Abstract
Children with epilepsy and normal structural MRI pose a particular challenge in localization of epileptic foci for surgical resection. Many of these patients have subtle structural lesions such as mild cortical dysplasia that can be missed by conventional MRI but may become detectable by optimized and advanced MRI acquisitions and post-processing. Specificity of objective analytic techniques such as voxel-based morphometry remains an issue. Combination of MRI with functional imaging approaches can improve the accuracy of detecting epileptogenic brain regions. Analysis of glucose positron emission tomography (PET) combined with high-resolution MRI can optimize detection of hypometabolic cortex associated with subtle cortical malformations and can also enhance presurgical evaluation in children with epileptic spasms. Additional PET tracers may detect subtle epileptogenic lesions and cortex with enhanced specificity in carefully selected subgroups with various etiologies; i.e., increased tryptophan uptake can identify epileptogenic cortical dysplasia in the interictal state. Subtraction ictal SPECT can be also useful to delineate ictal foci in those with non-localizing PET or after failed surgical resection. Presurgical delineation of language and motor cortex and the corresponding white matter tracts is increasingly reliable by functional MRI and DTI techniques; with careful preparation, these can be useful even in young and sedated children. While evidence-based pediatric guidelines are still lacking, the data accumulated in the last decade strongly indicate that multimodal imaging with combined analysis of MRI, PET, and/or ictal SPECT data can optimize the detection of subtle epileptogenic lesions and facilitate seizure-free outcome while minimizing the postsurgical functional deficit in children with normal conventional MRI.
Keywords: pediatric epilepsy, epilepsy surgery, cortical dysplasia, magnetic resonance imaging, positron emission tomography, single photon emission computed tomography
Diagnostic brain imaging has been widely utilized and plays a pivotal role in the presurgical evaluation of children with epilepsy. Despite a daunting amount of data accumulated in the past three decades, evidence-based guidelines for the use of various imaging modalities in the clinical management of pediatric epilepsy are missing. A report of ILAE Task Forces and Commissions in 2014 on diagnostic tests in the evaluation for resective surgery in epileptic children cited several shortcomings of the available data, including the lack of class 1 and 2 evidence establishing the relative utility of each test in specific clinicopathologic cohorts [1]. Many of the published studies included both pediatric and adult subjects and utilized highly variable, center-specific inclusion and exclusion criteria, image acquisition, and post-processing and data analysis protocols. Many single-center studies have reported on a limited number of cases and were underpowered for multivariate analyses. One major issue in collecting large, prospective, multicentric data sets over a longer period of time is the rapid development of imaging protocols and sequences, and steady advances in processing and quantification approaches. The following sections focus on major findings and advances, as well as updates and remaining issues in neuroimaging in children with non-lesional epilepsy, who often present a challenge for detecting the epileptogenic brain regions during presurgical evaluation. Due to the lack of a well-defined lesion, these children often end up with large, multi-lobar or hemispheric resections that pose major risks for postoperative deficits. Optimization of presurgical diagnostic imaging, often with a combined, multimodal approach, has the potential of limiting the extent of resection and, thus, minimizing postoperative physical and/or neuropsychological deficits while not compromising seizure outcome [2].
Structural MR imaging in detection of subtle epileptogenic lesions
A focal cortical lesion, detected by MRI in children with drug-resistant epilepsy, is one of the most reliable biomarkers of the general location of the epileptogenic region; however, the exact location and extent of the seizure onset zone may be quite different (often more extensive) than the MRI-detected lesion. In order to maximize the chance to detect such lesions, children with epilepsy undergoing presurgical evaluation should have a high-quality, high-resolution MRI, preferably on a 3T scanner [1,3]. The 2014 report of the Task Force for Paediatric Epilepsy Surgery and the Diagnostic Commission of the ILAE [1] notes the lack of a general agreement on the specifics of MRI sequences for presurgical evaluation, and the report made recommendations for imaging children while emphasizing some key differences of the protocols between older (age >2 years) and younger subjects (see details in Table 1). To be classified as MRI negative, the report recommended a 2-step review process: all MRI studies should be reported blind as normal, and then should undergo a post hoc review by a skilled, experienced neuroradiologist after other functional localization data are available, in an effort to identify occult focal cortical dysplasia (FCD) [1,4]. The report also expressed concern that focusing attention on only a specific suspect location might lead to elevated sensitivity (detection of “lesion”) at the cost of diminished specificity. To minimize this risk, they recommended that attempts should be made to exclude any other lesion elsewhere in the brain at similar sensitivity thresholds [1].
Table 1.
MRI sequences recommended for children undergoing presurgical evaluation for drug-resistant epilepsy (adopted from Jayakar et al., 2014 [1])
| Age >2 years | Age <2 years |
|---|---|
| 3D T1 GRE, 1-1.5 mm | 3D T1 GRE, 1-1.5 mm# |
| T2 axial + coronal, 3-4 mm slices* | T2 axial/coronal or 3D, ≤2 mm slices |
| T2 oblique coronal, high-resolution§ | T2 oblique coronal, high-resolution§ |
| FLAIR axial (coronal optional) | FLAIR axial |
: 2 mm suggested for subtle FCD;
: less useful for children <1 year of age
: fast or turbo spin echo weighted, for imaging the hippocampus
Abbreviations: GRE: gradient-recalled-echo; FLAIR: fluid-attenuated inversion recovery
Despite the above efforts to optimize the yield of MRI, subtle FCD, particularly FCD type I, can remain undetected in a substantial number of cases by conventional imaging. In such cases, additional MRI sequences such as magnetization transfer imaging, and various postprocessing methods, including curvilinear reformatting and morphometric analysis of T1-weighted and T2-FLAIR images may be able to detect subtle structural abnormalities [5–12]. However, centers use different acquisition and post-processing protocols that can yield highly variable findings that are difficult to compare. To address this issue, in a recent study, investigators compared four, commonly used MRI analytic approaches on the same image data set to detect subtle lesions by vox el-based morphometry (VBM) applied on T1 (gray matter volume, gray matter concentration, junction maps) and T2-FLAIR images (normalized FLAIR) in both MRI-positive (n=15, consistent with FCD) and MRI-negative (n=129) epilepsy cases, as compared to images of 50 healthy controls [11]. For detecting FCD, only normalized FLAIR showed sufficient specificity (90%, combined with 55% sensitivity), while the T1-based maps had poor specificity with high rates of positive findings in controls. The accuracy of the maps was lower in the MRI-negative group. Concordant findings of normalized FLAIR with the resection area were associated with a favorable postsurgical outcome with an odds ratio of 7.3. The authors note that their T2-FLAIR images were suboptimal with an anisotropic 2D dataset with 5 mm slice thickness, and the applied algorithms are not widely available. The use of high-resolution isotropic T2-FLAIR sequences along with the application of novel features such as metrics of surface morphology [13] can improve the accuracy of objective detection of FCD.
Regional cortical thickness abnormalities have been also reported in non-lesional epilepsy and linked to specific epileptogenic pathology as well as surgical outcome. For example, in frontal lobe epilepsy associated with cortical dysplasia, patients with type II FCD showed cortical thickening, while type I was associated with thinning [14]. By using a machine learning approach of regional cortical thickness data, the authors were able to lateralize the epileptic focus (with 92% accuracy in FCD type I and 86% accuracy in FCD type II) and predict surgical outcome (accuracy: 92% in type I and 82% in type II) in the majority of individual subjects. Consistent with these findings, our group reported that neocortical thinning in the epileptic hemisphere, most robust in the frontal lobe, predicted one-year postsurgical seizure freedom with 93% sensitivity and 73% specificity in a pediatric population; these numbers were even better in a subgroup of children above 6 years of age (100%/92%, respectively) [15].
Altogether, quantitative post-processing of high-resolution MR images has the ability to extract subtle morphological features that can be linked to specific epileptogenic pathology and show promise in outcome prediction in individual patients. Still, the variable specificity of various analytic approaches highlights the limitation of the use of stand-alone MR images to detect epileptogenic regions reliably. One way to overcome this limitation is the combination of MRI with functional imaging, such as positron emission tomography (PET) or ictal single photon emission computed tomography (SPECT), as discussed in the following sections.
PET imaging
PET scanning of the brain allows imaging and quantifying various biological processes after injection of a radiolabeled compound. PET radiotracers are labeled by a positron-emitting isotope generated by a cyclotron. After injection, the tracers reach various organs in the body, including the brain, where they are transported through the blood-brain barrier and accumulate in tissues in a way that reflects the biological behavior of the radiotracer. The emitted positrons at the accumulation site generate paired γ-photons that are detected by the PET scanner via coincidence detection, which allows PET to have a spatial resolution in the mm range as opposed to SPECT, which has poorer resolution. By using specifically designed radiotracers, the PET system can measure a wide range of functions, such as blood flow, metabolism, transport rates, synthesis rates of various molecules (such as protein, DNA), or receptor density. PET imaging in epilepsy also has the advantage of providing localizing information in the interictal state, while SPECT imaging typically requires the capture of seizures (see details below).
While the number of potential PET tracers is large, imaging brain glucose metabolism using 2-deoxy-2[18F]fluoro-D-glucose (FDG) remains the most commonly utilized PET approach in clinical practice, including localization of epileptic foci. The glucose metabolic pattern visualized on FDG-PET reflects glucose transport and tissue trapping during a prolonged period of time (approximately 30 min), thus representing a summation of cellular metabolic processes during the uptake period. Therefore, FDG-PET is not suitable to measure short-term neuronal processes, which are better detected by blood flow imaging via PET (e.g., by using O-15 labeled water) or, more commonly in recent years, by functional MRI (fMRI). The FDG uptake pattern can be affected by ongoing or recent seizure(s) and active interictal spiking, making such images difficult to interpret in some cases. Scalp EEG recording during the FDG uptake period is useful to recognize such epileptic patterns. Non-FDG PET tracers can provide additional localizing and also potential etiologic information even when MRI and FDG-PET fail to detect an epileptogenic lesion; these are briefly summarized later.
FDG-PET in children with non-localizing MRI and mild cortical developmental malformations.
