Abstract
Background.
Non-invasive markers of Type-2 inflammation are needed to identify children and adolescents who might benefit from personalized biologic therapy.
Objective.
We hypothesized that blood eosinophil counts would predict one or more acute visits for asthma and that prediction could be improved with the addition of a second, non-invasive Type-2 inflammatory biomarker.
Methods.
Children and adolescents 5 to 21 years (N=589) with an asthma exacerbation necessitating systemic corticosteroid treatment in the previous year completed a characterization visit and telephone calls at 6 and 12 months. The primary outcome was an acute visit for asthma with receipt of systemic corticosteroids. Acute visits were verified by medical record review. Exploratory outcomes included time to first acute visit and hospitalization.
Results.
Acute visits occurred in 106 (35.5%) children and 72 (24.8%) adolescents. Elevated blood eosinophils were associated with increased odds and shorter time to first acute visit, but optimal cut-points differed by age (≥150 vs. ≥300 cells/microliter for children vs. adolescents, respectively). Addition of a second marker of Type-2 inflammation did not improve prediction in children, but increased the odds and hazard of an acute visit up to 16.2% and 11.9%, respectively, in adolescents. Similar trends were noted for hospitalizations.
Conclusion.
Blood eosinophils and other non-invasive markers of Type-2 inflammation may be useful in the clinical assessment of children and adolescents with asthma. However, features of Type-2 inflammation vary by age. Whether children and adolescents also respond differently to management of Type-2 inflammation is unclear and warrants further evaluation.
Keywords: Asthma control, Asthma exacerbation, Biomarker, Eosinophil, Type 2 inflammation, Allergic sensitization
Introduction
Asthma symptom control is suboptimal in the majority of children worldwide.1 Even in developed nations with widespread availability of asthma controller medications such as inhaled corticosteroids (ICS) and standardized treatment guidelines,2 more than 50% of children with asthma experience an exacerbation each year.3 While the factors responsible for asthma exacerbations in children are complex and include limited access to care,4 poor adherence to ICS5, 6 and exposures to environmental allergens and irritants such as tobacco smoke,7–9 it is also recognized that children with exacerbation-prone asthma are a heterogeneous group10 with differing biological mechanisms or “endotypes” that contribute to varied clinical outcomes and longitudinal disease trajectories.11 However, the precise biological mechanisms that contribute to exacerbations in children are not well understood.
Type-2 inflammation, which is driven by interleukin (IL)-4, IL-5, and IL-13 and characterized by high immunoglobulin E (IgE) antibody titers and eosinophilia,12 is thought to be a pivotal risk factor for persistent and severe wheezing and airflow obstruction in children with asthma13–16 that may be amenable to treatment with personalized biologic therapy. However, studies of Type-2 inflammation in children are quite limited. Whereas the majority of studies of Type-2 inflammation in adults have focused on gene expression in invasive airway samples obtained for research purposes,17, 18 bronchoscopy for research purposes is typically not performed in pediatric asthma patients, particularly in children without severe uncontrolled asthma.19 Furthermore, while other studies of Type-2 inflammation in adults have utilized induced sputum samples,20, 21 the induced sputum procedure is not feasible in younger children and has a relatively poor success rate (i.e., sample yield) in pre-adolescents.22, 23 Non-invasive markers of Type-2 inflammation are therefore needed in children with exacerbation-prone asthma to identify those patients who might benefit from personalized biologic therapy.
We therefore analyzed 12 month longitudinal data from a cohort of children and adolescents with exacerbation-prone asthma participating in asthma research studies in Atlanta, Georgia. We hypothesized that blood eosinophil counts would predict one or more acute visits for asthma at 6 or 12 months post-measurement, defined as an emergency department visit or unscheduled healthcare visit with receipt of systemic corticosteroids, and that prediction of acute events could be improved with the addition of a second, non-invasive Type-2 inflammatory biomarker.
Methods
This study was a secondary analysis of children and adolescents with exacerbation-prone asthma participating in asthma research studies at Emory University in Atlanta, Georgia, between January 2007 and December 2016. Children and adolescents 5 to 21 years of age were eligible for inclusion if they had a physician diagnosis of asthma, an exacerbation necessitating systemic corticosteroid treatment in the previous year, and either ≥ 12% reversibility in the forced expiratory volume in one second (FEV1) after bronchodilator administration or airway hyperresponsiveness to methacholine evidenced by a provocative concentration of methacholine causing a 20% decline in FEV1 of 16 mg/mL or less. Exclusion criteria included premature birth before 35 weeks gestation, current smoking, or other airway disorders mimicking asthma such as pulmonary aspiration or vocal cord dysfunction. Permission to proceed with this study was granted by the Emory University Institutional Review Board. Informed written consent and assent were obtained.
Study design and procedures.
Participants completed a baseline study visit that was postponed if acute illness or an asthma exacerbation treated with systemic corticosteroids was reported within the preceding four weeks. At the study visit, participants completed questionnaires pertaining to demographics, medical history and symptoms. Neighborhood characteristics were obtained from the 2010–2014 American Community Survey available at www.factfinder.census.gov.24 Spirometry (KoKo® PDS, Ferraris, Louisville, CO) was performed as previously described following a bronchodilator withhold.25 The best of three forced vital capacity (FVC) maneuvers was interpreted according to population reference equations26 before and after 4 inhalations of albuterol sulfate (90 microgram/actuation) delivered through a metered dose inhaler. Airway hyperresponsiveness to methacholine was also measured in a subset of participants with <12% FEV1 reversibility to ensure study eligibility as described previously.27 Exhaled nitric oxide concentrations were measured with a commercial device (NIOX MINO®, Circrassia Pharmaceuticals, Chicago, IL).28 Aeroallergen sensitization was assessed by specific IgE testing (Children’s Healthcare of Atlanta, Atlanta, GA) or skin prick testing with the following extracts: tree mix (Quercus alba, Ulmus americana, Platanus acerifolia, Salix caprea, Populus deltoides), grass mix (Cynodon dactylon, Lolium perenne, Phleum pratense, Poa pratensis, Sorghum halepense, Paspalum notatum), weed mix (Artemisia vulgaris, Chrysanthemum leucanthemum, Taraxacum vulgare, Solidago virgaurea), common ragweed (Ambrosia artemisiifolia), Alternaria alternata, Aspergillus fumagatis, Cladosporium herbarum, dog dander, cat dander, German cockroach (Blatella germanica), Dermatophagoides farinae, and Dermatophagoides pteronyssinus (Greer® Laboratories, Lenoir, NC). Venipuncture was also performed for quantification of blood eosinophils and total IgE (Children’s Healthcare of Atlanta). Participants were also telephoned at 6 and 12 months after the study visit and questioned about asthma-related healthcare utilization.
