Abstract
Background:
Congenital limb deficiencies (CLDs) are a relatively common group of birth defects whose etiology is mostly unknown. Recent studies suggest maternal air pollution exposure as a potential risk factor.
Aim:
To investigate the relationship between ambient air pollution exposure during early pregnancy and offspring CLDs.
Methods:
The study population was identified from the National Birth Defects Prevention Study, a population-based multi-center case-control study, and consisted of 615 CLD cases and 5,701 controls with due dates during 1997 through 2006. Daily averages and/or maxima of six criteria air pollutants (particulate matter < 2.5 μm [PM2.5], particulate matter < 10 μm [PM10], nitrogen dioxide [NO2], sulfur dioxide [SO2], carbon monoxide [CO], and ozone [O3]) were averaged over gestational weeks 2–8, as well as for individual weeks during this period, using data from EPA air monitors nearest to the maternal address. Logistic regression was used to estimate odds ratios (aORs) and 95% confidence intervals (CIs) adjusted for maternal age, race/ethnicity, education, and study center. We estimated aORs for any CLD and CLD subtypes (i.e., transverse, longitudinal, and preaxial). Potential confounding by co-pollutant was assessed by adjusting for one additional air pollutant. Using the single pollutant model, we further investigated effect measure modification by body mass index, cigarette smoking, and folic acid use. Sensitivity analyses were conducted restricting to those with a residence closer to an air monitor.
Results:
We observed near-null aORs for CLDs per interquartile range (IQR) increase in PM10, PM2.5, and O3. However, weekly averages of the daily average NO2 and SO2, and daily max NO2, SO2, and CO concentrations were associated with increased odds of CLDs. The crude ORs ranged from 1.03 to 1.12 per IQR increase in these air pollution concentrations, and consistently elevated aORs were observed for CO. Stronger associations were observed for SO2 and O3 in subtype analysis (preaxial). In co-pollutant adjusted models, associations with CO remained elevated (aORs: 1.02–1.30); but aORs for SO2 and NO2 became near-null. The aORs for CO remained elevated among mothers who lived within 20 km of an air monitor. The aORs varied by maternal BMI, smoking status, and folic acid use.
Conclusion:
We observed modest associations between CLDs and air pollution exposures during pregnancy, including CO, SO2, and NO2, though replication through further epidemiologic research is warranted.
Keywords: Congenital limb deficiencies, Air pollution, Carbon monoxide, Sulfur dioxide, Nitrogen dioxide
1. Introduction
Congenital limb deficiencies (CLDs) are major structural birth defects characterized by complete or partial absence of limbs, specifically upper arm, lower arm, wrist, hand, fingers, thigh, lower leg, ankle, foot, or toes (NBDPN, 2015). The birth prevalence of CLDs in the United States ranges from 3 to 7 per 10,000 births per year (Ephraim et al., 2003; Parker et al., 2010). However, the causes of CLDs remain largely unknown (CDC, 2018). Thalidomide is an established teratogen (Therapontos et al., 2009), and increasing evidence supports genetic factors (Gold et al., 2011), maternal smoking during pregnancy (Caspers et al., 2013; Källén, 1997), and other factors including substance abuse during pregnancy and maternal diabetes (Åberg et al., 2001; Gold et al., 2011), as potential risk factors. Since the thalidomide epidemic in the 1960’s, there have been increased efforts to identify other modifiable environmental teratogens related to CLDs. A study in Boston that classified CLD cases according to their suspected etiology (Gold et al., 2011) reported that genetic or syndromic risk factors accounted for only 33% of the cases, with the remaining majority attributed to unknown (35%), vascular disruption (28%), or teratogenic (4%) etiologies.
Ambient air pollution exposures may be possible teratogens for CLDs, as they are known risk factors for some adverse birth outcomes including fetal growth and preterm delivery (Glinianaia et al., 2004; Sram, 1999; Šrám et al., 2005). In recent years, there is a growing body of literature linking air pollution exposure to birth defects including congenital heart defects and oral clefts (Gilboa et al., 2005; Padula et al., 2013a, 2013b; Stingone et al., 2014; Vrijheid et al., 2010). After searching PubMed for English language studies we found one study that had the central aim to evaluate associations between CLDs and air pollution (Lin et al., 2014) and 6 other studies providing effect estimates for CLDs and air pollution (Dolk et al., 2009; Padula et al., 2013c; Pedersen et al., 2017; Schembari et al., 2014; Vinceti et al., 2016; Vinikoor-Imler et al., 2013). The 6 other studies were conducted with the purpose of screening potential effects of air pollution on a wide range of birth defects including CLDs. Results were inconsistent across studies, and most studies had a focus on particulate matter (PM), ozone (O3) and/or nitrogen dioxide (NO2) with limited information regarding carbon monoxide (CO) or sulfur dioxide (SO2). Such selective focus on PM, O3, and NO2 could be in part due to their reported associations with other congenital anomalies (Vrijheid et al., 2011) and due to publically available exposure models that are frequently used for observational studies such as CMAQ (Appel et al., 2017) and LUR (Hoek et al., 2008). Limited research on CO and SO2 in relation to CLDs could be due to their low ambient concentrations and challenges in exposure assessment given their spatio-temporal variability as compared to the other criteria air pollutants. Due to the sparse evidence coupled with the need to identify potentially modifiable risk factors for CLDs, the relationship between air pollutants and CLDs merits further research.
We sought to investigate possible relationships between CLDs and exposure to six criteria air pollutants at maternal residence during pregnancy using a large US population-based case-control study. In addition, we aimed to explore critical windows of exposure to these pollutants during pregnancy and assess potential effect measure modification by factors that can interfere with the underlying biological pathway such as folic acid or BMI.
