Abstract
Background
A lower prevalence of atrial fibrillation (AF), but paradoxically higher burden of cardiovascular disease risk factors, has been observed among African Americans compared to Whites in studies of AF identified by mostly 12-lead electrocardiograms (ECGs), and clinically.
Methods
We performed 48-hour ambulatory electrocardiography (aECG) in a biracial sample of 1193 participants in the Atherosclerosis Risk in Communities (ARIC) (mean age=78 years, 62% African Americans, 64% female). Atrial fibrillation was identified from aECG, study visit ECGs, and discharge codes from cohort hospitalizations. We used covariate-adjusted logistic regression to estimate prevalence odds ratios for AF in African Americans versus Whites, with adjustment for sampling and non-response.
Results
African Americans were more likely than Whites to have hypertension and diabetes, but less likely to have CHD. The prevalence of AF detected by aECG or ARIC study ECG (adjusted for age and CHD) was lower in African Americans than Whites (2.7% versus 5.0%). White men had a higher (although not significant) AF prevalence of 7.8% compared to the other race and gender groups at 2.3–2.8%. The adjusted OR for AF was 0.49 (0.24–0.99) comparing African-Americans to Whites. Findings were similar when AF was defined to include prior AF hospitalizations (OR=0.42, 0.25–0.72). There were no significant differences by race for asymptomatic or paroxysmal AF.
Conclusions
Atrial fibrillation was less prevalent in African American than White older adults, regardless of detection method. Although overall detection of new AF cases with aECG was low, future studies should consider longer term monitoring to characterize AF by race.
Keywords: race and ethnicity, atrial fibrillation, electrocardiography, epidemiology
Journal Subject Terms: Atrial Fibrillation, Epidemiology, Race and Ethnicity
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide and a known risk factor for stroke, myocardial infarction and mortality.1–4 Despite an adverse risk profile for AF and a much higher risk of stroke and other outcomes in African Americans compared to Whites, a lower prevalence of AF in African Americans has been reported from multiple sources.5–9 It has been theorized that this paradox may be due to differential detection of AF by race.10 Because AF can be paroxysmal and asymptomatic, ambulatory electrocardiography is considered optimal for accurate ascertainment of AF. Most prior studies, however, have defined AF based on self-report, hospitalizations, administrative claims data, and/or 12-lead electrocardiograms (ECG), which may be insensitive to subclinical and intermittent AF.
The National Heart Lung and Blood Institute (NHLBI) therefore called for advancements in AF epidemiology through increased surveillance of AF in longitudinal studies, especially among non-White ethnic groups. In response, we examined the prevalence of AF in African Americans, including underreported, subclinical and manifest AF, in a study nested in the ongoing, mostly bi-ethnic and population-based Atherosclerosis Risk in Communities (ARIC) cohort, using 48 hour aECG monitoring for AF detection. To this study we added repeat 48-hour monitoring in a subset of participants to determine the degree to which AF prevalence estimates are influenced by length of aECG monitoring.
Methods
The data that support the findings of this study are available from the corresponding author or the ARIC study upon reasonable request. Research reported here was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (Loehr). The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract. Additional support was provided by American Heart Association (Alonso), and from National Heart, Lung, and Blood Institute grants (Chen). Molly Wen assisted with the programming for the analysis for this manuscript.
Study population
This study is ancillary to the ARIC study, a longitudinal population-based cohort study of cardiovascular disease and its risk factors ongoing since 1987.11 Selected participants in the ARIC study were invited to participate in this ancillary study that included a brief clinic visit followed by 48-hour aECG monitoring. The ancillary study took place between 2014 and 2016 at two of the four ARIC sites, Forsyth County, NC, and Jackson, MS. Participants were selected from among those that attended the fifth study visit (V5, 2011–2013) by a stratified random design that enriched for African Americans, and those with risk factors for AF. This feature increased efficiency and the precision of the estimates of AF prevalence. The sampling weights used in the analysis correspond to the (inverse of) the sampling fractions in order to estimate the prevalence in the source visit 5 ARIC study population. Eligible participants were African American and White participants from one of the two sites who had a V5 echocardiogram and electrocardiogram.12 Our goal was to recruit 825 African American and 400 White participants by stratified random sampling to enrich the study population with African Americans and individuals at high risk of AF. To this end, ARIC cohort members from both sites with AF risk factors considered to be high risk for AF (including a prior hospitalization for heart failure, a reduced ejection fraction (<50 %), or enlarged left atrial size (LAVI ≥32) on echocardiography) were all invited to participate in the study. Others that did not have one of the above mentioned AF risk factors were sampled at varying fractions. At the Forsyth County site, 100% of African Americans were invited, and 16–17% of White participants. At the Jackson ARIC cohort, which is exclusively African American, 61% of those not enriched for AF risk factors were invited to participate. See the analysis section regarding weighting of the analysis for sampling and nonresponse in order to estimate AF prevalence in the original ARIC study population.
