Abstract
Youth with elevated psychopathic traits exhibit a number of comparable neurocognitive deficits as adult psychopathic offenders, including error-related processing deficits. Subregions of the basal ganglia play an important, though indirect, role in error-related processing through connections with cortical areas including the anterior cingulate cortex. A number of recent structural and functional magnetic resonance imaging (fMRI) studies have associated basal ganglia dysfunction in youth with elevated psychopathic traits, but these studies have not examined whether dysfunction occurring within subregions of the basal ganglia help contribute to error-related processing deficits previously observed in such at-risk youth. Here, we investigated error-related processing using a response inhibition Go/NoGo fMRI experimental paradigm in a large sample of incarcerated male adolescent offenders (n = 182). In the current report, psychopathy scores (measured via the Psychopathy Checklist: Youth Version (PCL:YV)) were negatively related to hemodynamic activity within input nuclei of the basal ganglia (i.e., the caudate and nucleus accumbens), as well as intrinsic/output nuclei (i.e., the globus pallidus and substantia nigra) and related nuclei (i.e., the subthalamic nucleus) during error-related processing. This is the first evidence to suggest that error-related dysfunction previously observed in youth with elevated psychopathic traits may be related to underlying abnormalities occurring within subregions of the basal ganglia.
Keywords: juvenile delinquency, callous-unemotional traits, functional magnetic resonance imaging, error-related processing, basal ganglia
Adult psychopathic offenders are characterized by their lack of moral emotions, and an impulsive, irresponsible lifestyle, increasing the likelihood of future incarceration (Hare, 1991, 2003). While only affecting 0.5 – 1% of the general population, the base rate of psychopathy increases tremendously in incarcerated settings, with 15 – 25% of offenders meeting the diagnostic criteria for psychopathy (Hare, 2003). This population has often proven resistant to treatment intervention approaches, as highlighted by their increased propensity towards violent recidivism (Hemphill, Hare, & Wong, 1998; Rice & Harris, 1997). Given the enormous societal cost of psychopathy, researchers have attempted to delineate the adolescent manifestation of this condition, as personality traits are still in nascent stages during development. Indeed, intervention efforts targeted specifically towards youth appear to have a better chance of altering otherwise life-course persistent antisocial behavior (Caldwell, 2011; Caldwell, McCormick, Umstead, & Van Rybroek, 2007).
Youth with elevated psychopathic traits exhibit comparable neurocognitive dysfunction as adult psychopathic offenders, including deficits within passive avoidance learning (Finger et al., 2008), response modulation (Budhani & Blair, 2005; Dadds et al., 2006), and error-related processing (Maurer, Steele, Cope, et al., 2016). The attentional bottleneck hypothesis suggests that individuals scoring high on psychopathy have difficulty reallocating attention, becoming hyper-focused on a single aspect of a task at the price of subsequent cognitive functioning, making it difficult to move beyond the current cognitive process (Newman & Baskin-Sommers, 2012, though see Blair, 2013, for evidence contrary to this hypothesis). This attentional abnormality has recently been applied to the investigation of error-related processing in youth with elevated psychopathic traits. Specifically, youth scoring high on the Psychopathy Checklist: Youth Version (PCL:YV) exhibit comparable amplitude of the error-related negativity (ERN/Ne) event-related potential (ERP) component to youth scoring low on the PCL:YV (Maurer, Steele, Cope, et al., 2016). Comparable amplitude of the ERN/Ne between groups suggests that youth with elevated psychopathic traits do not exhibit deficits in regard to the initial identification of an error. However, youth scoring higher on the PCL:YV exhibit reduced amplitude of the error-related positivity (Pe) ERP component compared to youth scoring low on the PCL:YV (Maurer, Steele, Cope, et al., 2016), suggesting deficits in fully processing the motivational (Ullsperger, Harsay, Wessel, & Ridderinkhof, 2010) or affective (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005) significance of error-related information in youth scoring higher on the PCL:YV. Therefore, youth scoring higher on the PCL:YV devote attentional resources to the initial identification of an error, but fail to fully evaluate the motivational or affective significance of error-related information, which is consistent with the attentional bottleneck hypothesis (Newman & Baskin-Sommers, 2012), and is similar to what has been observed in adult samples (Brazil et al., 2009; Maurer, Steele, Edwards, et al., 2016; Steele, Maurer, Bernat, Calhoun, & Kiehl, 2016). This attentional abnormality with respect to error-related processing may have serious implications in regards for the tendency for youth with elevated psychopathic traits to engage in impulsive, reckless behavior, without fully considering the consequences of their mistakes (Edens, Campbell, & Weir, 2007). While ERPs provide excellent temporal resolution on the order of milliseconds, the methodology is severely limited with respect to spatial resolution. To date, there have not been any previous studies that have integrated functional magnetic resonance imaging (fMRI) to identify the brain regions that may help contribute to error-related dysfunction in youth with elevated psychopathic traits.
Subregions of the basal ganglia have been shown to play a significant, though indirect role, in error-related processing. The basal ganglia can be best described as a subcortical relay station located at the base of the forebrain typically involved in reward processing (Haber & Knutson, 2010). Subregions of the basal ganglia consist of input nuclei (which receive information from the cortex and thalamus), output nuclei (which send information to the thalamus for further processing), and intrinsic nuclei that are located between input and output nuclei of the basal ganglia, which help further process information received from input nuclei. Intrinsic nuclei of the basal ganglia include the caudate and putamen, which together form the dorsal striatum, and the nucleus accumbens [NAcc]. Output and intrinsic nuclei of the basal ganglia are comprised of different portions of the globus pallidus and substantia nigra. For example, output nuclei of the basal ganglia consist of the internal segment of the globus pallidus and the substantia nigra pars reticulata, whereas intrinsic nuclei of the basal ganglia include the external segment of the globus pallidus and the substantia nigra pars compacta. Nearby nuclei include structures located in the diencephalon, though still considered to be part of the basal ganglia, including the subthalamic nucleus (Lanciego, Luquin, & Obeso, 2012).
Error-related information processed by the input and intrinsic nuclei are sent to the thalamus via output nuclei of the basal ganglia, which relay information to regions considered central to error-related processing, including the dorsal anterior cingulate cortex (dACC), where the ERN/Ne ERP component is believed to be generated; the Pe is believed to arise from the rostral division of the ACC (Edwards, Calhoun, & Kiehl, 2012; Hermann, Rommler, Ehlis, Heidrich, & Fallgatter, 2004; van Veen & Carter, 2002). The various subregions of the basal ganglia continually monitor and steadily predict the result of ongoing events (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Mathalon, Whitfield, & Ford, 2003), determining whether the end result of events will be favorable or not (Holroyd & Coles, 2002). When encountering events that deviate from expectations, including the commission of errors, the basal ganglia relays information to the ACC via the thalamus; the ACC, as part of the salience network, integrates this information, communicating with the executive control network, signaling the need for increased cognitive control (Ham, Leff, de Boissezon, Joffe, & Sharp, 2013; Kennerley, Walton, Behrens, Buckley, & Rushworth, 2006). Interactions between the ACC, specifically the dACC, and lateral prefrontal structures are often associated with subsequent behavioral changes (Egner, 2009).