2. Materials and methods
We identified the study population from nine participating centers of the National Birth Defects Prevention Study (NBDPS; 1997–2012): Arkansas (AR), California (CA), Georgia (GA), Iowa (IA), Massachusetts (MA), North Carolina (NC), New York (NY), Texas (TX), and Utah (UT). Briefly, NBDPS is a multi-state population-based case-control study conducted to investigate a range of risk factors for over 30 major structural birth defects. Participating centers identified cases meeting eligibility criteria among live births, stillbirths, and elective terminations. The current study obtained data on birth defect cases classified as CLDs who met the eligibility criteria. Eligible cases of CLDs were determined by a clinical geneticist, excluding 1) infants with absent, partially absent, or missing bony elements of the extremities; 2) cases of CLDs with a known chromosomal anomaly; and 3) infants whose mothers said they had diabetes before they were pregnant. The clinical geneticist classified CLDs into isolated (i.e., CLD was the only major structural birth defect) or non-isolated (additional co-occurring major birth defect(s)) cases, and further categorized CLDs into subtypes including longitudinal and transverse. Cases of transverse (ICD-9 codes: 755.20, 755.24, 755.30, 755.34), longitudinal (755.25–755.27, 755.35–37), and preaxial limb deficiencies (755.26, 755.36; a subtype of longitudinal) were used in the current study for additional analyses by subtypes. Controls were live births without major birth defects, randomly selected from birth certificates or hospital records. Case and control mothers were invited to participate in a computer-assisted telephone interview to provide information about pregnancy, socio-demographics, lifestyle, and residential history over the course of pregnancy. Further details about the NBDPS were published previously (Reefhuis et al., 2015).
The initial NBDPS sample for this study consisted of 751 CLD cases and 7,127 controls with due dates during 1997 through 2006. We excluded women without reported residential address during days 8–56 post-conception or without an active air monitor within 50 km of residential address (excluded 135 cases and 1,411 controls), with donor egg/embryo/sperm (excluded 1 case and 14 controls), and live births whose gestational age was less than 20 weeks (excluded 1 control). The final study population consisted of 615 CLD cases and 5,701 controls who had exposure data on at least one of the six criteria air pollutants (i.e., O3, CO, SO2, NO2, PM < 10 μm [PM10], and PM < 2.5 μm [PM2.5]). For each air pollution exposure – CLDs analysis, separate analytic samples were created by removing from the final study population the participants missing the gestational-period-specific air pollution exposure specific to each analysis.
Daily air pollution concentrations were obtained from all available monitors reporting to the Environmental Protection Agency Air Quality System, including both the background/regional monitors and the local/near-source monitors (EPA, 2019). Specifically, we used 24-h measurements of PM10 and PM2.5, daily maximum 8-h moving average for O3, daily 1-h maximum measurements for CO, SO2, and NO2, and additionally used daily average measurements for SO2 and NO2 (values using daily maximums are referred to as SO2 max and NO2 max).
All study participants’ gestational-period-specific air pollution exposures was characterized with the eight daily metrics of air pollution, by linking active air monitoring locations based on mothers’ self-reported residences during gestational weeks 2–8. The air pollution exposure window included the critical time-window for limb development (gestational weeks 3–8; Gold et al., 2011) and 1 week before for any lagged effects of air pollution (gestational week 2). Gestational ages of cases and controls were estimated based on clinician-provided estimated delivery dates (“due dates”) reported by mothers during interviews. If mothers did not know her due dates, medical record abstraction was used to estimate their due dates. Mothers’ gestational-age-specific addresses were centrally geocoded and linked to their nearest active air monitor within 50 km (Stingone et al., 2014). From the linked monitors, the eight metrics of daily air pollution concentrations were assigned to mothers to calculate weekly and overall average exposures during gestational weeks 2–8. The gestational-period-specific air pollution concentrations were calculated only if daily values constituting the gestational period were available for 75% or more of the time, for all pollutants except PM10 and PM2.5. Since PM monitors generally operate every 6 days, this information was considered in addition to the 75% criteria for the quality control of PM data. Data for PM2.5 concentrations became available in 1999 and were not available for participants with exposure windows during 1997 and 1998.
Descriptive characteristics of covariates for cases and controls were compared. Correlations were calculated between air pollution concentrations, across the eight air pollution metrics and gestational weeks. The association between interquartile range (IQR) increase in air pollution concentrations and overall CLDs was assessed separately for each of the eight metrics of average air pollution concentrations using logistic regression models. We separately examined weekly and overall average (i.e., weeks 2–8) exposure windows. The relationship between air pollution concentrations and specific CLD phenotypes were separately assessed using logistic regression. Adjustment variables (confounders and risk factors for CLDs) were selected a priori based on a directed acyclic graph, and included maternal age (< 20; 20–29; ≥30 years), maternal years of education (< 12; 12; 13–15; ≥16), maternal race/ethnicity (Non-Hispanic White; Non-Hispanic Black; Hispanic; Other), and study center (AR, CA, GA, IA, MA, NC, NY, TX, UT). Additionally, potential confounding by seasonality was considered by including in the logistic regression models quadratic terms for the day of year at conception. Sensitivity analyses were conducted by restricting the data to mothers living within 20 km of an air monitor to reduce air pollution misclassification due to large distance (50 km).
Potential confounding by a co-pollutant was assessed by comparing single-pollutant model results with co-pollutant models that were adjusted for the same gestational-period-specific concentration of an additional air pollutant for air pollutants with the most consistent and highest magnitude odds ratios (ORs). Changes in effect estimates attributable to restriction in sample size (i.e., those with available information on both pollutants) was assessed by re-examining the single pollutant model after restricting the sample to those included in co-pollutant models.
Possible effect measure modification was assessed in models with the interaction terms between the exposure and modifier, and by examining the p-values of the interaction term using an alpha = 0.1 criterion. Potential effect measure modifiers included maternal pre-pregnancy body mass index (BMI) reported at baseline interview, folic acid supplement use during 3 months before conception until date of birth, and cigarette smoking status during 3 months before conception until 1 month after conception.
The NBDPS and this analysis have approval from the Institutional Review Boards from the Centers for Disease Control and Prevention (CDC) and all participating centers. All analyses were conducted using SAS 9.4 (SAS, 2012).
3. Results
The study population consisted of 5,701 controls and 615 cases, 449 of which were isolated cases. The 615 CLD cases were further sub-classified into 355 transverse, 234 longitudinal, 138 preaxial, 27 intercalary and 15 were ‘not otherwise specified’ limb deficiencies (LDs).
The majority of the cases and controls were singletons and live births (Table 1). The majority of case and control mothers had a high school education or higher and were non-Hispanic Whites. Most of the mothers used folic acid supplements and did not smoke during pregnancy, with slightly higher percentages in the controls compared to cases. The case group consisted of a higher percentage of males. Family history of CLDs in first-degree relatives was present in six CLD cases (1%) and six controls (0.1%).
Table 1.