Of the 1205 participants that consented for this study (68% of those contacted consented from Jackson, 53% of those contacted consented from Forsyth), 1193 were included in these analyses (742 African American, and 451 White). The following were applied: aECG transmission errors or drop-out (n=4), >5% time with noise on aECG (n=3), and aECG worn <46 hours (n=6); these categories are not mutually exclusive. Participants provided written informed consent, and the study was approved by the Institutional Review Boards at the field centers, Collaborative Studies Coordinating Center at the University of North Carolina at Chapel Hill, and the Epidemiologic Cardiology Research Center (EPICARE) at the Wake Forest University School of Medicine (Winston-Salem, NC, USA). A total of 101 participants consented to repeat aECG within two months following the same protocol although other study measures such as anthropometrics or blood pressure, were not repeated. Dr. Loehr has full access to all of the data and takes full responsibility for the integrity of this manuscript and the associated data analysis.
Study Measures
Anthropometric and blood pressure measurements were collected. Medications (names; strengths; units) used within two weeks of the exam were inventoried and therapeutically classified per V5 study protocol. In addition, questionnaires were interviewer administered to obtain medical history relevant to atrial fibrillation and arrhythmia. Participants reported the occurrence of AF signs and symptoms while wearing the aECG monitor with a standardized questionnaire. Body weight was measured to the nearest 0.1 kilogram, and height was recorded to the nearest centimeter. Three seated blood pressures were measured after a five-minute rest using an automated, oscillometric sphygmomanometer, and the last two measurements were averaged. Hypertension was defined as SBP ≥140 mm/Hg, diastolic blood pressure (DBP) ≥90 mm/Hg, or anti-hypertensive medication use. Creatinine was measured with a creatinase enzymatic method, and was used to estimate glomerular filtration rate (eGFR) using the 2009 CKD-EPID (CKD Epidemiology Collaboration) creatinine equation.13 Diabetes was defined as fasting glucose ≥ 6.99 mmol/L (126 mg/dL), non-fasting glucose ≥ 11.10 mmol/L (200 mg/dL), anti-diabetic medication use, or self-reported physician diagnosis of diabetes. Prevalent coronary heart disease (CHD) was defined using the ARIC study visit data and ARIC cohort events surveillance through the date of the ancillary study visit. It includes myocardial infarction, coronary artery bypass surgery, and coronary angioplasty adjudicated by a panel of reviewers.14 Twelve lead ECGs were performed at all study visits and read at the EPICARE. Burden of premature atrial contractions (PACs) was defined from 48 hour aECG as presence of PACs for <1%, 1–5% (occasional) and >5% (frequent) of the 48 hour recording time period.
Ambulatory ECG
The aECG monitors were SEER Light Extend Compact Digital AECG Recorder (GE, Milwaukee, WI). Study staff attached 7 electrodes to the participant and placed the aECG in a carrying case that the participant could connect to a belt or wear using a strap across their body. Participants were instructed to continue with usual activities, but avoid getting the monitor wet. After 48 hours of wear, participants returned to the field center where the aECG data were downloaded and digitally transferred to the EPICARE Center for standardized processing with MARS™ Ambulatory ECG System Software, Version 8.0.2 (GE, Milwaukee, WI), manual verification of atrial fibrillation/flutter, and determination of the percent of the 48-hour recording period during which participants were in atrial fibrillation/flutter.