Only a handful of studies to date have been performed investigating the relationship between adolescent psychopathic traits and subregions of the basal ganglia, which have incorporated both structural magnetic resonance imaging (sMRI) (Yang et al., 2015) and functional MRI using various experimental paradigms, including pain perception (Marsh et al., 2013), drug cue processing (Vincent, Cope, King, Nyalakanti, & Kiehl, 2017), and passive avoidance learning (Finger et al., 2011). These studies have reported increased gray matter in the putamen (Yang et al., 2015), as well as reduced hemodynamic activity in the putamen during pain perception (Marsh et al., 2013), the caudate during early stimulus-reinforcement exposure (Finger et al., 2011), and the caudate, globus pallidus, and putamen during drug cue processing (Vincent et al., 2017) in youth scoring high on measures of psychopathic traits. Furthermore, dysfunction within subregions of the basal ganglia can help contribute to many of the deficits associated with youth with elevated psychopathic traits. For example, damage to the caudate has been associated with perseveration deficits (Nys, van Zandvoort, van der Worp, Kappelle, & de Haan, 2006), a deficit typically associated with youth with elevated psychopathic traits (Budhani & Blair, 2005; Dadds et al., 2006; Finger et al., 2008; Roussy & Toupin, 2000), and damage to the globus pallidus is associated with poor motivation on behavioral tasks (Vijayaraghavan, Vaidya, Humphreys, Beglinger, & Paradoso, 2008). Additionally, the substantia nigra is associated with the processing of salient information (Bunzeck & Duzel, 2006). Dysfunction within subregions of the basal ganglia could contribute to many of the deficits associated with youth with elevated psychopathic traits, including error-related processing deficits. For example, such youth may not fully process salient information, including errors, and may exhibit perseveration deficits, failing to process the motivational or affective significance of error-related information after initially identifying an error’s occurrence.
To date, no study has investigated whether adolescent psychopathic traits are associated with dysfunction occurring within subregions of the basal ganglia during experimental paradigms designed to evaluate error processing, including a Go/NoGo task. Furthermore, previous studies not have investigated whether dysfunction occurring in subregions of the basal ganglia could help contribute to error-related processing deficits previously observed in youth with elevated psychopathic traits (i.e., reduced Pe ERP amplitude) (Maurer, Steele, Cope, et al., 2016). In addition, two subregions of the basal ganglia (the substantia nigra and subthalamic nucleus) have never been investigated in relation to adolescent psychopathic traits via region of interest (ROI)-based analyses. Here, we address these issues by reporting on error-related processing using a response inhibition Go/NoGo fMRI experimental paradigm known to elicit basal ganglia activity in healthy individuals (Steele et al., 2014) in a sample of incarcerated male adolescent offenders. Psychopathic traits were assessed via the PCL:YV (Forth, Kosson, & Hare, 2003), a downward extension of the Hare Psychopathy Checklist – Revised (PCL-R) (Hare, 2003). We specifically hypothesized adolescent psychopathy scores would be negatively related to error-related hemodynamic activity within input nuclei (i.e., the caudate and putamen) and intrinsic/output nuclei of the basal ganglia (i.e., the globus pallidus), as previous studies have reported abnormalities within these regions in relation to adolescent psychopathy scores during various experimental paradigms (Finger et al., 2011; Marsh et al., 2013; Vincent et al., 2017; Yang et al., 2015). Analyses investigating the relationship between adolescent psychopathy scores and error-related hemodynamic activity in the NAcc, substantia nigra, and subthalamic nucleus remained exploratory in nature.
Method
Participants
Participants included n = 182 incarcerated male adolescent offenders recruited from a maximum-security juvenile detention center in New Mexico who participated in a larger overall study (Southwest Advanced Neuroimaging Cohort – Youth (SWANC-Y), RO1 MH071896: PI: Kiehl), ranging from 14 to 20 years of age (M age = 17.6 years, SD = 1.11 years) at the time of MRI data collection. The sample was predominantly right-handed (10% reported being left-hand dominant). Participants largely self-identified as Hispanic/Latino (77%), with the remaining self-identifying as White (14%), American Indian or Alaskan Native (6%), Black or African American (1%), or Native Hawaiian or Pacific Islander (1%). One percent of the sample chose not to disclose their ethnicity. Initial contact was made with potential study participants and informed consent was obtained. Individuals 18 years of age or older provided written informed consent and individuals younger than 18 years of age provided written informed assent in conjunction with parent/guardian informed consent. Participants were informed of their right to terminate participation at any point, the lack of direct institutional benefits, and that their participation would not affect their facility status or parole. Participants received remuneration at the hourly labor wage of the facility (roughly $1 per hour). All research protocols were approved by Ethical and Independent Review Services (E&I), the Office for Human Research Protections (OHRP), and the juvenile detention center where data collection occurred.
Assessments
Psychopathy Checklist: Youth Version (PCL:YV)
Psychopathic traits were assessed using the PCL:YV (Forth et al., 2003), which assesses interpersonal, affective, lifestyle, and antisocial/developmental features related to the adolescent manifestation of psychopathy. Each of the twenty items of the PCL:YV is scored on the following criteria: a score of zero reflects the item does not apply to the individual, a score of one implies the item applies somewhat to the individual, and a score of two means that the item definitely applies to the individual. PCL:YV total scores can potentially range from 0 to 40. The mean PCL:YV total score for the sample was 23.50 (SD = 6.11) (range 2 to 35). The Cronbach’s alpha for the PCL:YV (all items) was .82 in the current sample, reflecting good internal consistency. Inter-rater reliability (IRR) for PCL:YV total scores calculated on 40 double-rated cases was excellent (ICC = .96). In addition to PCL:YV total scores, we incorporated the use of a two-factor model of psychopathic traits, with Factor 1 comprising interpersonal and affective traits, and Factor 2 consisting of lifestyle and antisocial/developmental traits (Neumann et al., 2006). The mean PCL:YV Factor 1 score was 6.55 (SD = 3.00) and the mean PCL:YV Factor 2 score was 14.67 (SD = 3.37) for the sample. PCL:YV Factor 1 and 2 scores were significantly positively correlated (r = .50, p < .001), consistent with previous reports (Mailloux, Forth, & Kroner, 1997). See Table 1 for the remainder of correlations between PCL:YV scores and covariate measures. We also examined a four-facet model of adolescent psychopathic traits, with four latent facets representing the underlying dimensions of the adolescent expression of psychopathy: interpersonal (Facet 1), affective (Facet 2), lifestyle (Facet 3), and antisocial/developmental (Facet 4) traits, respectively (Neumann, Kosson, Forth, & Hare, 2006). We investigated factor and facet scores in addition to PCL:YV total scores in the current report, as there is accumulating literature revealing unique correlates of interpersonal/affective elements and lifestyle/behavioral foundations of psychopathy. We expected factor and facet scores on the PCL:YV to reveal more specific directional and stable effects than those with PCL:YV total scores alone. For example, factors and facets of the PCL-R are associated with dimensional traits that have often revealed discrete (sometimes opposing) relationships with physiological measures (Anderson et al., 2015; Juarez et al., 2013; Maurer et al., 2016; Patrick et al., 1993; Phillip et al., 2015; Steele et al., 2016).
Table 1.
Correlations between PCL:YV Variables and Covariate Measures
| PCL: YV Total |
PCL: YV Factor 1 |
PCL: YV Factor 2 |
PCL: YV Facet 1 |
PCL: YV Facet 2 |
PCL: YV Facet 3 |
PCL: YV Facet 4 |
IQ | Age | Sub. Use |
|
|---|---|---|---|---|---|---|---|---|---|---|
| PCL:YV Total | __ | |||||||||
| PCL:YV Factor 1 | .835** | __ | ||||||||
| PCL:YV Factor 2 | .878** | .502** | __ | |||||||
| PCL:YV Facet 1 | .632** | .843** | .301** | __ | ||||||
| PCL:YV Facet 2 | .773** | .832** | .547** | .408** | __ | |||||
| PCL:YV Facet 3 | .788** | .449** | .896** | .294** | .465** | __ | ||||
| PCL:YV Facet 4 | .749** | .418** | .862** | .217** | .491** | .550** | __ | |||
| IQ | −.051 | .083 | −.156 | .159* | −.009 | −.124 | −.162* | __ | ||
| Age | −.031 | −.001 | −.047 | .033 | −.027 | −.081 | .003 | −.118 | __ | |
| Sub. Use | .293** | .194* | .290** | .167* | .166* | .272** | .234** | .086 | −.029 | __ |
Note. n = 182 participants. Sub. Use corresponds to Number of substance dependencies.
p < .05
p < .01.
Additional Assessments
In addition to psychopathic traits, additional assessments were administered to assess intelligence quotient (IQ), substance dependence, psychopathology, and traumatic brain injury (TBI). Exclusionary criteria for the study included: a full-scale IQ less than 70, TBI accompanied with a significant loss of consciousness, or personal or familial bipolar or psychotic disorders. None of the 182 participants met any of these potential exclusionary criteria and all were thus retained in final analyses.