Outcome measures.
The primary outcome measure was one or more acute visits for asthma (defined as an emergency department visit or unscheduled healthcare visit) with receipt of systemic corticosteroids at 6 or 12 months post-measurement.29 Exploratory outcomes included time to first acute visit and hospitalization. Acute visits and hospitalizations were verified by a review of medical records.
Statistical analyses.
Data were analyzed with SPSS® Statistics (Version 24, IBM, Armonk, NY) with stratification by age group (5–11 vs. 12–21 years). Complete demographic data, asthma medical history data, lung function data and outcome data were available for all participants (N = 589). However, blood eosinophil counts, serum IgE, exhaled nitric oxide and aeroallergen sensitization were missing in 35 (5.9%), 55 (9.3%), 30 (5.1%) and 50 (8.5%) of participants and were subjected to multiple imputation to retain variables in the models. Little’s chi square tests were performed on the expectation-maximum covariance matrix of key study variables (age, sex, race, household education, asthma controller medications, FEV1 % predicted value, blood eosinophil counts, serum IgE, exhaled nitric oxide, and aeroallergen sensitization) to confirm that values were missing completely at random. Multiple imputation was performed using a fully conditional specification (Markov Chain Monte Carlo algorithm) with 5 iterations. Data presented are pooled estimates.
Multivariate logistic regression was performed to obtain odds ratios for acute visits within 12 months. Cox proportional hazards regression was used to determine time to first acute visit. Predictors included blood eosinophils (≥150, ≥200, ≥250 and ≥300 cells/microliter), exhaled nitric oxide (≥35 ppb for children 5–11 years and ≥50 ppb for adolescents 12–21 years),28 serum IgE concentration (≥100, ≥200, and ≥300 kU/L)30 and aeroallergen sensitization (any sensitization and multiple sensitization). Models were adjusted for sex, ethnicity, race, household education level, tobacco smoke exposure, and ICS use.
Results
Five hundred eighty nine children (N = 299) and adolescents (N = 290) with an asthma exacerbation in the previous year were enrolled. Demographic features of the participants are shown in Table 1.
Table 1.
Features of the participants. Data represent the mean ± standard deviation or the number of participants (%).
| All participants N = 589 |
Children Age 5–11 years N = 299 |
Adolescents Age 12–21 years N = 290 |
|
|---|---|---|---|
| Age (years) | 12.6 ± 4.4 | 9.1 ± 1.8 | 16.2 ± 3.2 |
| Asthma duration (years) | 9.0 ± 4.3 | 6.6 ± 2.8 | 11.8 ± 4.2 |
| Males | 334 (56.7) | 178 (59.5) | 156 (53.8) |
| Race | |||
| White | 196 (33.3) | 77 (25.8) | 119 (41.0) |
| Black | 332 (56.4) | 196 (65.6) | 136 (46.9) |
| More than one race | 55 (9.3) | 24 (8.6) | 31 (10.7) |
| Other | 6 (1.0) | 2 (0.7) | 4 (1.4) |
| Hispanic ethnicity | 43 (7.3) | 21 (7.0) | 22 (7.6) |
| Household education level | |||
| Refused | 117 (19.9) | 52 (17.4) | 65 (22.4) |
| Did not complete high school | 20 (3.4) | 9 (3.0) | 11 (3.8) |
| High school diploma | 74 (12.6) | 42 (14.0) | 32 (11.0) |
| Some college or technical training | 175 (29.7) | 84 (28.1) | 91 (31.4) |
| College degree | 203 (34.5) | 112 (37.5) | 91 (31.4) |
| Family history | |||
| Father with asthma | 127 (21.6) | 73 (24.4) | 54 (18.6) |
| Mother with asthma | 205 (34.8) | 114 (38.1) | 91 (31.4) |
| Other medical conditions | |||
| Obesity | 150 (25.5) | 79 (26.5) | 71 (24.5) |
| Pneumonia (ever) | 254 (43.1) | 127 (42.5) | 127 (43.8) |
| Recurrent sinusitis | 155 (26.3) | 66 (22.1) | 89 (30.7) |
| Eczema | 322 (54.7) | 191 (63.9) | 131 (45.2) |
| Allergic rhinitis | 463 (78.6) | 242 (80.9) | 221 (76.2) |
| Nasal polyps | 32 (5.4) | 10 (3.3) | 22 (7.6) |
| Gastroesophageal reflux | 123 (20.9) | 49 (16.4) | 74 (25.5) |
| Asthma controller medications | |||
| Inhaled corticosteroid | 451 (76.6) | 258 (86.3) | 193 (66.6) |
| Long-acting beta agonist | 328 (55.7) | 157 (52.5) | 171 (59.0) |
| Montelukast | 347 (58.9) | 195 (65.2) | 152 (52.4) |
| Omalizumab | 19 (3.2) | 7 (2.3) | 12 (2.3) |
| Indoor exposures | |||
| Cat | 79 (13.4) | 32 (10.7) | 47 (16.2) |
| Dog | 199 (33.8) | 97 (32.4) | 102 (35.2) |
| Tobacco smoke | 92 (15.6) | 33 (11.0) | 59 (20.3) |
| ZIP code features | |||
| Population (in thousands) | 34.4 ± 16.6 | 34.8 ± 17.1 | 33.8 ± 16.0 |
| Unemployment (% of adults) | 12.6 ± 5.8 | 12.6 ± 5.6 | 12.6 ± 6.1 |
| Bachelors degree (% of adults) | 31.9 ± 16.5 | 30.7 ± 15.5 | 33.4 ± 17.5 |
| Below poverty threshold (% of families) | 20.2 ± 11.5 | 19.8 ± 10.7 | 20.5 ± 12.4 |
| Asthma healthcare utilization | |||
| Emergency department (past year) | 276 (46.9) | 170 (56.9) | 106 (36.6) |
| Hospitalization (past year) | 151 (25.6) | 84 (28.1) | 67 (23.1) |
| Intubation for asthma (ever) | 78 (13.2) | 37 (12.4) | 41 (14.1) |
| Symptoms (past four weeks) | |||
| Nighttime symptoms >twice weekly | 112 (19.1) | 61 (20.5) | 51 (17.7) |
| Dyspnea >twice weekly | 142 (24.3) | 70 (23.6) | 72 (25.0) |
| Wheeze >twice weekly | 125 (21.4) | 60 (20.3) | 65 (22.6) |
| Lung function (% predicted value) | |||
| Baseline FVC | 100.6 ± 15.4 | 102.7 ± 14.5 | 98.5 ± 16.1 |
| Baseline FEV1 | 90.3 ± 17.8 | 93.6 ± 16.3 | 86.9 ± 18.6 |
| Post-bronchodilator FVC | 107.1 ± 15.4 | 110.3 ± 14.6 | 103.7 ± 15.6 |
| Post-bronchodilator FEV1 | 103.1 ± 16.4 | 107.2 ± 16.0 | 99.0 ± 15.7 |
Non-invasive Type-2 inflammatory markers.