Ascertainment of AF events by the ARIC study
The presence of AF prior to this ancillary study examination was ascertained by the ARIC study from ECGs from 5 prior study visits, and surveillance of hospitalization records since baseline (1987–1989) through 2013 for discharge diagnosis codes. All study visit ECGs identified as having AF were re-read by a trained cardiologist for confirmation. International Classification of Disease (ICD)-9-CM discharge codes were obtained from all hospitalizations. Hospitalizations were either self-reported or identified through ongoing hospital surveillance for cohort participant events. Hospitalizations with an ICD-9-CM discharge code for atrial fibrillation or atrial flutter (427.31 or 427.32) were considered an AF event. Hospitalizations with an AF ICD-9-CM code occurring at the same time as a code for cardiac surgery were not considered an AF event.
Definition of Atrial Fibrillation
Two definitions of AF were considered in this analysis: 1) ECG (12 lead study visit ECG or aECG) detected AF and 2) hospitalized or ECG detected AF. The purpose of the first definition was to define AF similarly with standardized ECG measures across the race and gender groups such that ascertainment bias would not be a problem. Then we were able to compare the results to the second AF definition which includes hospitalized AF to see if ascertainment bias might be a contributor to the racial differences in AF prevalence. Specifically, AF was defined by atrial fibrillation or atrial flutter of any duration on 48 hour aECG or study visit ECG (at visits 1 through 5). As a more comprehensive definition of prevalent AF, we also included hospitalizations with an International Classification of Disease (ICD-9-CM) discharge code for AF or atrial flutter (427.31 or 427.32) since study baseline (1987–1988), as defined above.
Asymptomatic AF was assessed from the questionnaires administered to study participants after wearing the monitor. Atrial fibrillation was considered asymptomatic if the participant did not report any of the following symptoms while wearing the monitor: dizziness, fainting, or an irregular or racing heartbeat. Paroxysmal AF was defined as AF on 48 hour Holter that occurred <99% of the recording time.
Statistical analysis
All analyses were weighted to account for sampling fractions and the percentage of non-response by site. Sampling fractions were thus based on race, study site, and presence of risk factors for atrial fibrillation and are described above under study population. The percentage of those contacted that chose not to participate (nonresponse) was ~32% from the Jackson site and ~47% from Forsyth County, NC. Weighted means, variances, and proportions (with 95% confidence intervals (CIs) were calculated for characteristics of the study population, by race. Frequencies of AF (95% CIs) were estimated by race and gender from predicted probabilities using weighted logistic regression that accounts for the study sampling design and non-response, and adjusted for age and history of coronary heart disease. The characteristics of AF were described by race, including the frequencies of those that were asymptomatic, or had persistent AF. Weighted logistic regression (SAS Proc Surveylogistic) was used to estimate odds ratios comparing race groups (African American to a referent group of Whites) for the prevalence of AF, asymptomatic AF, and persistent AF adjusted for gender, prevalent CHD, diabetes, hypertension and age. All analyses were performed using SAS version 9.4.
Results
Approximately 55% of white participants and 67% of African American participants were female (Table 1). The mean age was similar between the groups (77–78 years). A higher percentage of African Americans had obesity, diabetes, or hypertension, whereas coronary heart disease was less prevalent among African Americans. The prevalence of AF identified prior to placement of the aECG monitor, based on hospitalization ICD codes and ARIC study ECGs, was almost three times greater in Whites (8%) compared to African Americans (3%).
Table 1.
