Abstract
Background:
The histopathological assessment of pediatric liver tumors at presentation is critical to establish a diagnosis, guide treatment, and collect appropriate research samples. The purpose of this study was to evaluate complications associated with different approaches to liver biopsy for newly diagnosed hepatoblastoma.
Methods:
Children with hepatoblastoma were enrolled on Children’s Oncology Group study AHEP0731 (September 2009–March 2012). This analysis evaluated the study cohort of initially unresectable patients who therefore underwent a biopsy procedure at diagnosis. The primary endpoint was clinically significant post-biopsy hemorrhage, defined as requiring red blood cell transfusion.
Results:
We identified 121 children who underwent open (n=76, 63%), laparoscopic (n=17, 14%), or percutaneous (n=28, 23%) liver biopsies. All biopsy procedures yielded adequate tissue for diagnosis. Post-biopsy hemorrhage requiring transfusion occurred after 26% (n=31) of biopsies. Need for blood product transfusion most frequently occurred following open (n=27/76, 36%) and laparoscopic (n=4/17, 24%) biopsies, compared with percutaneous (n=0/28, 0%) biopsies (p<0.01).
Conclusions:
Pre-treatment biopsy of pediatric liver tumors via a percutaneous approach yielded the lowest frequency of clinically significant hemorrhage requiring transfusion, without evidence of sacrificing diagnostic accuracy.
Keywords: Hepatoblastoma, Biopsy, Intraoperative Complications, Minimally Invasive Surgical Procedures, Pediatrics, Oncology
INTRODUCTION
The initial histopathological assessment of pediatric liver tumors is critical to ensure accurate diagnosis, determine appropriate risk-based therapy, and allow for timely patient management. In this setting, tissue sample procurement is currently accomplished by open, laparoscopic, or percutaneous approaches. Documented major risks associated with biopsy include hemorrhage, bile leak, pneumothorax, arteriovenous fistula, and bowel perforation [1]. Complication rates between the different approaches to liver tumor biopsy in pediatric patients have not been directly evaluated or compared in a prospective manner.
In addition to establishing a diagnosis, adequate tissue sampling allows for the evaluation of molecular markers, which support the pathologic diagnosis as well as may provide prognostic information and potential therapeutic targets. Furthermore, obtaining and banking specimens for current and future research studies is essential, particularly in rare pediatric tumors. However, there may be an inherent conflict between the most diagnostic, highest-quality biopsy technique and the goal of minimizing patient morbidity for such a procedure.
Children diagnosed with hepatoblastoma who were enrolled on Children’s Oncology Group (COG) protocol AHEP0731 had data collected prospectively on the type of biopsy performed and associated complications. The purpose of this analysis was to evaluate factors associated with complications after liver biopsy, specifically post-biopsy hemorrhage. We hypothesized that the proportion of post-biopsy hemorrhage would be lower among children undergoing percutaneous liver biopsies, compared with open or laparoscopic approaches.
METHODS
COG study AHEP 0731 included newly diagnosed patients with all hepatoblastoma tumor stages. Children under age 21 years with histologically confirmed Evans stage 3 and 4 hepatoblastoma who enrolled between September, 2009 and March, 2012 were included in this analysis [2]. The study was originally designed to assess the impact of a risk-based treatment approach on event-free survival and chemotherapy-related complications [2]. Surgical guidelines were provided to assist in the initial surgical approach, based on PRETreatment EXTent of disease (PRETEXT) group (defined by subtracting the highest number of contiguous disease-free liver segments from 4) [3]. The ultimate surgical decisions were determined by the local treatment team. Central review of imaging studies was performed for all patients and access to one of six members of the study surgical team was provided, if desired to assist in operative planning. At the time of analysis, the study had been open for enrollment at 185 COG institutions. Patients in this present analysis underwent biopsies for diagnosis at a total of 74 centers. Approach to biopsy was determined by the local treating team. All image-guided biopsies were core needle biopsies. Patients were excluded from this analysis if they underwent diagnostic biopsies of only metastatic lesions or if they underwent primary hepatectomy without a preceding biopsy.
