Abstract
The fungal species Candida albicans is most frequently associated with biofilm formation in immune-and medically-compromised patients, and it is now firmly established that biofilm formation represents a major virulence factor during candidiasis. A growing body of evidence has demonstrated that C. albicans biofilm development is a highly regulated and coordinated process, where adhesive interactions, morphogenetic conversions and consortial behavior, play significant roles. Cells within the biofilms are protected from environmental stresses including host immune defenses and antifungal treatment, which carries important clinical consequences for the treatment of biofilm-associated infections. Dispersal of cells from biofilms represents one of the hallmarks of the biofilm life-style, and in the case of C. albicans dispersed cells are responsible for candidemia and dissemination leading to the establishment of invasive disease.
Keywords: Candida albicans, biofilm, dispersal, virulence
Introduction
Candida albicans remains the main etiological agent of candidiasis, the most common invasive fungal infection and now the third-to-fourth most frequent infection in hospitals worldwide[1,2]. A trait that greatly complicates treatment of these infections in an increasing number of patients is the ability of C. albicans to form an organized consortia of cells called biofilms, on indwelling medical devices [3]. Research on C. albicans biofilms is a little older than two decades; but the last few years have seen an increased interest by multiple groups of investigators that continue to contribute new aspects to our understanding of the biofilm life cycle and its clinical consequences. Here we provide a summary of some of these major contributions, with emphasis on some of the most recent work on this topic.
Candida albicans Biofilm Formation
C. albicans is normally a harmless member of the native human microbiota. However, it can take advantage of immune-and/or medically compromised patients and cause a variety of opportunistic infections, ranging from superficial dermal and mucosal infections to life-threatening systemic candidiasis[1]. It is now fully established that a majority of manifestations of candidiasis are associated with biofilm formation on the surface of biological or artificial surfaces[4,5]. Different biomaterials are able to support C. albicans biofilm formation and distressingly, the increased incidence of candidiasis has virtually paralleled the increasing use of a broad range of medical devices in the clinical practice. Biofilms are defined as highly organized attached microbial communities typically surrounded by a self-produced matrix of exoplymeric materials[6]. Biofilm formation complicates treatment and contributes to high morbidity and mortality rates, and as such represents one of the major virulence factors contributing to the pathogenesis of candidiasis[7,8].
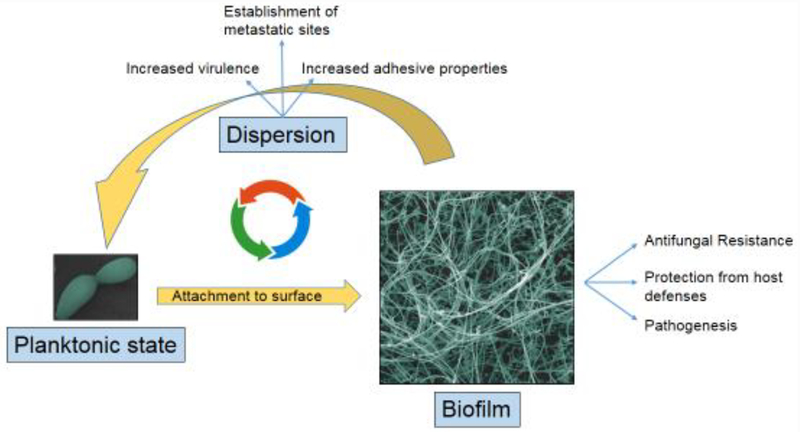
Overall, the C. albicans biofilm developmental process can be divided into four major phases: adherence, proliferation, maturation and dispersal[9,10]. In the early adherence phase, yeast cells attach to a material surface and form a basal layer that will anchor the biofilm to the surface. This is followed by a proliferation phase, which is characterized by the initiation of filamentation leading to the emergence of hyphal and pseudohyphal cells that continue to elongate during the entire biofilm developmental process forming a complicated network that contributes to the overall robustness of the biofilm. In the subsequent maturation phase, the hyphal scaffold become encased in a blanket of self-produced exopolymeric substances (EPS) that essentially act as an adhesive glue that holds the entire biofilm structure together. The C. albicans EPS is composed of carbohydrates, proteins, lipids and eDNA which interact with each other conferring the matrix properties of an amalgam[11,12]. As a part of this developmental process, C. albicans biofilms continuously release yeast cells of a unique elongated morphology that serve to seed new sites of infection. Dispersal stage ensures that the “biofilm life-cycle” can be repeated all over again[13]. Under most experimental conditions the entire process normally takes 24–48 hours, and a mature biofilm is typically several hundred micrometers in thickness. Overall, it is considered that this structural complexity represents the optimal spatial arrangement to facilitate the influx of nutrients, disposal of waste products and the establishment of microniches throughout the biofilm. Figure 1 shows the C. albicans transitions between the planktonic and biofilm states.