Baseline characteristics of Atherosclerosis Risk in Communities (ARIC) cohort members that participated in the ambulatory electrocardiography study, stratified by race, 2014–2016, N=2,434 (weighted* for sampling and non-response)
| White | African American | p-value | |
|---|---|---|---|
| Age in years, mean ± SD | 78 ± 8 | 77 ± 6 | 0.01 |
| Female, n(%) | 696 (55) | 778 (67) | <0.01 |
| Former smoker, n(%) | 593 (53) | 505 (48) | 0.02 |
| Obese, n(%) | 353 (28) | 552 (47) | <0.01 |
| Hypertension, n(%) | 828 (65) | 1004 (87) | <0.01 |
| Diabetes, n(%) | 339 (27) | 415 (36) | <0.01 |
| Enlarged left atrial volume index, n(%) | 218 (17) | 226 (19) | 0.16 |
| Prevalent coronary heart disease, n(%) | 226 (18) | 99 (8) | <0.01 |
| Estimated Glomerular Filtration Rate (eGFR) | |||
| eGFR = 0 to <30 ml/min/1.73 m2 | 21 (2) | 17 (1) | <0.01 |
| eGFR = 30 to <60 ml/min/1.73 m2 | 351 (28) | 254 (22) | |
| eGFR >=60 ml/min/1.73 m2 | 888 (70) | 882 (76) | |
| Premature Atrial Contractions (PAC) from 48 Hour Holter | |||
| PACs <1% (Referent) | 1034 (82) | 962 (83) | 0.32 |
| PACs 1 – 5% (Occasional) | 169 (13) | 158 (14) | |
| PACs >5% (Frequent) | 65 (5) | 45 (4) | |
| AF on ECG from any of 5 ARIC study visits, n(%) | 35 (3) | 9 (1) | <0.01 |
| AF from ARIC ECG or hospitalization discharge code, n(%) | 99 (8) | 38 (3) | <0.01 |
Analyses based on 1,193 participants, N=742 African Americans and N=451 Whites weighted for non-response and sampling design
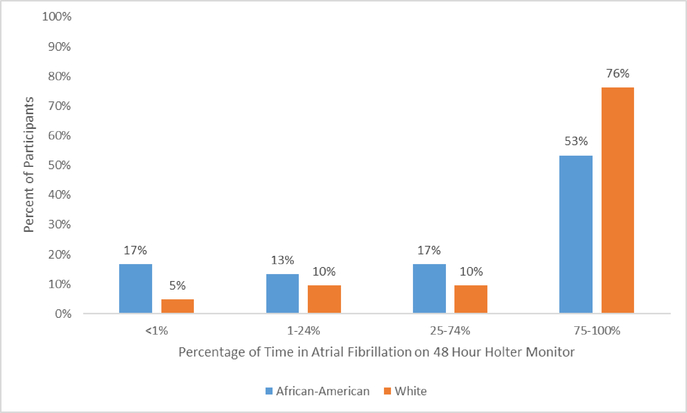
There were 61 occurrences of AF detected from 48 hour aECG recordings in White participants, and 28 in African American participants. Among those with AF on aECG, the percent of time in AF over the 48 hours is shown in Figure 1, stratified by race. The majority of AF events occurred for 75–100% of the 48 hour recording time period. As seen in Figure 1, more White participants than African American participants were observed in the group with more persistent AF (75–100% of the time). Of note (data not shown), the hourly mean heart rate was over 100 beats per minute for at least one hour in 2 out of 26 White participants with AF and 3 out of 17 African American participants with AF. Among those with AF on aECG, 19% of Whites and 18% of African Americans reported experiencing symptoms during the time that they wore the aECG monitor (data not shown).
Figure 1.
Time in atrial fibrillation (% of the 48-hour recording period) among those with atrial fibrillation detected by 48 hour ambulatory electrocardiography, stratified by race, 2014–2016, N=2,434 (weighted)
As part of the repeatability study, 101 participants returned for repeat 48 hour aECG monitoring. Atrial fibrillation was detected in 4 participants on repeat aECG monitoring; all were observed to have AF at the time of initial aECG monitoring, and there was none with AF initially that was not detected on repeat monitoring. Originally, we had planned to calibrate AF prevalence estimates based on the findings from this repeatability study (to address misclassification); calibration was not needed however, since no additional AF events were detected with extended ECG monitoring.
The prevalence of AF (defined broadly with ECG, aECG, and hospitalizations) adjusted for age and previously detected coronary heart disease was 11.0 % (95% CI: 8.1–14.9) in Whites, which was nearly two-fold higher compared to 5.8 % (95% CI: 3.9–8.5) in African Americans. White men had the highest AF prevalence at 12.7% (95% CI 8.7–18.4) followed by White women, then African American women, and African American men (Table 2). Considering AF that is present for 75–100% of the 48 hour, White men showed a higher frequency at 5.7% (95% CI: 3.3–9.9) compared to the next highest group of White women at 1.6% (95% CI: 0.5–3.5), thus largely contributing to the differences seen here in by race (Figure). The prevalence of parosymsal AF (defined as <99% of time in AF) and asymptomatic AF was similar across the race and gender groups, although still marginally higher in White men. We also considered the prevalence of AF defined by aECG or ARIC study visit ECG in order to define AF consistently with standardized ECG measures across the race and gender groups and avoid ascertainment bias that might be introduced when hospitalizations are considered in the definition of AF (Table 2). When stratified by race and gender, we observed a similar relationship with this definition of AF prevalence, however three of the race and gender groups were in a narrow range of AF prevalence (2.3–2.8%), whereas white men had a higher prevalence at 7.8%. Of note, these stratified estimates were not precise and the confidence intervals overlap when comparing across race and gender.