In children with presurgical evaluation for epilepsy surgery, the most common clinical application of FDG-PET is to obtain lateralizing and localizing information regarding epileptogenic cortex when MRI does not show a clear focal lesion and scalp EEG does not provide sufficient localization either. In addition, FDG-PET imaging can be helpful in cases where structural imaging shows subtle or obvious (or even multiple) lesions, but these are discordant with the electro-clinical findings. These foci are most common in neocortical epilepsy often associated with mild cortical developmental malformations that can be missed by structural imaging. Early PET studies demonstrated the ability of FDG-PET to identify hypometabolic regions in the lobe(s) of cortical seizure onset in non-lesional cases [16–19]. In 11 studies reporting on surgical series including patients with extratemporal lobe epilepsy and normal MRI, localized FDG-PET abnormalities were reported in 36% to 93% of patients, with a 71% mean detection rate [20]. The sensitivity of FDG-PET can be increased by co-registration of color-coded PET images with structural MRI [21]. With this approach, mild type I dysplasia could be detected in up to 98% of the cases [22]. The use of FDG-PET/MRI co-registration added useful localizing value for 1/3 of patients with non-concordant EEG and MRI findings, and 82% of their patients became seizure-free after resection guided by the MRI/PET findings and electrocorticography (ECoG). In a subsequent study of 48 children, FDG-PET showed a clear advantage over MRI in detecting mild malformations of cortical development, which were missed in about 2/3 of the cases but detected by PET in 77% [23]. While some previous studies utilized suboptimal MRI, 1.5 T scanners, and/or lower resolution scans, a recent study used 3T MRI with high-resolution images (1 mm3 isotropic voxels) combined with co-registered FDG-PET in patients with non-localizing conventional MRI [24]. This approach detected a previously missed MRI lesion in 46% and confirmed a suspicious subtle lesion in 12% of the cases, thus supporting the importance of high-resolution MRI combined with PET imaging; as a result, the rate of detected focal abnormalities increased to 94% by PET/MRI fusion as compared to 68% by PET alone. The increasing availability of hybrid PET/MRI scanners may facilitate the combined, simultaneous evaluation of FDG-PET and high-resolution MR images, although simultaneous acquisition may not necessarily improve co-localization considering the static nature of the brain. Nevertheless, the use of such hybrid machines may have the advantage of limiting the length of sedation required for two separate studies in young children.
A recent study reported a practical and effective multimodal imaging approach to detect subtle epileptic abnormalities by combined, quantitative MRI and FDG-PET analyses in patients with negative preoperative MRI [12]. MRI scans underwent morphometric analysis to detect subtle FCD causing blurring of the grey/white matter junction. Complete resection of MRI/PET-positive regions was associated with seizure-free outcome, and this effect was more robust in the extratemporal subgroup (p<0.01) as compared to those with temporal lobe epilepsy (p=0.054). The resected regions showed mostly FCD type I, and he study provided further confirmation that multimodal imaging and combined analysis of MRI and PET data can optimize the detection of subtle epileptogenic lesions and facilitate an improved postsurgical seizure outcome.
Objective comparisons of PET hypometabolism vs. ECoG-defined epileptic cortex.
An early study from our group compared the exact locations of objectively defined cortical hypometabolic regions on PET to ictal and interictal epileptiform abnormalities from subdural EEG recordings [25]. The overlap detected between hypometabolic areas and seizure onset electrodes was found to be partial at best: onset electrodes often extended beyond hypometabolic cortex, while glucose metabolic abnormalities expanded to non-epileptogenic regions. This observation has been confirmed by subsequent studies utilizing various PET analytic methods [26,27]. Overall, these data demonstrated that interictal hypometabolism on PET is best at regionalizing the epileptic cortex, mostly on the lobar level. In a study with statistical parametric mapping (SPM), an objective voxel-based method to detect cortical metabolic abnormalities as compared to a control group, we found that SPM-detected hypometabolic lobes had accuracy around 70%, similar to that of expert visual assessment [28]. In some cases, mild medial frontal abnormalities were correctly detected by SPM where visual assessment missed some of those abnormalities. Epileptic foci in the medial cortex can be associated with generalized epileptiform activity on scalp EEG due to secondary bilateral synchrony; thus, these foci may be difficult to lateralize without supporting imaging abnormalities. PET images in patients with suspected focal epilepsy associated with generalized epileptiform discharges on EEG can be helpful to evaluate medial cortex. Review of both axial and coronal PET images and objective image analysis can be helpful in such cases and also to improve detection of hypometabolic cortex localized to deep inferior cortical regions such as the orbital cortex (Figure 1). The findings may prompt intracranial EEG monitoring including interhemispheric electrodes.
Figure 1.

Detection of a left inferior frontal epileptic focus in a 12-year old child whose MRI was read as normal originally. FDG PET showed a clearly hypometabolic area (arrow), best appreciated on coronal planes, in the inferior frontal cortex. A secondary review of T1-weighted and FLAIR images, co-registered with the PET images, showed a small suspicious area of cortical malformation in the same region (asterisks on images in the bottom row). Subsequent intracranial EEG showed seizure onset from this region, which was resected.
The accuracy of objective detection of PET metabolic abnormalities in children can be improved with the use of age-matched (pediatric) PET templates and comparisons of individual scans to age-matched control groups. Since PET scans from completely healthy children are not available due to ethical reasons, several studies validated the use of brain PET scans of “pseudo-normal” groups, such as children with extra-cranial disease [29–31] or those with epilepsy and normal PET as defined by careful visual assessment [27,32,33]. Using this concept, our group created PET templates for children above 1 year of age, and we have recently defined normal, age-specific regional cortical asymmetries in children age 1-18 years [34]. These latter studies demonstrated that regional glucose uptake is most symmetric at around 1 year of age, and asymmetries emerge as the brain matures in a region-specific manner: several frontal regions show an increase in left>right metabolic asymmetry, while selected regions in the temporal, parietal and occipital cortex show a right>left asymmetry by teenage years.
Even with these advances of PET image evaluation, a portion of children with epilepsy show a normal glucose metabolic pattern and could be further evaluated by other PET tracers or other functional imaging modalities, such as ictal SPECT (see details below) and magnetoencephalography (MEG). A study of 26 children with normal or subtle changes on MRI demonstrated that FDG-PET and MEG often show complementary localizing findings as compared to surgical resection sites [35]. The added impact of incorporation of both FDG-PET and MEG and high-resolution 3T MRI in the presurgical evaluation has been reported in a subsequent study [36]. In this study, a new, improved multimodal strategy may have contributed to the increase in seizure-free outcome from 57% to 78% in those with malformations of cortical development. Since multiple changes have been introduced simultaneously, the relative impact of individual modalities could not be evaluated separately.
FDG-PET in the presurgical evaluation of epileptic spasms.
Children with epileptic spasms pose a particular challenge in presurgical evaluation, as their seizure semiology and EEG often does not provide strong clues for focal-onset seizures. Early studies in such children demonstrated areas of cortical hypometabolism that provided target areas for surgical resection. The affected regions often showed cortical dysplasia on histopathology [37]. A larger, follow-up study found that detection of focal cortical metabolic abnormalities can raise the number of surgically remediable cases [38]. These data, along with subsequent advances in MR imaging of subtle cortical malformations, have led to an increase of successful surgical treatment of West syndrome [39]. The surgical outcome data of a recent, single-center study from Detroit supported the important role of neuroimaging in the presurgical evaluation of children with epileptic spasms [40]. In this series of 65 children, seizure-free outcome was associated with shorter duration of epilepsy and presence of a lesion on MRI. Interestingly, FDG-PET was positive in the vast majority of the cases, thus helping in surgical decision making and guiding subdural grid placements in those with non-lesional MRI. Importantly, epilepsy surgery in this group may reverse developmental delay [41,42]. On the other hand, children with spasms and diffuse metabolic abnormalities are unlikely to be surgical candidates and may need to undergo detailed studies for genetic and metabolic disorders.
The role of other PET tracers in presurgical evaluation of children with epilepsy.
In the past three decades, numerous additional PET tracers have been tested for their ability of detecting epileptic foci. While several of these radioligands showed some promise in selected patient groups, none of them has gained widespread clinical use so far. One of the major reasons for this is that most of the tested tracers have been labeled with carbon-11, a positron-emitting isotope with a short half-life (20 min), requiring an on-site cyclotron. Among the most interesting and tested radiotracers, [11C]flumazenil (FMZ) has been studied widely. FMZ is the analog of the gamma amino-butyric acidA (GABAA) receptor antagonist flumazenil, and it gained attention after the initial report demonstrating decreased FMZ binding in human epileptic foci [43]. While initial studies focused on adult temporal lobe epilepsy, subsequent reports included subjects with normal MRI associated with neocortical epilepsy [44–49]. Although direct comparisons suggested that FMZ abnormalities may be more specific for epileptic cortex than areas of glucose hypometabolism [46,47], some studies reported the presence of FMZ binding abnormalities in remote projection areas [49] and in apparently non-epileptic regions [50]. Although initial clinical applications with longer-half life F-18-labeled flumazenil derivatives have been promising [51, 52,53], applications of this PET approach in the presurgical evaluation of epilepsy have been incompletely explored.
Among other PET radiotracers, α-[11C]methyl-L-tryptophan (AMT) has been studied intensely since the late 1990s and showed exciting localizing data for detection of epileptogenic tubers and certain types of epileptogenic cortical malformations [54–60] (Figure 2). This radiotracer has been originally developed to image and quantify brain serotonin synthesis in vivo [61]. The rationale of AMT-PET imaging in focal epilepsy came from reports of increased serotonin content in human epileptic cortex [62]. Subsequent studies from epileptogenic tubers and other epileptic lesions suggested that the trapping of the tracer may be more related to metabolism of tryptophan via the inflammatory (and immunosuppressive) kynurenine pathway [63,64]. Thus, high AMT accumulation may be an imaging marker of chronic neuroinflammation in epileptic foci [65]. The main advantage of AMT-PET is its ability to identify epileptogenic lesions and cortex as an area with increased uptake in the interictal state. This ability is particularly valuable in cases with extensive non-epileptic hypometabolism, multiple lesions (common in tuberous sclerosis; Figure 2), and in cases with failed epilepsy surgery when reoperation is considered [66]. Definite increases in AMT uptake within or around tubers indicate epileptogenicity with a high specificity [58,67]. In a cohort of 191 patients, ictal scalp EEG and AMT-PET showed an excellent agreement with seizure focus lateralization, and AMT-PET was more localizing in 41% of the cases [60]. Importantly, AMT-PET was localizing in 10 of 17 patients with non-lateralized ictal scalp EEG. In children with intractable focal epilepsy not associated with tuberous sclerosis, focal cortical increases of AMT uptake were relatively common in those with cortical developmental malformations, and, in particular, in those with type IIB cortical dysplasia [57,59,68], even if MRI was non-localizing (Figure 2). On detailed comparisons with subdural EEG data, epileptic cortex was not always confined to the AMT hot spots but also extended to nearby cortex [57].