Figure 1.
C. albicans transitions between the planktonic and biofilm life-styles and their major implications contributing to pathogenesis.
Most of the information on C. albicans biofilms has come from in vitro models. Although initial models were somewhat cumbersone and required expert handling and the use of specialized equipment[14], the development of a 96-well microtiter plate model biofilm formation represented a significant step towards the simplification and standardization of C. albicans biofilm for mation[15–17]. This technique involves the formation of multiple equivalent fungal biofilms on the bottom of wells of micro titer plates, coupled with some type of colorimetric read-out to allow for the estimation of the extent of biofilm formation or metabolic activity of cells within the biofilms. As such, the method is simple, flexible, relatively inexpensive, accurate and robust; and because of its advantages it has been subsequently adopted by many different groups around the world and democratized fungal biofilm research despite some inherent limitations. Other models involve the formation of C. albicans biofilms under conditions of flow, that more closely mimic physiological conditions within the human body[18]. More recently, a series of articles have described the development of nano-biofilm arrays, in which as many as one thousand equivalent miniaturized biofilms, each of approximately 30 nanoliters in volume can be formed on a single modified microscope slide, allowing for high throughput applications[19,20]. Notably, any and all these in vitro biofilm models reflect architectural features and properties of biofilms formed in vivo, in different animal models and also from those recovered from clinical samples, providing validation to these in vitro models[14].
The entire process of biofilm formation is highly regulated at the molecular level. In the past decade molecular studies have begun to shed light on the signaling processes underlying the biofilm mode of growth in C. albicans. Early studies demonstrated a key role for morphogenetic transitions, adhesive interactions and quorum sensing in the formation of C. albicans biofilms[21,22]. Seminal work by the Mitchell group started to dissect the contributions of individual genes/proteins to biofilm formation and maintenance, leading to the identification of key transcriptional factors and adhesins involved in biofilm formation[9,23,24]. For example, the C. albicans transcription factor mutants Δefg1 and Δtec1 are defective in biofilm formation due to their inability to filament. Similarly another transcription factor Bcr1 was identified as a major regulator of biofilm formation[25] due to its control of several hyphal adhesins including Als3 and Hwp1 whose complementary functions provide cohesiveness to the biofilm structure[25–27]At the same time, different groups of investigators used powerful transcriptomic techniques to examine global patterns of gene expression in C. albicans biofilms as compared to their planktonic counterparts, which mostly highlighted the role of metabolism in biofilm growth[10,28]. Subsequently, a seminal report by Nobile and colleagues revealed that a core network of nine interwoven transcription regulators (Bcr1, Brg1, Efg1, Ndt80, Rob1, Tec1, Flo8, Gal4 and Rfx2) are required for normal biofilm formation[29,30]. Together, these recently evolved regulators control the expression of about one thousand genes, representing approximately 15% of the entire C. albicans genome. Of note, several of these transcription factors also control the yeast-to-hyphae morphogenetic conversion, thereby establishing firmly that filamentation and biofilm growth are intimately linked.