Demographic information, Evans tumor stage, biopsy characteristics, tumor relapse, biopsy procedural reports, and pathology (institutional and central pathologic review) were prospectively collected. PRETEXT group was reported by local institutions and by central review by study radiologists and surgeons. Procedural details and periprocedural outcomes were independently evaluated by review of detailed operative records (C.B.W.). The primary endpoint was clinically significant post-biopsy hemorrhage, defined as requiring transfusion of red blood cells. The occurrence of clinically significant post-biopsy hemorrhage was presented as prevalence of patients with hemorrhage requiring red blood cell transfusion within each biopsy approach group. The criteria for requiring such a transfusion was determined by the local treating group. Additionally, a secondary endpoint of any hemorrhage was reported, which was also determined by the local treating group. Event-free survival (EFS) over the course of this trial was evaluated to assess if the type of biopsy procedure performed was associated with EFS. EFS was defined as the time from study enrollment to disease progression, death, diagnosis of a second malignancy or last follow-up, whichever came first. Patients who experienced disease progression, death or diagnosis with a second malignancy were considered to have experienced an EFS event; otherwise, the patient was censored at last follow-up.
Categorical variables were compared between the different approaches to biopsy using Pearson’s chi-square test (or exact test, when appropriate) [4]. Continuous variables were compared between the different approaches to biopsy using ANOVA (or Kruskal–Wallis test [5], when appropriate). The cumulative incidence of disease relapse was calculated according to the method of Gray [6]; death or second malignancy before relapse were considered competing events. A two-tailed p value <0.05 was considered statistically significant. Data were analyzed using SAS version 9.3 (SAS Institute, Inc., Cary, NC) or R version 3.4.1 (www.R-project.org).
RESULTS
Data current to December 31, 2017 were used in this analysis. A total of 137 children with intermediate-risk Evans stage 3 (n=105) and high-risk Evans stage 4 (n=32) hepatoblastoma were considered for enrollment on the study. Two patients were ineligible for enrollment due to failure to meet organ function criteria. Two patients with clinical hepatoblastoma were deemed clinically unsafe for biopsy and therefore were not biopsied as recommended per protocol. Two patients had the diagnosis made by biopsy of metastatic lung lesions. Nine patients had primary tumor resection and were intermediate risk due to institutional pathologic diagnosis of small cell undifferentiated histology or tumor-involved lymph nodes. One patient had metastatic tumor resection before enrollment. These 16 patients were excluded and, as a result, a total of 121 participants underwent hepatic biopsy for diagnosis and form the basis for this analysis. The median age at enrollment was 18 months (range: 1 month–16 years). PRETEXT group central review was: I=0 (0%), II=36 (30%), III=60 (50%), and IV=25 (21%). PRETEXT annotation factor venous involvement using the AHEP0731 central review definitions was: V(neg)=8 (7%), V0=9 (7%), V1=18 (15%), V2=83 (69%), and V3=3 (2%). Portal involvement was: P(neg)=7 (6%), P0=10 (8%), P1=18 (15%), P2=77 (64%), and P3=9 (7%). Details of patient characteristics are reported in Table 1.
Table 1.
Overall Characteristics of Children Who Underwent Liver Tumor Biopsy
| Variable | Number (%) or median (range) |
|---|---|
| Overall | 121 (100%) |
| Age at diagnosis, months | 18 (1-190) |
| <3 years | 101 (83%) |
| 3-8 years | 15 (12%) |
| >8 years | 5 (4%) |
| PRETEXTa | |
| II | 36 (30%) |
| III | 60 (50%) |
| IV | 25 (21%) |
| Venous involvement | |
| V(neg) | 8 (7%) |
| V0 | 9 (7%) |
| V1 | 18 (15%) |
| V2 | 83 (69%) |
| V3 | 3 (2%) |
| Portal involvement | |
| P(neg) | 7 (6%) |
| P0 | 10 (8%) |
| P1 | 18 (15%) |
| P2 | 77 (64%) |
| P3 | 9 (7%) |
| Biopsy | |
| Percutaneous | 28 (23%) |
| Laparoscopic | 17 (14%) |
| Open | 76 (63%) |
| Post biopsy complication | |
| Bleeding | 47 (39%) |
| Bleeding requiring transfusion | 31 (26%) |
| Days from biopsy to treatment | 4 (1 - 19) |
Determined by central review
Hepatic biopsies were performed via open (n=76, 63%), laparoscopic (n=17, 14%), or percutaneous (n=28, 23%) approach (Table 2). No patient had more than one biopsy approach or underwent a repeat biopsy procedure. The median age at enrollment was not significantly different for children undergoing percutaneous (23 months), compared to open (16 months) or laparoscopic (17 months) biopsies (p=0.08). There was no difference in approach to biopsy based on PRETEXT group (p=0.52). There was no difference in (median: range) time to initiating treatment after biopsy between the percutaneous (4: 2-15 days), laparoscopic (4: 2-19 days), and open (5: 1-15 days) groups (p=0.59). The number of biopsy procedures performed at each center ranged from 1 to 6 (median: 1). All biopsy procedures allowed for a pathologic diagnosis of hepatoblastoma but an evaluation of the quality of the tumor tissue was beyond the scope of this protocol and paper.