Figure 2.

Detection of an epileptogenic tuber (A-C) and focal cortical dysplasia (D-F) by high α-[11C]methyl-L-tryptophan (AMT) uptake on PET imaging. (A) Axial FLAIR MRI showed multiple lesions in both hemispheres (arrows indicate six of the lesions). (B) All of these lesions were severely hypometabolic on FDG-PET. (C) AMT-PET showed increased tracer uptake in the left frontal hypometabolic tuber (orange arrows), suggesting epileptogenicity in this lesion. In a young child with suspected frontal lobe seizures but no clear lesion detected by MRI, although mild blurring of the gray/white matter junction was noticed in the left frontal lobe (D), FDG-PET (E) showed mild hypometabolism in the left frontal cortex (arrow); in the same area, AMT-PET showed increased interictal uptake (arrow) suggesting epileptogenic cortex.
The main limitations of AMT-PET include the short half-life (20min) of C-11 and the low sensitivity of this imaging modality to detect epileptogenic lesions and cortex; in non-lesional cases, the sensitivity of AMT-PET is below 50%. The former limitation could be overcome by testing the clinical use of F-18-labeled tryptophan derivatives that can track tryptophan metabolism via the kynurenine pathway. Recent studies demonstrated the feasibility of this approach [69–71], but the new PET tracers will have to be tested in humans to determine if they have a similar (or better) ability to detect epileptic foci than AMT. In addition to AMT, there have been additional PET radiotracers showing increased interictal uptake in some epileptic foci; e.g., radiotracers targeting activated microglia, histamine or certain types of opiate receptors [72,73]; however, these tracers have not been tested in larger cohorts of children undergoing presurgical evaluation.
Ictal SPECT imaging
Seizures can increase blood flow in the epileptic region by up to 300% [74]; these regions can be detected as areas of hyperperfusion on ictal SPECT studies. Areas of interictal hypoperfusion can be mild and difficult to detect and interpret. In contrast, ictal increases are often prominent and can identify seizure onset areas if the tracer is injected as early as possible after the seizure initiation to obtain ictal SPECT images. The accuracy of ictal SPECT can be increased by applying Subtraction Ictal SPECT Co-registered to MRI (SISCOM), where the interictal SPECT images are subtracted from ictal images, and the difference areas are superimposed on coregistered MR images of the patient for better anatomical localization [75]. Late injection (>45 sec) of the radiotracer is more likely associated with false or non-localizing SISCOM [76]. Beside injection time, selection of the optimal difference threshold is also crucial for reliable SISCOM results. In clinical practice, a z score of 2 is commonly used; however, a recent study of 26 epileptic patients found that a z score of 1.5 had the highest sensitivity and specificity (85% and 94%, respectively) in localizing the epileptic focus [77]. Thus, adjustment of the threshold to detect relative ictal increases may provide complementary information and enhance the accuracy of correct focus localization.
An early study of children with intractable epilepsy showed a high concordance between the SISCOM focus and seizure onset defined by EEG and MRI in those with seizures lasting more than 10 seconds; in addition, 6 of 40 patients focal hyperperfusion was detected despite non-localizing MRI and electroclinical findings [78]. In a subsequent study, presurgical SISCOM imaging was performed in 15 children with intractable focal epilepsy associated with focal cortical dysplasia, where SISCOM was concordant in 8 children, and non-concordant (n=5) or non-localizing (n=2) in 7 [79]. In 4 patients with normal MRI, 3 had localized SISCOM findings who were seizure-free after surgery. In a review of ictal SPECT studies, Lerner et al. [21] found this modality to be localizing in slightly more than 50% of surgical patients with epilepsy associated with cortical dysplasia. Complete or even partial resection of the SISCOM-detected focus was associated with seizure-free outcome in the majority of patients, although postoperative seizures can occur in some cases even after the SISCOM lesions have been completely removed [80]. Nevertheless, ictal SPECT may not be useful in extratemporal epilepsy (common in children) when seizures are brief and rapidly propagate to remote areas. Overall, the comparative diagnostic performance of FDG-PET vs. ictal SPECT has been variable, depending on the cohort involved and the study design [80–83]. Many centers are more experienced in one modality than the other, and there could be other preferences and bias that affect the overall results. Between these two modalities, interictal FDG-PET is easier to plan and perform than ictal SPECT, and the former has been proposed (following MRI) as the initial test for presumed cortical dysplasia and for non-lesional cases [1]. On the other hand, ictal SPECT may have a better yield in cases with multiple lesions (such as tubers) and in patients with a large acquired lesion or previous resection(s) (Figure 3).
Figure 3.

Localization of a right frontal epileptic focus by SISCOM (subtraction ictal SPECT co-registered to MRI) in a patient with a large fronto-parietal infarct due to a childhood head injury. FDG-PET showed severe hypometabolism in the infarct region and mild hypometabolism inferior to that region but did not localize the epileptic cortex located in front of the infarcted area.
Ictal SPECT can also be considered if the PET findings are discordant with the MRI. In a recent pediatric cohort, where the majority of patients had both PET and subtraction ictal SPECT available, patients whose co-registered images were used for presurgical evaluation were less likely to undergo invasive EEG monitoring and were more likely seizure-free one and two years after surgery [84]. These data again support the clinical benefit of performing multimodal imaging in pediatric epilepsy surgery.
Functional MRI
Functional MRI is most commonly utilized in presurgical evaluation of patients with drug-resistant epilepsy to localize eloquent brain areas such as language and motor areas in and around the epileptic focus, with the intent to reduce postsurgical functional impairment. Although epilepsy is the most common serious neurological disease in childhood, the number of studies focusing on the use of fMRI in pediatric epileptic surgery population is small without clear standardized guidance for clinicians [85]. A recent review comparing invasive and non-invasive presurgical brain mapping tools recommended the use of non-invasive functional imaging methods, including fMRI, first, for the purposes of presurgical functional mapping, while invasive studies should be only performed if the non-invasive methods provide ambiguous or unconvincing result [86].
Functional MRI shows signal alterations based on magnetic resonance blood oxygen level–dependent (BOLD) signal changes, as the activated brain region shows increased blood flow with an elevation in the ratio of oxygenated to deoxyhemoglobin [87]. The brain activation can be provoked by various activities depending on which functional area is being tested. In the pediatric population, fMRI performance can be challenging due to extensive motion effects or poor performance of the different tasks due to young age and/or developmental impairment. A large study of 409 children found significantly lower, but still reasonable success rates in children with neurological diseases (70-80% depending on etiology; for epilepsy, the success rate was 80%) as compared to typically developing children (87%) [88]. They also reported lower success rates in younger children (age 4-6-year) when compared to 10-18-year old children. On the other hand, Shurtleff et al. [89] reported that, with proper preparation and practice, fMRI could be performed adequately even in chronologically or developmentally very young children. Here, we briefly overview the role of fMRI in pediatric presurgical evaluation and treatment planning along with other invasive or non-invasive modalities.
Language area evaluation.
Detection of language areas before resective surgery is essential to minimize postoperative language deficit. Language evaluations can target the “expressive” aspect of language using verbal fluency tests or the “receptive” aspect using auditory or reading comprehension tasks [90]; as well as using the semantic decision task, where, in the active condition, patients have to decide whether the words presented match the previously determined category [91]. In the past, functional language evaluation required invasive methods, such as the intracarotid amytal test (IAT or Wada test) and electrocortical stimulation mapping (ESM). However, in the past two decades, fMRI has been increasingly reported to be a reliable, non-invasive alternative for language mapping in both adults [90,92–94] and children [95,96]. A recent study used several types of language tasks and both qualitative and quantitative methods in evaluation of frontal and temporal lobe for preoperative language mapping in 20 children as young as 7 years of age [97]. Although the gold standard IAT was more likely to lateralize than fMRI (the latter showing bilateral language activation in at least 30%), the authors reported a good concordance between fMRI and both with IAT and ESM and recommended the invasive testing only in cases with bilateral fMRI findings. Another study found a threshold-dependent good sensitivity (100%), but low specificity (69%) of fMRI compared with ESM in 8 children with left frontal or temporal epilepsy during presurgical sentence generation task; 5 of them underwent surgery with no postoperative deficit [98] (Figure 4). They concluded that fMRI could be a useful tool for the planning of electrode placement and increase the yield of stimulations.
Figure 4.

Accurate co-localization of an fMRI activation cluster with the location of depth electrodes detecting language disruption (yellow circles) in the pars opercularis of the left inferior frontal gyrus (A-C). Postoperative MRI showing the resection with the pars opercularis intact. The child was seizure-free and had no language deficit 1 year after surgery (From Ribaupierre et al. [98] with permission).
Two recent studies investigating a mixed (pediatric and adult) epileptic population reported that combination of various techniques with complementary aspects (such as fMRI, magnetoencephalography, and ECoG) may provide additional localization information and increase the rate of informative language or somatosensory mapping results [99,100]. Furthermore, this multimodal approach can predict postsurgical language outcome and help to select optimal surgical plan using support vector regression method [100].
Norrelgen et al. [101] reported dichotic listening task as a new approach for language lateralization in 19 epileptic children. Dichotic listening is a behavioral assessment of language lateralization based on the principle that contralateral auditory cortical projections are stronger than ipsilateral projections [102]; thus, when two competing speech stimuli are presented in each ear simultaneously, the response to the stimuli presented to the contralateral ear of the language dominant hemisphere will show an advantage over stimuli to the ipsilateral ear [101]. The authors achieved conclusive language dominance in 14 patients (74%) in which dichotic listening provided critical data in 3 cases, while the result was contradictory in 2 patients [101]. However, this approach needs further confirmation. Another approach, cerebellar language mapping, was suggested by Gelinas et al. [103] who found that this method could contribute to comprehensive preoperative evaluation of language lateralization in 46 children (age: 7-19 years); moreover, patients with atypical cerebellar language activation may possess a risk for having atypical cerebral language organization, thus they have a greater risk for postsurgical deficit. Subsequently, this method was also reported to have an additional diagnostic feature, particularly in cases of uncertainty, to determine language dominance in adults with brain tumor [104].
Receptive language tasks can be helpful to localize language-related areas in sedated children while using various sedating agents such as propofol [105] or chloral hydrate [106] (Figure 5). Significant BOLD signal increases in the language cortex could be generated in about half of the patients. However, a study examining auditory cortex in awake and propofol-sedated 5-8-year-old children reported that sedated children exhibited a different auditory fMRI cortical activation pattern from the age-matched non-sedated children, as the former group showed lesser activation of Wernicke’s area and did not show activation in Broca’s [105]. This information might be helpful for neuroradiologists who need to interpret fMRI in sedated patients.