Role of C. albicans Biofilms in Virulence
The NIH estimates that approximately 80% of infections in the United States are associated with a biofilm aetiology, and this also holds true for C. albicans infections[4,5]. As mentioned before, the formation of biofilms by C. albicans carries notable clinical repercussions, contributing to higher mortality rates. Thus, it is now widely accepted that biofilm formation represents one of the main virulence traits associated with the pathogenesis of candidiasis[31]. Biofilms provide a safe haven for fungal cells and can act as reservoirs for persistent sources of infections. From a clinical perspective, the two major consequences of biofilm formation negatively impacting the management of patients with these infections are the increased resistance of cells within the biofilms against antifungal therapy and their protection from host defenses[3,32].
There are three main classes of antifungals used to treat candidiasis: azoles, polyenes and echinocandins[33]. C. albicans cells within a biofilm display high-levels of resistance to azoles and polyenes[33,34], with biofilms formed under flow conditions exhibiting even higher resistance [18]. Bofilms are intrinsically resistance to fluconazole and other azole derivatives. The anti-biofilm activity of polyenes occurs at high concentrations which generally are considered toxic and unsafe; although liposomal formulations show increased activity[35]. In contrast echinocandins, the newest class of antifungal agents targeting the cell wall component beta-1,3 glucans, display excellent activity against C. albicans biofilms at therapeutic concentrations, and used as first line therapy against these infections[33,35]. Multiple mechanisms contribute to the high levels of antifungal drug resistance exhibited by C. albicans biofilms, and for details readers are referred to an excellent review on this topic[36]. Briefly, the biofilm extracellular matrix is a major contributor to resistance, sequestering antifungal molecules and preventing their penetration into the depths of the biofilm. Among other contributors to resistance are the increased cell density (i.e. safety in numbers), overexpression efflux pumps linked to drug resistance, changes in the sterol composition of the cell membrane, and presence of a subpopulation of persister cells that can tolerate high concentrations of antifungals[36].
Although much less is known about mechanisms of protection of C. albicans biofilm from host defenses, the last few years have seen an increasing number of articles devoted to this topic. Distinct components exposed on the surface of the biofilm matrix as compared to those on the fungal cell wall are likely responsible for differences in the interaction with pattern recognition receptors in host immune cells contributing to immune evasion[11,32]. In particular, cells within biofilms are protected from killing by neutrophils, macrophages and monocytes, which normally play important roles in the immune response against disseminated candidiasis[32]. For example, compared to planktonic organisms, C. albicans biofilms inhibit the release of neutrophil extracellular traps (NETs) and impair the generation of reactive oxygen species (ROS) by neutrophils[37,38]. C. albicans biofilm formation also dampens macrophage migration, which is likely independent of the matrix and related to its physical structure[39]. Monocytes fail to phagocytose biofilm-associated C. albicans also leading to an altered cytokine profile, particularly the downregulation of TNF-α, a cytokine which facilitates phagocyte activation and which plays a key role in the protection against candidiasis[40].
Further evidence for the role that C. albicans biofilms play during infection comes from recent reports on the development of anti-virulence approaches for the treatment of candidiasis. Pierce et al. described a large-scale screening assay of chemical libraries in search for new small molecule compounds that inhibited C. albicans biofilm formation[41]. Several hits were identified belonging to a novel series of diazaspiro-decane structural analogs. Further characterization of the leading compound from this series confirmed that it did not affect growth of C. albicans (unlike conventional antifungals) but rather inhibited the ability of the fungus to form biofilms[41]. The fact that treatment with this anti-biofilm compound was effective against both oral and systemic candidiasis using animal models of infection provides corroboration of the role of biofilms in the pathogenesis of C. albicans infections. Similar results have also been reported for other compounds with the ability to inhibit C. albicans filamentation and biofilm formation[42,43].