Table 2.
Adjusted prevalence* (95% CI) of atrial fibrillation detected by: 1) aECG, study visit electrocardiogram or prior hospitalization, 2) ambulatory electrocardiography (aECG) or prior study visit electrocardiogram, and for asymptomatic and paroxysmal atrial fibrillation for those with atrial fibrillation detected by aECG, stratified by race and by race and gender groups, 2014–2016.
| AF prevalence on Holter/ECG/Hospitalization % (95% CI) | AF prevalence on Holter or ECG % (95% CI) | Asymptomatic AF prevalence % (95% CI) | Prevalence of Paroxysmal AF** % (95% CI) | |
|---|---|---|---|---|
| Race Stratified | ||||
| White | 11.0 (8.1,14.9) | 5.0 (3.3,7.6) | 4.0 (2.5,6.4) | 1.6 (0.7,3.5) |
| African American | 5.8 (3.9,8.5) | 2.7 (1.5,4.7) | 2.4 (1.3,4.4) | 1.4 (0.6,3.2) |
| Race and Gender Stratified | ||||
| White Women | 9.4 (5.9,14.6) | 2.8 (1.3,5.9) | 2.5 (1.1,5.5) | 1.3 (0.4,4.1) |
| White Men | 12.7 (8.7,18.4) | 7.8 (4.8,12.3) | 5.9 (3.4,10) | 1.9 (0.7,5.2) |
| African American Women | 6 (3.8,9.5) | 2.6 (1.3,4.9) | 2.1 (1,4.3) | 1.1 (0.4,3.3) |
| African American Men | 4.7 (2.4,9.1) | 2.3 (0.9,5.9) | 2.4 (0.9,6.2) | 1.6 (0.5,5.1) |
Prevalence (%) adjusted for age, gender, and prevalent coronary heart disease for estimates stratified by race; prevalence (%) adjusted for age and prevalent coronary heart disease for estimates stratified by race and gender. Weighted for sampling design and non-response.
Paroxysmal AF was defined by time in AF <99%; time in AF does not include 0%.
Table 3 shows the results of multivariable, weighted logistic regression comparing African Americans to Whites for four different AF outcomes: 1) prevalent AF from any source (prior hospitalization, study visit ECG, or on 48 hour aECG), 2) AF from aECG or study visit ECG, 3) asymptomatic AF, or 4) paroxysmal AF (<99% of the time on aECG monitor). Models were adjusted for gender, prevalent CHD, age eGFR, hypertension, and diabetes. Prevalent AF, as well as persistent AF, were significantly lower in African Americans compared to Whites. P values for the interaction of gender with race for AF prevalence were not significant (p=0.4, results not shown). The prevalence OR for African Americans compared to Whites for the outcome of asymptomatic AF was not statistically significant, although the association was in the same direction for asymptomatic AF.
Table 3.
Prevalence odds ratio of atrial fibrillation among African Americans compared to Whites, according to two definitions of AF, and asymptomatic and paroxysmal atrial fibrillation, 2014–2016, N=2,434 (weighted for sampling design and non-response)
| Odds of AF | Odds Ratio (95% CI)* | |
|---|---|---|
| Model 1: Atrial Fibrillation defined on aECG, ARIC study visit ECG, or Hospitalization | ||
| African American | 50/1116 | 0.42 (0.25,0.72) |
| White | 124/1144 | 1. |
| Model 2: Atrial Fibrillation occurrence defined by aECG or ARIC study visit ECG | ||
| African American | 28/1138 | 0.48 (0.23,0.99) |
| White | 64/1204 | 1. |
| Model 3: Asymptomatic Atrial Fibrillation† defined on aECG | ||
| African American | 23/1139 | 0.54 (0.24, 1.22) |
| White | 48/1209 | 1. |
| Model 4: Paroxysmal Atrial Fibrillation defined on aECG‡ | ||
| African American | 13/1138 | 1.04 (0.29,3.72) |
| White | 18/1207 | 1 |
Odds ratios and 95% confidence intervals adjusted for gender, prevalent CHD, age, eGFR, hypertension and diabetes. Odds ratios that are bolded were statistically significant (P < 0.05).