Descriptive characteristics of the study participants by case/control status.
| Controls (n = 5,701) | Cases (n = 615) | ||||||
|---|---|---|---|---|---|---|---|
| Mean or N | Min, max or % | Missing (%) | Mean or N | Min, max or % | Missing (%) | ||
| Air pollution concentrationsa | PM2.5 | 13.5 | 3.5, 66.9 | 1247 (22) | 13.4 | 2.9, 71.9 | 157 (26) |
| PM10 | 27 | 2.8, 147.9 | 1047 (18) | 26.9 | 5.3, 86.6 | 88 (14) | |
| NO2 | 17.7 | 0.9, 47.5 | 1686 (30) | 18.3 | 2.7, 49.7 | 160 (26) | |
| NO2 max | 32.9 | 3.4, 77.8 | 1686 (30) | 33.4 | 7.3, 68.5 | 160 (26) | |
| SO2 | 3.8 | 0, 21.1 | 2064 (36) | 3.9 | 0.1, 14.4 | 237 (39) | |
| SO2 max | 11.2 | 0, 71.8 | 2064 (36) | 11.3 | 0.8, 44.3 | 237 (39) | |
| O3 | 42.5 | 10.0, 92.5 | 1527 (27) | 42.4 | 12.6, 88.7 | 176 (29) | |
| CO | 1.3 | 0.3, 5.6 | 1270 (22) | 1.4 | 0.3, 10.8 | 119 (19) | |
| Age at delivery (years) | 27.9 | 13, 47 | 0 | 27.5 | 14, 46 | 0 | |
| Gestational age (weeks) | 38.6 | 21, 44 | 0 | 36.8 | 14, 44 | 0 | |
| Center | Arkansas | 419 | 7.4 | 0 | 31 | 5 | 0 |
| California | 858 | 15.1 | 121 | 19.7 | |||
| Iowa | 617 | 10.8 | 73 | 11.9 | |||
| Massachusetts | 907 | 15.9 | 99 | 16.1 | |||
| New York | 616 | 10.8 | 56 | 9.1 | |||
| Texas | 643 | 11.3 | 77 | 12.5 | |||
| CDC/Atlanta | 752 | 13.2 | 78 | 12.7 | |||
| North Carolina | 451 | 7.9 | 21 | 3.4 | |||
| Utah | 438 | 7.7 | 59 | 9.6 | |||
| Estimated year of delivery | 1997 | 82 | 1.4 | 0 | 6 | 1 | 0 |
| 1998 | 509 | 8.9 | 69 | 11.2 | |||
| 1999 | 553 | 9.7 | 64 | 10.4 | |||
| 2000 | 602 | 10.6 | 79 | 12.9 | |||
| 2001 | 559 | 9.8 | 68 | 11.1 | |||
| 2002 | 518 | 9.1 | 53 | 8.6 | |||
| 2003 | 744 | 13.1 | 68 | 11.1 | |||
| 2004 | 796 | 14 | 78 | 12.7 | |||
| 2005 | 683 | 12 | 63 | 10.2 | |||
| 2006 | 655 | 11.5 | 67 | 10.9 | |||
| Birth outcome | Live Birth | 5700 | 100 | 1 | 580 | 94.3 | 0 |
| Stillbirth | 0 | 0 | 12 | 2 | |||
| Induced Abortion | 0 | 0 | 23 | 3.7 | |||
| Plurality | Singleton | 5532 | 97.1 | 6 | 574 | 93.3 | 0 |
| Multiple | 163 | 2.9 | 41 | 6.7 | |||
| Infant sex | Male | 2905 | 51.0 | 4 | 352 | 57.7 | 5 |
| Female | 2792 | 49.0 | 256 | 42 | |||
| Ambiguous | 0 | 0 | 2 | 0.3 | |||
| Mother’s years of education | 16 or more | 1880 | 33.2 | 34 | 172 | 28.2 | 4 |
| 13–15 | 1543 | 27.2 | 176 | 28.8 | |||
| 12 | 1319 | 23.3 | 156 | 25.5 | |||
| Less than 12 | 925 | 16.3 | 107 | 17.5 | |||
| Maternal race/ethnicity | NHWc | 3354 | 58.8 | 1 | 350 | 56.9 | 0 |
| NHBc | 640 | 11.2 | 54 | 8.8 | |||
| Hispanic | 1340 | 23.5 | 179 | 29.1 | |||
| Other | 366 | 6.4 | 32 | 5.2 | |||
| Maternal Obesityb | Underweight | 277 | 5.1 | 218 | 33 | 5.6 | 25 |
| Normal weight | 3056 | 55.7 | 302 | 51.2 | |||
| Overweight | 1257 | 22.9 | 144 | 24.4 | |||
| Obese | 893 | 16.3 | 111 | 18.8 | |||
| Folic acid use | Yes | 711 | 12.5 | 8 | 73 | 11.9 | 1 |
| No | 4982 | 87.5 | 541 | 88.1 | |||
| Smoking status | No | 4718 | 83.0 | 16 | 497 | 80.9 | 1 |
| Yes | 967 | 17.0 | 117 | 19.1 | |||
| Family history in 1st degree relative | No | 5659 | 99.9 | 0 | 609 | 99.0 | 0 |
| Yes | 6 | 0.1 | 6 | 1.0 | |||
Units: ppb for NO2, SO2, and O3; ppm for CO; μg/m3 for PM2.5 and PM10.
Using NIH BMI cutoffs (i.e., 18.5, 25, and 30) for classification.
NHW: non-Hispanic White; NHB: non-Hispanic Black.
The maternal exposures to outdoor air pollution during gestational weeks 2–8 were characterized using 480 (NO2) to 962 (PM10) monitors, whose median distance to maternal residence ranged from 19.3 km (PM2.5) to 23.2 km (SO2 and NO2). The averages of PM10 and O3 were higher in controls while the averages of PM2.5, NO2, NO2 max, SO2, SO2 max, and CO were higher among the cases. Overall average concentrations of air pollutants were moderately (i.e., 0.4–0.7) to highly (i.e., > 0.7) correlated with weekly averages (results not shown). When examining individual pollutants, weekly averages were moderately to highly correlated for NO2, NO2 max, SO2, O3, and CO, and low to moderate correlations were observed for PM2.5, PM10, and SO2 max. Moderate to weak correlations were observed between two different air pollutants during the same gestational week (STable 1).