Full-scale IQ was estimated using the Vocabulary and Matrix Reasoning sub-tests of the Wechsler Adult Intelligence Scale – 3rd Edition (WAIS-III) (Wechsler, 1997) for participants sixteen years of age or older and from the Wechsler Intelligence Scale for Children – 4th Edition (WISC-IV) (Wechsler, 2003) for participants younger than sixteen years of age (M IQ score = 93.05; SD = 11.86). IQ scores were included as a covariate in analyses as input nuclei of the basal ganglia, including the caudate, putamen, and NAcc, have been previously linked to intelligence (Burgaleta et al., 2014; Grazioplene et al., 2015). Psychopathology and substance dependence were assessed using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman, Birmaher, & Brent, 1997). Substance dependence was entered as a covariate in analyses as youth psychopathic traits are comorbid with substance use severity (Mailloux et al., 1997) and substance use has been previously linked to basal ganglia dysfunction (Yin & Knowlton, 2006). Number of substance dependencies were calculated by summing the total number of substances (both alcohol and drug) for which participants met lifetime dependence diagnoses (M = 2.32, SD = 1.74). See Table 2 for the remaining descriptive statistics for this sample.
Table 2.
Descriptive Statistics and Independent Samples t-tests
| All Participants (n = 182) |
PCL:YV Highest Quartile (n = 46) |
PCL:YV Lowest Quartile (n = 46) |
Group Diff. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean | SD | n | Mean | SD | n | Mean | SD | t | df | P |
| PCL:YV Total | 182 | 23.50 | 6.11 | 46 | 31.00 | 2.13 | 46 | 15.52 | 3.47 | 25.80 | 90 | < .001 |
| PCL:YV Factor 1 | 182 | 6.55 | 3.00 | 46 | 10.27 | 1.93 | 46 | 3.67 | 1.79 | 17.02 | 90 | < .001 |
| PCL:YV Factor 2 | 182 | 14.67 | 3.37 | 46 | 17.69 | 1.36 | 46 | 10.51 | 3.24 | 13.85 | 90 | < .001 |
| PCL:YV Facet 1 | 181 | 2.13 | 1.85 | 46 | 4.09 | 1.79 | 46 | 1.04 | 1.13 | 9.75 | 90 | < .001 |
| PCL:YV Facet 2 | 181 | 4.43 | 1.76 | 46 | 6.22 | 0.87 | 46 | 2.63 | 1.12 | 17.15 | 90 | < .001 |
| PCL:YV Facet 3 | 181 | 6.48 | 2.04 | 46 | 8.21 | 1.22 | 46 | 4.13 | 1.69 | 13.24 | 90 | < .001 |
| PCL:YV Facet 4 | 180 | 8.19 | 1.74 | 46 | 9.43 | 0.78 | 45 | 6.44 | 2.03 | 9.32 | 89 | < .001 |
| Age | 182 | 17.56 | 1.11 | 46 | 17.43 | 1.31 | 46 | 17.66 | 1.00 | −0.97 | 90 | .333 |
| IQ | 182 | 93.05 | 11.86 | 46 | 91.98 | 10.75 | 46 | 93.35 | 13.17 | −0.55 | 90 | .586 |
| Sub. Use | 182 | 2.32 | 1.74 | 46 | 2.70 | 1.64 | 46 | 1.70 | 1.74 | 2.84 | 90 | .006 |
| Hit RT | 182 | 445.22 | 50.81 | 46 | 446.90 | 52.70 | 46 | 439.65 | 47.26 | 0.70 | 90 | .489 |
| Mean Hits | 182 | 399.90 | 21.00 | 46 | 401.24 | 20.70 | 46 | 402.85 | 10.66 | 0.70 | 90 | .641 |
| False Alarm RT | 182 | 399.33 | 38.09 | 46 | 399.28 | 35.83 | 46 | 397.67 | 33.38 | −0.22 | 90 | .824 |
| Mean FA’s | 182 | 25.02 | 11.27 | 46 | 22.89 | 10.04 | 46 | 24.91 | 12.29 | 0.86 | 90 | .390 |
| Post-Error Slowing | 182 | 42.08 | 65.55 | 46 | 59.62 | 78.44 | 46 | 38.72 | 59.25 | 1.44 | 90 | .153 |
Note. n = 182 participants. Sub. Use corresponds to Number of substance dependencies.
Go/NoGo Experimental Paradigm
Participants performed a response inhibition Go/NoGo fMRI experimental paradigm (Kiehl, Liddle, & Hopfinger, 2000) consisting of two runs, each comprising 245 visual stimuli. The stimuli were presented to participants using the computer-controlled visual and auditory software package, Presentation (www.neurobs.com). Each stimulus appeared for 250 milliseconds (ms) in white text within a continuously displayed rectangular fixation box. Participants were instructed to respond as quickly and accurately as possible with their right index finger via a button box every time the target (“Go”) stimulus (a white “X”) appeared, and to withhold responding when the distracter (“NoGo”) (a white “K”) stimulus appeared. Targets appeared with higher frequency (84% of trials, 412 total trials, with 206 on each run) than distracters (16% of trials, 78 total trials, with 39 on each run) to establish a strong stimulus-response mapping on Go trials. Two NoGo stimuli were never presented sequentially. The stimuli were approximately 3 × 5 visual degrees on a black background. The interstimulus interval was jittered (1 – 3 seconds stimulus onset asynchrony [SOA], averaging 1.5 seconds). The SOA between Go stimuli varied pseudo-randomly between 1000, 2000, and 3000 ms, subject to the constraint that three Go stimuli were presented within each six second period. The NoGo stimuli were interspersed among the Go stimuli in a pseudo random manner subject to two constraints: the minimum SOA between Go and NoGo stimuli was 1000 ms, and the SOA between successive NoGo stimuli was in the range of eight to fourteen seconds. Hits were defined as successful behavioral responses to Go stimuli and Correct Rejects (CR’s) were defined as successful behavioral responses to NoGo stimuli, whereas False Alarms (FA’s) were defined as incorrect behavioral responses to NoGo stimuli. Prior to recording, each participant performed a block of ten practice trials to ensure task instructions were clearly understood.
Imaging Parameters and Preprocessing
Images were collected with the Mind Research Network’s mobile Siemens 1.5T Avanto stationed at the juvenile detention center with advanced SQ gradients (max slew rate 200 T/m/s 346 T/m/s vector summation, rise time 200 microseconds (μs)) equipped with a twelve-element head coil. The EPI gradient-echo pulse sequence (TR/TE 2000/39 ms, flip angle 75°, FOV 24 × 24 cm, 64 × 64 matrix, 3.4 × 3.4 mm in plane resolution, 5 mm slice thickness, 30 slices) effectively covers the entire brain (150 mm) in 2000 ms. Head motion was limited using padding and restraint, and was evaluated using INRIalign, a mutual information algorithm unbiased by local signal change (Freire & Mangin, 2001; Freire, Roche, & Mangin, 2002). No participants were excluded due to excessive motion, defined as motion greater than two SD’s away from the mean (i.e., greater than two mm translation, or 1.5° rotation).
Functional images were reconstructed offline at 16-bit resolution and manually reoriented to approximately the anterior commissure/posterior commissure (AC/PC) plane. Functional images were spatially normalized to the Montreal Neurological Institute (MNI) template via a parameter affine transformation using smooth basis functions to account for nonlinear differences, and spatially smoothed (8 mm full-width at half maximum) in the Statistical Parametric Mapping 12 (SPM12) software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) (Calhoun et al., 2017). Response types (CR’s, Flits, and FA’s) were modeled as separate events. Event-related responses were modeled using a synthetic response function composed of two gamma functions. The first gamma function modeled the hemodynamic response using a peak latency of six seconds. A term proportional to the derivative of this gamma function was used to model the small “overshoot” of the hemodynamic response on recovery. A latency variation amplitude-correction method was used to provide a more accurate estimate of the hemodynamic response for each condition that controlled for differences between slices and timing and variation across regions in the latency of the hemodynamic response (Calhoun, Stevens, Pearlson, & Kiehl, 2004). Second-level analyses examined associations between contrast estimates and adolescent psychopathic traits (via PCL:YV total, factor, and facet scores) and covariate measures (participant’s age, IQ, and number of substance dependencies).