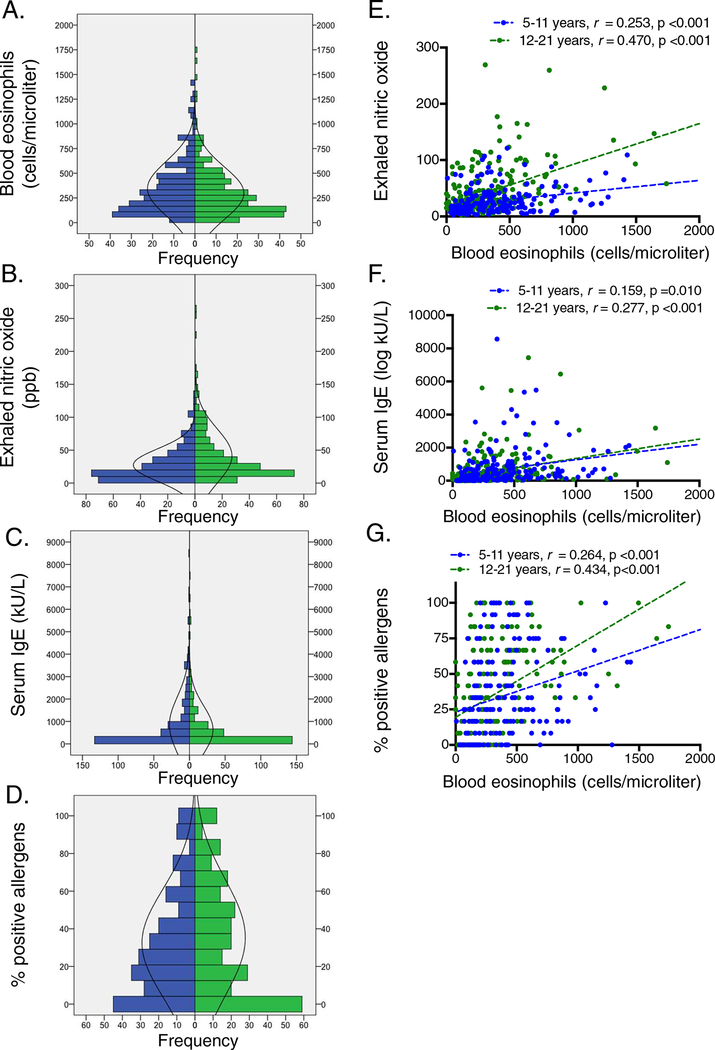
Blood eosinophil counts were 344.1 ± 274.5 cells/microliter in children and 296.5 ± 265.2 cells/microliter in adolescents. The distribution of blood eosinophil counts, exhaled nitric oxide, serum IgE, and the percentage of positive aeroallergens is shown in Figure 1A–D. In children and adolescents, patterns of sensitization were as follows: mold sensitization, 47.8% and 47.9%; pollen sensitization, 62.5% and 59.3%; cat sensitization, 32.2% and 39.5%; dog sensitization, 28.7% and 33.5%; dust mite sensitization, 63.9% and 60.7%; and cockroach sensitization, 25.3% and 30.4%, respectively. Associations between blood eosinophils, exhaled nitric oxide, serum IgE, and the percentage of positive allergens are shown in Figure 1E–G.
Figure 1.
Distribution of (A) blood eosinophils, (B) exhaled nitric oxide, (C) serum IgE, and (D) percentage of positive allergens and associations between blood eosinophils and (E) exhaled nitric oxide, (F) serum IgE, and (G) percentage of positive allergens in children 5–11 years (blue) and adolescents 12–21 years (green).
Acute visits.
The primary outcome of interest, an acute visit for an asthma exacerbation with receipt of systemic corticosteroids, occurred in 106 (35.5%) children and 72 (24.8%) adolescents. Among participants with an acute visit, the time to first acute visit was 140.5 ± 120.7 days in children and 122.3 ± 103.2 in adolescents. Hospitalizations occurred in 22 (20.8%) children and 19 (26.4%) adolescents.
Single biomarker prediction of acute events.
Crude and adjusted associations between acute visits and non-invasive Type-2 inflammatory markers are shown in Table 2. In children, blood eosinophil counts (≥150, ≥200 and ≥250 cells/microliter) and sensitization were significant predictors of acute visit after covariate adjustment, although blood eosinophils ≥150 cells/microliter were most strongly associated with acute visit. Frequency of daytime symptoms was also associated with acute visits but associations were of a lesser magnitude than those observed for the Type-2 inflammatory markers (adjusted odds ratio (OR): 1.23, 95% confidence interval (CI): 1.02, 1.47).
Table 2.