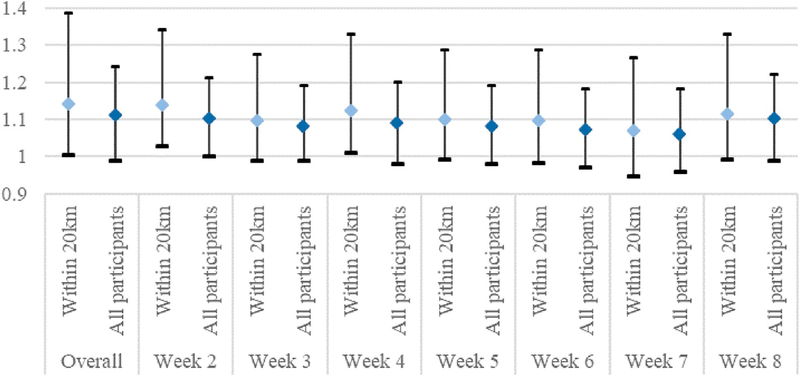
Near null association was observed between overall (Table 2) or weekly (STable 2 adjusted model 1) averages of O3 and PM2.5, after adjusting for maternal race/ethnicity, education, age, and study center. Inverse association was observed for PM10. However, increased odds of CLDs were observed for IQR increase in NO2, SO2, and CO concentration averaged across gestational weeks 2–8 (Table 2). The adjusted ORs for weekly averages ranged from 1.03 to 1.13, with the strongest associations observed with air pollution concentrations during gestational week 2 or 3 (STable 2 adjusted model 1). The effect estimates were larger for PM10, SO2 and O3 when CLDs were restricted to preaxial, but slightly attenuated for CO (Table 3). Additional adjustment for seasonality improved model fit and resulted in attenuated (NO2 and SO2 related; STable 2 adjusted model 2) or larger effect estimates (O3; STable 2 adjusted model 2). However, the association between CO and CLDs remained after consideration of seasonality (STable 2 adjusted model 2) or restriction to residential addresses within 20 km of a monitor (Fig. 1).
Table 2.
Crude and adjusted odds ratios (ORs) of overall CLDs per interquartile range (IQR) increase in air pollution levels, averaged across gestational weeks 2–8, from single-pollutant and co-pollutant models.
| Pollutanta | IQR | Single-pollutant models | Adjusted ORs (95% CI) from co-pollutant modelsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjustedb OR (95% CI) | Model 1: CO + NO2 | Model 2: CO + NO2 max | Model 3: CO + SO2 | Model 4: CO + SO2max | Model 5: CO+PM2.5 | Model 6: CO+PM10 | Model 7: CO + O3 | ||
| CO | 0.8 | 1.11 (0.99, 1.24) | 1.11 (0.99, 1.24) | 1.12 (0.96, 1.29) | 1.15 (1.00, 1.32) | 1.23 (1.06, 1.42) | 1.23 (1.07, 1.43) | 1.20 (1.00, 1.43) | 1.16 (1.03, 1.32) | 1.07 (0.93, 1.24) |
| NO2 | 8.5 | 1.12 (0.99, 1.26) | 1.10 (0.96, 1.26) | 0.99 (0.83, 1.18) | . | . | . | . | . | . |
| NO2 max | 12.6 | 1.08 (0.94, 1.23) | 1.04 (0.90, 1.21) | . | 0.92 (0.77, 1.10) | . | . | . | . | . |
| SO2 | 2.6 | 1.04 (0.93, 1.15) | 1.10 (0.97, 1.25) | . | . | 1.01 (0.88, 1.17) | . | . | . | . |
| SO2 max | 8.3 | 1.03 (0.91, 1.17) | 1.10 (0.95, 1.27) | . | . | . | 1.00 (0.84, 1.18) | . | . | . |
| PM2.5 | 5.6 | 0.99 (0.91, 1.08) | 0.95 (0.86, 1.04) | . | . | . | . | 0.88 (0.79, 0.98) | . | . |
| PM10 | 11.3 | 0.99 (0.90, 1.08) | 0.89 (0.80, 0.98) | . | . | . | . | . | 0.84 (0.75, 0.95) | . |
| O3 | 19.9 | 0.99 (0.86, 1.13) | 0.98 (0.84, 1.13) | . | . | . | . | . | . | 1.02 (0.87, 1.21) |
Daily average values unless otherwise specified; units in ppb (NO2, SO2, and O3), ppm (CO) or μg/m3 (PM2.5 and PM10).
Adjusted for study center, maternal age, race/ethnicity, and education.
Adjusted for study center, maternal age, race/ethnicity, education, and one additional air pollutant.
Table 3.
ORs of CLDs per IQR increase in air pollution levels averaged across gestational weeks 2–8 by CLD subtypes using single-pollutant models adjusting for study center, maternal age, race/ethnicity, and education.
| Air pollutant | IQR | Overall | Transverse | Longitudinal | Preaxial |
|---|---|---|---|---|---|
| CO | 0.8 ppb | 1.11 (0.99, 1.24) | 1.12 (0.97, 1.29) | 1.07 (0.88, 1.29) | 1.06 (0.83, 1.35) |
| NO2 | 8.5 ppb | 1.10 (0.96, 1.26) | 1.02 (0.85, 1.22) | 1.14 (0.92, 1.41) | . |
| NO2 max | 12.6 ppb | 1.04 (0.90, 1.21) | 1.02 (0.85, 1.24) | 1.00 (0.79, 1.26) | . |
| SO2 | 2.6 ppb | 1.10 (0.97, 1.25) | 1.07 (0.91, 1.25) | 1.14 (0.93, 1.38) | 1.22 (0.95, 1.56) |
| SO2 max | 8.3 ppb | 1.10 (0.95, 1.27) | 1.13 (0.95, 1.34) | 1.07 (0.85, 1.35) | 1.08 (0.81, 1.46) |
| PM2.5 | 5.6 μg/m3 | 0.95 (0.86, 1.04) | 0.96 (0.86, 1.08) | 0.90 (0.77, 1.05) | 0.92 (0.75, 1.12) |
| PM10 | 11.3 μg/m3 | 0.89 (0.80, 0.98) | 0.85 (0.74, 0.97) | 0.94 (0.81, 1.10) | 1.07 (0.89, 1.29) |
| O3 | 19.9 ppb | 0.98 (0.84, 1.13) | 0.95 (0.79, 1.14) | 1.02 (0.81, 1.29) | 1.27 (0.94, 1.72) |
Fig. 1.