Region of Interest (ROI) Analyses
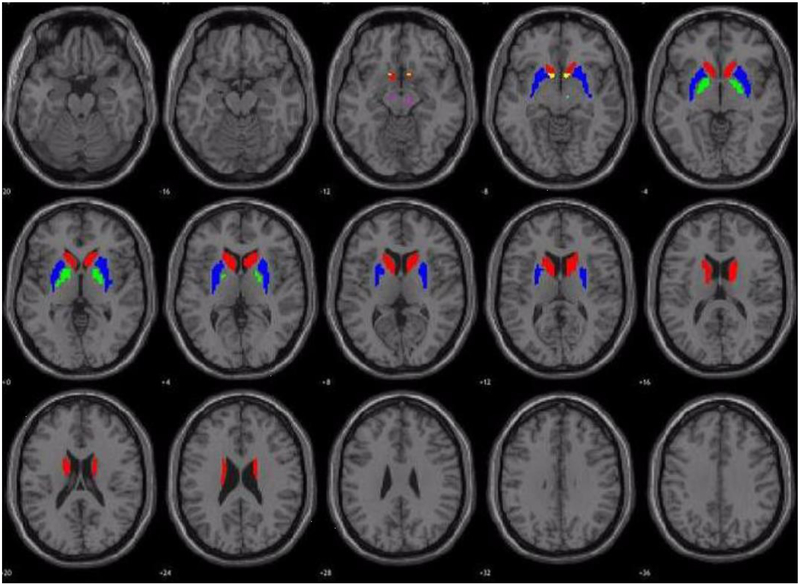
Several a priori ROIs were selected from subregions of the basal ganglia previously investigated in relation to youth psychopathic traits, including the caudate, putamen, globus pallidus, and NAcc (Finger et al., 2011; Marsh et al., 2013; Vincent et al., 2017; Yang et al., 2015). In addition, two additional ROIs were selected which have never been previously investigated in relation to adolescent psychopathy scores: the subthalamic nucleus and substantia nigra. For computational purposes, ROIs in the current study were defined primarily by actual anatomical boundaries of brain regions contributing to the regions described above. Boundaries were defined by automated anatomical labels (AAL) featured in the Wake Forest University PickAtlas toolbox available in SPM12 to generate ROIs for the caudate, putamen, globus pallidus, subthalamic nucleus, and substantia nigra (Maldjian, Laurienti, & Burdette, 2004; Maldjian, Laurienti, Kraft, & Burdette, 2003). The NAcc ROI was made with a hand-drawn mask in a similar manner to a previous publication from our laboratory (Cope et al., 2014). See Figure 1 for a visual representation of the a priori ROIs.
Figure 1.
Visual representation of the a priori ROIs included in analyses. Red refers to the caudate, green refers to the globus pallidus, blue refers to the putamen, yellow refers to the nucleus accumbens [NAcc], magenta refers to the substantia nigra, and cyan refers to the subthalamic nucleus.
Data Analysis
The contrast of interest (FA’s vs. Hits) was evaluated for linear relationships with PCL:YV scores (either total, two-factor, or four-facet scores) in the a priori ROIs. All main effects presented are whole-brain corrected for Family-Wise Error (FWE) rate. All effects were then small-volume peak corrected with an FWE multiple comparison correction within each anatomically-defined ROI. Effects related to PCL:YV total scores (Model 1) were examined separately from PCL:YV factor scores (Model 2) and facet scores (Model 3). Model 2 included both PCL:YV factor scores and Model 3 included all four PCL:YV facet scores in order to account for unique effects of the individual factors and facets, holding the other factors and facets constant. These three models accounted for participant’s age, IQ, and number of substance dependencies, holding these variables constant. We did not control for ethnicity in the current analyses, as participants who identified as Hispanic/Latino (n = 142) and those who did not identify as Hispanic/Latino (n = 40) did not significantly differ in error-related hemodynamic activity in any of the a priori ROIs in independent samples t-tests.
Results
Behavioral Results
Response times (RTs) and frequency for Hit and FA stimuli were analyzed. As expected, participants responded faster to NoGo stimuli (M = 399 ms, SD = 36 ms) compared to Go stimuli (M = 455 ms, SD = 50 ms), t(181) = 18.81, p < .001. Participants made at least six errors to ensure a reliable error-related signal (range 6 – 55 FA’s) (Steele, Anderson, et al., 2016). Additionally, participants made significantly more errors to NoGo stimuli (M = 25.02, SD = 11.27) compared to Go stimuli (M = 12.10, SD = 21.00), t(181) = 7.91, p < .001. There was a main effect for post-error slowing (PES) (M = 42 ms, SD = 65.55 ms), t(181) = 8.66, p < .001, defined as the behavioral slowing that occurs after one commits an incorrect behavioral response, to help prevent future errors from occurring (Rabbit, 1981). Participants did indeed respond more slowly after error trials (M = 453 ms, SD = 90 ms) than after correct trials (M = 411 ms, SD = 61 ms).
Correlational Analyses
PCL:YV total, factor, and facet scores were significantly positively correlated with number of substance dependencies (see Table 1), consistent with previous studies (Mailloux et al., 1997). IQ scores and several PCL:YV scores were significantly correlated, including between IQ and PCL:YV Factor 2 scores (r = −.16, p = .035), and Facet 1 scores (r = .16, p = .033), and Facet 4 scores (r = −.16, p = .030). IQ scores were not significantly correlated with any of the remaining PCL:YV scores or covariate measures. See Table 1 for the remainder of correlations between PCL:YV scores and covariate measures.
PCL:YV Facet 1 scores were significantly negatively related to number of FA’s (r = −.15, p = .041) and IQ scores were significantly negatively related to number of Hits (r = −.16, p = .036). No other significant correlations emerged between PCL:YV scores or covariate measures with number of Hits or FA’s, or with Hit or FA Response Time. Additionally, PCL:YV scores or covariate measures did not significantly correlate with PES.
fMRI Analyses: FA vs. Hits Contrast
Main Effects:
This contrast produced significant activation in bilateral regions of the caudate, putamen, globus pallidus, NAcc, subthalamic nucleus, and substantia nigra (see Table 3 for peaks).
Table 3.
Main Effects: False Alarms vs. Hits condition
| Region | x | y | z | FWE p- value |
Peak Voxel t-value |
Cohen’s d | Cluster Size |
|---|---|---|---|---|---|---|---|
| Left Caudate | −6 | 0 | 9 | < .001 | 7.52 | 1.12 | 293 |
| Right Caudate | 9 | 0 | 12 | < .001 | 7.15 | 1.06 | 287 |
| Left Putamen | −33 | 6 | −6 | < .001 | 16.89 | 2.51 | 293 |
| Right Putamen | 30 | 12 | −9 | < .001 | 15.64 | 2.33 | 312 |
| Left Globus Pallidus | −12 | −3 | 0 | < .001 | 7.20 | 1.07 | 86 |
| Right Globus Pallidus | 15 | −3 | −6 | < .001 | 8.14 | 1.21 | 82 |
| Left NAcc | −12 | 6 | −6 | < .001 | 3.97 | 0.59 | 17 |
| Right NAcc | 12 | 6 | −6 | < .001 | 4.89 | 0.73 | 17 |
| Left Subthalamic Nucleus | −9 | −15 | −9 | < .001 | 11.73 | 1.74 | 5 |
| Right Subthalamic Nucleus | 9 | −12 | −9 | < .001 | 12.54 | 1.86 | 7 |
| Left Substantia Nigra | −6 | −15 | −12 | < .001 | 13.14 | 1.95 | 7 |
| Right Substantia Nigra | 9 | −21 | −12 | < .001 | 12.91 | 1.92 | 7 |
Note. All p’s < .001 and results were whole-brain corrected for Family-Wise Error Rate (FWE).