Table 2.
Factors Associated with Approach to Liver Tumor Biopsy
| Variable | Laparoscopic | Open | Percutaneous | P- value |
|---|---|---|---|---|
| Overall | 17 (14%) | 76 (63%) | 28 (23%) | |
| Age at diagnosis, months | 0.21 | |||
| <3 years | 13 (76%) | 66 (87%) | 22 (79%) | |
| 3-8 years | 2 (12%) | 9 (12%) | 4 (14%) | |
| >8 years | 2 (12%) | 1 (1%) | 2 (7%) | |
| 0.52 | ||||
| PRETEXTa | ||||
| II | 6 (35%) | 24 (32%) | 6 (21%) | |
| III | 8 (47%) | 39 (51%) | 13 (46%) | |
| IV | 3 (18%) | 13 (17%) | 9 (32%) | |
| 0.77 | ||||
| Venous involvement | ||||
| V(neg) | 0 (0%) | 6 (8%) | 2 (7%) | |
| V0 | 2 (12%) | 4 (5%) | 3 (11%) | |
| V1 | 3 (18%) | 12 (16%) | 3 (11%) | |
| V2 | 11 (65%) | 52 (68%) | 20 (71%) | |
| V3 | 1 (6%) | 2 (3%) | 0 (0%) | |
| 0.64 | ||||
| Portal involvement | ||||
| P(neg) | 1 (6%) | 4 (5%) | 2 (7%) | |
| P0 | 2 (12%) | 4 (5%) | 4 (14%) | |
| P1 | 2 (12%) | 12 (16%) | 4 (14%) | |
| P2 | 10 (59%) | 52 (68%) | 15 (54%) | |
| P3 | 2 (12%) | 4 (5%) | 3 (11%) | |
| Days from biopsy to treatment | 4 (2 - 19) | 5 (1 - 15) | 4 (2 - 15) | 0.59 |
| Post-biopsy complication | ||||
| Bleeding | 7 (41%) | 38 (50%) | 2 (7%) | <0.01 |
| Bleeding requiring transfusion | 4 (24%) | 27 (36%) | 0 (0%) | <0.01 |
Number (%) or median (range)
Determined by central review
PRETEXT, pretreatment extent of disease
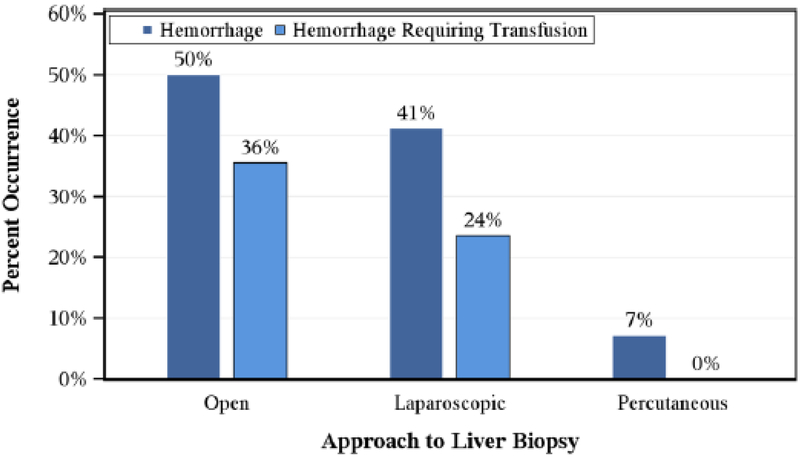
The primary outcome of post-biopsy hemorrhage requiring transfusion occurred after 31 (26%) biopsies (Figure 1). Blood product transfusion occurred significantly more frequently following open (n=27/76, 36%) and laparoscopic (n=4/17, 24%) biopsies, compared with percutaneous (n=0/28, 0%) biopsies (p<0.01). There was no difference in age (p=0.81) or PRETEXT group (p=0.22) between patients who did or did not require post-biopsy transfusion (Table 3). The time interval (median: range) before beginning treatment was 5 (1-12) days for patients who developed post-biopsy hemorrhage requiring transfusion and 4 (1-19) days for those who did not develop post-biopsy hemorrhage requiring transfusion. No patient required reoperation for complications related to biopsy. We next analyzed the frequency of tumor relapse in relation to the type of pre-treatment biopsy undertaken. In the overall cohort, the 6-year cumulative incidence (standard error) of tumor relapse was 18% (4%). Regarding the three different approaches to biopsy, the 6-year cumulative incidence (standard error) was 19% (10%) in the laparoscopy group, 19% (5%) in the open group, and 14% (7%) in the percutaneous group. The sites of disease relapse were solitary lung (n=11); solitary liver (n=2); lung and liver (n=4); lung, liver, and lymph nodes (n=1); bone and lung (n=1); lung and brain (n=1); and lymph nodes (n=1).