Associations between single non-invasive Type-2 inflammatory markers and an acute visit for asthma by 12 months (primary outcome).
| Age group | Predictor | % with outcome | Crude OR (95% CI) |
Adjusted OR1 (95% CI) |
Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Children 5–11 years | Elevated blood eosinophils | |||||
| ≥ 150 cells/microliter | 40.4 | 2.68 (2.07, 3.46)* | 2.39 (1.83, 3.13)* | 0.848 | 0.312 | |
| ≥ 200 cells/microliter | 40.4 | 1.81 (1.47, 2.22)* | 1.66 (1.33, 2.06)* | 0.707 | 0.437 | |
| ≥ 250 cells/microliter | 41.1 | 1.74 (1.43, 2.12)* | 1.51 (1.23, 1.87)* | 0.627 | 0.509 | |
| ≥ 300 cells/microliter | 39.8 | 1.42 (1.17, 1.73)* | 1.19 (0.97, 1.46) | 0.510 | 0.578 | |
| Elevated exhaled nitric oxide2 | 35.2 | 0.99 (0.80, 1.22) | 0.97 (0.78, 1.22) | 0.293 | 0.708 | |
| Elevated serum IgE | ||||||
| ≥ 100 kU/L | 35.7 | 1.06 (0.84, 1.35) | 0.99 (0.77, 1.28) | 0.777 | 0.246 | |
| ≥ 200 kU/L | 35.5 | 1.00 (0.82, 1.23) | 0.86 (0.69, 1.08) | 0.648 | 0.360 | |
| ≥ 300 kU/L | 35.8 | 1.04 (0.85, 1.26) | 0.92 (0.75, 1.14) | 0.547 | 0.465 | |
| Aeroallergen sensitization | ||||||
| Any sensitization | 37.4 | 1.84 (1.37, 2.47)* | 1.65 (1.21, 2.25)* | 0.893 | 0.188 | |
| Multiple sensitization | 38.2 | 1.64 (1.30, 2.08)* | 1.49 (1.17, 1.91)* | 0.694 | 0.426 | |
| Adolescents 12–21 years | Elevated blood eosinophils | |||||
| ≥ 150 cells/microliter | 28.7 | 1.98 (1.54, 2.55)* | 1.50 (1.13, 1.98)* | 0.772 | 0.392 | |
| ≥ 200 cells/microliter | 31.5 | 2.46 (1.93, 3.12)* | 1.82 (1.40, 2.36)* | 0.727 | 0.492 | |
| ≥ 250 cells/microliter | 32.5 | 2.24 (1.79, 2.80)* | 1.61 (1.26, 2.07)* | 0.621 | 0.586 | |
| ≥ 300 cells/microliter | 35.7 | 2.53 (2.03, 3.16)* | 2.04 (1.60, 2.60)* | 0.555 | 0.687 | |
| Elevated exhaled nitric oxide2 | 31.3 | 1.59 (1.26, 2.00)* | 1.24 (0.95, 1.61) | 0.354 | 0.757 | |
| Elevated serum IgE | ||||||
| ≥ 100 kU/L | 28.3 | 2.00 (1.54, 2.61)* | 1.43 (1.06, 1.92)* | 0.769 | 0.361 | |
| ≥ 200 kU/L | 27.7 | 1.47 (1.17, 1.85)* | 1.04 (0.81, 1.35) | 0.628 | 0.461 | |
| ≥ 300 kU/L | 28.0 | 1.44 (1.16, 1.80)* | 1.07 (0.84, 1.37) | 0.555 | 0.535 | |
| Aeroallergen sensitization | ||||||
| Any sensitization | 29.3 | 4.69 (3.17, 6.93)* | 2.39 (1.54, 3.71)* | 0.928 | 0.263 | |
| Multiple sensitization | 30.8 | 4.06 (2.95, 5.58)* | 2.31 (1.63, 3.28)* | 0.730 | 0.438 |
p < 0.05
OR = odds ratio, CI = confidence interval
Adjusted for sex, ethnicity, race, household education level, tobacco smoke exposure, and ICS use.
Defined as ≥35 ppb for children 5–11 years and ≥50 ppb for adolescents 12–21 years
In adolescents, blood eosinophil counts (≥150, ≥200, ≥250 and ≥350 cells/microliter), serum IgE ≥100 kU/L and sensitization were significant predictors of acute visit after covariate adjustment. However, in contrast to children, blood eosinophils ≥300 cells/microliter were most strongly associated with the odds of having an acute visit for asthma. Frequency of daytime symptoms was also associated with acute visits but associations were again of a lesser magnitude than those observed for the Type-2 inflammatory markers (adjusted OR: 1.85, 95% CI: 1.47, 2.33). However, for each of the Type-2 inflammatory predictors irrespective of age group, specificity remained poor (Table 2).
Time to first acute visit analyses for children and adolescents also showed similar trends (Table E1).
Multiple biomarker prediction of acute events.
To determine whether blood eosinophils plus a second non-invasive marker of Type-2 inflammation provided better detection of acute visits for asthma over 12 months, blood eosinophils, serum IgE and sensitization were modeled as interaction terms. The results are provided in Table 3. In children, the interaction of blood eosinophils with serum IgE ≥100 kU/L and sensitization was associated with increased odds of acute visit by 12 months, but the corresponding odds ratios were not increased compared to blood eosinophils as a single predictor. In subset analysis of children with symptoms more than twice weekly, similar results were noted and there was again no additional benefit of a second non-invasive marker of Type-2 inflammation compared to blood eosinophils alone (Table 3).
Table 3.
Associations between multiple non-invasive Type-2 inflammatory markers1 and an acute visit for asthma by 12 months.
| Group | Model | Adjusted OR2 (95% CI) |
Change in OR relative to blood eosinophils |
|---|---|---|---|
| Children 5–11 years | |||
| All patients (N = 299) | Blood eosinophils ≥150 cells/microliter | 2.39 (1.83, 3.13) | -- |
| Blood eosinophils ≥150 + IgE > 100 kU/L | 1.51 (1.22, 1.88) | −36.8% | |
| Blood eosinophils ≥150 + any sensitization | 2.07 (1.64, 2.62) | −13.4% | |
| Blood eosinophils ≥150 + multiple sensitization | 1.85 (1.48, 2.30) | −22.6% | |
| Symptoms > twice weekly (N = 70) | Blood eosinophils ≥150 cells/microliter | 4.34 (2.36, 7.98) | -- |
| Blood eosinophils ≥150 + IgE > 100 kU/L | 1.82 (1.09, 3.03) | −58.1% | |
| Blood eosinophils ≥150 + any sensitization | 2.32 (1.42, 3.79) | −46.5% | |
| Blood eosinophils ≥150 + multiple sensitization | 1.65 (1.07, 2.54) | −62.0% | |
| Adolescents 12–21 years | |||
| All patients (N = 290) | Blood eosinophils ≥300 cells/microliter | 2.04 (1.60, 2.60) | -- |
| Blood eosinophils ≥300 + IgE > 100 kU/L | 1.68 (1.31, 2.14) | −17.6% | |
| Blood eosinophils ≥300 + any sensitization | 2.27 (1.78, 2.91) | 11.3% | |
| Blood eosinophils ≥300 + multiple sensitization | 2.37 (1.86, 3.02) | 16.2% | |
| Symptoms > twice weekly (N = 72) | Blood eosinophils ≥300 cells/microliter | 2.44 (1.70, 3.51) | -- |
| Blood eosinophils ≥300 + IgE > 100 kU/L | 2.43 (1.60, 3.69) | −0.4% | |
| Blood eosinophils ≥300 + any sensitization | 2.72 (1.88, 3.94) | 11.5% | |
| Blood eosinophils ≥300 + multiple sensitization | 2.54 (1.69, 3.82) | 4.1% | |
OR = odds ratio, CI = confidence interval
Modeled as interaction terms
Adjusted for sex, ethnicity, race, household education level, tobacco smoke exposure, and ICS use.