Model 1:
In this model, participant’s age, IQ, and number of substance dependencies were included as covariates in statistical analyses along with PCL:YV total scores. PCL:YV total scores were significantly negatively related to error-related hemodynamic activity in the subthalamic nucleus bilaterally and the right substantia nigra (see Table 4). In addition, IQ scores were significantly negatively related to error-related hemodynamic activity in the right substantia nigra, right NAcc, right caudate, and left globus pallidus (see Table 4). No significant associations emerged with participant’s age or number of substance dependencies with any of the a priori ROIs.
Table 4.
Model 1: PCL:YV Total and IQ scores negatively related to error-related hemodynamic activity in subregions of the basal ganglia.
| Variable | Region | x | y | z | FWE p- value |
Peak Voxel t- value |
Cohen’s d |
Cluster Size |
|---|---|---|---|---|---|---|---|---|
| PCL:YV Total | Right Substantia Nigra | 15 | −21 | −6 | 0.014 | 2.61 | 0.39 | 7 |
| Left Subthalamic Nucleus | −12 | −12 | −3 | 0.036 | 2.19 | 0.32 | 5 | |
| Right Subthalamic Nucleus | 12 | −15 | −6 | 0.022 | 2.42 | 0.36 | 7 | |
| IQ | Right Caudate | 18 | 24 | −9 | 0.027 | 3.18 | 0.47 | 287 |
| Left Globus Pallidus | −15 | −3 | −6 | 0.023 | 2.89 | 0.43 | 86 | |
| Right NAcc | 9 | 6 | −9 | 0.009 | 2.87 | 0.42 | 17 | |
| Right Substantia Nigra | 15 | −21 | −9 | 0.035 | 2.25 | 0.33 | 7 |
Note. Investigating error-related processing in the contrast False Alarms (FA’s) vs. Hits. Coordinates are given in MNI space. Results were small-volume peak corrected for the ROI (FWE-corrected). No significant associations were found with ROIs and age or number of substance dependencies. Analyses performed with n = 182 participants.
Model 2:
In this model, the same three covariate measures from Model 1 were entered with PCL:YV Factor 1 scores (reflecting interpersonal and affective traits) and Factor 2 scores (measuring lifestyle and antisocial/developmental traits) from the two-factor model (Neumann & Hare, 2008). In Model 2, neither PCL:YV Factor 1 or 2 scores, or participant’s age or number of substance dependencies were significantly associated with any of the a priori ROIs. Instead, IQ scores were significantly negatively related to error-related hemodynamic activity in the right NAcc and left globus pallidus.
Model 3:
In this model, the same three covariate measures included in Models 1 and 2 were entered with PCL:YV facet scores (Facet 1: interpersonal traits, Facet 2: affective traits, Facet 3: lifestyle traits, and Facet 4: antisocial/developmental traits, respectively) from the four-facet model (Neumann & Hare, 2008). In Model 3, PCL:YV Facet 1 scores were significantly negatively related to error-related hemodynamic activity in the left caudate and right subthalamic nucleus, Facet 3 scores were significantly negatively related to error-related hemodynamic activity in the left subthalamic nucleus, and Facet 4 scores were significantly negatively related to error-related hemodynamic activity in the right NAcc, right caudate, and right globus pallidus (see Table 5). As observed in the two prior models, IQ scores were significantly negatively related to error-related hemodynamic activity in the right NAcc and left globus pallidus (see Table 5). No other significant associations emerged with PCL:YV Facet 2 scores, participant’s age, or number of substance dependencies in any of the a priori ROIs.
Table 5.
Model 3: PCL:YV Facet scores from the four-facet model and IQ scores negatively related to error-related hemodynamic activity in subregions of the basal ganglia.
| Variable | Region | x | y | z | p-value | t-value | Cohen’s d | Cluster Size |
|---|---|---|---|---|---|---|---|---|
| PCL:YV Facet 1 | Left Caudate | −21 | −18 | 21 | 0.009 | 3.52 | 0.52 | 293 |
| Right Subthalamic Nucleus | 12 | −12 | −3 | 0.045 | 2.11 | 0.31 | 7 | |
| PCL:YV Facet 3 | Left Subthalamic Nucleus | −12 | −12 | −3 | 0.013 | 2.58 | 0.38 | 5 |
| PCL:YV Facet 4 | Right Caudate | 6 | 9 | −3 | 0.025 | 3.17 | 0.47 | 287 |
| Right Globus Pallidus | 12 | 3 | −3 | 0.019 | 2.91 | 0.43 | 82 | |
| Right NAcc | 9 | 6 | −6 | 0.005 | 3.08 | 0.46 | 17 | |
| IQ | Left Globus Pallidus | −15 | −3 | −6 | 0.019 | 2.92 | 0.43 | 86 |
| Right NAcc | 9 | 6 | −9 | 0.011 | 2.78 | 0.41 | 17 |
Note. Investigating error-related processing in the contrast False Alarms (FA’s) vs. Hits. Coordinates are given in MNI space. Results were small-volume peak corrected for the ROI (FWE-corrected). No significant associations were found with ROIs and age or number of substance dependencies. No significant associations were found with ROIs and PCL:YV Factor 2 scores, age, or number of substance dependencies. Analyses performed with n = 182 participants.
Discussion
Youth with elevated psychopathic traits perseverate during experimental learning paradigms, failing to adjust their behavior to meet the demands established by external sources (Budhani & Blair, 2005; Dadds et al., 2006; Finger et al., 2008; Roussy & Toupin, 2000). Such youth exhibit a number of comparable neurocognitive deficits as adult psychopathic offenders, including response modulation (Budhani & Blair, 2005; Dadds et al., 2006) and error-related processing deficits (Maurer, Steele, Cope, et al., 2016). In the current report, we sought to investigate whether dysfunction occurring in subregions of the basal ganglia could help contribute to error-related processing deficits previously observed in youth with elevated psychopathic traits using ERPs (Maurer, Steele, Cope, et al., 2016).
Here, we investigated error-related processing using a response inhibition Go/NoGo fMRI experimental paradigm in a sample of incarcerated male adolescent offenders (n = 182). In the current report, adolescent psychopathy scores measured via the PCL:YV were negatively related to error-related hemodynamic activity within input nuclei (the caudate and NAcc), intrinsic/output nuclei (the globus pallidus and substantia nigra), and related nuclei (the subthalamic nucleus) of the basal ganglia. Dysfunction occurring early within subregions of the basal ganglia has important implications for error-related processing in general. Subregions of the basal ganglia have been shown to play an important, though indirect role, in error-related processing, as they help monitor and steadily predict the result of ongoing events (Botvinick et al., 2001; Mathalon et al., 2003), determining whether the end result of events will be favorable or not (Holroyd & Coles, 2002). When encountering events that deviate from expectations, including the commission of errors, the basal ganglia relays information to regions of the salience network, including the dorsal ACC (Ham et al., 2013; Kennerley et al., 2006) via the thalamus. The ACC helps signal the need for increased cognitive control to regions involved in the executive control network, including lateral prefrontal structures (Egner, 2009). Results obtained in the current report suggest that error-related processing deficits previously observed in youth with elevated psychopathic traits (i.e., reduced Pe ERP amplitude (Maurer et al., 2016)), may be due in part to underlying dysfunction occurring downstream within subregions of the basal ganglia.
In the current report, PCL:YV Facet 1 scores (i.e., traits reflecting impression management, pathological lying, manipulation for personal gain, and a grandiose sense of self-worth) were negatively related to error-related hemodynamic activity within input nuclei of the basal ganglia, including the caudate. Previous studies have associated increased caudate volume (Yang et al., 2015) and reduced hemodynamic activity in the caudate during drug cue processing (Vincent et al., 2017) and early stimulus-reinforcement exposure (Finger et al., 2011) in youth scoring high on psychopathy. The caudate is typically involved in the shifting of attentional sets (Middleton & Strick, 2000; Ravizza & Ciranni, 2002), with damage resulting in increased stimulus-bound perseverative behavior (Nys, van Zandvoort, van der Worp, Kappelle, & de Haan, 2006). Caudate dysfunction may result in perseveration deficits typically associated with youth with elevated psychopathic traits (Budhani & Blair, 2005; Dadds et al., 2006; Finger et al., 2008; Roussy & Toupin, 2000), and could easily be related to error dysfunction, including failing to engage in further stages of error-related processing, such as evaluating the motivational significance of error-related information (Ullsperger et al., 2010), consistent with the aforementioned attentional bottleneck hypothesis (Newman & Baskin-Sommers, 2012).