Figure 1.
Percent occurrence of hemorrhage (p<0.01) and hemorrhage requiring transfusion (p<0.01) by surgical approach to biopsy.
Table 3.
Factors Associated with Bleeding Requiring Transfusion after Liver Biopsy
| Variable |
No transfusion |
Transfusion | P- value |
|---|---|---|---|
| Overall | 90 (74%) | 31 (26%) | |
| Age at enrollment | 0.81 | ||
| <3 years | 76 (84%) | 25 (81%) | |
| 3-8 years | 10 (11%) | 5 (16%) | |
| >8 years | 4 (4%) | 1 (3%) | |
| 0.22 | |||
| PRETEXTa | |||
| II | 25 (28%) | 11 (35%) | |
| III | 43 (48%) | 17 (55%) | |
| IV | 22 (24%) | 3 (10%) | |
| IV | 22 (24%) | 3 (10%) | |
| 0.96 | |||
| Venous involvement | |||
| V(neg) | 6 (7%) | 2 (6%) | |
| V0 | 6 (7%) | 3 (10%) | |
| V1 | 14 (16%) | 4 (13%) | |
| V2 | 62 (69%) | 21 (68%) | |
| V3 | 2 (2%) | 1 (3%) | |
| 0.77 | |||
| Portal involvement | |||
| P(neg) | 6 (7%) | 1 (3%) | |
| P0 | 7 (8%) | 3 (10%) | |
| P1 | 12 (13%) | 6 (19%) | |
| P2 | 57 (63%) | 20 (65%) | |
| P3 | 8 (9%) | 1 (3%) | |
| Biopsy type | |||
| <0.01 | |||
| Laparoscopic | 13 (14%) | 4 (13%) | |
| Open | 49 (54%) | 27 (87%) | |
| Percutaneous | 28 (31%) | 0 (0%) | |
| Days from biopsy to treatment | 4 (1 - 19) | 5 (1 - 12) | 0.16 |
Number (%) or median (range)
Determined by central review
PRETEXT, pretreatment extent of disease
DISCUSSION
Among 121 children who underwent pre-treatment diagnostic biopsy of hepatoblastoma, 26% developed a bleeding complication. Proportion of clinically significant hemorrhage varied substantially according to biopsy approach, ranging from 0-36%. Percutaneous biopsy was associated with the lowest frequency of clinically significant hemorrhage requiring red blood cell transfusion, compared with open or laparoscopic biopsies. All biopsy approaches always yielded a pathologic diagnosis. Characterization of the amount and quality of tissue samples was beyond the scope of this analysis, but is an important consideration and could be evaluated in future trials. It should be noted that there was not evidence of an increased risk of local tumor relapse in this study cohort, regardless of biopsy type.
Histopathological diagnosis determines therapy, risk stratification, and prognosis [7,8]. As such, the optimal approach to obtain tumor tissue must be determined to ensure diagnostic accuracy, provide for the procurement of diagnostic materials, and minimize patient toxicity [9]. When utilized, percutaneous biopsy should be performed under imaging guidance [10]. In the SIOPEL-1 study, conducted by the Société Internationale d'Oncologie Pédiatrique (SIOP) – Epithelial Liver Study Group, children with hepatoblastoma underwent liver biopsy by percutaneous (n=63) or open (n=30) approaches.[11] Of those, 5% and 3% developed bleeding, respectively [11]. Schnater reported the Dutch experience and retrospectively described biopsy complications in 1 of 32 (3%) pediatric patients with liver tumors [12]. Despite the high-risk population and clinical importance of liver biopsy among children with hepatic tumors, few direct comparisons of approach for liver biopsy exist in the literature.