Dispersal of cells from biofilms
Once established, biofilms initiate or prolong infections by providing a safe sanctuary from which organisms can disperse and seed new infection sites. Recent reports have described that biofilm-dispersed cells arise mostly from the top-most hyphal layers of biofilms, and have a unique phenotype (elongated yeast cells)[13]. The frequency of dispersal is directly dependent on the carbon source and pH of growth media, wherein glucose induces higher frequencies of lateral yeast cells than alternative carbon sources. Importantly, in comparison to age-matched planktonic yeast cells, dispersed lateral yeast cells display enhanced adhesion to and damage of endothelial cells, increased filamentation and formation of denser biofilms, higher fluconazole resistance, and enhanced virulence in a murine model of hematogenously disseminated candidiasis[13]. RNAseq analysis on age-matched biofilm hyphae, dispersed cells, and planktonic yeast cells revealed that despite their yeast morphology, >60% of the differentially regulated genes in the dispersed cells were similar in expression to parent hyphae, making them a unique cell type. Consistent with their virulent phenotype, dis-persed cells upregulate genes involved in adhesion (ALS5, ALS6, ECM33), drug resistance (MDR1, QDR1, ERG genes), nutrient acquisition (ZRT1, ZRT2, ZAP1) and pathogenesis (SAP genes), compared to age-matched planktonic yeast cells[44]. These results show that increased virulence of dispersed yeast cells is likely due to increased expression of genes associated with virulence. Reports by the Uppuluri group has demonstrated that the hyphae-to-yeast transition is paramount for dispersal; dispersed cells are yeast cells released from the lateral septal regions of hyphae present in the biofilm. These “lateral yeast cells” are regulated by PES1, an essential gene conserved in all eukaryotes[13]. Negative genetic regulation of PES1 expression either in an in vitro flow biofilm model or in vivo, in a catheter (implanted in the jugular vein of mice), blocked production of lateral yeast cells from biofilm hyphae and abrogated biofilm-associated disseminated candidiasis[13,44]. Thus, dispersed cells are virulent entities that ensure that a surface-bound drug resistant community of cells can further propagate into distal sites of infection. Approaches to inhibit the process of lateral yeast dispersal from drug resistant biofilms could serve as an alternative strategy to seal the biofilm reservoir and reduce dissemination.
Conclusions
Over the last approximately two decades there has been an increasing appreciation of the role that biofilm formation plays in the biology and pathogenicity of C. albicans, and concomitantly, research on this topic has gained increasing momentum. Pioneering studies on the development of models for C. albicans biofilm formation and the description of their structural characteristics were followed by more in depth mechanistic studies to understand biofilm development and its regulation at the molecular level. Likewise, the increased resistance of C. albicans biofilms against most conventional antifungal drugs has spurred new investigations in search for novel anti-biofilm agents targeting biofilms at various steps of its development. This drug-discovery process has been substantially facilitated by our increasing understanding of molecular mechanisms underpinning biofilm growth and drug resistance. A big hurdle yet to overcome is to identifying how the immune cells can be potentiated to combat biofilms. This may be just the tip of the iceberg as there are many other directions, such as the expansion of these studies to other non-albicans Candida species fully capable of biofilm development, and to the study of much more structurally complex and even more recalcitrant polymicrobial biofilms. It is our hope that this acquired knowledge will soon translate into the development of novel and/or alternative approaches to successfully combat the threat of C. albicans biofilm infections.
HIGHLIGHTS.