Figure 5.

BOLD signal increases in the left hemisphere in response to a speech-based auditory task in a child under anesthesia with chloral hydrate. (From Ives-Deliperi et al. [106] with permission).
Motor area evaluation.
Motor decline after surgery is a major concern for clinicians and families. To localize motor cortex activation, most studies utilized finger/hand or foot tapping tasks [89,106–109]. Motor cortex activation can be achieved using passive (under sedation or in paretic extremities) or active tasks where the patient is awake. Preoperative motor fMRI can help in surgery planning [106,108] and in prediction of surgical outcome [109]. Multiple studies reported the feasibility and reliability of passive-motion fMRI in epileptic patients under sedation [106,108]. One of these studies investigated motor cortex activation during passive hand and foot movement under general anesthesia (mostly using propofol alone) in 62 fMRI studies and reported successful motor cortex localization even in children younger than 2 years thus aiding the surgical planning [108] (Figure 6).
Figure 6.

(A) Axial MR images showing the overlay of cortical fMRI activation maps for passive movement of the left lower extremity (pink) in a 16-year-old girl with a history of left functional hemispherectomy showing orthotopic cortical activation in the posterior/superior aspect of the right paracentral lobule. (B) Activation maps for passive movement of the right lower extremity (red) are in a more posterior and inferior location in the right (ipsilateral) hemisphere. Parasagittal (C) and coronal-oblique (D) images show the relationship between the area of activation for both lower extremities. (From Choudri et al. [108] with permission).
Another study sedated 9 children using chloral hydrate as a light sedating agent during assisted finger or foot tapping tasks and reported significant BOLD signal increases in all but one case in hand motor task, and in all patients in lower extremity task suggesting that this imaging method can be useful in uncooperative patients [106]. Despite good reliability of passive-motion fMRI in young and uncooperative children, sedation or general anesthesia always involve some risk for the patients. Shurtleff et al. [89] successfully performed preoperative active motor fMRI in 7 patients under 8 years of age (mostly 5-6 years old or developmentally younger) by using a variety of preparation and co-operation-enhancing strategies and emphasized that although the proper training of this population is often time-consuming, these younger patients should not be rejected as subjects for active motor fMRI.
To investigate capacity of motor fMRI for predicting severe motor decline after surgery, a recent study of 25 children (age: 1-18 years) with functional hemispherectomy reported that none of the patients with ipsilateral motor cortex activation before surgery had severe motor deficit in the affected side, while all patients with presurgical contralateral activation presented severely weaker hand movement after surgery; thus suggesting an excellent prediction value of this imaging technique [109].
A longitudinal study investigated 7 children with unilateral hemispheric pathology who underwent hemispherectomy using pre-and postsurgical fMRI and diffusion tensor imaging (DTI) in order to understand timing and possible patterns of function relocation/reorganization [107]. They performed anatomical hemispherectomy in case of complete loss of brain function demonstrated by fMRI in the affected hemisphere; otherwise, they chose functional hemispherectomy. Among the 7 patients, six had ipsilateral motor cortex activation during hand motor task after surgery, but 4 of them presented ipsilateral hand motor area also before surgery. This suggests that relocation of motor cortex to the ipsilateral hemisphere can occur both before and after surgery, but in some cases the relocation does not occur. Brain plasticity in association with extensive surgical resection, such as hemispherectomy, depends on multiple variables including the patient’s age at the time of the insult, the size, location, and etiology of the brain lesion, the maturity of the brain, integrity of the brain surrounding and contralateral to the lesion, severity of epilepsy, and medication effects [110].
Evaluation of the epileptogenic zone by EEG-triggered fMRI.
Simultaneous EEG-fMRI has been shown to be useful to define the epileptic focus, as interictal epileptic discharges (IED) may be associated with BOLD response on fMRI. Adequate patient selection is crucial for this diagnostic tool, and children with poorly-controlled focal epilepsy have higher chance to generate IEDs during an EEG-fMRI study resulting a higher yield. Although this method has limitations, e.g., the need for special equipment (such as MRI-compatible EEG cap), low yield due to the unpredictable nature of IEDs, long study duration, and sensitivity to motion, Centeno et al. [111] demonstrated that a good quality EEG-fMRI can be obtained in children as young as six years of age without sedation using a child friendly stimulus such as movies. They reported an overall high success rate, as 83% of the children could complete the whole protocol, while they had only two failed scans (due to the intolerance of the EEG cap application and fear from the MRI scanner). The authors achieved a modest concordance of 63% with the suggested epileptogenic zone based on presurgical tests, including cases with no structural lesion on MRI. Their subsequent extended study used simultaneous EEG-fMRI data to derive EEG-fMRI and electrical source imaging (ESI) maps to localize the epileptogenic zone and predict seizure outcome in 53 children with lesional or non-lesional drug-resistant epilepsy [112]. They reported that the EEG-fMRI maps alone had a good yield (89%) but low sensitivity (50%) for the correct localization of the epileptogenic zone. However, the combination of EEG-fMRI with ESI maps resulted the highest sensitivity both in localization (92%) and prediction of surgical outcome (91%) even in non-lesional cases. Furthermore, these tests were reported to be more spatially accurate than most of the current non-invasive presurgical tests such as PET or SPECT [112].
Resting-state fMRI – evaluation of brain connectivity.
Resting-state fMRI (rs-fMRI) is a task-free fMRI acquisition where the patients are tested with eyes closed at rest or under sedation (“resting state”). During this resting state, low frequency fluctuations (0.01–0.08 Hz) in BOLD signals related to spontaneous neural activity across brain regions result different resting-state networks (RSN), including sensorimotor, visual, auditory, and default mode network, which can be visualized by rs-fMRI. The most commonly used method is the seed-based analysis, which estimates changes in BOLD signal of a particular region of interest to the whole-brain connectivity. Several pediatric studies have demonstrated evidence that patterns of RSN can change in various epileptic diseases, such as absence [113] and partial epilepsy [114], including temporal lobe epilepsy [115], showing mostly decreased connectivity compared to a group of healthy control subjects. The connectivity changes observed in such group analyses encouraged investigators to apply rs-fMRI in the clinical practice and report preliminary experiences in individual patients [116–118]. A review of the methodology and role of rs-fMRI in pediatric epilepsy, including also two case reports, concluded that rs-fMRI may have significant utility in preoperative evaluation of pediatric children, especially in cases where task-based non-invasive functional mapping is not possible [117]. A recent study evaluated the clinical role of rs-fMRI for surgical planning in 20 therapy-resistant epileptic children [118]. The authors used machine learning algorithm as an automated rs-fMRI analysis pipeline for RSN localization and concluded that rs-fMRI may be a useful tool in combination with other modalities, such as task-based fMRI, diffusion tensor imaging, and ECoG. Although sedation can reduce rs-fMRI signals, it does not interfere with cortical network detection and RSN localization remains feasible in sedated patients [117,118].
Diffusion tensor imaging
Diffusion tensor imaging can be used to evaluate white matter integrity and visualize and quantify white matter tracts based on the diffusivity of water molecules. Main diffusion values determined by DTI include the mean diffusivity (MD) and fractional anisotropy (FA). While MD provides information about the overall magnitude of molecular diffusion, FA characterizes the degree to which a single direction of water motion dominates the overall diffusivity in a voxel. DTI tractography identifies the directional diffusivity of water within the voxel and connects it to neighboring voxels with similar diffusion directionalities, thus generating a tract of voxels with similar diffusivities. As a result of tractography, the volume of specific white matter tracts can be calculated. DTI tractography can be performed by application of various acquisitions and analytic techniques, including deterministic fiber tracking protocols [119], voxel-based methods (e.g., tract-based spatial statistics [TBSS]) [120], as well as advanced approaches such as high angular resolution (HARDI) [121]. HARDI can provide superior qualitative data to demonstrate multiple crossing fibers in the three-dimensional space. Unfortunately, HARDI requires acquisition of at least 60 gradient directions at high b-value that prolongs study time, complicating application in pediatric population where sedation is needed, and HARDI-based measures are yet to make a mark in routine clinical applications [122]. In addition, the high variability of acquisition and processing techniques across studies causes difficulties in the comparison and interpretation of published results; further standardized and prospective studies are needed to help assess the full impact of DTI in neurosurgical planning [123]. On the other hand, multiple reviews agreed that standard DTI can be beneficial in patients (children and adults) undergoing diagnostic evaluation as a part of multimodal epilepsy surgery planning [124,125]. Here, we are focusing on the use of DTI in language lateralization, motor fiber tracking, and its ability of detecting preoperative changes and predicting postoperative deficits in children with epilepsy.
Utility of DTI in language lateralization.
Detection of language network tracts, especially the arcuate fasciculus, which connects the two main language areas of the brain (Broca’s and Wernicke’s), is important in language lateralization. The arcuate fasciculus visualized by DTI was colocalized well with the anterior language areas detected by cortical stimulation, but less so with the posterior areas, suggesting that the latter may be more spatially dispersed [126].
One study comparing DTI and fMRI with the Wada test in adult epilepsy patients found that the language dominant hemisphere contained significantly more high anisotropy arcuate fasciculus pathways than the non-dominant hemisphere, thus, it had significant predictive power for determining language laterality compared to the Wada test [127]. This asymmetric distribution in the dominant hemisphere may reflect enhanced connectivity between frontal and temporal sites to support fluent language processes, while asymmetry of the uncinate and inferior longitudinal fasciculi appeared unrelated to hemispheric language dominance [127]. A subsequent study, analyzing the arcuate fasciculus in 13 children with intractable epilepsy, showed tract volumes to be significantly larger in the language dominant side, but both the volume and FA lateralized 1-1 patient falsely compared to the Wada test [128]. A study of 10 healthy children, comparing DTI with fMRI, showed that laterality index of the volume of the arcuate fasciculus measured correlated well with laterality index of fMRI suggesting an association between language function and anatomical structures [129]. The authors concluded that DTI can be a good alternative to fMRI for language lateralization in children.
On the other hand, another study comparing diverse language pathways in 33 epileptic children with cortical malformation found significant differences of scalar metrics (lower MD and higher FA) measured in the left uncinate, inferior fronto-occipital, and arcuate fasciculi between children with normal and impaired language function [130]. A more recent study of the same group, analyzing 37 epileptic children, also reported that the failure to identify the left arcuate fasciculus was a highly specific marker for language dysfunction in pediatric epilepsy patients with cortical malformations [131].