Additionally, PCL:YV Facet 4 scores (i.e., traits reflecting poor anger control, early behavioral problems, serious criminal behavior and violation of conditional release, and criminal versatility) were negatively related to error-related hemodynamic activity within input nuclei of the basal ganglia, including the caudate and NAcc. To date, hemodynamic activity within the NAcc has never been significantly related to adolescent psychopathic traits in previously published reports. The NAcc plays a significant role in the mesocorticolimbic reward pathway in the brain, and is highly implicated with substance use disorders (SUDs) (Weissman et al., 2015), due to the important connection between NAcc and dopaminergic functioning. Psychopathic traits, particularly Facet 4 traits, have been shown to be comorbid with substance use proclivity (Kennealy, Hicks, & Patrick, 2007). The NAcc is also involved in processing rewarding and reinforcing information, including errors (Dalley et al., 2007). With reduced error-related hemodynamic activity in the NAcc, this may potentially explain why youth scoring high on Facet 4 traits have an increased propensity towards substance use, incarceration, and eventual recidivism (Edens et al., 2007), by failing to fully evaluate rewarding and reinforcing information, including errors, to the extent as youth scoring lower on these traits.
Adolescent psychopathy scores in the current sample were also associated with reduced error-related hemodynamic activity within intrinsic/output nuclei in the basal ganglia. Specifically, PCL:YV total scores were negatively related to error-related hemodynamic activity in the substantia nigra, and Facet 4 scores were negatively related to error-related hemodynamic activity in the globus pallidus. The substantia nigra is typically involved in information processing, particularly for behaviorally salient and novel information (Bunzeck & Duzel, 2006), and has never been investigated in relation to youth with elevated psychopathic traits. Errors are typically described as behaviorally salient events, as they are quite novel, deviating from normal processing (Harsay, Spaan, Wijnen, & Ridderinkhof, 2012). The globus pallidus is involved in the generation of voluntary movements, with lesions specific to this region being associated with poor motivation on behavioral tasks (Vijayaraghavan, Vaidya, Humphreys, Beglinger, & Paradoso, 2008). Reduced error-related hemodynamic activity within the substantia nigra and globus pallidus therefore suggests youth with elevated psychopathic traits may exhibit a specific deficit in processing the motivational significance of error-related information (Ullsperger et al., 2010).
Finally, PCL:YV total, Facet 1 and Facet 3 (i.e., traits reflecting stimulation seeking, impulsivity, irresponsibility, parasitic orientation, and a lack of realistic, long-term goals) scores were negatively related to error-related hemodynamic activity within related nuclei of the basal ganglia in the subthalamic nucleus. This region is typically involved in the phenomenon of post-error slowing (Cavanagh, Sanguinetti, Allen, Sherman, & Frank, 2014; Siegert et al., 2014), defined as the behavioral slowing down after an incorrect behavioral response in order to prevent future errors from occurring (Rabbit, 1981). In the current report, we did not find any significant relations between adolescent psychopathy scores and PES. Typically, PES is best observed in experimental paradigms that have a short response-stimulus interval (RSI) (Danielmeier & Ullsperger, 2011). As the current study had longer RSIs than the optimal RSI for the detection of PES, we may not be able to accurately speak of PES effects. To date, the subthalamic nucleus has never been investigated in relation to youth with elevated psychopathic traits. Interestingly, damage to the subthalamic nucleus has been shown to be associated with increases in impulsivity (Uslaner & Robinson, 2006), a key PCL:YV Facet 3 trait. Thus, reduced error-related hemodynamic activity in the subthalamic nucleus may play a role in the impulsive nature that exemplifies youth with elevated psychopathic traits, who typically act on the spur of the moment, without fully considering the consequences of their actions (Forth et al., 2003).
In addition to adolescent psychopathy scores, IQ scores were negatively related to error-related hemodynamic activity in several regions encompassing the basal ganglia, including the caudate, NAcc, substantia nigra, and globus pallidus. Several neuroimaging studies support the neural efficiency hypothesis, whereby those characterized by higher intelligence exhibit reduced hemodynamic activity compared to less intelligent individuals on cognitive tasks of low to moderate difficulty (Neubauer & Fink, 2009). Individuals with higher intelligence show more efficient processing by blocking out interfering information and display lower energy consumption in the brain (Haier et al., 1998) compared to those characterized by lower intelligence. In addition, compared to other response inhibition paradigms, including Stop-Signal or Flanker tasks, the Go/NoGo task used in the current report focuses specifically on response inhibition processes, without any additional working memory-related confounds (Rubia et al., 2001). Task difficulty often moderates neural efficiency. For example, IQ scores are often negatively related to hemodynamic activity during relatively easy experimental paradigms, whereas opposing findings are often observed for more difficult tasks (Doppelmayr et al., 2005). As our Go/NoGo experimental paradigm focuses solely on cognitive control processes, including error-related processing, and not other, more complicated cognitive processes, including working memory or abstract problem processing, participants in the current study characterized by higher intelligence may not engage subregions of the basal ganglia to the extent needed for individuals characterized by lower intelligence.
Limitations
To our knowledge, this is the first evidence to suggest that dysfunction occurring within subregions of the basal ganglia help contribute to error-related processing deficits previously associated with youth with elevated psychopathic traits using ERPs, even when controlling for substance dependence, age, and IQ. Still, several limitations in the current study should be noted. First, psychopathic traits, at least at moderate to high levels detected early in life, often reduce naturally for most youth (Frick et al., 2003; Hawes et al., in press; Hemphälä, Kosson, Westerman, & Hodgins, 2015; Lee, Klaver, Hart, Moretti, & Douglas, 2009; Lynam, Caspi, Moffitt, Loeber, & Stouthamer-Loeber, 2007). Some evidence of continuity from adolescence to adulthood comes from longitudinal research incorporating both self-report and interview-based measures of psychopathic traits, showing only moderate stability from age 13 to 23 (Lynam et al., 2007). As such, there exists the possibility that many youth in our current study may not grow up to meet the established criteria for psychopathic personality as adults. Longitudinal research is needed to see whether reduced error-related hemodynamic activity in youth samples can serve as a potential biological vulnerability marker for the future development of psychopathic personality.
Second, our study recruited participants from a maximum-security correctional facility. While recruiting participants from incarcerated settings compared to community samples leads to a substantially higher base rate of psychopathic traits (Neumann & Hare, 2008), youth in incarcerated settings differ on a number of variables compared to youth from general community samples. Such differences include substance use history, general intelligence, and trait anxiety (Foley, 2001; Wasserman, McReynolds, Lucas, Fisher, & Santos, 2002). We note in the current study we had PCL:YV total scores ranging from the low to extreme range of scores, with means in line with previously published incarcerated youth samples. Thus, our sample should be considered to have clinical levels of psychopathic traits, which may not extrapolate to samples with lower overall psychopathy scores.
Finally, our study incorporated the use of a 1.5T mobile MRI scanner to investigate error-related processing in a sample of incarcerated male adolescent offenders. While the use of such a scanner leads to unprecedented access to investigate a high-risk incarcerated sample, it should be noted that the lower field strength of a 1.5T MRI scanner makes it more difficult to reliably distinguish between smaller regions encompassing the basal ganglia, including the substantia nigra and subthalamic nucleus, from clusters of nearby, surrounding structures. Thus, future studies should attempt to replicate our findings with MRI scanners of higher field strength, including 3T and 7T scanners. Furthermore, future studies should incorporate the use of agnostic, data-driven approaches, including Independent Component Analysis (ICA), to help identify brain networks related to error-related processing implicated in youth with elevated psychopathic traits, to see if deficits within the basal ganglia help contribute to dysfunction observed in higher-order brain regions, which we could not test in the current study.