The proportion of patients who developed hemorrhage observed in this series is somewhat higher than that reported in the literature, and that is likely in part due to the strength of the analysis for this study which had a designated case report form which required prospective collection on whether or not patients had bleeding and if they required a transfusion. This analysis is the first to prospectively collect data directly comparing biopsy techniques among children enrolled in a study evaluating the treatment of advanced-stage hepatoblastoma. This contemporary, multi-center COG study includes a relatively large cohort of 121 patients, over a relatively short three-year study period largely reducing variability of techniques as a contributing variable. As such, complications may be less prone to temporal trends, compared with other single-institution case series.
In addition to temporal trends and study design factors, the frequency of complications depends on the underlying patient population, as biopsy in the setting of oncologic diagnosis may be a risk factor for post-procedure hemorrhage [13,14]. Westheim and colleagues reported a 1.1% major (requiring re-intervention) bleeding frequency among 190 children who underwent 275 percutaneous liver biopsies at a single institution between 2000 and 2011 [14]. Risk factors for post-procedure hemorrhage were biopsy of a focal lesion and treatment with low-molecular weight heparin [14].
One limitation of our series is that the prospectively collected information was limited to reoperation, clinical evidence of hemorrhage, and the need for blood product transfusion. However, other complications may occur following liver biopsy as well. Transient abdominal pain or pain referred to the right shoulder may occur following as many as 20% of liver biopsies [15]. Persistent and progressive pain warrants immediate evaluation for hemorrhage. In the adult literature, complications from liver biopsy generally have been found to occur early, with 60% and 96% of complications presenting within 2 and 24 hours post-procedure, respectively [13]. Similarly, among one study by Gonzalez-Vallina and colleagues of pediatric patients who underwent 184 outpatient percutaneous liver biopsies, the authors report that bleeding complications occurred among 1% of children and presented within 4 hours of biopsy [16]. Apart from hemorrhage, other complications of liver biopsy may include infection, pneumothorax, and injury to visceral organs [17]. Several potentially important covariates were not available, including laboratory tests of coagulation, hematocrit, and platelet count; needle gauge; number of biopsy passes, and type of biopsy (e.g., wedge vs. core). As such, post-procedure change in hemoglobin was unavailable for the cohort. Instead, the local treating physician’s judgment of need for transfusion was employed as a surrogate end-point for clinically significant hemorrhage. There may have been misclassification of the outcomes of hemorrhage or hemorrhage requiring transfusion, which we expect would be distributed non-differentially between approaches to biopsy.
Another limitation in our study is that the ability to generalize the results of this cohort to other populations of children and disease states may be limited, given that all included participants were children with advanced stage hepatoblastoma who were enrolled on a COG protocol. However, in addition to providing much needed information about this subset of children with a rare tumor, the homogeneity of the cohort confers improved internal validity to this study. Outcomes were limited to the early post-operative period. However, based on the aforementioned literature, late post-operative complications in this population are rare [13,16]. Finally, we were unable to evaluate the effect of experience (e.g., by case volume) of participating hospitals and surgeons. Consideration of all of these factors will be helpful in designing future surgical study aims and associated data collection forms.
In this analysis, we report a lower frequency of post-procedure hemorrhage with percutaneous liver biopsy, compared with open or laparoscopic biopsy. However, the approach to biopsy must ultimately depend on individual patient characteristics and the treating center’s capabilities and experience, as there are advantages and disadvantages to each technique. In addition to less frequent post-procedure hemorrhagic complications after percutaneous biopsy as noted in our study, the literature reports improved recovery time, with some centers reporting early data on success with outpatient percutaneous biopsy [18]. Similarly, when feasible, biopsy procedure and port placement may be able to be combined in a single anesthetic setting. An image-guided percutaneous approach may also allow for sampling of several different tumors or different areas of the same tumor, in addition to tumors deeper in the parenchyma that may not able to be defined by simply inspecting the liver surface. Obtaining a percutaneous biopsy with a zone of overlying normal hepatic parenchyma may limit bleeding and help to prevent potential peritoneal tumor seeding. While this practice must be replicated before widespread adoption, percutaneous approach with a reduced period of post-procedural observation would be a potentially promising solution for diagnostic liver biopsy as the patient might not need inpatient hospitalization during the initial workup and determination of final pathology. Finally, the percutaneous approach is favored and recommended by the Childhood Liver Tumour Strategy Group (SIOPEL) for the additional reason that this approach may minimize the development of adhesions between tumor and the abdominal wall [19].