In contrast, in adolescents, the interaction of blood eosinophils with sensitization increased the odds of acute visit for asthma by up to 16.2% compared to blood eosinophils alone (Table 3). In subset analysis of adolescents with symptoms more than twice weekly, sensitization also provided some additive effects (Table 3).
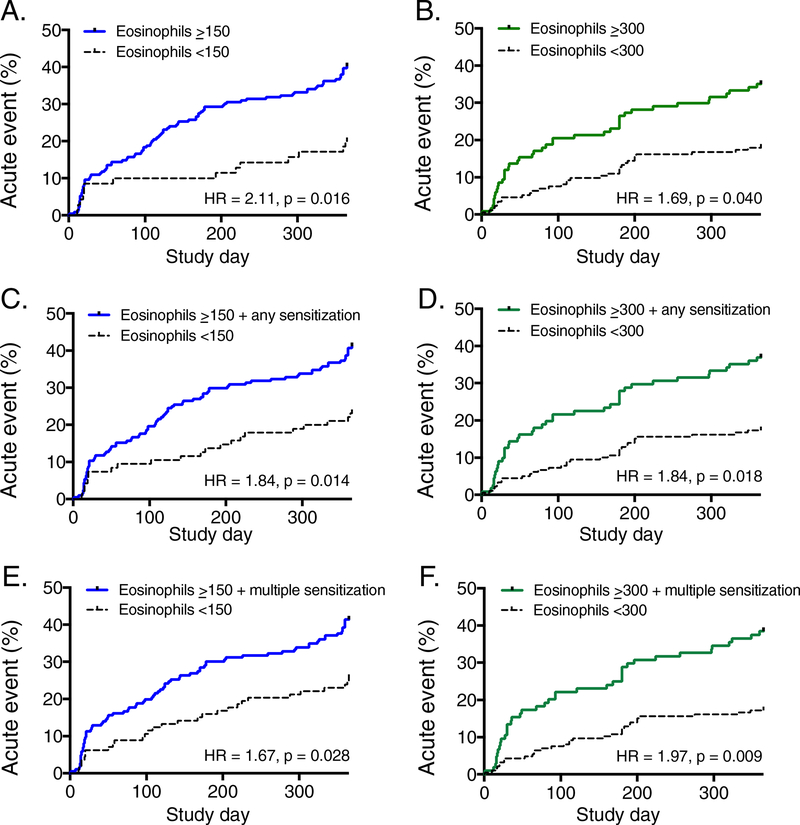
Time to first acute visit analyses showed similar trends in children and adolescents (Table E2), with a significantly shorter time to first acute visit in adolescents with elevated blood eosinophils (≥300 cells/microliter) and sensitization (Figure 2). In subset analysis of adolescents with symptoms more than twice weekly, a significant interaction between blood eosinophils ≥300 cells/microliter and any sensitization was again noted and this association was stronger than that noted for blood eosinophils as a single predictor (Table E2).
Figure 2.
Time to first acute visit in children age 5–11 years (left panels, blue) and adolescents (right panels, green) based on elevated blood eosinophils alone (panels A-B), elevated blood eosinophils plus any sensitization (panels C-D), and elevated blood eosinophils plus multiple sensitization (panels E-F). Models were adjusted for sex, ethnicity, race, household education level, tobacco smoke exposure, and inhaled corticosteroid use.
Hospitalization.
In children, blood eosinophils ≥150 cells/microliter were associated with increased odds of hospitalization (adjusted OR: 2.50, 95% CI: 1.23, 5.07). The interaction of blood eosinophils with sensitization did not reach the level of significance (eosinophils ≥150 cells/microliter plus any sensitization, adjusted OR: 1.42, 95% CI: 0.37, 5.42; eosinophils ≥150 cells/microliter plus multiple sensitization, adjusted OR: 1.38, 95% CI: 0.42, 4.51). In adolescents, blood eosinophils ≥300 cells/microliter were similarly associated with increased odds of hospitalization (adjusted OR: 4.79, 95% CI: 1.28, 17.93). The interaction of blood eosinophils with sensitization was also associated with increased odds of hospitalization in adolescents (eosinophils ≥300 cells/microliter plus any sensitization, adjusted OR: 4.79, 95% CI: 1.28, 17.93; eosinophils ≥300 cells/microliter plus multiple sensitization, adjusted OR: 5.10, 95% CI: 1.36, 19.08), although lack of precision in the point estimates was noted.
Discussion
In this cohort of children and adolescents with exacerbation-prone asthma in Atlanta, Georgia, elevated blood eosinophils were associated with acute visits for asthma exacerbations over 12 months of follow-up in both univariate and adjusted analyses. However, optimal cut-points for blood eosinophils differed by age and were lower for children 5–11 years (≥150 cells/microliter) than for adolescents (≥300 cells/microliter). Furthermore, the addition of a second non-invasive marker of Type-2 inflammation (i.e., sensitization) was not useful in children but improved prediction of acute events in adolescents. These observations suggest that single measures of blood eosinophils and other non-invasive markers of Type-2 inflammation can aid clinical assessment of children and adolescents with asthma. However, these data also suggest that features of Type-2 inflammation vary by age.
