the primary series of wP vaccine was enhanced in HEU due to lower levels of transplacental acquired maternal antibody as we had previously observed in this cohort [10], which could interfere with primary immune responses. However, the same mechanism would not explain the higher post-boost GMCs to FHA (and DT) in HEU compared to HIV-unexposed children. Consequently, further research is warranted to explain the basis for the more the seemingly more robust memory responses to wP and DT in HEU compared to HIV-unexposed children.
Limitations to our study include that we were not powered to assess break-through infections; therefore, the functionality of induced antibodies against preventing disease cannot be definitively determined. Also, despite having used whole-cell pertussis vaccine, we measured antibody to PT and FHA rather than using the whole-cell pertussis ELISA assay, as this was more practical for inclusion in the Luminex multiplex immunoassay. The PT and FHA antibody responses, although providing a relative measure of immunogenicity of the whole-cell vaccine, are not necessarily a complete measure of immunity induced by it, although antibody against PT is strongly correlated with protection against severe pertussis disease [1]. The differences in responses between the PT and FHA observed in this study, could be attributable to the acellular vaccine antigens used in the luminex assay, as previously suggested by Cherry et al that the type of ELISA used may affect the antibody titers detected [37].
In conclusion, our results show that vaccine induced memory responses and humoral immunity is maintained among HIV-infected children initiated on ART during receipt of their primary series of DTwP. In contrast, deferring ART initiation in HIV-infected children attenuated their booster responses to TT, PT and HBV compared to HIV-unexposed children, despite the HIV-infected children having establishing normal CD4+-lymphocytes counts [10]. This suggests a need for further booster doses of TT, PT and HBV among HIV-infected children not initiated on ART during the time of their primary vaccination series.
Supplementary Material
KEY ISSUES.
Early ART initiation during the first year of infancy had continued effects on the immune responses to booster-dose of most of the vaccines epitopes tested.
Although the ART-Def group began ART during the first year of life and had similar CD4+-lymphocytes counts compared to the ART-Immed group at the time of booster, they had an attenuated booster response to most vaccine epitopes compared to HIV-unexposed (for TT, DT and PT) and ART-Immed children (for TT and DT).
Memory responses and antibody persistence were generally similar between the ART-Immed and the HIV-unexposed children.
ART interruption at 12-months of age compared to interruption at 24-months did not negatively impact response to DTwP booster vaccination or persistence of antibodies to the evaluated vaccine epitopes.
ART-Def children were unable to maintain HBsAg antibody concentrations ≥10IU/ml at 24-months of age in contrast to the other study groups.
ACKNOWLEDGEMENTS
Collaborators and Centers for study: South Africa: Avy Violari, James McIntyre, Wilma Pelser, Ravindre Panchia, Kennedy Otwombe, Afaaf Liberty, Nastassja Choolinal (Perinatal HIV Research Unit); Mark F Cotton, Helena Rabie, Anita Janse van Rensburg, Els Dobbels, George Fourie, Marietjie Bester, Wilma Orange, Ronelle Arendze, Catherine Andrea, Marlize Smuts, Kurt Smith, Theresa Louw, Alec Abrahams, Kenny Kelly, Amelia Bohle, Irene Mong, Jodie Howard, Tanya Cyster, Genevieve Solomon, Galroy Benjamin, Jennifer Mkhalipi, Edward Barnes (Children’s Infectious Diseases Clinical Research Unit); Peter Adrian; Shabir A Madhi; Nadia van Niekerk (Respiratory and Meningeal Pathogens Research Unit). United States of America: Karen Reese, Patrick Jean-Philippe (HJF-DAIDS). United Kingdom: Diana M Gibb, Abdel Babiker (Medical Research Council Clinical Trials Unit, London).
REFERENCES
*Of importance
**Of considerable importance
- 1.Plotkin SA. Correlates of protection induced by vaccination. Clinical and Vaccine Immunology. 2010;17(7):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pensieroso S, Cagigi A, Palma P, et al. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children [Research Support, Non-U.S. Gov’t]. Proceedings of the National Academy of Sciences of the United States of America. 2009. May 12;106(19):7939–7944. doi: 10.1073/pnas.0901702106.Timing of HAART initiation is a major predictor of longevity of B cell epitopes in vaccination of HIV-1 infected children.