Biofilm formation is a major contributor to pathogenesis during Candida infection
C. albicans biofilm formation is a multi-step, highly regulated process
Cells in biofilms are protected from environmental stresses and host defenses
Dispersal is critical for propagation of cells into the bloodstream
Acknowledgements
Work in the PU laboratory is funded by American Heart Association 16SDG30830012. Biofilm-related work in the JLL-R laboratory is supported by grants numbered R01DE023510 and R01AI119554 from the National Institute of Dental and Craniofacial Research and the National Institute of Allergy and Infectious Diseases, respectively to JLLR. Additional support was provided by the Margaret Batts Tobin Foundation, San Antonio, TX. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC: Hidden killers: human fungal infections. Sci Transl Med 2012, 4:165rv113. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ: Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007, 20:133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uppuluri P, Pierce CG, Lopez-Ribot JL: Candida albicans biofilm formation and its clinical consequences. Future Microbiol 2009, 4:1235–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojic EM, Darouiche RO: Candida infections of medical devices. Clin Microbiol Rev 2004, 17:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramage G, Martinez JP, Lopez-Ribot JL: Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 2006, 6:979–986. [DOI] [PubMed] [Google Scholar]
- 6.Donlan RM: Biofilms: microbial life on surfaces. Emerg Infect Dis 2002, 8:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson EM, Hanson MF, Williams C, Munro CA, Jones BJ, Ramage G: Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection-Scotland, 2012–2013. Clin Microbiol Infect 2016, 22:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]; *The authors demonstrate in a prospective study that the biofilm-forming ability of Candida clinical isolates from patients with candidemia correlates with higher mortality rates.
- 8.Tumbarello M, Fiori B, Trecarichi EM, Posteraro P, Losito AR, De Luca A, Sanguinetti M, Fadda G, Cauda R, Posteraro B: Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 2012, 7:e33705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankenship JR, Mitchell AP: How to build a biofilm: a fungal perspective. Curr Opin Microbiol 2006, 9:588–594. [DOI] [PubMed] [Google Scholar]
- 10.Lohse MB, Gulati M, Johnson AD, Nobile CJ: Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol 2018, 16:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]; **A rather comprehensive, insightful, up to date and exquisitely well written review article on C. albicans biofilms.
- 11.Mitchell KF, Zarnowski R, Andes DR: The Extracellular Matrix of Fungal Biofilms. Adv Exp Med Biol 2016, 931:21–35. [DOI] [PubMed] [Google Scholar]; *In this excellent review the authors provide a summary on the composition and function of the extracellular matrix of fungal biofilms, with emphasis on C. albicans.
- 12.Pierce CG, Vila T, Romo JA, Montelongo-Jauregui D, Wall G, Ramasubramanian A, Lopez-Ribot JL: The Candida albicans Biofilm Matrix: Composition, Structure and Function. J Fungi (Basel) 2017, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Kohler JR, Kadosh D, Lopez-Ribot JL: Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 2010, 6:e1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dijck P, Sjollema J, Cammue BP, Lagrou K, Berman J, d’Enfert C, Andes DR, Arendrup MC, Brakhage AA, Calderone R, et al. : Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb Cell 2018, 5:300–326. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This represents a group effort to summarize and bring the field to a consensus in the techniques used for antifungal susceptibility testing of biofilms, both in vitro and in vivo.
- 15.Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr., Mowat E, Ramage G, Lopez-Ribot JL: A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 2008, 3:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierce CG, Uppuluri P, Tummala S, Lopez-Ribot JL: A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J Vis Exp 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL: Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 2001, 45:2475–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uppuluri P, Chaturvedi AK, Lopez-Ribot JL: Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia 2009, 168:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan A, Leung KP, Lopez-Ribot JL, Ramasubramanian AK: High-throughput nano-biofilm microarray for antifungal drug discovery. MBio 2013, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan A, Uppuluri P, Lopez-Ribot J, Ramasubramanian AK: Development of a High-Throughput Candida albicans Biofilm Chip. PLoS One 2011, 6:e19036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL: Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 2002, 68:5459–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL: The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett 2002, 214:95–100. [DOI] [PubMed] [Google Scholar]
- 23.Finkel JS, Mitchell AP: Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 2011, 9:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobile CJ, Mitchell AP: Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol 2006, 8:1382–1391. [DOI] [PubMed] [Google Scholar]
- 25.Nobile CJ, Mitchell AP: Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Curr Biol 2005, 15:1150–1155. [DOI] [PubMed] [Google Scholar]
- 26.Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP: Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog 2006, 2:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP: Complementary adhesin function in C. albicans biofilm formation. Curr Biol 2008, 18:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong PP, Chin VK, Wong WF, Madhavan P, Yong VC, Looi CY: Transcriptomic and Genomic Approaches for Unravelling Candida albicans Biofilm Formation and Drug Resistance-An Update. Genes (Basel) 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox EP, Bui CK, Nett JE, Hartooni N, Mui MC, Andes DR, Nobile CJ, Johnson AD: An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol Microbiol 2015, 96:1226–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD: A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012, 148:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsui C, Kong EF, Jabra-Rizk MA: Pathogenesis of Candida albicans biofilm. Pathog Dis 2016, 74:ftw018. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Insightful summary of the role that C. albicans biofilms play in the pathogeness of the different forms of candidiasis.