DTI fiber tracking can be validated by complementary methods defining language areas. Our study using diffusion-weighted imaging (DWI) tractography in children with focal epilepsy found 77% accuracy of a DWI-maximum a posteriori probability map (DWI-MAP, which can automatically sort individual fibers originating from fMRI language areas or ESM-defined language areas) in prediction of language areas compared with fMRI and 82% accuracy compared with ESM [132]. Importantly, decreased volumes of DWI-MAP-defined pathways after surgery were associated with postoperative language deficit. In addition, a longitudinal study evaluating the arcuate fasciculus pre- and post-surgery in 10 children, who underwent resection of the left arcuate fasciculus, found a marked interval enlargement and normalization of the right (contralateral) arcuate fasciculus volume following surgery in 8 patients. These data suggested a postsurgical compensatory reorganization of the right arcuate fasciculus [133]. However, the functional correlates of this contralateral reorganization for language improvement remained to be determined.
Motor fiber tracking and prediction of postoperative motor outcome.
Preoperative evaluation of the integrity of the motor cortex and the corticospinal tract (CST) may help predict motor outcome after epilepsy surgery. While CST has been considered to originate from the primer motor area, located in the precentral gyrus (Brodmann area 4), DTI studies reported that the origin of CST was not confined to the precentral gyrus in children; rather, it was originated from both pre-and postcentral gyri in 71% of subjects, with younger children more likely to have CST derived from precentral gyrus exclusively [134]. The origin and course of CST can be modified due to early structural abnormalities affecting the motor network. For example, in a 22-year-old right-handed man with extensive right-sided subcortical nodular heterotopia with fMRI activation seen both anterior and posterior to the lesion, DTI detected a modified CST passing around the malformation in concordance with the fMRI [135].
Preoperative asymmetry of the CST can predict postsurgical motor outcome. For example, in a longitudinal study of 4 patients with an early structural damage, those with a strongly lateralized CST showed a stable postoperative motor performance after resective surgery. In contrast, a patient with a bilateral pattern showed worsening of the upper limb function. For all cases, fMRI activations shifted to the intact hemisphere, demonstrating recruitment of ipsilateral motor networks to preserve function in the affected extremities [136]. Another recent study utilized motor fMRI and DTI to predict postoperative motor function in 25 children undergoing hemispherectomy [109]. Bilateral robust CST on DTI was associated with severe postsurgical motor function with 86% sensitivity and 100% specificity; therefore, patients with high risk for motor function loss can be identified prior to the surgery using the combination of the two non-invasive techniques.
In a recent study focusing on a mixed intractable epilepsy patient group (mean age: 12±14.5 years, range 0–55 years) treated with functional hemispherectomy, the authors found that preoperative absolute FA values of the affected CSTs and of commissural fibers were significantly higher in patients with postoperative loss of muscle strength compared to patients with unchanged strength [137]. Similar findings were observed for interhemispheric CST FA ratios, which predicted loss of muscle strength with 80% sensitivity and 70% specificity [137].
While routine fiber tracking approaches detect CST as a whole with a limited sensitivity and the inability to identify the entire tract volume, advanced post-processing techniques can separate functionally relevant CST segments associated with mouth/lip, finger, and leg areas. This has been demonstrated by our group with the application of an independent component analysis combined with a ball-stick model (ICA-BSM) [138] (Figure 7). The accuracy of the detected segments was validated against both fMRI and ESM in children with intractable epilepsy; thus, this approach can effectively identify the locations of motor areas and motor fibers for epilepsy surgery planning even in the absence of fMRI or ESM data.
Figure 7.
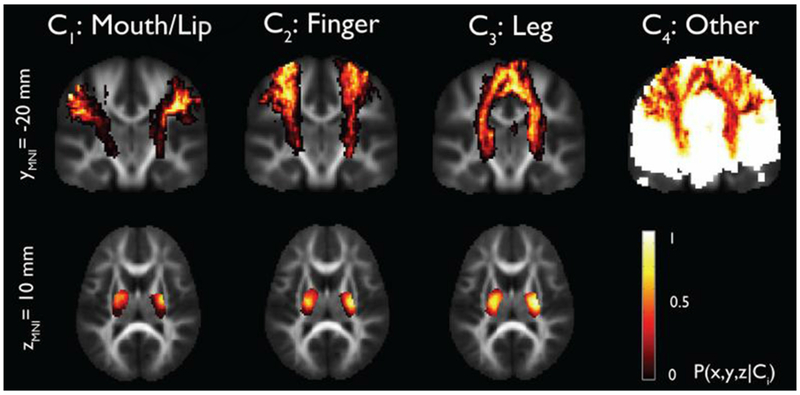
Stereotaxic probability maps of “mouth/lip”, “fingers”, and “leg” pathways from ICA+BSM tractography. Each map shows the probability of a voxel that belongs to mouth/lip, finger, and leg pathways in a group of healthy children (n=17, age 4.3-17.8 years). (Reproduced with permission from Jeong et al. [138]).
Detecting intact transcallosal connections after failed hemispherectomy.
Presurgical work-up of children with recurrent seizures who underwent previous hemispherectomy can be challenging, and surgical options are limited. DTI tractography of transcallosal fibers can be useful to identify remaining interhemispheric connections after surgery (Figure 8). Kiehna et al. investigated 8 patients following functional hemispherectomy to detect residual fiber connections between the two hemispheres as the potential reason for the surgical failure [139]. In all patients, DTI showed limited but definite residual connections, primarily across the rostrum/genu of the corpus callosum, and repeated surgery resulted in seizure freedom in 5 patients with 3 having Engel Class II after a minimum of 24 months follow-up. The utility of intra- and postoperative DTI to evaluate completeness of hemispheric disconnection has been also demonstrated in other small studies [140–142]; however, the overall accuracy of this approach as compared to the use of conventional high-resolution structural MRI has not been established.
Figure 8.

Example of a residual transcallosal connection documented by DTI fiber tracking (and superimposed on an axial T1-weighted image) in a right-handed boy with Sturge-Weber syndrome who underwent right functional hemispherectomy at age 1 year due to intractable seizures. He became seizure-free for 6 years but then started having seizures emerging from the right centro-temporal region. DTI 9 years after the initial surgery showed residual interhemispheric connections through the genu of the corpus callosum (arrow). He underwent a subsequent right anatomical hemispherectomy that resulted in seizure freedom.
Conclusions
In conclusion, the data reviewed above demonstrate the clinical value of various imaging modalities to detect epileptogenic brain regions and, in some cases, contribute to improved surgical outcome in children with non-lesional pediatric epilepsy. While no single modality provides optimal localizing information in all cases, the choice of one or multiple imaging approaches can be tailored to the individual patient according to the presumed underlying etiology and electro-clinical features. Multimodal imaging with combined analysis of MRI, PET, and/or ictal SPECT data can optimize the detection of subtle epileptogenic lesions such as cortical dysplasia and facilitate an improved postsurgical seizure outcome. In addition, fMRI and DTI, as a part of multidisciplinary presurgical evaluation, are useful tools for detection of eloquent and, in some cases, epileptogenic zones in the pediatric epileptic population. These modalities can assist tailoring neocortical epilepsy surgery and predicting postoperative neurological deficits by determining the locations and connections of language and motor areas. However, in cases of inconclusive or ambiguous neuroimaging findings, invasive techniques are still recommended before surgery. Going forward, a major challenge will be to design prospective, multicenter studies to evaluate the comparative value and combined utility of multiple imaging modalities while using standardized acquisition protocols and objective analytic approaches to obtain definitive and reproducible data sets that can be incorporated into future diagnostic guidelines for pediatric epilepsy surgery.
Highlights.