Gestational-period-specific CO concentrations and odds of CLDs in the restricted (residential address within 20 km of air monitoring station) and original analyses, adjusting for maternal education, race/ethnicity, age, and study center.
The positive associations between CO and CLDs observed in the single-pollutant models were generally robust to co-pollutant adjustment (Table 2, STable 3). However, the positive effect estimates for SO2 and NO2 observed in the single-pollutant models were attenuated to the null when CO was included in the co-pollutant models (Table 2, STable 3). The single-pollutant models restricted to participants included in the co-pollutant models yielded similar results to the original single-pollutant models (results not shown).
The strength of association between average air pollution concentrations during pregnancy and CLDs in single-pollutant models varied by BMI, cigarette smoking status, and folic acid intake. Stronger associations were observed in participants whose mothers were obese (SFig. 1-a), non-smokers (SFig. 1-b), or did not use folic acid during pregnancy (SFig. 1-c), while near-null associations were observed in the other subpopulations.
4. Discussion
In this large population-based case-control study, we observed that maternal exposure to higher levels of some criteria air pollutants during early pregnancy was associated with increased odds of CLDs. In fully-adjusted single-pollutant models, average CO, NO2, and SO2 during gestational weeks 2–8 were associated with increased odds of CLDs, while PM10 was inversely associated with CLDs. The ORs of CLDs for NO2 and SO2 were attenuated towards the null when adjusting for CO, while the association between CO and CLDs was robust to co-pollutant adjustment. The ORs of CLDs per IQR increase in CO became slightly larger when participants were restricted to those within 20 km of an active CO monitor. We observed suggestive evidence of effect measure modification of the relationship between CO and CLDs by folic acid intake. We also observed differences in ORs of CLDs and SO2 by BMI and smoking status; however, we advise caution interpreting these results as the models did not adjust for co-exposure to CO. The CO-CLDs association estimated in the single-pollutant model remained relatively unchanged in additional analyses by subtypes, though the association was slightly attenuated in evaluation of longitudinal LDs. Elevated odds of preaxial LDs were observed per IQR increase in PM10, SO2, and O3 using single-pollutant models.
The associations observed between CO and CLDs in the current study are not directly comparable to results from the limited number of previous studies. Only two previous studies reported ORs for CO concentrations during pregnancy and CLDs (Lin et al., 2014; Padula et al., 2013c). Contrary to our findings, CO was inversely associated with longitudinal or transverse LDs (Padula et al., 2013c) and near-null associations were shown for reduction deformities (Lin et al., 2014). Neither study reported co-pollutant adjusted estimates for CO. Although Lin et al. included results from co-pollutant models for other air pollutants such as SO2, O3, and PM10 (Lin et al., 2014), they did not observe confounding by CO. Lin et al. observed increased odds of reduction deformities with SO2 averaged across gestational weeks 9–12 in a single-pollutant model (OR per 1 ppb increase = 1.02, 95% CI = 1.00–1.05) and CO-adjusted model (OR per 1 ppb increase = 1.02, 95% CI = 1.00–1.05). Increased odds of limb reduction in association with SO2 concentration has been reported in another study in England (OR per increase from 10th to the 90th centile of the annual average SO2 concentration = 1.06, 95% CI = 0.85–1.32) (Dolk et al., 2009).
The discrepancies in the results on CO across studies may be due to differences in how exposures were measured. We estimated week-specific and overall average of air pollution concentrations during the critical period of limb development (weeks 2–8) considering maternal residential history, while Lin et al. did not consider residential history and estimated month-specific average pollutant concentrations including gestational periods outside the critical period of limb development (i.e., weeks 9–12) and Padula et al. averaged across the first two months of pregnancy considering residential history. The mean CO concentrations are much lower in our study population compared to those reported in Taiwan. The participants included in the study by Padula were from the NBDPS California center and therefore overlap with the current study. However, the average CO concentrations in our study, which included 8 additional NBDPS centers, was higher than that reported in San Joaquin Valley, CA. Given the differences in CO mean concentrations, the observed differences in ORs may be due to a nonlinear dose-response relationship as suggested previously (Ritz, 2010).
Another difference is in the ascertainment of CLD cases. Lin et al. relied on singleton live birth cases (ICD-9755.20 and 755.30) identified from passive surveillance and excluded cases reporting smoking during pregnancy, while our population included non-chromosomal CLDs (ICD-9755.20–755.28 and 755.30–755.38) occurring among live births, stillbirths, and elective terminations through active surveillance and clinical confirmation by clinical geneticists. Restriction of cases to livebirths in the study by Lin et al. may be prone to selection bias (Tinker et al., 2015), given the higher proportion of termination (33%) reported in prenatally identified CLDs (Dicke et al., 2015). Such bias may be associated with socioeconomic status (SES), since access to healthcare will influence prenatal identification of CLDs and socioeconomically disadvantaged individuals may be subject to higher air pollution (Jerrett et al., 2001). Furthermore, the prevalence of CLDs are 30–40 times higher in stillbirths compared to livebirths (Ephraim et al., 2003) and air pollution may increase the risk of stillbirths (Faiz et al., 2012; Hwang et al., 2011).
Lastly, statistical analyses were also different across studies, where we evaluated single-pollutant and co-pollutant models, and observed potential confounding by CO in the CLDs - SO2 and CLDs - NO2 relationships. Lin et al. also included a co-pollutant model adjusting for CO. In this previous study, CO-adjustment did not influence the association between limb reduction and SO2 association (OR per 1 ppb increase SO2 = 1.02, 95% CI = 1.00–1.05), although the single-pollutant model result was similar to that observed in our study (OR per 1 ppb increase SO2 = 1.02, 95% CI = 1.00–1.05). The discrepancies in the potential confounding by CO may be due to differences in exposure characteristics and in model specifications. The SO2 concentrations were higher in Taiwan when compared to concentrations measured in our study, and the correlation with CO was also different. In terms of co-pollutant models, we used logistic regression models to separately evaluate week-specific air pollution concentration averages, adjusting for maternal age, race/ethnicity, education, study center and one additional air pollutant average during the same time-window. On the other hand, Lin et al. included all three month-specific averages along with 1st trimester average during pregnancy in the same model, adjusting for age, district-level SES, and one additional air pollutant (time-window not specified).