In adults, numerous studies have shown associations between elevated blood eosinophils, poorer asthma control and asthma exacerbations in general asthma populations,31–33 which are a primary driver of asthma-related healthcare utilization and cost.34 For example, adults with elevated blood eosinophils (i.e., ≥350 or ≥400 cells/microliter) have a greater risk of hospital admission35, 36 and hospital readmission within the following year37, as well as nearly 2-fold increased healthcare costs.38 Our findings are similar to these studies and others that have noted an increased frequency of exacerbation or increased prevalence of severe asthma exacerbation in children and adolescents with higher blood eosinophil counts.39, 40 However, to our knowledge, our observation of age-related differences in non-invasive Type-2 inflammatory markers between pre-adolescent children and adolescents has not been previously reported.
Although a few other studies have also examined multiple versus single non-invasive Type-2 biomarkers in pediatric populations, there are important methodological differences worth mentioning. For example, whereas Konradsen et al.41 noted improved discrimination of exacerbations in children with “high” exhaled nitric oxide and “high” blood eosinophils based on median split of the sample, this analysis was cross-sectional in nature and did not address potential sources of variable confounding.41 A similar analysis of National Health and Nutrition Examination Survey results33 likewise noted higher odds of asthma exacerbations and emergency department visits in participants with exhaled nitric oxide concentrations ≥50 ppb and blood eosinophils ≥500 cells/microliter, but this analysis spanned a broad age range (6 to 80 years) and utilized biomarker cut-points that are more suitable for adults than children28 and therefore have unclear sensitivity and specificity for outcome detection in pediatric populations. In an unrelated study, Malinovschi et al.42 also noted an increased prevalence of uncontrolled asthma and frequent asthma exacerbations in patients with simultaneous increases in exhaled nitric oxide concentrations (≥20 ppb) and blood eosinophil counts (≥300 cells/microliter) after covariate adjustment, but this study again spanned a wide age range (10 −35 years) and was cross-sectional in nature.
Strengths of the present study include the detailed clinical characterization and follow-up of enrolled participants with verification of outcomes (i.e., acute events) through medical records review. The larger nature of the sample also permitted stratification by age group and adjustment for key covariates (sex, ethnicity, race, household education level, tobacco smoke exposure, and ICS use) which are known to be associated with the primary outcome of interest (acute visits for asthma).43 The inclusion criterion of an exacerbation treated with systemic corticosteroids in the previous year also ensured a study population with sufficient disease burden at greatest risk for the primary outcome, since several prior studies in children have identified a recent exacerbation treated with systemic corticosteroids as the strongest risk factor for subsequent exacerbations regardless of disease severity or use of controller medications.40, 44–47 This may explain why other investigators have found no associations between non-invasive Type-2 inflammatory markers and clinical outcomes in milder, general asthma populations irrespective of prior exacerbation history.48 Indeed, the prevalence of acute visits in our study was similar to that observed in other studies of children and adolescents with difficult-to-treat asthma.49, 50
Nonetheless, this study does have limitations. Recognizing that biomarker cut-points are ideal for practicing clinicians, we elected not to analyze blood eosinophil counts as a continuous measure (converted to the log scale). However, the optimal cut-point of blood eosinophils for clinical detection of Type-2 inflammation is still not clear. One retrospective study of children 5–11 years in a large medical care organization compared blood eosinophil cutoff points of 150 cells/microliter, 200 cells/microliter, 300 cells/microliter and 400 cells/microliter and their associations with the rate and risk of future exacerbation.51 That analysis identified an ideal cutpoint of 300 cells/microliter, which was associated with a 52% increase in asthma exacerbation rate and 40% increase in the frequency of any exacerbation.51 It is unclear why the 300 cells/microliter cut-point was not significant in our study, although this finding could be due to differences in sociodemographics between the study populations. Our observation of a higher optimal blood eosinophil cut-point (≥300 cells/microliter) in adolescents is in keeping with other studies of older populations. For example, one study of patients ≥12 years with severe uncontrolled asthma found that a blood eosinophils ≥400 cells/microliter was a significant risk factor for 2 or more asthma exacerbations and any emergency department visit or hospitalization in the outcome year after covariate adjustment.52 Similarly, in adults 18–64 years with persistent asthma, a blood eosinophil cut-point of 400 cells/microliter also predicted the annualized rate of subsequent exacerbations and short-acting beta agonist pharmacy dispenses.53 Nonetheless, other studies have argued for differing cut-points of blood eosinophils for the initiation of biologic therapy. Secondary analyses of adults treated with mepolizumab (i.e., anti-IL-5) have shown reduction of exacerbation rates by 52% in patients with baseline blood eosinophil counts of at least 150 cells/microliter.54 In that same study, exacerbation rates were reduced by 70% in patients with baseline blood eosinophil counts ≥500 cells/microliter.54 However, in other biologic studies, adults with blood eosinophil counts ≥300 cells/microliter treated with either dupilumab (i.e., anti-IL-4 receptor a) or benralizumab (i.e., anti-IL-5 receptor a) had the greatest reduction in the risk of severe asthma exacerbations.55, 56
Other important limitations of the study include the measurement of Type-2 biomarkers at a single time point, which may not be optimal for assessment of Type-2 inflammation. For example, one analysis of adults with severe asthma found that “same day” measurements of blood eosinophils and IgE identified only half of the eosinophilic group.36 A separate study of adults with mild asthma also noted marked within-subject variability in blood eosinophil counts measured on four different occasions that were not solely due to seasonal fluctuations or asthma medication use.57 However, other studies have shown that multiple measures of blood eosinophils only marginally increase the sensitivity of the biomarker.58 It is also possible that blood eosinophils may not adequately reflect the degree of Type-2 inflammation in the airways. However, unlike blood eosinophils, airway eosinophils have not been associated with responsiveness to mepolizumab therapy in adults.58, 59 Blood eosinophils have been shown to predict sputum eosinophilia60 and offer several advantages over airway eosinophil measurement, which is invasive and difficult to perform in general populations of children. Furthermore, although periostin been shown to correlate with blood and airway eosinophils and exhaled nitric oxide concentrations in adults with asthma,61, 62 periostin concentrations were not available in the present study for comparison. Studies of periostin are also very limited in children with asthma, with little conclusive data,63 since periostin levels are typically higher in children than in adults due to osteoblast stimulation during linear growth.64 Finally, the observed associations between blood eosinophils and acute visits in the present study may potentially be due to unmeasured confounding, despite adjustment of models for known cofounders such as demographic features, household education level, tobacco smoke exposure and ICS use. For example, frequency of systemic corticosteroid use prior to study enrollment was not adequately documented and could potentially impact blood eosinophil counts. Other factors such as lung function, which are also associated with exacerbations, were also not accounted for by the model and could have also contributed to our observations.