Advanced MRI can detect subtle epileptogenic lesions missed by conventional MRI
Interictal PET, ictal SPECT can delineate epileptogenic regions even if MRI is normal
Multimodal imaging can optimize presurgical detection of epileptic cortex
Functional MRI can localize motor and speech regions and assist focus localization
DTI helps in presurgical localization of motor fibers, language lateralization
Acknowledgments
Funding:
Data presented in this review were based on work supported by the National Institutes of Health (grant numbers NS041922, NS034488, NS064989).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH; Task Force for Paediatric Epilepsy Surgery, Commission for Paediatrics, and the Diagnostic Commission of the International League Against Epilepsy. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55:507–18. [DOI] [PubMed] [Google Scholar]
- [2].Hyslop A, Miller I, Bhatia S, Resnick T, Duchowny M, Jayakar P. Minimally resective epilepsy surgery in MRI-negative children. Epileptic Disord. 2015;17:263–74. [DOI] [PubMed] [Google Scholar]
- [3].Craven IJ, Griffiths PD, Bhattacharyya D, Grunewald RA, Hodgson T, Connolly DJ, et al. 3.0 T MRI of 2000 consecutive patients with localisation-related epilepsy. Br J Radiol. 2012;85:1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gaillard WD, Chiron C, Cross JH, Harvey AS, Kuzniecky R, Hertz-Pannier L, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009;50:2147–2153. [DOI] [PubMed] [Google Scholar]
- [5].Huppertz HJ, Wellmer J, Staack AM, Altenmuller DM, Urbach H, Kroll J. Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia. 2008;49:772–85. [DOI] [PubMed] [Google Scholar]
- [6].Focke NK, Bonelli SB, Yogarajah M, Scott C, Symms MR, Duncan JS. Automated normalized FLAIR imaging in MRI-negative patients with refractory focal epilepsy. Epilepsia. 2009;50:1484–90. [DOI] [PubMed] [Google Scholar]
- [7].Vézina LG. MRI-negative epilepsy: protocols to optimize lesion detection. Epilepsia. 2011;52 Suppl 4:25–7. [DOI] [PubMed] [Google Scholar]
- [8].Wagner J, Weber B, Urbach H, Elger CE, Huppertz HJ. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011;134:2844–54. [DOI] [PubMed] [Google Scholar]
- [9].Mellerio C, Labeyrie MA, Chassoux F, Roca P, Alami O, Plat M, et al. 3T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia. 2014;55:117–22. [DOI] [PubMed] [Google Scholar]
- [10].Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13:1114–1126. [DOI] [PubMed] [Google Scholar]
- [11].Martin P, Winston GP, Bartlett P, de Tisi J, Duncan JS, Focke NK. Voxel-based magnetic resonance image postprocessing in epilepsy. Epilepsia. 2017;58:1653–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lin Y, Fang YD, Wu G, Jones SE, Prayson RA, Moosa ANV, et al. Quantitative positron emission tomography-guided magnetic resonance imaging postprocessing in magnetic resonance imaging-negative epilepsies. Epilepsia. 2018;59:1583–1594. [DOI] [PubMed] [Google Scholar]
- [13].Adler S, Wagstyl K, Gunny R, Ronan L, Carmichael D, Cross JH, et al. Novel surface features for automated detection of focal cortical dysplasias in paediatric epilepsy. Neuroimage Clin. 2017;14:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hong SJ, Bernhardt BC, Schrader DS, Bernasconi N, Bernasconi A. Whole-brain MRI phenotyping in dysplasia-related frontal lobe epilepsy. Neurology. 2016;86:643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kamson DO, Pilli VK, Asano E, Jeong JW, Sood S, Juhasz C, et al. Cortical thickness asymmetries and surgical outcome in neocortical epilepsy. J Neurol Sci. 2016;368:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Swartz BE, Halgren E, Delgado-Escueta AV, Mandelkern M, Gee M, Quinones N, et al. Neuroimaging in patients with seizures of probable frontal lobe origin. Epilepsia. 1989;30:547–58. [DOI] [PubMed] [Google Scholar]
- [17].Henry TR, Sutherling WW, Engel J Jr, Risinger MW, Levesque MF, Mazziotta JC, et al. Interictal cerebral metabolism in partial epilepsies of neocortical origin. Epilepsy Res. 1991;10:174–82. [DOI] [PubMed] [Google Scholar]
- [18].da Silva EA, Chugani DC, Muzik O, Chugani HT. Identification of frontal lobe epileptic foci in children using positron emission tomography. Epilepsia. 1997;38:1198–208. [DOI] [PubMed] [Google Scholar]
- [19].Schlaug G, Antke C, Holthausen H, Arnold S, Ebner A, Tuxhorn I, et al. Ictal motor signs and interictal regional cerebral hypometabolism. Neurology. 1997;49:341–50. [DOI] [PubMed] [Google Scholar]
- [20].Juhasz C, Chugani HT. Positron emission tomography: glucose metabolism in extratemporal lobe epilepsy In: Chugani HT, editor. Neuroimaging in Epilepsy, New York: Oxford University Press; 2011, p. 141–155. [Google Scholar]
- [21].Lerner JT, Salamon N, Hauptman JS, Velasco TR, Hemb M, Wu JY, et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: A critical review and the UCLA experience. Epilepsia. 2009;50:1310–35. [DOI] [PubMed] [Google Scholar]
- [22].Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, et al. FDG-PET/MRI co-registration improves detection of cortical dysplasia in epilepsy patients. Neurology. 2008;71:1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim YH, Kang HC, Kim DS, Kim SH, Shim KW, Kim HD, et al. Neuroimaging in identifying focal cortical dysplasia and prognostic factors in pediatric and adolescent epilepsy surgery. Epilepsia. 2011;52:722–7. [DOI] [PubMed] [Google Scholar]
- [24].Ding Y, Zhu Y, Jiang B, Zhou Y, Jin B, Hou H, et al. 18F-FDG PET and high-resolution MRI co-registration for pre-surgical evaluation of patients with conventional MRI-negative refractory extra-temporal lobe epilepsy. Eur J Nucl Med Mol Imaging. 2018;45:1567–1572. [DOI] [PubMed] [Google Scholar]
- [25].Juhász C, Chugani DC, Muzik O, Watson C, Shah J, Shah A, et al. Is epileptogenic cortex truly hypometabolic on interictal positron emission tomography? Ann Neurol. 2000;48:88–96. [PubMed] [Google Scholar]
- [26].Alkonyi B, Juhasz C, Muzik O, Asano E, Saporta A, Shah A, et al. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jeong JW, Asano E, Pilli V, Nakai Y, Chugani HT, Juhász C. Objective 3D surface evaluation of intracranial electrophysiologic correlates of cerebral glucose metabolic abnormalities in children with focal epilepsy. Hum Brain Mapp. 2017;38:3098–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kumar A, Juhász C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51:1901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shan ZY, Leiker AJ, Onar-Thomas A, Li Y, Feng T, Reddick WE, et al. Cerebral glucose metabolism on positron emission tomography of children. Hum Brain Mapp. 2014;35:2297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hua C, Merchant TE, Li X, Li Y, Shulkin BL. Establishing age-associated normative ranges of the cerebral 18F-FDG uptake ratio in children. J Nucl Med. 2015;56:575–9. [DOI] [PubMed] [Google Scholar]
- [31].London K, Howman-Giles R. Voxel-based analysis of normal cerebral [18F]FDG uptake during childhood using statistical parametric mapping. Neuroimage. 2015;106:264–71. [DOI] [PubMed] [Google Scholar]
- [32].Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–97. [DOI] [PubMed] [Google Scholar]
- [33].Archambaud F, Bouilleret V, Hertz-Pannier L, Chaumet-Riffaud P, Rodrigo S, Dulac O, et al. Optimizing statistical parametric mapping analysis of 18F-FDG PET in children. EJNMMI Res. 2013;3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pilli VK, Jeong JW, Konka P, Kumar A, Chugani HT, Juhász C. Objective PET study of glucose metabolism asymmetries in children with epilepsy: Implications for normal brain development. Hum Brain Mapp. 2019;40:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Widjaja E, Shammas A, Vali R, Otsubo H, Ochi A, Snead OC, et al. FDG-PET and magnetoencephalography in presurgical workup of children with localization-related nonlesional epilepsy. Epilepsia. 2013;54:691–9. [DOI] [PubMed] [Google Scholar]
- [36].Rubinger L, Chan C, D’Arco F, Moineddin R, Muthaffar O, Rutka JT, et al. Change in presurgical diagnostic imaging evaluation affects subsequent pediatric epilepsy surgery outcome. Epilepsia. 2016;57:32–40. [DOI] [PubMed] [Google Scholar]
- [37].Chugani HT, Shields WD, Shewmon DA, Olson DM, Phelps ME, Peacock WJ. Infantile spasms: I. PET identifies focal cortical dysgenesis in cryptogenic cases for surgical treatment. Ann Neurol. 1990;27:406–13. [DOI] [PubMed] [Google Scholar]
- [38].Chugani HT, Conti JR. Etiologic classification of infantile spasms in 140 cases: role of positron emission tomography. J Child Neurol. 1996;11:44–8. [DOI] [PubMed] [Google Scholar]
- [39].Chugani HT, Asano E, Sood S. Infantile spasms: who are the ideal surgical candidates? Epilepsia. 2010;51 Suppl 1:94–6. [DOI] [PubMed] [Google Scholar]
- [40].Chugani HT, Ilyas M, Kumar A, Juhász C, Kupsky WJ, Sood S, et al. Surgical treatment for refractory epileptic spasms: The Detroit series. Epilepsia. 2015;56:1941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Asarnow RF, LoPresti C, Guthrie D, Elliott T, Cynn V, Shields WD, et al. Developmental outcomes in children receiving resection surgery for medically intractable infantile spasms. Dev Med Child Neurol. 1997;39:430–40. [DOI] [PubMed] [Google Scholar]
- [42].Jonas R, Asarnow RF, LoPresti C, Yudovin S, Koh S, Wu JY, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology. 2005;64:746–50. [DOI] [PubMed] [Google Scholar]
- [43].Savic I, Persson A, Roland P, Pauli S, Sedvall G, Widén L. In-vivo demonstration of reduced benzodiazepine receptor binding in human epileptic foci. Lancet. 1988;2:863–6. [DOI] [PubMed] [Google Scholar]
- [44].Savic I, Thorell JO, Roland P. [11C]flumazenil positron emission tomography visualizes frontal epileptogenic regions. Epilepsia. 1995;36:1225–32. [DOI] [PubMed] [Google Scholar]
- [45].Ryvlin P, Bouvard S, Le Bars D, De Laémerie G, Grégoire MC, Kahane P, et al. Clinical utility of flumazenil-PET versus [18F]fluorodeoxyglucose-PET and MRI in refractory partial epilepsy. A prospective study in 100 patients. Brain. 1998;121:2067–81. [DOI] [PubMed] [Google Scholar]
- [46].Muzik O, da Silva EA, Juhasz C, Chugani DC, Shah J, Nagy F, et al. Intracranial EEG versus flumazenil and glucose PET in children with extratemporal lobe epilepsy. Neurology. 2000;54:171–9. [DOI] [PubMed] [Google Scholar]
- [47].Juhász C, Chugani DC, Muzik O, Shah A, Shah J, Watson C, et al. Relationship of flumazenil and glucose PET abnormalities to neocortical epilepsy surgery outcome. Neurology. 2001;56:1650–8. [DOI] [PubMed] [Google Scholar]