A near-null association observed between NO2 and CLDs in the current study is consistent with results reported in previous studies (Lin et al., 2014; Pedersen et al., 2017; Schembari et al., 2014). An imprecise positive association was observed for the middle two quartiles compared to the lowest quartile of NO2 exposures with regards to transverse but not longitudinal LDs (Padula et al., 2013c). Associations between PM or ozone, and CLDs are inconsistent across previous literature. Some reported positive associations (Dolk et al., 2009; Lin et al., 2014; Padula et al., 2013c; Pedersen et al., 2017; Vinceti et al., 2016; Vinikoor-Imler et al., 2013; Vinikoor-Imler et al., 2015), while others reported negative associations (Padula et al., 2013c; Schembari et al., 2014; Vinikoor-Imler et al., 2015).
In the analysis by subtype, the odds of preaxial LDs remained elevated with an increase in CO. We additionally observed non-statistically significant increases in preaxial LDs ORs for PM10 (OR: 1.07; 95% CI: 0.89, 1.29), SO2 (OR: 1.22; 95% CI: 0.95, 1.56), and O3 (OR: 1.27; 95% CI: 0.94, 1.72), although co-pollutant adjustment was not available due to quasi-complete separation in models. ORs of preaxial LDs have not been reported thus far, although small differences have been observed between longitudinal and transverse LDs (Padula et al., 2013c), and upper and lower LDs (Vinikoor-Imler et al., 2013). Observed differences in our results on preaxial LDs as compared to a larger grouping of CLDs (e.g., transverse, longitudinal, and overall) may in part be due to potential heterogeneity in the underlying etiology of the subtypes. Although we were able to explore preaxial LDs as a distinct type of longitudinal LDs, other sub-classifications (e.g., postaxial longitudinal, transverse with or without nubbins (Gardiner and Holmes, 2012)) or further sub-classification of preaxial LDs (e.g., unilateral and bilateral) were unavailable due to limited sample size or data availability. Since etiologic heterogeneity may exist by further sub-classification, as in the case of thalidomide (Källén et al., 1984), future investigation with adequate power to analyze further sub-classification of CLDs would be useful to better characterize the relationship between air pollution and CLDs.
We observed suggestive modification of the relationship between air pollution exposures and CLDs by maternal folic acid supplement, pre-pregnancy BMI, and smoking status. The ORs for CO-CLDs were higher in children born to mothers who did not use folic acid supplement during pregnancy; and the ORs for SO2 max – CLDs were higher in children born to mothers who were obese or were non-smokers. Maternal BMI and folic acid supplement use may interact with the hypothesized biological pathway by which air pollution may affect human health (i.e., inflammatory reactions activated by increased oxidative stress (Chuang et al., 2007; Kelly, 2003)). Increased markers of inflammations were reported in overweight or obese individuals (Vgontzas et al., 2000), and obesity has been suggested as a risk factor for CLDs (Persson et al., 2017; Stothard et al., 2009; Waller et al., 2007) and other birth defects (Marengo et al., 2013). Folic acid is known to have anti-inflammatory effects (Zhao et al., 2013) and folic acid supplementation has been suggested as a primary prevention for structural defects including CLDs (Czeizel, 2000).
Given the association observed between CO and CLDs in the current study, the underlying potential biological mechanism for this relationship is worth discussion. Ambient air CO originates from incomplete combustion of carbon-containing fuel, and the major source is on-road vehicles (EPA, 2010). Abnormally high concentrations of CO may influence fetal health via hypoxia: less oxygen is available for placental and fetal tissues due to CO binding of hemoglobin (EPA, 2010) and crossing the placental barrier (Friedman et al., 2015). Experimental studies identified CO as a teratogen to mice that could induce congenital spinal deformities (Loder et al., 2000). Low-levels of CO exposure could also influence the fetus, as reported by Garvey and Longo (1978). Consistent with experimental evidence, epidemiologic studies have reported increased risk of spontaneous abortion (Grippo et al., 2018), congenital heart defects (Dadvand et al., 2011; Ritz et al., 2002; Stingone et al., 2014), low birth weight (Ritz and Yu, 1999; Salam et al., 2005), and fetal growth retardation (Salam et al., 2005) with increased CO concentrations.
Interpretations of our study results are strengthened by the large population-based study design and ten years of accumulated data from the NBDPS, which allowed us to further investigate the association by CLD subtypes. We used refined outcome data, which better captures major structural birth defects through active surveillance and multiple levels of clinical review and classification. Another strength of the current study comes from high-quality covariate information from maternal interviews, such as smoking status, folic acid use, and pre-pregnancy BMI, which otherwise are not available in studies relying on birth certificates or birth defect surveillance data. Specifically, we generated week-specific ambient air pollution concentrations during critical weeks of embryogenesis considering residential mobility.
One limitation of our study is measurement error in the air pollution exposure term. The air pollution exposure terms were modeled using the nearest air monitors within 50 km radius, which may not capture small-area-variability of some air pollutants. Larger measurement error is expected for local air pollutants, which spatially peaks around sources. For example, CO is a chemically stable gas with higher concentrations measured near sources, such as motor vehicles, which may disperse rapidly within a 100 m radius (Zhu et al., 2002). However, such measurement error can be viewed as Berkson error, where associations are attenuated yet not biased (Zeger et al., 2000). Although we accounted for residential mobility during pregnancy in our study design, we did not have measurements of indoor air pollution concentrations and daily activity patterns to characterize personal air pollution exposures. Measuring such sources of exposure variability remains a challenge without prospectively collected data via personal sampling, which is infeasible in retrospective study designs for outcomes as rare as birth defects. Indoor exposure to outdoor CO has been shown to be similar to outdoor exposure to CO (Polidori et al., 2012), since CO is a non-reactive gas, so time spent indoors is not expected to affect the results substantially. Since we identified CO as a possible risk factor for CLDs using a simple exposure assessment approach, further research should be conducted utilizing alternative modeling techniques that better estimate spatial CO exposure variability.
There is a potential for selection bias in our analytic sample due to excluding cases and controls without air pollution exposure data. A total of 18% of cases and 20% of controls were excluded due to lack of 1) residential address reporting or 2) an active monitor within 50 km of residential address. The socio-demographic characteristics of the analytic sample (5,701 controls and 615 cases), however, was similar to the original sample (7,127 controls and 751 cases).