Asymptomatic AF defined by AF on Holter and no symptoms on post-test questionnaire. Symptoms defined by any of the following self-reported symptoms while wearing the Holter: racing or irregular heartbeat, dizziness, or fainting.
Paroxysmal AF was defined by time in AF <99%; time in AF does not include 0%
Discussion
To our knowledge this is the first study to evaluate the paradoxical low frequency of AF in African Americans with a comparison group of Whites using a standardized measurement of 48 hour aECG. We also defined the prevalence of AF including extant ARIC study data resources, including 25 years of follow-up for hospitalizations, and twelve lead ECGs from five prior ARIC study visits. The prevalence of AF on aECG or twelve lead ECG in our population aged 69–92 years was lower in African Americans than Whites, and also statistically significantly lower when compared using multivariable adjustment. When the definition of the prevalence of AF was expanded to also include hospitalizations for AF, this finding persisted; therefore, ascertainment bias is unlikely to account for low AF in African-Americans. As expected, the burden of AF risk factors in this older cohort was greater among its African American than White members. Prior studies have shown that obesity and hypertension were primary contributors to the population attributable fraction for AF.15, 16 These conditions were observed to be more frequent among African Americans in this study population. In addition, kidney disease is more common in African-Americans and has been associated with risk of AF, however adjustment for eGFR did not change our inferences.17
The observed low prevalence of AF in African Americans is puzzling, and several theories have been proposed to account for this paradox. An important consideration is ascertainment bias, possibly induced by differential access to health care and thus to cardiac monitoring. This concern was addressed by a report on heart failure patients in which similar outpatient utilization rates were found for African Americans and Whites prior to hospitalization for AF.18 A higher frequency of subclinical AF among African Americans represents an alternate interpretation, yet not one supported here by prolonged aECG monitoring. As part of an avenue less explored, ancestry-informative markers from a genome wide array suggest that ancestral genetic architecture is only modestly predictive of AF.19 More recently, a single-nucleotide polymorphism was found associated with AF in Whites more so than in African Americans, although authors conclude that likely other genetic and environmental risk factors are influential in this association.20 Survival bias must also be considered as a possible interpretation, positing that fewer African Americans survive AF than Whites; similarly, differential mortality from conditions such as myocardial infarction (MI) that increase risk for AF is yet another plausible interpretation. While higher mortality post-MI in African Americans has been reported, the difference by race is not sufficient to explain the AF paradox10. We considered the prevalence of AF when our definition included hospitalizations as compared to study visit ECG and aECG, and found the inferences were the same across race groups further indicating that ascertainment bias is an unlikely source of race differences in AF prevalence.
An unexpected finding from our study of older adults is that the burden of AF in White men was higher than that of all the other race and gender groups, although not statistically significantly different. A higher AF burden in men was not observed in African-Americans; although not statistically significant, the prevalence for African-American women was slightly higher than for men. Prior research has shown a higher age-adjusted prevalence of AF in men compared to women in both developed and developing countries21. This observation should be investigated further in studies powered to study such differences.