- [48].Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003;126:1300–18. [DOI] [PubMed] [Google Scholar]
- [49].Juhász C, Asano E, Shah A, Chugani DC, Batista CE, Muzik O, et al. Focal decreases of cortical GABAA receptor binding remote from the primary seizure focus: what do they indicate? Epilepsia. 2009;50:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Koepp MJ. [11C]Flumazenil positron emission tomography In: Chugani HT, editor. Neuroimaging in Epilepsy, New York: Oxford University Press; 2011, p. 174–185. [Google Scholar]
- [51].Levêque P, Sanabria-Bohorquez S, Bol A, De Voider A, Labar D, Van Rijckevorsel K, et al. Quantification of human brain benzodiazepine receptors using [18F]fluoroethylflumazenil: a first report in volunteers and epileptic patients Eur J Nucl Med Mol Imaging. 2003;30:1630–6. [DOI] [PubMed] [Google Scholar]
- [52].Vivash L, Gregoire MC, Lau EW, Ware RE, Binns D, Roselt P, et al. 18F-flumazenil: a γ-aminobutyric acid A-specific PET radiotracer for the localization of drug-resistant temporal lobe epilepsy. J Nucl Med. 2013;54:1270–7. [DOI] [PubMed] [Google Scholar]
- [53].Hodolic M, Topakian R, Pichler R. 18F-fluorodeoxyglucose and 18F-flumazenil positron emission tomography in patients with refractory epilepsy. Radiol Oncol. 2016;50:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chugani DC, Chugani HT, Muzik O, Shah JR, Shah AK, Canady A, et al. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-1-tryptophan positron emission tomography. Ann Neurol. 1998;44:858–66. [DOI] [PubMed] [Google Scholar]
- [55].Asano E, Chugani DC, Muzik O, Shen C, Juhasz C, Janisse J, et al. Multimodality imaging for improved detection of epileptogenic foci in tuberous sclerosis complex. Neurology. 2000;54:1976–84. [DOI] [PubMed] [Google Scholar]
- [56].Fedi M, Reutens D, Okazawa H, Andermann F, Boling W, Dubeau F, et al. Localizing value of alpha-methyl-l-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57:1629–36. [DOI] [PubMed] [Google Scholar]
- [57].Juhász C, Chugani DC, Muzik O, Shah A, Asano E, Mangner TJ, et al. Alpha-methyl-l-tryptophan PET detects epileptogenic cortex in children with intractable epilepsy. Neurology. 2003;60:960–8. [DOI] [PubMed] [Google Scholar]
- [58].Kagawa K, Chugani DC, Asano E, Juhász C, Muzik O, Shah A, et al. Epilepsy surgery outcome in children with tuberous sclerosis complex evaluated with alpha-[11C]methyl-l-tryptophan positron emission tomography (PET). J Child Neurol. 2005;20:429–38. [DOI] [PubMed] [Google Scholar]
- [59].Wakamoto H, Chugani DC, Juhász C, Muzik O, Kupsky WJ, Chugani HT. Alpha-methyl-l-tryptophan positron emission tomography in epilepsy with cortical developmental malformations. Pediatr Neurol. 2008;39:181–8. [DOI] [PubMed] [Google Scholar]
- [60].Chugani HT, Luat AF, Kumar A, Govindan R, Pawlik K, Asano E. α-[11C]-Methyl-L-tryptophan-PET in 191 patients with tuberous sclerosis complex. Neurology. 2013;81:674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Diksic M, Nagahiro S, Sourkes TL, Yamamoto YL A new method to measure brain serotonin synthesis in vivo. I. Theory and basic data for a biological model. J Cereb Blood Flow Metab 1990;10:1–12. [DOI] [PubMed] [Google Scholar]
- [62].Trottier S, Evrard B, Vignal JP, Scarabin JM, Chauvel P. The serotonergic innervation of the cerebral cortex in man and its changes in focal cortical dysplasia. Epilepsy Res. 1996;25:79–106. [DOI] [PubMed] [Google Scholar]
- [63].Chugani DC, Muzik O. Alpha[C-ll] methyl-l-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab. 2000;20:2–9. [DOI] [PubMed] [Google Scholar]
- [64].Juhász C, Buth A, Chugani DC, Kupsky WJ, Chugani HT, Shah AK, et al. Successful surgical treatment of an inflammatory lesion associated with new-onset refractory status epilepticus. Neurosurg Focus. 2013;34:E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Juhász C, Mittal S. Mapping metabolism and inflammation in epilepsy Chapter 9, in: Bernasconi A, Bernasconi N, Koepp MJ (eds.): Imaging Biomarkers in Epilepsy. Cambridge University Press, Cambridge, UK: 2019; 95–107. 10.1017/9781316257951.010 [DOI] [Google Scholar]
- [66].Juhász C, Chugani DC, Padhye UN, Muzik O, Shah A, Asano E, et al. Evaluation with alpha-[11C]methyl-L-tryptophan positron emission tomography for reoperation after failed epilepsy surgery. Epilepsia. 2004;45:124–30. [DOI] [PubMed] [Google Scholar]
- [67].Rubí S, Costes N, Heckemann RA, Bouvard S, Hammers A, Marti Fuster B, et al. Positron emission tomography with α-[11C]methyl-L-tryptophan in tuberous sclerosis complex-related epilepsy. Epilepsia. 2013;54:2143–50. [DOI] [PubMed] [Google Scholar]
- [68].Chugani HT, Kumar A, Kupsky W, Asano E, Sood S, Juhasz C. Clinical and histopathologic correlates of 11C-alpha-methyl-L-tryptophan (AMT) PET abnormalities in children with intractable epilepsy. Epilepsia. 2011;52:1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Henrottin J, Zervosen A, Lemaire C, Sapunaric F, Laurent S, Van den Eynde B, et al. N (1)-Fluoroalkyltryptophan analogues: Synthesis and in vitro study as potential substrates for indoleamine 2,3-dioxygenase. ACS Med Chem Lett. 2015;6:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Giglio BC, Fei H, Wang M, Wang H, He L, Feng H, et al. Synthesis of 5-[18F]Fluoro-α-methyl tryptophan: New Trp based PET agents. Theranostics. 2017;7:1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Xin Y, Cai H. Improved radiosynthesis and biological evaluations of L- and D-l-[18F]fluoroethyl-tryptophan for PET imaging of IDO-mediated kynurenine pathway of tryptophan metabolism. Mol Imaging Biol. 2017;19:589–598. [DOI] [PubMed] [Google Scholar]
- [72].Kumar A, Chugani HT. The role of radionuclide imaging in epilepsy, Part 1: Sporadic temporal and extratemporal lobe epilepsy. J Nucl Med Technol. 2017;45:14–21. [DOI] [PubMed] [Google Scholar]
- [73].Scott G, Mahmud M, Owen DR, Johnson MR . Microglial positron emission tomography (PET) imaging in epilepsy: Applications, opportunities and pitfalls. Seizure. 2017;44:42–47. [DOI] [PubMed] [Google Scholar]
- [74].Hougaard K, Oikawa T, Sveinsdottir E, Skinoj E, Ingvar DH, Lassen NA. Regional cerebral blood flow in focal cortical epilepsy. Arch Neurol. 1976;33:527–535. [DOI] [PubMed] [Google Scholar]
- [75].O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Jack CR Jr, et al. Subtraction SPECT co-registered to MRI improves postictal SPECT localization of seizure foci. Neurology. 1999;52:137–146. [DOI] [PubMed] [Google Scholar]
- [76].O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–54. [DOI] [PubMed] [Google Scholar]
- [77].Newey CR, Wong C, Wang ZI, Chen X, Wu G, Alexopoulos AV. Optimizing SPECT SISCOM analysis to localize seizure-onset zone by using varying z scores. Epilepsia. 2013;54:793–800. [DOI] [PubMed] [Google Scholar]
- [78].Chiron C, Vera P, Kaminska A, Hollo A, Cieuta C, Ville D, et al. Single-photon emission computed tomography: ictal perfusion in childhood epilepsies. Brain Dev. 1999;21:444–6. [DOI] [PubMed] [Google Scholar]
- [79].Gupta A, Raja S, Kotagal P, Lachhwani D, Wyllie E, Bingaman WB. Ictal SPECT in children with partial epilepsy due to focal cortical dysplasia. Pediatr Neurol. 2004;31:89–95. [DOI] [PubMed] [Google Scholar]
- [80].Jalota A, Rossi MA, Pylypyuk V, Stein M, Stoub T, Balabanov A, et al. Resecting critical nodes from an epileptogenic circuit in refractory focal-onset epilepsy patients using subtraction ictal SPECT coregistered to MRI. J Neurosurg. 2016;125:1565–1576. [DOI] [PubMed] [Google Scholar]
- [81].Sturm JW, Newton MR, Chinvarun Y, Berlangieri SU, Berkovic SF. Ictal SPECT and interictal PET in the localization of occipital lobe epilepsy. Epilepsia. 2000;41:463–466. [DOI] [PubMed] [Google Scholar]
- [82].Kim SK, Lee DS, Lee SK, Kim YK, Kang KW, Chung CK, et al. Diagnostic performance of [18F]FDG-PET and ictal [99mTc]-HMPAO SPECT in occipital lobe epilepsy. Epilepsia. 2001;42:1531–1540. [DOI] [PubMed] [Google Scholar]
- [83].Seo JH, Holland K, Rose D, Rozhkov L, Fujiwara H, Byars A, et al. Multimodality imaging in the surgical treatment of children with nonlesional epilepsy. Neurology. 2011;76:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Perry MS, Bailey L, Freedman D, Donahue D, Malik S, Head H, et al. Coregistration of multimodal imaging is associated with favourable two-year seizure outcome after paediatric epilepsy surgery. Epileptic Disord. 2017;19:40–48. [DOI] [PubMed] [Google Scholar]
- [85].Collinge S, Prendergast G, Mayers ST, Marshall D, Siddell P, Neilly E, et al. Pre-surgical mapping of eloquent cortex for paediatric epilepsy surgery candidates: Evidence from a review of advanced functional neuroimaging. Seizure. 2017;52:136–146. [DOI] [PubMed] [Google Scholar]
- [86].Papanicolaou AC, Rezaie R, Narayana S, Choudhri AF, Abbas-Babajani-Feremi, Boop FA, et al. On the relative merits of invasive and non-invasive pre-surgical brain mapping: New tools in ablative epilepsy surgery. Epilepsy Res. 2018;142:153–155. [DOI] [PubMed] [Google Scholar]
- [87].Gaillard WD. Structural and functional imaging in children with partial epilepsy. Ment Retard Dev Disabil Res Rev. 2000;6:220–6. [DOI] [PubMed] [Google Scholar]
- [88].Yerys BE, Jankowski KF, Shook D, Rosenberger LR, Barnes KA, Berl MM, et al. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp. 2009;30:3426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Shurtleff H, Warner M, Poliakov A, Bournival B, Shaw DW, Ishak G, et al. Functional magnetic resonance imaging for presurgical evaluation of very young pediatric patients with epilepsy. J Neurosurg Pediatr. 2010;5:500–6. [DOI] [PubMed] [Google Scholar]
- [90].Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–8. [DOI] [PubMed] [Google Scholar]
- [91].Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–71. [DOI] [PubMed] [Google Scholar]
- [92].Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. [DOI] [PubMed] [Google Scholar]
- [93].Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Todd Constable R. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50:2225–41. [DOI] [PubMed] [Google Scholar]
- [94].Ota T, Kamada K, Kawai K, Yumoto M, Aoki S, Saito N. Refined analysis of complex language representations by non-invasive neuroimaging techniques. Br J Neurosurg. 2011;25:197–202. [DOI] [PubMed] [Google Scholar]
- [95].Liégeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, Baldeweg T. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17:1861–7. [DOI] [PubMed] [Google Scholar]
- [96].Jones SE, Mahmoud SY, Phillips MD. A practical clinical method to quantify language lateralization in fMRI using whole-brain analysis. Neuroimage. 2011;54:2937–49. [DOI] [PubMed] [Google Scholar]