- 32.Kernien JF, Snarr BD, Sheppard DC, Nett JE: The Interface between Fungal Biofilms and Innate Immunity. Front Immunol 2017, 8:1968. [DOI] [PMC free article] [PubMed] [Google Scholar]; *The authors review the unique host response to fungal biofilms, focusing on how they are recognized by the immune system.
- 33.Pierce CG, Srinivasan A, Uppuluri P, Ramasubramanian AK, Lopez-Ribot JL: Antifungal therapy with an emphasis on biofilms. Curr Opin Pharmacol 2013, 13:726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn DM, Ghannoum MA: Candida biofilms: antifungal resistance and emerging therapeutic options. Curr Opin Investig Drugs 2004, 5:186–197. [PubMed] [Google Scholar]
- 35.Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA: Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002, 46:1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taff HT, Mitchell KF, Edward JA, Andes DR: Mechanisms of Candida biofilm drug resistance. Future Microbiol 2013, 8:1325–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson CJ, Cabezas-Olcoz J, Kernien JF, Wang SX, Beebe DJ, Huttenlocher A, Ansari H, Nett JE: The Extracellular Matrix of Candida albicans Biofilms Impairs Formation of Neutrophil Extracellular Traps. PLoS Pathog 2016, 12:e1005884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kernien JF, Johnson CJ, Nett JE: Conserved Inhibition of Neutrophil Extracellular Trap Release by Clinical Candida albicans Biofilms. J Fungi (Basel) 2017, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alonso MF, Gow NAR, Erwig LP, Bain JM: Macrophage Migration Is Impaired within Candida albicans Biofilms. J Fungi (Basel) 2017, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katragkou A, Kruhlak MJ, Simitsopoulou M, Chatzimoschou A, Taparkou A, Cotten CJ, Paliogianni F, Diza-Mataftsi E, Tsantali C, Walsh TJ, et al. : Interactions between human phagocytes and Candida albicans biofilms alone and in combination with antifungal agents. J Infect Dis 2010, 201:1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pierce CG, Chaturvedi AK, Lazzell AL, Powell AT, Saville SP, McHardy SF, Lopez-Ribot JL: A Novel Small Molecule Inhibitor of Candida albicans Biofilm Formation, Filamentation and Virulence with Low Potential for the Development of Resistance. NPJ Biofilms Microbiomes 2015, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazly A, Jain C, Dehner AC, Issi L, Lilly EA, Ali A, Cao H, Fidel PL Jr., Rao RP, Kaufman PD: Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc Natl Acad Sci U S A 2013, 110:13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romo JA, Pierce CG, Chaturvedi AK, Lazzell AL, McHardy SF, Saville SP, Lopez-Ribot JL: Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation. MBio 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Perhaps the strongest proof of concept to date that anti-virulence approaches, specifically those targeting filamentation and biofilm formation, can be used for the management of C. albicans infections.
- 44.Uppuluri P, Acosta Zaldivar M, Anderson MZ, Dunn MJ, Berman J, Lopez Ribot JL, Kohler JR: Candida albicans Dispersed Cells Are Developmentally Distinct from Biofilm and Planktonic Cells. MBio 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]