Prior studies of AF in African-Americans in clinical populations have almost exclusively defined AF based on self-report, twelve lead ECGs, administrative claims, and more recently implanted devices. One study of 430,317 members of a large health maintenance organization (60 years and older) found that the prevalence of AF among African Americans (3.5%) was about half that of Whites (8%).5 More recently, race differences in rates of AF were studied using claims data of those with newly implanted pacemakers with the goal of having similar monitoring by race group. A lower rate of AF in African Americans was observed.22 In a recent study of 2,580 hypertensive participants without known AF and with implanted monitoring devices (72 were African), it was found that those of non-European ancestry had a lower incidence of AF over 2.5 years of follow-up, although the estimates were imprecise due to small numbers.23 We add to this literature by studying participants in a community based observational cohort study. In our study, the population is aged 69 years and older and thus in the age range at which the racial difference in AF prevalence was previously observed.24
Multiple studies have attempted to quantify AF that is asymptomatic.25–28 In the AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) Study, 12% of 4,060 trial participants had AF and were asymptomatic, whereas the Framingham Heart Study reported 40% (228/562) of AF to be asymptomatic on routine biennial EKGs.26,28 Asymptomatic AF is thought to convey the same risks of thromboembolic events as does symptomatic AF; thus, studies that do not ascertain asymptomatic AF could grossly underestimate the prevalence and prognostic impact of AF.29 The NHLBI has recommended that cohort studies add AF as an endpoint and furthermore, distinguish between asymptomatic and symptomatic events to better characterize the clinical course of AF.30 While we were able to distinguish symptomatic from asymptomatic AF in this population the numbers were small. Considering this constraint, we observed a lower frequency of asymptomatic AF in African Americans compared to Whites; this difference was not statistically significant, although similar magnitude and direction to other associations.
Our study is innovative in its use of 48-hour aECG monitoring in a well-characterized largely minority population sampled from within the ARIC study. Prior work in the ARIC study has observed a lower frequency of AF in African Americans compared to Whites at a mean age of 54 years at baseline, and lower AF incidence rates over 21 years of follow-up.9 Among prior studies of AF in other community cohorts, the Cardiovascular Health Study (CHS) and the Framingham Heart Study performed 24-hour monitoring on a subset of volunteers during several different study visits.31 Based on small numbers of African Americans at the baseline visit (N=244), the CHS found a lower prevalence of both self-reported AF, and AF from the baseline study ECGs among African Americans.31,32
A potential weakness of our study is that longer monitoring might have detected additional cases of paroxysmal AF, although no additional episodes of AF were detected on repeat monitoring in our repeatability study. In addition, we were preempted from studying atrial flutter separately from atrial fibrillation by the small number of individuals with atrial flutter in this study (n=2). There are reports indicating that atrial flutter may be more common in African-Americans than Whites33, which has led to a call to study atrial flutter and atrial fibrillation as separate outcomes.34 Among the strengths of this study are the standardized and rigorous methodology used to detect AF, and the use of 48-hour aECG to detect intermittent or asymptomatic AF. We had similar findings using aECG and clinically defined AF from hospitalizations, indicating that ascertainment bias is unlikely to explain the differences in AF prevalence observed by race. Studies of AF burden by race should consider stratification by race and gender given that our results imply (although non-significant) a higher burden in White men compared to White women that is not seen in African-Americans. Future work from the ARIC study will include up to 14 days of aECG monitoring, and may contribute insights into the paradoxical lower frequency of AF among African Americans.
Acknowledgments
Funding Sources
Research reported here was supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under award number R01HL116900. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. Additional support was provided by American Heart Association grant 16EIA26410001 (Alonso), and from National Heart, Lung, and Blood Institute grants R01HL126637 and R01HL141288 (Chen). The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosure
The authors do not have any conflicts of interest.
Conflict of interest statement: The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 2.Soliman EZ, Lopez F, O’Neal WT, Chen LY, Bengtson L, Zhang ZM, Loehr L, Cushman M and Alonso A. Atrial Fibrillation and Risk of ST-Segment-Elevation Versus Non-ST-Segment-Elevation Myocardial Infarction: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;131:1843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB and Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 4.Conen D, Chae CU, Glynn RJ, Tedrow UB, Everett BM, Buring JE and Albert CM. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen AY, Contreras R, Sobnosky S, Shah AI, Ichiuji AM, Jorgensen MB, Brar SS and Chen W. Racial/ethnic differences in the prevalence of atrial fibrillation among older adults--a cross-sectional study. J Natl Med Assoc. 2010;102:906–13. [DOI] [PubMed] [Google Scholar]