- [97].Rodin D, Bar-Yosef O, Smith ML, Kerr E, Morris D, Donner EJ. Language dominance in children with epilepsy: concordance of fMRI with intracarotid amytal testing and cortical stimulation. Epilepsy Behav. 2013;29:7–12. [DOI] [PubMed] [Google Scholar]
- [98].de Ribaupierre S, Fohlen M, Bulteau C, Dorfmuller G, Delalande O, Dulac O, et al. Presurgical language mapping in children with epilepsy: clinical usefulness of functional magnetic resonance imaging for the planning of cortical stimulation. Epilepsia. 2012;53:67–78. [DOI] [PubMed] [Google Scholar]
- [99].Genetti M, Tyrand R, Grouiller F, Lascano AM, Vulliemoz S, Spinelli L, et al. Comparison of high gamma electrocorticography and fMRI with electrocortical stimulation for localization of somatosensory and language cortex. Clin Neurophysiol. 2015;126:121–30. [DOI] [PubMed] [Google Scholar]
- [100].Babajani-Feremi A, Holder CM, Narayana S, Fulton SP, Choudhri AF, Boop FA, et al. Predicting postoperative language outcome using presurgical fMRI, MEG, TMS, and high gamma ECoG. Clin Neurophysiol. 2018;129:560–571. [DOI] [PubMed] [Google Scholar]
- [101].Norrelgen F, Lilja A, Ingvar M, Åmark P, Fransson P. Presurgical language lateralization assessment by fMRI and dichotic listening of pediatric patients with intractable epilepsy. Neuroimage Clin. 2014;7:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Rosenzweig MR. Representations of the two ears at the auditory cortex. Am J Physiol. 1951;167:147–58. [DOI] [PubMed] [Google Scholar]
- [103].Gelinas JN, Fitzpatrick KP, Kim HC, Bjornson BH. Cerebellar language mapping and cerebral language dominance in pediatric epilepsy surgery patients. Neuroimage Clin. 2014;6:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Méndez Orellana C, Visch-Brink E, Vernooij M, Kalloe S, Satoer D, Vincent A, et al. Crossed cerebrocerebellar language lateralization: an additional diagnostic feature for assessing atypical language representation in presurgical functional MR imaging. AJNR Am J Neuroradiol. 2015;36:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gemma M, Scola E, Baldoli C, Mucchetti M, Pontesilli S, De Vitis A, et al. Auditory functional magnetic resonance in awake (nonsedated) and propofol-sedated children. Paediatr Anaesth. 2016;26:521–30. [DOI] [PubMed] [Google Scholar]
- [106].Ives-Deliperi VL, Butler JT. Functional mapping in pediatric epilepsy surgical candidates: Functional magnetic resonance imaging under sedation with chloral hydrate. Pediatr Neurol. 2015;53:478–84. [DOI] [PubMed] [Google Scholar]
- [107].Zhang J, Mei S, Liu Q, Liu W, Chen H, Xia H, et al. fMRI and DTI assessment of patients undergoing radical epilepsy surgery. Epilepsy Res. 2013;104:253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Choudhri AF, Patel RM, Siddiqui A, Whitehead MT, Wheless JW. Cortical activation through passive-motion functional MRI. AJNR Am J Neuroradiol. 2015;36:1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang AC, Ibrahim GM, Poliakov AV, Wang PI, Fallah A, Mathern GW, et al. Corticospinal tract atrophy and motor fMRI predict motor preservation after functional cerebral hemispherectomy. J Neurosurg Pediatr. 2018;21:81–89. [DOI] [PubMed] [Google Scholar]
- [110].Chugani HT, Muller RA, Chugani DC. Functional brain reorganization in children. Brain Dev. 1996;18:347–56. [DOI] [PubMed] [Google Scholar]
- [111].Centeno M, Tierney TM, Perani S, Shamshiri EA, St Pier K, Wilkinson C, et al. Optimising EEG-fMRI for localisation of focal epilepsy in children. PLoS One. 2016;11:e0149048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Centeno M, Tierney TM, Perani S, Shamshiri EA, St Pier K, Wilkinson C, et al. Combined electroencephalography-functional magnetic resonance imaging and electrical source imaging improves localization of pediatric focal epilepsy. Ann Neurol. 2017;82:278–287. [DOI] [PubMed] [Google Scholar]
- [113].Masterton RA, Carney PW, Jackson GD. Cortical and thalamic resting-state functional connectivity is altered in childhood absence epilepsy. Epilepsy Res. 2012;99:327–34. [DOI] [PubMed] [Google Scholar]
- [114].Luo C, Qiu C, Guo Z, Fang J, Li Q, Lei X, et al. Disrupted functional brain connectivity in partial epilepsy: a resting-state fMRI study. PLoS One. 2011;7:e28196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Grassia F, Poliakov AV, Poliachik SL, Casimo K, Friedman SD, Shurtleff H, et al. Changes in resting-state connectivity in pediatric temporal lobe epilepsy. J Neurosurg Pediatr. 2018;22:270–275. [DOI] [PubMed] [Google Scholar]
- [116].Pizoli CE, Shah MN, Snyder AZ, Shimony JS, Limbrick DD, Raichle ME, et al. Resting-state activity in development and maintenance of normal brain function. Proc Natl Acad Sci U S A. 2011;108:11638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Vadivelu S, Wolf VL, Bollo RJ, Wilfong A, Curry DJ. Resting-state functional MRI in pediatric epilepsy surgery. Pediatr Neurosurg. 2013;49:261–73. [DOI] [PubMed] [Google Scholar]
- [118].Roland JL, Griffin N, Hacker CD, Vellimana AK, Akbari SH, Shimony JS, et al. Resting-state functional magnetic resonance imaging for surgical planning in pediatric patients: a preliminary experience. J Neurosurg Pediatr. 2017;20:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–9. [DOI] [PubMed] [Google Scholar]
- [120].Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–505. [DOI] [PubMed] [Google Scholar]
- [121].Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577–82. [DOI] [PubMed] [Google Scholar]
- [122].Descoteaux M High Angular Resolution Diffusion Imaging (HARDI). doi: 10.1002/047134608X.W8258. In book: Wiley Encyclopedia of Electrical and Electronics Engineering; 2015. [DOI] [Google Scholar]
- [123].Essayed WI, Zhang F, Unadkat P, Cosgrove GR, Golby AJ, O’Donnell LJ. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. Neuroimage Clin. 2017;15:659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Chaudhary UJ, Duncan JS. Applications of blood-oxygen-level-dependent functional magnetic resonance imaging and diffusion tensor imaging in epilepsy. Neuroimaging Clin N Am. 2014;24(4):671–94. [DOI] [PubMed] [Google Scholar]
- [125].Szmuda M, Szmuda T, Springer J, et al. Diffusion tensor tractography imaging in pediatric epilepsy - A systematic review. Neurol Neurochir Pol. 2016;50(1):1–6. [DOI] [PubMed] [Google Scholar]
- [126].Diehl B, Piao Z, Tkach J, et al. Cortical stimulation for language mapping in focal epilepsy: correlations with tractography of the arcuate fasciculus. Epilepsia. 2010;51(4):639–46. [DOI] [PubMed] [Google Scholar]
- [127].Ellmore TM, Beauchamp MS, Breier JI, et al. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. Neuroimage. 2010;49(3):2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Tiwari VN, Jeong JW, Asano E, Rothermel R, Juhasz C, Chugani HT. A sensitive diffusion tensor imaging quantification method to detect language laterality in children: correlation with the Wada test. J Child Neurol. 2011;26(12):1516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sreedharan RM, Menon AC, James JS, Kesavadas C, Thomas SV. Arcuate fasciculus laterality by diffusion tensor imaging correlates with language laterality by functional MRI in preadolescent children. Neuroradiology. 2015;57(3):291–7. [DOI] [PubMed] [Google Scholar]
- [130].Paldino MJ, Hedges K, Zhang W. Independent contribution of individual white matter pathways to language function in pediatric epilepsy patients. Neuroimage Clin. 2014;6:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Paldino MJ, Hedges K, Golriz F. The arcuate fasciculus and language development in a cohort of pediatric patients with malformations of cortical development. AJNR Am J Neuroradiol. 2016;37(1):169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jeong JW, Asano E, Juhász C, Chugani HT. Localization of specific language pathways using diffusion-weighted imaging tractography for presurgical planning of children with intractable epilepsy. Epilepsia. 2015;56(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Goradia D, Chugani HT, Govindan RM, Behen M, Juhász C, Sood S. Reorganization of the right arcuate fasciculus following left arcuate fasciculus resection in children with intractable epilepsy. J Child Neurol. 2011;26(10):1246–51. [DOI] [PubMed] [Google Scholar]
- [134].Kumar A, Juhasz C, Asano E, et al. Diffusion tensor imaging study of the cortical origin and course of the corticospinal tract in healthy children. AJNR Am J Neuroradiol. 2009;30(10):1963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wieshmann UC, Krakow K, Symms MR, et al. Combined functional magnetic resonance imaging and diffusion tensor imaging demonstrate widespread modified organisation in malformation of cortical development. J Neurol Neurosurg Psychiatry. 2001;70(4):521–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Rosazza C, Deleo F, D’lncerti L, et al. Tracking the re-organization of motor functions after disconnective surgery: A longitudinal fMRI and DTI study. Front Neurol. 2018;9:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Nelles M, Urbach H, Sassen R, et al. Functional hemispherectomy: postoperative motor state and correlation to preoperative DTI. Neuroradiology. 2015;57(11):1093–102. [DOI] [PubMed] [Google Scholar]
- [138].Jeong JW, Asano E, Brown EC, Tiwari VN, Chugani DC, Chugani HT. Automatic detection of primary motor areas using diffusion MRI tractography: comparison with functional MRI and electrical stimulation mapping. Epilepsia. 2013;54(8):1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kiehna EN, Widjaja E, Holowka S, et al. Utility of diffusion tensor imaging studies linked to neuronavigation and other modalities in repeat hemispherotomy for intractable epilepsy. J Neurosurg Pediatr. 2016;17(4):483–90. [DOI] [PubMed] [Google Scholar]
- [140].Toda K, Baba H, Ono T, Ono K. The utility of diffusion tensor imaging tractography for post-operative evaluation of a patient with hemispherotomy performed for intractable epilepsy. Brain Dev. 2014;36:641–4. [DOI] [PubMed] [Google Scholar]
- [141].Ho AL, Pendharkar AV, Sussman ES, Casazza M, Grant GA. Diffusion tensor imaging in an infant undergoing functional hemispherectomy: A surgical aid. Cureus. 2017;9:e1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Kim GH, Seo JH, Schroff S, Chen PC, Lee KH, Baumgartner J. Impact of intraoperative 3-T MRI with diffusion tensor imaging on hemispherectomy. J Neurosurg Pediatr. 2017;19:63–69. [DOI] [PubMed] [Google Scholar]