- 3.Palma P, Romiti ML, Cancrini C, et al. Delayed early antiretroviral treatment is associated with an HIV-specific long-term cellular response in HIV-1 vertically infected infants. Vaccine. 2008. September 19;26(40):5196–201. doi: 10.1016/j.vaccine.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Feyen O, Jebran AF, et al. Memory B cell function in HIV-infected children--Decreased memory B cells despite ART. Pediatric Research. 2009;66(2):185–190. [DOI] [PubMed] [Google Scholar]
- 5.Moir S, Fauci AS. Pathogenic mechanisms of B lymphocyte dysfunction in HIV disease. Journal of Allergy and Clinical Immunology. 2008;122(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MA, Rathore MH. Immunization in special populations. Advances in Pediatrics. 2012;59(1):95–136. [DOI] [PubMed] [Google Scholar]
- 7.Iwajomo OH, Moons P, Nkhata R, et al. Delayed reconstitution of B cell immunity to pneumococcus in HIV-infected Malawian children on antiretriviral therapy. The Journal of Infection. 2015;70(6):616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir S, Buckner CM, Ho J, et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116(25):5571–5579.Describes the overall effects of changes in the B cell compartment decreases their capacity to proliferate in response to B cell stimuli, neoantigens and recall antigens, as well as decreased capacity to generate antigen-specific memory B cells.
- 9.Bekker V, Scherpbier H, Pajkrt D, et al. Persistent humoral immune defect in highly active antiretroviral therapy-treated children with HIV-1 infection: loss of specific antibodies against attenuated vaccine strains and natural viral infection. Pediatrics. 2006;118(2):e315–e322.Describe loss of specific antibodies to live attenuated vaccines during HAART treatment.
- 10.Simani OE, Izu A, Violari A, et al. Effects of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS. 2014;28(4):531–541.Describes response to primary vaccination in HIV infected children of different ART treatment strategies who are either on early ART or Deferred ART.
- 11.Cagigi A, Cotugno N, Giaquinto C, et al. Immune reconstitution and vaccination outcome in HIV-1 infected children. Present knowledge and future directions. Human Vaccines and Immunotherapeutics. 2012;8(12):1784–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madhi SA, Adrian P, Cotton MF, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants [Research Support, N.I.H., Extramural]. The Journal of infectious diseases. 2010. August 15;202:355–361. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hygino J, Lima PG, Filho RGS, et al. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clinical Immunology. 2008;127(3):340–347. [DOI] [PubMed] [Google Scholar]
- 14.Faye A, Pornprasert S, Mary JY, et al. Characterization of the main placental cytokine profiles from HIV-1-infected pregnant women treated with anti-retroviral drugs in France. Clinical and Experimental Immunology. 2007;149(3):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feiterna-Sperling C, Weizsaecker K, Bührer C, et al. Hematologic effects of maternal antiretroviral therapy and transmission prophylaxis in HIV-1-exposed uninfected newborn infants. Journal of Acquired Immunodeficiency Syndromes. 2007;45(1):43–51. [DOI] [PubMed] [Google Scholar]
- 16.Borges-Almeida E, Milanez HMBM, Vilela MMS, et al. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infectious Diseases. 2011;11:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunders M, Thorne C, Newell ML, et al. Maternal and infant factors and lymphocyte, CD4 and CD8 cell counts in uninfected children of HIV-1-infected mothers. AIDS. 2005;19(10):1071–1079. [DOI] [PubMed] [Google Scholar]
- 18.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96(12):3866–3871. [PubMed] [Google Scholar]
- 19.Cotton MF, Violari A, Otwombe K, et al. Early time-limited anitiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382(9904):1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008. November 20;359(21):2233–44. doi: 359/21/2233 [pii] 10.1056/NEJMoa0800971.Describes in detail the selection criteria of study participants and randomization strategies used in ART treatment interruption of HIV infected children within the CHER clinical trial.