In summary, elevated blood eosinophils were associated with increased odds and shorter time to first acute visit for asthma in children and adolescents, particularly in patients with frequent symptoms, but optimal cut-points for blood eosinophils differed by age. Addition of a second marker of Type-2 inflammation improved prediction of acute events, but only in adolescents. Similar trends were noted for asthma hospitalizations. Blood eosinophils and other non-invasive markers of Type-2 inflammation may be therefore useful in the clinical assessment of children and adolescents with asthma. Whether children and adolescents also respond differently to management strategies for Type-2 inflammation is unclear and warrants further evaluation.
Supplementary Material
Highlights Box.
What is already known about this topic?
Studies have demonstrated associations between elevated blood eosinophils, poor symptom control, and exacerbations in adults with asthma. The utility of blood eosinophils in the prediction of asthma exacerbations in children is unclear.
What does this article add to our knowledge?
Elevated blood eosinophils were associated with acute visits for asthma, but optimal blood eosinophil cut-points differed by age group. Addition of a second Type-2 inflammatory biomarker improved prediction of an acute visit only in adolescents.
How does this study impact current management guidelines?
Blood eosinophils and other non-invasive markers of Type-2 inflammation may be useful in the clinical assessment of children and adolescents with asthma, but features of Type-2 inflammation vary by age.
Acknowledgments
This study was supported in part by R01NR013700, R01NR012021, R01HL69170, UL1TR000454, and the Children’s Healthcare of Atlanta Pediatric Research Alliance Center for Clinical Outcomes Research and Public Health
Abbreviations
- CI
Confidence interval
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- HR
Hazard ratio
- ICS
Inhaled corticosteroid
- IgE
Immunoglobulin E
- IL
Interleukin
- OR
Odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could appect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures:
Samar P. Shah, M.D
Nothing to disclose.
Jocelyn Grunwell, M.D., Ph.D.
Nothing to disclose.
Jennifer Shih, M.D.
Dr. Shih reports service on advisory boards for Teva and AstraZeneca, outside the submitted work.
Susan Stephenson, Ph.D.
Nothing to disclose.
Anne M. Fitzpatrick, Ph.D.
Nothing to disclose.
REFERENCES
- 1.Fischer GB, Sarria EE, Camargos P, Mocelin HT, Soto-Quiroz M, Cruz AA, et al. Childhood asthma in low and middle-income countries: Where are we now? Paediatr Respir Rev 2018. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, et al. National Prevalence of Poor Asthma Control and Associated Outcomes Among School-Aged Children in the United States. J Allergy Clin Immunol Pract 2018; 6:536–44 e1. [DOI] [PubMed] [Google Scholar]
- 3.2016 National Health Interview Survey (NHIS) Data, Centers for Disease Control and Prevention. Available at http://www.cdc.gov/asthma/most_recent_data.htm. Last accessed February 26, 2019. [Google Scholar]
- 4.Kodjebacheva GD, Sabo T, Parker S. Influences of asthma on reported health indicators and access to health care among children. Ann Allergy Asthma Immunol 2016; 116:126–33. [DOI] [PubMed] [Google Scholar]
- 5.Kit BK, Simon AE, Ogden CL, Akinbami LJ. Trends in preventive asthma medication use among children and adolescents, 1988–2008. Pediatrics 2012; 129:62–9. [DOI] [PubMed] [Google Scholar]
- 6.Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin SL, Globe G, et al. Asthma control in the United States, 2008–2010: indicators of poor asthma control. J Allergy Clin Immunol 2014; 133:1579–87. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, May SM, Charoenlap S, Pyle R, Ott NL, Mohammed K, et al. Effects of secondhand smoke exposure on asthma morbidity and health care utilization in children: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2015; 115:396–401 e2. [DOI] [PubMed] [Google Scholar]
- 8.Sala KA, Carroll CL, Tang YS, Aglio T, Dressler AM, Schramm CM. Factors associated with the development of severe asthma exacerbations in children. J Asthma 2011; 48:558–64. [DOI] [PubMed] [Google Scholar]
- 9.Dick S, Doust E, Cowie H, Ayres JG, Turner S. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open 2014; 4:e003827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011; 127:382–9 e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Just J, Bourgoin-Heck M, Amat F. Clinical phenotypes in asthma during childhood. Clin Exp Allergy 2017; 47:848–55. [DOI] [PubMed] [Google Scholar]
- 12.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol 2015; 15:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995; 332:133–8. [DOI] [PubMed] [Google Scholar]
- 14.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol 2003; 111:661–75; quiz 76. [DOI] [PubMed] [Google Scholar]
- 15.Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol 2011; 127:1505–12 e14. [DOI] [PubMed] [Google Scholar]
- 16.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax 2008; 63:974–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlidis S, Takahashi K, Ng Kee Kwong F, Xie J, Hoda U, Sun K, et al. “T2-high” in severe asthma related to blood eosinophil, exhaled nitric oxide and serum periostin. Eur Respir J 2019; 53. [DOI] [PubMed] [Google Scholar]
- 18.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safety and ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child 2001; 84:423–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, et al. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol 2019; 143:104–13 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters MC, Ringel L, Dyjack N, Herrin R, Woodruff PG, Rios C, et al. A Transcriptomic Method to Determine Airway Immune Dysfunction in T2-High and T2-Low Asthma. Am J Respir Crit Care Med 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract 2018; 6:545–54 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson NM, Bridge P, Spanevello A, Silverman M. Induced sputum in children: feasibility, repeatability, and relation of findings to asthma severity. Thorax 2000; 55:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unites States Census Bureau. 2010–2014 American Community Survey 5-Year Estimates. Available from: http://www.factfinder.census.gov. Last accessed January 26. [Google Scholar]
- 25.Shams MR, Bruce AC, Fitzpatrick AM. Anxiety Contributes to Poorer Asthma Outcomes in Inner-City Black Adolescents. J Allergy Clin Immunol Pract 2018; 6:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–87. [DOI] [PubMed] [Google Scholar]