Another limitation of our study may arise from possible recall bias in maternal interviews. As the maternal interviews were administered between 6 weeks and 24 months after the estimated date of delivery and hence after case diagnoses. Thus, there is a potential for recall bias differential by outcome status. However, we do not expect covariate recall to differ by ambient air pollution concentrations since mothers were unaware of their ambient air pollution concentrations and we used EPA monitor values to assign maternal air pollution exposure.
We cannot rule out residual confounding by other traffic-related air pollutants, such as polycyclic aromatic hydrocarbons (PAH) and ultra-fine particles (Ritz and Wilhelm, 2008; Sioutas et al., 2005). Previous studies reported associations between occupational exposures to PAH and other birth defects such as neural tube defects (Langlois et al., 2012) or gastroschisis (Lupo et al., 2012), and ambient levels during pregnancy and adverse birth outcomes (Yuan et al., 2013). As a next step, future studies that utilize concentrations of multiple air pollutants, including but not limited to routinely measured criteria air pollutants and multipollutant measurements obtained near roads, would be particularly useful to further characterize the observed associations.
5. Conclusion
Modest associations were observed between CLDs and ambient air pollution concentrations during gestational weeks 2–8 (CO, NO2, and SO2), when using single-pollutant models. However, the associations for NO2 and SO2 became near-null when adjusted for CO. The association for CO was robust to co-pollutant adjustment and consistent across sensitivity analyses including individual types of CLDs.
Supplementary Material
Acknowledgments
This project was supported through funding from the Centers for Disease Control and Prevention (CDC) to the North Carolina Center for Birth Defects Research and Prevention) at the University of North Carolina at Chapel Hill (grant number U01DD001231), and through CDC cooperative agreements under PA #96043, PA #02081, FOA #DD09–001, FOA #DD13–003, and NOFO #DD18–001 to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study (NBDPS) and/or the Birth Defects Study To Evaluate Pregnancy exposureS (BD-STEPS). This work was also supported in part by a training grant from the National Institute of Environmental Health Sciences [T32ES007018].
We would like to thank Richard Olney (clinical geneticist at CDC) for conducting classifications of children with congenital limb deficiencies and Lew Holmes for reviewing the manuscript. We thank the California Department of Public Health, Maternal Child and Adolescent Health Division for providing surveillance data from California for this study. We thank Achal Patel for the replication of this study.
We acknowledge Jennifer Richmond-Bryant (air pollution exposure scientist at EPA) for her feedback on exposure assessments in the discussion section, and Breanna Alman (EPA) and Kristen Rappazzo (EPA) for technical review of the manuscript.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2019.108716.
Publisher's Disclaimer: Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the EPA, the Centers for Disease Control and Prevention, or the California Department of Public Health.
Declarations of conflict of interests
None.
References
- Åberg A, et al. , 2001. Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum. Dev 61, 85–95. [DOI] [PubMed] [Google Scholar]
- Appel KW, et al. , 2017. Description and evaluation of the Community Multiscale Air Quality (CMAQ) modeling system version 5.1. Geosci. Model Dev. (GMD) 10, 1703–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers KM, et al. , 2013. Maternal periconceptional exposure to cigarette smoking and congenital limb deficiencies. Paediatr. Perinat. Epidemiol 27, 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2018. Facts about Upper and Lower Limb Reduction Defects. CDC Birth Defects Homepage CDC Birth Defects Homepage. [Google Scholar]
- Chuang K-J, et al. , 2007. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am. J. Respir. Crit. Care Med 176, 370–376. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, 2000. Primary prevention of neural-tube defects and some other major congenital abnormalities. Paediatr. drugs 2, 437–449. [DOI] [PubMed] [Google Scholar]
- Dadvand P, et al. , 2011. Ambient air pollution and congenital heart disease: a register-based study. Environ. Res 111, 435–441. [DOI] [PubMed] [Google Scholar]
- Dicke JM, et al. , 2015. The utility of ultrasound for the detection of fetal limb abnormalities–a 20-year single-center experience. Prenat. Diagn 35, 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolk H, et al. , 2009. Ambient Air Pollution and Risk of Congenital Anomalies in England, 1991–99. Occupational and Environmental Medicine. , 045997 oem. 2009. [DOI] [PubMed] [Google Scholar]
- EPA, 2019. EPA Air Quality System (AQS).
- EPA, 2010. Integrated Science Assessment for Carbon Monoxide. US EPA, Washington, DC. [Google Scholar]
- Ephraim PL, et al. , 2003. Epidemiology of limb loss and congenital limb deficiency: a review of the literature. Arch. Phys. Med. Rehabil 84, 747–761. [DOI] [PubMed] [Google Scholar]
- Faiz AS, et al. , 2012. Ambient air pollution and the risk of stillbirth. Am. J. Epidemiol 176, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman P, et al. , 2015. Carbon monoxide exposure during pregnancy. Obstet. Gynecol. Surv 70, 705–712. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Holmes LB, 2012. Hypothesis: terminal transverse limb defects with “nubbins” represent a regenerative process during limb development in human fetuses. Birth Defects Res. A Clin. Mol. Teratol 94, 129–133. [DOI] [PubMed] [Google Scholar]
- Garvey DJ, Longo LD, 1978. Chronic low level maternal carbon monoxide exposure and fetal growth and development. Biol. Reprod 19, 8–14. [DOI] [PubMed] [Google Scholar]
- Gilboa S, et al. , 2005. Relation between ambient air quality and selected birth defects, seven county study, Texas, 1997–2000. Am. J. Epidemiol 162, 238–252. [DOI] [PubMed] [Google Scholar]
- Glinianaia SV, et al. , 2004. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology 15, 36–45. [DOI] [PubMed] [Google Scholar]
- Gold NB, et al. , 2011. Anatomic and etiological classification of congenital limb deficiencies. Am. J. Med. Genet 155A, 1225–1235. [DOI] [PubMed] [Google Scholar]
- Grippo A, et al. , 2018. Air Pollution Exposure during Pregnancy and Spontaneous Abortion and Stillbirth. Reviews on environmental health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, et al. , 2008. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos. Environ 42, 7561–7578. [Google Scholar]
- Hwang BF, et al. , 2011. Air pollution and stillbirth: a population-based case-control study in Taiwan. Environ. Health Perspect 119, 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, et al. , 2001. A GIS–environmental justice analysis of particulate air pollution in Hamilton, Canada. Environ. Plan 33, 955–973. [Google Scholar]
- Källén B, et al. , 1984. Infants with congenital limb reduction registered in the Swedish Register of Congenital Malformations. Teratology 29, 73–85. [DOI] [PubMed] [Google Scholar]