- 6.Manolio TA, Burke GL, Psaty BM, Newman AB, Haan M, Powe N, Tracy RP and O’Leary DH. Black-white differences in subclinical cardiovascular disease among older adults: the Cardiovascular Health Study. CHS Collaborative Research Group. Journal of clinical epidemiology. 1995;48:1141–52. [DOI] [PubMed] [Google Scholar]
- 7.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE and Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–45. [DOI] [PubMed] [Google Scholar]
- 8.Soliman EZ and Prineas RJ. The paradox of atrial fibrillation in African Americans. J Electrocardiol. 2014;47:804–8. [DOI] [PubMed] [Google Scholar]
- 9.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR and Alonso A. Racial Differences in Atrial Fibrillation-Related Cardiovascular Disease and Mortality: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soliman EZ, Alonso A and Goff DC Jr. Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–56. [DOI] [PubMed] [Google Scholar]
- 11.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR and Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J and Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez CJ, Swett K, Agarwal SK, Folsom AR, Fox ER, Loehr LR, Ni H, Rosamond WD and Chang PP. Systolic blood pressure levels among adults with hypertension and incident cardiovascular events: the atherosclerosis risk in communities study. JAMA Intern Med. 2014;174:1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez F, Stefanick ML, Greenland P, Soliman EZ, Manson JE, Parikh N, Martin LW, Larson JC, Hlatky M, Nassir R, Cene CW, Rodriguez BL, Albert C and Perez MV. Racial and ethnic differences in atrial fibrillation risk factors and predictors in women: Findings from the Women’s Health Initiative. Am Heart J. 2016;176:70–7. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal N, Zelnick LR, Alonso A, Benjamin EJ, de Boer IH, Deo R, Katz R, Kestenbaum B, Mathew J, Robinson-Cohen C, Sarnak MJ, Shlipak MG, Sotoodehnia N, Young B and Heckbert SR. eGFR and Albuminuria in Relation to Risk of Incident Atrial Fibrillation: A Meta-Analysis of the Jackson Heart Study, the Multi-Ethnic Study of Atherosclerosis, and the Cardiovascular Health Study. Clin J Am Soc Nephrol. 2017;12:1386–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruo B, Capra AM, Jensvold NG and Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–35. [DOI] [PubMed] [Google Scholar]
- 19.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR and Candidate-Gene Association Resource S. European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts JD, Hu D, Heckbert SR, Alonso A, Dewland TA, Vittinghoff E, Liu Y, Psaty BM, Olgin JE, Magnani JW, Huntsman S, Burchard EG, Arking DE, Bibbins-Domingo K, Harris TB, Perez MV, Ziv E and Marcus GM. Genetic Investigation Into the Differential Risk of Atrial Fibrillation Among Black and White Individuals. JAMA Cardiol. 2016;1:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M and Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamel H, Kleindorfer DO, Bhave PD, Cushman M, Levitan EB, Howard G and Soliman EZ. Rates of Atrial Fibrillation in Black Versus White Patients With Pacemakers. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau CP, Gbadebo TD, Connolly SJ, Van Gelder IC, Capucci A, Gold MR, Israel CW, Morillo CA, Siu CW, Abe H, Carlson M, Tse HF, Hohnloser SH, Healey JS and investigators A. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J Cardiovasc Electrophysiol. 2013;24:381–7. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV and Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 25.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB and Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 26.Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R, Mickel M and Barrell P. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:657–63. [DOI] [PubMed] [Google Scholar]
- 27.Martin A, Benbow LJ, Butrous GS, Leach C and Camm AJ. Five-year follow-up of 101 elderly subjects by means of long-term ambulatory cardiac monitoring. European heart journal. 1984;5:592–6. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ and Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. Jama. 1994;271:840–4. [PubMed] [Google Scholar]
- 29.Rho RW and Page RL. Asymptomatic atrial fibrillation. Progress in cardiovascular diseases. 2005;48:79–87. [DOI] [PubMed] [Google Scholar]
- 30.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd-Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL and Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manolio TA, Furberg CD, Rautaharju PM, Siscovick D, Newman AB, Borhani NO, Gardin JM and Tabatznik B. Cardiac arrhythmias on 24-h ambulatory electrocardiography in older women and men: the Cardiovascular Health Study. J Am Coll Cardiol. 1994;23:916–25. [DOI] [PubMed] [Google Scholar]
- 32.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD and Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61. [DOI] [PubMed] [Google Scholar]
- 33.Dewland TA, Olgin JE, Vittinghoff E and Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–7. [DOI] [PubMed] [Google Scholar]
- 34.Soliman EZ. Race and atrial flutter: a needed update to understand the atrial fibrillation race paradox. Future Cardiol. 2017. [DOI] [PubMed] [Google Scholar]