- 27.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med 2017; 195:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184:602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012; 129:S34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005; 115:233–42. [DOI] [PubMed] [Google Scholar]
- 31.Blakey JD, Price DB, Pizzichini E, Popov TA, Dimitrov BD, Postma DS, et al. Identifying Risk of Future Asthma Attacks Using UK Medical Record Data: A Respiratory Effectiveness Group Initiative. J Allergy Clin Immunol Pract 2017; 5:1015–24 e8. [DOI] [PubMed] [Google Scholar]
- 32.Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med 2015; 3:849–58. [DOI] [PubMed] [Google Scholar]
- 33.Malinovschi A, Fonseca JA, Jacinto T, Alving K, Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol 2013; 132:821–7 e1–5. [DOI] [PubMed] [Google Scholar]
- 34.Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, et al. Utilization and Costs of Severe Uncontrolled Asthma in a Managed-Care Setting. J Allergy Clin Immunol Pract 2016; 4:120–9 e3. [DOI] [PubMed] [Google Scholar]
- 35.Casciano J, Krishnan JA, Small MB, Buck PO, Gopalan G, Li C, et al. Burden of asthma with elevated blood eosinophil levels. BMC Pulm Med 2016; 16:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haughney J, Morice A, Blyth KG, Lee AJ, Coutts A, McKnight E, et al. A retrospective cohort study in severe asthma describing commonly measured biomarkers: Eosinophil count and IgE levels. Respir Med 2018; 134:117–23. [DOI] [PubMed] [Google Scholar]
- 37.Kerkhof M, Tran TN, van den Berge M, Brusselle GG, Gopalan G, Jones RCM, et al. Association between blood eosinophil count and risk of readmission for patients with asthma: Historical cohort study. PLoS One 2018; 13:e0201143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casciano J, Krishnan J, Dotiwala Z, Li C, Sun SX. Clinical and Economic Burden of Elevated Blood Eosinophils in Patients With and Without Uncontrolled Asthma. J Manag Care Spec Pharm 2017; 23:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran TN, Khatry DB, Ke X, Ward CK, Gossage D. High blood eosinophil count is associated with more frequent asthma attacks in asthma patients. Ann Allergy Asthma Immunol 2014; 113:19–24. [DOI] [PubMed] [Google Scholar]
- 40.Wu AC, Tantisira K, Li L, Schuemann B, Weiss ST, Fuhlbrigge AL, et al. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest 2011; 140:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konradsen JR, Skantz E, Nordlund B, Lidegran M, James A, Ono J, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr Allergy Immunol 2015; 26:772–9. [DOI] [PubMed] [Google Scholar]
- 42.Malinovschi A, Janson C, Borres M, Alving K. Simultaneously increased fraction of exhaled nitric oxide levels and blood eosinophil counts relate to increased asthma morbidity. J Allergy Clin Immunol 2016; 138:1301–8 e2. [DOI] [PubMed] [Google Scholar]
- 43.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol 2016; 138:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puranik S, Forno E, Bush A, Celedon JC. Predicting Severe Asthma Exacerbations in Children. Am J Respir Crit Care Med 2017; 195:854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forno E, Celedon JC. Predicting asthma exacerbations in children. Curr Opin Pulm Med 2012; 18:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol 2008; 122:741–7 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haselkorn T, Zeiger RS, Chipps BE, Mink DR, Szefler SJ, Simons FE, et al. Recent asthma exacerbations predict future exacerbations in children with severe or difficult-to-treat asthma. J Allergy Clin Immunol 2009; 124:921–7. [DOI] [PubMed] [Google Scholar]
- 48.Semprini R, Williams M, Semprini A, McDouall A, Fingleton J, Holweg C, et al. Type 2 Biomarkers and Prediction of Future Exacerbations and Lung Function Decline in Adult Asthma. J Allergy Clin Immunol Pract 2018; 6:1982–8 e1. [DOI] [PubMed] [Google Scholar]
- 49.Chipps BE, Haselkorn T, Rosen K, Mink DR, Trzaskoma BL, Luskin AT. Asthma Exacerbations and Triggers in Children in TENOR: Impact on Quality of Life. J Allergy Clin Immunol Pract 2018; 6:169–76 e2. [DOI] [PubMed] [Google Scholar]
- 50.Chipps BE, Zeiger RS, Borish L, Wenzel SE, Yegin A, Hayden ML, et al. Key findings and clinical implications from The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol 2012; 130:332–42 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. The association of blood eosinophil counts to future asthma exacerbations in children with persistent asthma. J Allergy Clin Immunol Pract 2015; 3:283–7 e4. [DOI] [PubMed] [Google Scholar]
- 52.Zeiger RS, Schatz M, Dalal AA, Chen W, Sadikova E, Suruki RY, et al. Blood Eosinophil Count and Outcomes in Severe Uncontrolled Asthma: A Prospective Study. J Allergy Clin Immunol Pract 2017; 5:144–53 e8. [DOI] [PubMed] [Google Scholar]
- 53.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract 2014; 2:741–50. [DOI] [PubMed] [Google Scholar]
- 54.Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016; 4:549–56. [DOI] [PubMed] [Google Scholar]
- 55.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med 2018; 378:2486–96. [DOI] [PubMed] [Google Scholar]
- 56.Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2014; 2:879–90. [DOI] [PubMed] [Google Scholar]
- 57.Mathur SK, Fichtinger PS, Evans MD, Schwantes EA, Jarjour NN. Variability of blood eosinophil count as an asthma biomarker. Ann Allergy Asthma Immunol 2016; 117:551–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 2014; 11:531–6. [DOI] [PubMed] [Google Scholar]
- 59.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380:651–9. [DOI] [PubMed] [Google Scholar]
- 60.Zhang XY, Simpson JL, Powell H, Yang IA, Upham JW, Reynolds PN, et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin Exp Allergy 2014; 44:1137–45. [DOI] [PubMed] [Google Scholar]
- 61.Johansson MW, Evans MD, Crisafi GM, Holweg CTJ, Matthews JG, Jarjour NN. Serum periostin is associated with type 2 immunity in severe asthma. J Allergy Clin Immunol 2016; 137:1904–7 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 2012; 130:647–54 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez-Garcia S, Habernau Mena A, Quirce S. Biomarkers in inflammometry pediatric asthma: utility in daily clinical practice. Eur Clin Respir J 2017; 4:1356160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonnet N, Garnero P, Ferrari S. Periostin action in bone. Mol Cell Endocrinol 2016; 432:75–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.