Despite a higher proportion of patients who developed clinically significant hemorrhage, there may be benefits to laparoscopic or open surgical liver biopsy in certain settings. First, non-percutaneous surgical biopsy permits visual inspection of the liver parenchyma as well as of the peritoneal cavity for evaluation of extra-hepatic disease [15]. Second, with percutaneous biopsy, unlike open biopsy, immediate discovery and intervention for intra-procedural bleeding is not as readily possible [13,20]. Furthermore, especially among certain malignancies (e.g. hepatocellular carcinoma) there is a theoretical risk of seeding the biopsy tract that must be considered with percutaneous biopsy. However for hepatoblastoma, tract seeding resulting in local tumor relapse has been found to be exceedingly infrequent, especially under image-guidance and biopsy core needle passage through a buffer zone of overlying hepatic parenchyma [19,21]. Furthermore, this risk may be mitigated by embolization of the core needle tract which may both prevent tumor seeding outside of the liver and hemorrhagic complications [22]. Finally, simultaneous sampling of ascitic fluid is an added benefit of laparoscopic or open biopsy, although this could also potentially be performed under image guidance if a reasonable volume of ascitic fluid were present [13].
In conclusion, pre-treatment percutaneous biopsy of pediatric liver tumors yielded the lowest proportion of clinically significant hemorrhage requiring blood product transfusion, without sacrificing diagnostic accuracy. When possible, diagnostic percutaneous liver biopsy should be considered among children with hepatoblastoma. Details of best practices for performing a percutaneous core needle biopsy in pediatric liver tumors have recently been published [22]. Further studies of biopsy procedures and associated complications for liver tumors can help further define the optimal approach to obtaining diagnostic pathologic material as well as material for biological studies, a primary necessity in the current world of targeted therapies.
Acknowledgments
Funding support: Supported by the Chair’s Grant No. U10 CA98543, the National Clinical Trials Network (NCTN) Operations Center Grant No. U10 CA180886, the NCTN Statistics and Data Center Grant No. U10 CA180899, the Statistics and Data Center Grant No. U10 CA98413 of the Children’s Oncology Group, the Imaging and Radiation Oncology Core (IROC-RI, formerly QARC) Grant No. U10 CA29511 from the National Cancer Institute, the St. Baldrick’s Foundation, and Children’s Oncology Group AHEP 0731. A complete listing of grant support for research conducted by Children’s Cancer Group and Pediatric Oncology Group before initiation of the Children’s Oncology Group grant in 2003 is available online at: https://www.childrensoncologygroup.org/index.php/research-funding.
Footnotes
Presented at: The International Society of Pediatric Surgical Oncology Annual Meeting, October 2016, Dublin, Ireland
Conflict of interest disclosures: the authors make no disclosures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Kader HA, Bellah R, Maller ES, Mamula P, Piccoli DA, Markowitz JE. The utility of ultrasound site selection for pediatric percutaneous liver biopsy. J Pediatr Gastroenterol Nutr 2003;36:364–7. [DOI] [PubMed] [Google Scholar]
- [2].National Cancer Institute; Children’s Oncology Group. Risk-Based Therapy in Treating Younger Patients With Newly Diagnosed Liver Cancer. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US) 2000- [cited 2012 July 2]. n.d. [Google Scholar]
- [3].Meyers RL, Tiao G, de Ville de Goyet J, Superina R, Aronson DC. Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 2014;26:29–36. doi: 10.1097/MOP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- [4].Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3rd ed. Hoboken, N.J: J. Wiley; 2003. [Google Scholar]
- [5].Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance Analysis. J Am Stat Assoc 1952;47:583. doi: 10.2307/2280779. [DOI] [Google Scholar]