- 21.WHO. Anteretroviral therapy of HIV infection in infants and children: Towards universal access Recommendations for a public health approach. Geneva: World Health Organization; 2006. [cited 2016 1 December] Available from: http://who.int/hiv/pub/paediatric/infants/en [PubMed] [Google Scholar]
- 22.Simani OE, Adrian PV, Violari A, et al. Effect of in-utero HIV exposure and antiretroviral treatment strategies on measles susceptibility and immunogenicity of measles-vaccine. AIDS. 2013;27(10):1583–1591. [DOI] [PubMed] [Google Scholar]
- 23.Madhi SA, Koen A, Cutland C, et al. Antibody persistence and booster vaccination of fully liquid hexavalent vaccine coadministered with measles/mumps/rubella and varicella vaccines at 15–18 months of age in healthy South African infants. Pediatr Infect Dis J. 2013;32(8):889–897. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney FG, Kane M. Hepatitis B vaccine In: Plotkin SA, Orenstein WA, editors. Vaccines. Philadelphia: Sanders Company; 1999. p. 158–182. [Google Scholar]
- 25.Zaccarelli-Filho CA, Ono E, Machado DM, et al. HIV-1-infected children on HAART: Immunologic features of three different levels of viral suppression. Cytometry Part B, Clinical Cytometry. 2007;72(1):14–21. [DOI] [PubMed] [Google Scholar]
- 26.Rigaud M, Borkowsky W, Muresan P, et al. Impaired immunity to recall antigens and neoantigens in severely immunocompromised children and adolescents during the first year of effective highly active antiretroviral therapy. The Journal of Infectious Diseases. 2008;198(8):1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melvin AJ, Mohan KM. Response to immunization with measles, tetanus, and Haemophilus influenzae type b vaccines in children who have human immunodeficiency virus type 1 infection and are treated with highly active antiretroviral therapy. Pediatrics. 2003;11(6):e641–e644. [DOI] [PubMed] [Google Scholar]
- 28.Kerneis S, Launay O, Turbelin C, et al. Long-term immune responses to vaccination in IV-infecte patients: A systemic review and meta-analysis. Clinical Infectious Diseases. 2014;58(8):1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenblatt HM, Song LY, Nachman SA, et al. Tetanus immunity after diphtheria, tetanus toxoids, and acellular pertussi vaccination in children with clinically stable HIV infection. The Journal of Allergy and Clinical Immunology. 2005;116(3):698–703. [DOI] [PubMed] [Google Scholar]
- 30.Menson EN, Mellado MJ, Bamford A, et al. Guidance on vaccination of HIV-infected children in Europe. HIV Medicine. 2012;13(6):333–336. [DOI] [PubMed] [Google Scholar]
- 31.Lin TY, Wang YH, Huang YC, et al. One-year post-primary antibody persistence and booster immune response to a fully liquid five-component acellular pertussis, diphtheria, tetanus, inactivated poliomyelitis, Haemophilus influenzae type b conjugate vaccine. International Journal of Infectious Diseases. 2007;11(6):488–495. [DOI] [PubMed] [Google Scholar]
- 32.Melvin JA, Scheller EV, Miller JF, et al. Bordetella pertussis pathogenesis: current and future challenges. Nature reviews Microbiology. 2014;12(4):274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abzug MJ, Song LY, Fenton T, et al. Pertussis booster vaccination in HIV-infected children receiving highly active antiretroviral therapy. Pediatrics. 2007;120(5):e1190–e1202. [DOI] [PubMed] [Google Scholar]
- 34.Abzug MJ, Warshaw M, Rosenblatt HM, et al. Immunogenicity and immunologic memory after hepatitis B virus booster vaccination in HIV-infected children receiving Highly Active Antiretroviral therapy. The Journal of Infectious Diseases. 2009;200(6):935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phung B-C, Sogni P, Launay O. Hepatitis B and human immunodeficiency virus co-infection. World Journal of Gastroenterology : WJG. 2014;20(46):17360–17367. doi: 10.3748/wjg.v20.i46.17360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reikie BA, Naidoo S, Ruck CE, et al. Antibody responses to vaccination among South African HIV-exposed and uninfected infants during the first 2 years of life. Clinical and Vaccine Immunology. 2013;20(1):33–38.Comparison of antibody response to childhood vaccines within the South African EPI between HIV exposed uninfected and HIV unexposed uninfected children during the first 2 years of life.
- 37.Cherry JD, Heininger U, Richards DM, et al. Antibody response patterns to Bordetella pertussis antigens in vaccinated (primed) and unvaccinated (unprimed) young children with pertussis. Clinical and vaccine immunology : CVI. 2010;17(5):741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.