Conclusions
Error-related dysfunction within input nuclei (caudate and NAcc), intrinsic/output nuclei (globus pallidus and substantia nigra), and related nuclei (subthalamic nucleus) of the basal ganglia could help contribute to some of the common characteristics associated with youth with elevated psychopathic traits. For example, dysfunction within the subthalamic nucleus could contribute to the impulsive, stimulation-seeking nature of youth with elevated psychopathic traits, whereas caudate dysfunction could help give rise to the central perseveration deficits associated with this population. Furthermore, dysfunction in the substantia nigra, NAcc, and globus pallidus could help contribute to the profile of an individual who suffers from an attentional abnormality, whereby they do not fully process the motivational significance of novel, salient events that have the potential to be quite reinforcing, including errors. These error-related processing deficits occurring in subregions of the basal ganglia could have tremendous implications in terms of the heightened propensity for youth with elevated psychopathic traits to engage in reckless, impulsive behavior, without fully processing the consequences of their errors. Lack of consideration of such consequences could potentially result in an increased propensity towards substance use, incarceration, and recidivism that typifies youth with elevated psychopathic traits (Edens et al., 2007).
Acknowledgments
This study was funded by the National Institute of Mental Health (NIMH) grant R01 MH071896 (PI: Kiehl), the National Institute of Child Health and Human Development (NICHD) grant R01 HD082257 (PI: Kiehl), the National Institute on Drug Abuse (NIDA) DA026502 (PI: Vincent), and the National Institute of General Medical Sciences (NIGMS) P20GM103472 (PI: Calhoun). JMM is supported by NIDA through grant number F31 DA043328. VRS is supported by the Intramural Research Program of NIDA, National Institutes of Health, Baltimore, Maryland. We are grateful to the staff and clients (and parents) at the Youth Diagnostic and Development Center and the New Mexico Children, Youth, and Families Department for their support and assistance in making this research possible. The authors report no biomedical financial interests or potential conflicts of interest. The authors thank Prashanth Nyalakanti for his assistance in data analysis.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Anderson NE, Steele VR, Maurer JM, Bernat EM, & Kiehl KA (2015). Psychopathy, attention, and oddball target detection: New insights from PCL-R facet scores. Psychophysiology, 52(9), 1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, & Cohen JD (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. [DOI] [PubMed] [Google Scholar]
- Brazil IA, de Bruijn ER, Bulten BH, von Borries AK, van Lankveld JJ, Buitelaar JK, & Verkes RJ (2009). Early and late components of error monitoring in violent offenders with psychopathy. Biological Psychiatry, 65(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Budhani S, & Blair RJR (2005). Response reversal and children with psychopathic tendencies: Success is a function of salience of contingency change. Journal of Child Psychology and Psychiatry, 46(9), 972–981. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, & Duzel E (2006). Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron, 51(369-379), 369. [DOI] [PubMed] [Google Scholar]
- Burgaleta M, MacDonald PA, Martínez K, Román FJ, Álvarez-Linera J, Ramos González A, … Colom R (2014). Subcortical regional morphology correlates with fluid and spatial intelligence. Human Brain Mapping, 35(5), 1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell MF (2011). Treatment-related changes in behavioral outcomes of psychopathy facets in adolescent offenders. Law and Human Behavior, 35(4), 275–187. [DOI] [PubMed] [Google Scholar]
- Caldwell MF, McCormick DJ, Umstead D, & Van Rybroek GJ (2007). Evidence of treatment progress and therapeutic outcomes among adolescents with psychopathic features. Criminal Justice and Behavior, 34(5), 573–587. [Google Scholar]
- Calhoun VD, Stevens M, Pearlson GD, & Kiehl KA (2004). fMRI analysis with the general linear model: Removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. NeuroImage, 22, 252–257. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Wager TD, Krishnan A, Rosch KS, Seymour KE, Nebel MB, … Kiehl KA (2017). The impact of T1 versus EPI spatial normalization templates for fMRI data analysis. Human Brain Mapping, 38(11), 5331–5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Sanguinetti JL, Allen JJB, Sherman SJ, & Frank MJ (2014). The subthalamic nucleus contributes to post-error slowing. The Journal of Neuroscience, 26(11), 2637–2644. [DOI] [PubMed] [Google Scholar]
- Cope LM, Vincent GM, Jobelius JL, Nyalakanti P, Calhoun VD, & Kiehl KA (2014). Psychopathic traits modulate brain responses to drug cues in incarcerated offenders. Frontiers in Human Neuroscience, 8(87), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, Merz S, Riddell AC, Haines DJ, … Abeygunawardane AI (2006). Attention to the eyes and fear-recognition deficits in child psychopathy. The British Journal of Psychiatry, 189(3), 280–281. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, … Robbins TW (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science, 315(5816), 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, & Ullsperger M (2011). Post-error adjustments. Frontiers in Psychology, 2(233), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Sauseng P, Hodlmoser K, Stadler W, & Hanslmayr S (2005). Intelligence related differences in EEG badpower. Neuroscience Letters, 381(3), 309–313. [DOI] [PubMed] [Google Scholar]
- Edens JF, Campbell JS, & Weir JM (2007). Youth psychopathy and criminal recidivism: A meta-analysis of the psychopathy checklist measures. Law and Human Behavior, 31(1), 53–75. [DOI] [PubMed] [Google Scholar]
- Edwards BG, Calhoun VD, & Kiehl KA (2012). Joint ICA of ERP and fMRI during error-monitoring. NeuroImage, 59(2), 1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T (2009). Prefrontal cortex and cognitive control: Motivating functional hierarchies. Nature Neuroscience, 12, 821–822. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, … Blair RJ (2011). Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry, 168(2), 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, … Blair JR (2008). Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives in General Psychiatry, 65(5), 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RM (2001). Academic characteristics of incarcerated youth and correctional educational programs: A literature review. Journal of Emotional and Behavioral Disorders, 9(4), 248–259. [Google Scholar]
- Forth AE, Kosson DS, & Hare RD (2003). The Psychopathy Checklist: Youth Version. Toronto, ON, Canada: Multi-Health Systems. [Google Scholar]
- Freire L, & Mangin JF (2001). Motion correction algorithms may create spurious brain activations in the absence of subject motion. NeuroImage, 14, 709–722. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, & Mangin JF (2002). What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging, 21, 470–484. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, & Loney BR (2003). Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology, 39(2), 246–260. [DOI] [PubMed] [Google Scholar]
- Grazioplene RG, Ryman SG, Gray JR, Rustichini A, Jung RE, & DeYoung CG (2015). Subcortical intelligence: Caudate volume predicts IQ in healthy adults. Human Brain Mapping, 36(4), 1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, & Paek J (1992). Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence, 12, 199–217. [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, & Sharp DJ (2013). Cognitive control and the salience network: An investigation of error processing and effective connectivity. Journal of Neuroscience, 33(16), 7091–7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD (1991). The Hare Psychopathy Checklist - Revised Manual. Multi-Health Systems: Toronto. [Google Scholar]
- Hare RD (2003). Manual for the Hare Psychopathy Checklist - Revised (2nd Ed). Toronto, Canada: Multi-Health Systems. [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, & Ridderinkhof KR (2012). Error awareness and salience processing in the oddball task: Shared neural mechanisms. Frontiers in Human Neuroscience, 6(246), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes SW, Byrd AL, Kelley SE, Gonzalez R, Edens JF, & Pardini DA (in press). Psychopathic features across development: Assessing longitudinal invariance among Caucasian and African American youths. Journal of Research in Personality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphälä M, Kosson DS, Westerman J, & Hodgins S (2015). Stability and predictors of psychopathic traits from mid-adolescence through early adulthood. Scandanavian Journal of Psychology, 56(6), 649–658. [DOI] [PubMed] [Google Scholar]