- [6].Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988;16:1141–54. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- [7].Haas JE, Feusner JH, Finegold MJ. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer 2001;92:3130–4. [DOI] [PubMed] [Google Scholar]
- [8].López-Terrada D, Alaggio R, de Davila MT, Czauderna P, Hiyama E, Katzenstein H, et al. Towards an international pediatric liver tumor consensus classification: proceedings of the Los Angeles COG liver tumors symposium. Mod Pathol Off J U S Can Acad Pathol Inc 2014;27:472–91. doi: 10.1038/modpathol.2013.80. [DOI] [PubMed] [Google Scholar]
- [9].Tapper EB, Lok AS-F. Use of Liver Imaging and Biopsy in Clinical Practice. N Engl J Med 2017;377:756–68. doi: 10.1056/NEJMra1610570. [DOI] [PubMed] [Google Scholar]
- [10].Nobili V, Comparcola D, Sartorelli MR, Natali G, Monti L, Falappa P, et al. Blind and ultrasound-guided percutaneous liver biopsy in children. Pediatr Radiol 2003;33:772–5. doi: 10.1007/s00247-003-1044-0. [DOI] [PubMed] [Google Scholar]
- [11].Schnater JM, Aronson DC, Plaschkes J, Perilongo G, Brown J, Otte J-B, et al. Surgical view of the treatment of patients with hepatoblastoma: results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group. Cancer 2002;94:1111–20. [PubMed] [Google Scholar]
- [12].Schnater JM, Kuijper CF, Zsiros J, Heij HA, Aronson DC. Pre-operative diagnostic biopsy and surgery in paediatric liver tumours--the Amsterdam experience. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2005;31:1160–5. doi: 10.1016/j.ejso.2005.07.012. [DOI] [PubMed] [Google Scholar]
- [13].Dezsőfi A, Baumann U, Dhawan A, Durmaz O, Fischler B, Hadzic N, et al. Liver biopsy in children: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2015;60:408–20. doi: 10.1097/MPG.0000000000000632. [DOI] [PubMed] [Google Scholar]
- [14].Westheim BH, Østensen AB, Aagenæs I, Sanengen T, Almaas R. Evaluation of risk factors for bleeding after liver biopsy in children. J Pediatr Gastroenterol Nutr 2012;55:82–7. doi: 10.1097/MPG.0b013e318249c12a. [DOI] [PubMed] [Google Scholar]
- [15].Ovchinsky N, Moreira RK, Lefkowitch JH, Lavine JE. Liver biopsy in modern clinical practice: a pediatric point-of-view. Adv Anat Pathol 2012;19:250–62. doi: 10.1097/PAP.0b013e31825c6a20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gonzalez-Vallina R, Alonso EM, Rand E, Black DD, Whitington PF. Outpatient percutaneous liver biopsy in children. J Pediatr Gastroenterol Nutr 1993; 17:370–5. [DOI] [PubMed] [Google Scholar]
- [17].Dezsőfi A, Knisely AS. Liver biopsy in children 2014: who, whom, what, when, where, why? Clin Res Hepatol Gastroenterol 2014;38:395–8. doi: 10.1016/j.clinre.2014.05.002. [DOI] [PubMed] [Google Scholar]
- [18].El-Shabrawi MH, El-Karaksy HM, Okahsa SH, Kamal NM, El-Batran G, Badr KA. Outpatient blind percutaneous liver biopsy in infants and children: is it safe? Saudi J Gastroenterol Off J Saudi Gastroenterol Assoc 2012;18:26–33. doi: 10.4103/1319-3767.91735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Czauderna P, Otte JB, Aronson DC, Gauthier F, Mackinlay G, Roebuck D, et al. Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer Oxf Engl 1990 2005;41:1031–6. doi: 10.1016/j.ejca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- [20].Esposito C, Garipoli V, Vecchione R, Raia V, Vajro P. Laparoscopy-guided biopsy in diagnosis of liver disorders in children. Liver 1997;17:288–92. [DOI] [PubMed] [Google Scholar]
- [21].Davies JQ, de la Hall PM, Kaschula ROC, Sinclair-Smith CC, Hartley P, Rode H, et al. Hepatoblastoma--evolution of management and outcome and significance of histology of the resected tumor. A 31-year experience with 40 cases. J Pediatr Surg 2004;39:1321–7. [DOI] [PubMed] [Google Scholar]
- [22].Hawkins CM, Towbin AJ, Roebuck DJ, Monroe EJ, Gill AE, Thakor AS, et al. Role of interventional radiology in managing pediatric liver tumors : Part 2: percutaneous interventions. Pediatr Radiol 2018;48:565–80. doi: 10.1007/s00247-018-4072-5. [DOI] [PubMed] [Google Scholar]