- Källén K, 1997. Maternal smoking during pregnancy and limb reduction malformations in Sweden. Am. J. Public Health 87, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FJ, 2003. Oxidative stress: its role in air pollution and adverse health effects. Occup. Environ. Med 60, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois PH, et al. , 2012. Maternal occupational exposure to polycyclic aromatic hydrocarbons and risk of neural tube defect-affected pregnancies. Birth Defects Res. Part A Clin. Mol. Teratol 94, 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-T, et al. , 2014. Air pollution and limb defects: a matched-pairs case-control study in Taiwan. Environ. Res 132, 273–280. [DOI] [PubMed] [Google Scholar]
- Loder RT, et al. , 2000. The induction of congenital spinal deformities in mice by maternal carbon monoxide exposure. J. Pediatr. Orthop 20, 662–666. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, et al. , 2012. Maternal occupational exposure to polycyclic aromatic hydrocarbons: effects on gastroschisis among offspring in the National Birth Defects Prevention Study. Environ. Health Perspect 120, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengo L, et al. , 2013. Body mass index and birth defects: Texas. 2005–2008. Matern. Child Health J 17, 1898–1907. [DOI] [PubMed] [Google Scholar]
- NBDPN, 2015. Guidelines for conducting birth defects surveillance. Appendix 3 (1), 52–54. [Google Scholar]
- Padula AM, et al. , 2013a. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am. J. Epidemiol 177, 1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, et al. , 2013b. Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin Valley of California. Paediatr. Perinat. Epidemiol 27, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AM, et al. , 2013c. Traffic-related air pollution and selected birth defects in the San Joaquin Valley of California. Birth Defects Res. A Clin. Mol. Teratol 97, 730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SE, et al. , 2010. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res. A Clin. Mol. Teratol 88, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Pedersen M, et al. , 2017. Exposure to air pollution and noise from road traffic and risk of congenital anomalies in the Danish National Birth Cohort. Environ. Res 159, 39–45. [DOI] [PubMed] [Google Scholar]
- Persson M, et al. , 2017. Risk of major congenital malformations in relation to maternal overweight and obesity severity: cohort study of 1.2 million singletons. Br. Med. J 357, j2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidori A, et al. , 2012. Indoor/outdoor relationships, trends, and carbonaceous content of fine particulate matter in retirement homes of the Los Angeles Basin. J. Air Waste Manag. Assoc 57, 366–379. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, et al. , 2015. The national birth defects prevention study: a review of the methods. Birth Defects Res. Part A Clin. Mol. Teratol 103, 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, 2010. Air pollution and congenital anomalies. Occup. Environ. Med 67, 221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M, 2008. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin. Pharmacol. Toxicol 102, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Yu F, 1999. The effect of ambient carbon monoxide on low birth weight among children born in southern California between 1989 and 1993. Environmental Health Perspectives 107, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, et al. , 2002. Ambient air pollution and risk of birth defects in Southern California. Am. J. Epidemiol 155, 17–25. [DOI] [PubMed] [Google Scholar]
- Salam MT, et al. , 2005. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children’s Health Study. Environ. Health Perspect. 113, 1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS, 2012. SAS 9.4 for Windows. SAS Institue Inc., Cary, NC, USA. [Google Scholar]
- Schembari A, et al. , 2014. Traffic-related air pollution and congenital anomalies in Barcelona. Environ. Health Perspect. 122, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioutas C, et al. , 2005. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environ. Health Perspect. 113, 947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sram R, 1999. Impact of air pollution on reproductive health. Environmental Health Perspectives 107, A542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šrám RJ, et al. , 2005. Ambient air pollution and pregnancy outcomes: a review of the literature. Environ. Health Perspect 113, 375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingone JA, et al. , 2014. Maternal exposure to criteria air pollutants and congenital heart defects in offspring: results from the national birth defects prevention study. Environ. Health Perspect 122, 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothard KJ, et al. , 2009. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. Jama 301, 636–650. [DOI] [PubMed] [Google Scholar]
- Therapontos C, et al. , 2009. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl. Acad. Sci 106, 8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker SC, et al. , 2015. Challenges in studying modifiable risk factors for birth defects. Curr. Epidemiol Rep 2, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, et al. , 2000. Chronic systemic inflammation in overweight and obese adults. Jama 283, 2235–2236. [DOI] [PubMed] [Google Scholar]
- Vinceti M, et al. , 2016. Does maternal exposure to benzene and PM10 during pregnancy increase the risk of congenital anomalies? A population-based case–control study. Sci. Total Environ 541, 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, et al. , 2015. An exploratory analysis of the relationship between ambient ozone and particulate matter concentrations during early pregnancy and selected birth defects in Texas. Environ. Pollut 202, 1–6. [DOI] [PubMed] [Google Scholar]
- Vinikoor-Imler LC, et al. , 2013. Early prenatal exposure to air pollution and its associations with birth defects in a state-wide birth cohort from North Carolina. Birth Defects Res. Part A Clin. Mol. Teratol 97, 696–701. [DOI] [PubMed] [Google Scholar]
- Vrijheid M, et al. , 2010. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ. Health Perspect 119, 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, et al. , 2011. Ambient air pollution and risk of congenital anomalies: a systematic review and meta-analysis. Environ. Health Perspect 119, 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller DK, et al. , 2007. Prepregnancy obesity as a risk factor for structural birth defects. Arch. Pediatr. Adolesc. Med 161, 745–750.17679655 [Google Scholar]
- Yuan Y, et al. , 2013. Levels of PAH–DNA adducts in placental tissue and the risk of fetal neural tube defects in a Chinese population. Reprod. Toxicol 37, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, et al. , 2000. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ. Health Perspect 108, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, et al. , 2013. Folic acid protects against lipopolysaccharide-induced preterm delivery and intrauterine growth restriction through its anti-inflammatory effect in mice. PLoS One 8, e82713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2002. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmos. Environ 36, 4323–4335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.