- Hemphill JF, Hare RD, & Wong S (1998). Psychopathy and recidivism: A review. Legal and Criminological Psychology, 3(1), 139–170. [Google Scholar]
- Hermann MJ, Rommler J, Ehlis AC, Heidrich A, & Fallgatter AJ (2004). Source localization (LORETA) of the error-related negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20(2), 294–299. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. [DOI] [PubMed] [Google Scholar]
- Juarez M, Kiehl KA, & Calhoun VD (2013). Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Human Brain Mapping, 34(8), 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, & Brent D (1997). Schedule for Affective Disorders and Schizophrenia for School-Aged Children: Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 37(7), 980–988. [DOI] [PubMed] [Google Scholar]
- Kennealy PJ, Hicks BM, & Patrick CJ (2007). Validity of factors of the Psychopathy Checklist-Revised in female prisoners: Discriminant relations with antisocial behavior, substance abuse, and personality. Assessment, 14(4), 323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, & Rushworth MFS (2006). Optimal decision making and the anterior cingulate cortex. Nature Neuroscience, 9, 940–947. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, & Hopfinger JB (2000). Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology, 37(2), 216–223. [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, & Obeso JA (2012). Functional neuroanatomy of the basal ganglia. Cold Spring Harbor Perspectives in Medicine, 5, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Z, Klaver JR, Hart SD, Moretti MM, & Douglas KS (2009). Short-term stability of psychopathic traits in adolescent offenders. Journal of Clinical Child and Adolescent Psychology, 38(5), 595–605. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Caspi A, Moffitt TE, Loeber R, & Stouthamer-Loeber M (2007). Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. Journal of Abnormal Psychology, 116, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux DL, Forth AE, & Kroner DG (1997). Psychopathy and substance use in adolescent male offenders. Psychological Reports, 81(2), 529–530. [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, & Burdette JH (2004). Precentral gyrus discrepency in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–455. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz IT, Schechter JC, … Blair RJ (2013). Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of Child Psychology and Psychiatry, 54(8), 900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, & Ford JM (2003). Anatomy of an error: ERP and fMRI. Biological Psychology, 64(1-2), 119–141. [DOI] [PubMed] [Google Scholar]
- Maurer JM, Steele VR, Cope LM, Vincent GM, Stephen JM, Calhoun VD, & Kiehl KA (2016). Dysfunctional error-related processing in incarcerated youth with elevated psychopathic traits. Developmental Cognitive Neuroscience, 19, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer JM, Steele VR, Edwards BG, Bernat EM, Calhoun VD, & Kiehl KA (2016). Dysfunctional error-related processing in female psychopathy. Social Cognitive and Affective Neuroscience, 11(7), 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, & Strick PL (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research Reviews, 31(2-3), 236–250. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, & Fink A (2009). Intelligence and neural efficiency. Neuroscience & Biobehavioral Reviews, 33(1004-1023), 1004. [DOI] [PubMed] [Google Scholar]
- Neumann CS, & Hare RD (2008). Psychopathic traits in a large community sample: Links to violence, alcohol use, and intelligence. Journal of Consulting and Clinical Psychology, 76(5), 893–899. [DOI] [PubMed] [Google Scholar]
- Neumann CS, Kosson DS, Forth AE, & Hare RD (2006). Factor structure of the Hare Psychopathy Checklist: Youth Version (PCL:YV) in incarcerated adolescents. Psychological Assessment, 18(2), 142–154. [DOI] [PubMed] [Google Scholar]
- Newman JP, & Baskin-Sommers AR (2012). Early selective attention abnormalities in psychopathy: Implications for self-regulation In Posner MI (Ed.), Cognitive Neuroscience of Attention (2 ed., pp 421–439). United States of America: The Guilford Press. [Google Scholar]
- Nys GMS, van Zandvoort MJE, van der Worp HB, Kappelle LJ, & de Haan EHF (2006).Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain, 129, 2148–2157. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, & Ridderinkhof KR (2005). Dissociable components of error processing. Journal of Psychophysiology, 19(4), 319–329. doi: 10.1027/0269-8803.19.4.319 [DOI] [Google Scholar]
- Patrick CJ, Bradley MM, & Lang PJ (1993). Emotion in the criminal psychopath: Startle reflex modulation. Journal of Abnormal Psychology, 102(1), 82. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Pujara MS, Motzkin JC, Newman J, Kiehl KA, & Koenigs M (2015). Altered resting-state functional connectivity in cortical networks in psychopathy. The Journal of Neuroscience, 35(15), 6068–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbit PMA (1981). Sequential reactions In Holding D (Ed.), Human Skills (pp. 153–175). New York: Wiley. [Google Scholar]
- Ravizza SM, & Ciranni MA (2002). Contributions of the prefrontal cortex and basal ganglia to set shifting. Journal of Cognitive Neuroscience, 14(3), 472–483. [DOI] [PubMed] [Google Scholar]
- Rice ME, & Harris GT (1997). Cross-validation and extension of the Violence Risk Appraisal Guide for child molestors and rapists. Law and Human Behavior, 21(2), 231–241. [DOI] [PubMed] [Google Scholar]
- Roussy S, & Toupin J (2000). Behavioral inhibition deficits in juvenile psychopaths. Aggressive Behavior, 26(6), 413–424. [Google Scholar]
- Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, … Taylor E (2001). Mapping motor inhibition: Conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage, 13(2), 250–261. [DOI] [PubMed] [Google Scholar]
- Siegert S, Herrojo Ruiz M, Brucke C, Huebl J, Schneider G-H, Ullsperger M, & Kuhn AA (2014). Error signals in the subthalamic nucleus are related to post-error slowing in patients with Parkinson's Disease. Cortex, 60, 103–120. [DOI] [PubMed] [Google Scholar]
- Steele VR, Anderson NE, Claus ED, Bernat EM, Rao V, Assaf M, … Kiehl KA (2016). Neuroimaging measures of error-processing: Extracting reliable signals from event-related potentials and functional magnetic resonance imaging. NeuroImage, 132, 247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Claus ED, Aharoni E, Harenski C, Calhoun VD, Pearlson GD, & Kiehl KA (2014). A large scale (n = 102) functional neuroimaging study of error processing in a Go/NoGo task. Behavioural Brain Science, 268, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele VR, Maurer JM, Bernat EM, Calhoun VD, & Kiehl KA (2016). Error-related processing in adult males with elevated psychopathic traits. Personality Disorders: Theory, Research, and Treatment, 7(1), 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, & Ridderinkhof KR (2010). Conscious perception of errors and its relation to the anterior insula. Brain Structure & Function, 214(5-6), 629–643. doi: 10.1007/s00429-010-0261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, & Robinson TE (2006). Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - Mediation by enchanced incentive motivation? European Journal of Neuroscience, 24(8), 2345–2354. [DOI] [PubMed] [Google Scholar]
- van Veen V, & Carter CS (2002). The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience, 14(4), 593–602. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan L, Vaidya JG, Humphreys CT, Beglinger LJ, & Paradoso S (2008). Emotional and motivational changes after bilateral lesions of the globus pallidus. Neuropsychology, 22, 412–418. [DOI] [PubMed] [Google Scholar]
- Vincent GM, Cope LM, King J, Nyalakanti P, & Kiehl KA (2017). Callous-unemotional traits modulate brain drug craving response in high-risk young offenders. Journal of Abnormal Child Psychology, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, McReynolds LS, Lucas CP, Fisher P, & Santos L (2002). The Voice DISC-IV with incarcerated male youths: Prevalence of disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 41(3), 314–321. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997). WAIS-III: Wechsler Adult Intelligence Scale (3rd Edition). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2003). Wechsler Intelligence Scale for Children - 4th Edition. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Weissman DG, Schriber RA, Fassbender C, Atherton O, Krafft C, Robins RW, … Guyer AE (2015). Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Developmental Cognitive Neuroscience, 16, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Narr KL, Baker LA, Joshi SH, Jahanshad N, Raine A, & Thompson PM (2015). Frontal and striatal alterations associated with psychopathic traits in adolescence. Psychiatry Research: Neuroimaging, 231(3), 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, & Knowlton BJ (2006). The role of the basal ganglia in habit formation. Nature Reviews Neuroscience, 7, 464–476. [DOI] [PubMed] [Google Scholar]