Abstract
The gut associated lymphoid tissue has effective mechanisms in place to maintain tolerance to food antigens. These can be exploited to induce antigen-specific tolerance for the prevention and treatment of autoimmune diseases and severe allergies and to prevent serious immune responses in protein replacement therapies for genetic diseases. An oral tolerance approach for the prevention of peanut allergy in infants proved highly efficacious and advances in treatment of peanut allergy have brought forth an oral immunotherapy drug that is currently awaiting FDA approval. Several other protein antigens made in plant cells are in clinical development. Plant cell-made proteins are protected in the stomach from acids and enzymes after their oral delivery because of bioencapsulation within plant cell wall, but are released to the immune system upon digestion by gut microbes. Utilization of fusion protein technologies facilitates their delivery to the immune system, oral tolerance induction at low antigen doses, resulting in efficient induction of FoxP3+ and latency-associated peptide (LAP)+ regulatory T cells that express immune suppressive cytokines such as IL-10. LAP and IL-10 expression represent potential biomarkers for plant-based oral tolerance. Efficacy studies in hemophilia dogs support clinical development of oral delivery of bioencapsulated antigens to prevent anti-drug antibody formation. Production of clinical grade materials in cGMP facilities, stability of antigens in lyophilized plant cells for several years when stored at ambient temperature, efficacy of oral delivery of human doses in large animal models and lack of toxicity augur well for clinical advancement of this novel drug delivery concept.
Keywords: Allergy, Peanut, Hemophilia, Transgenic plants, Chloroplast, Immune tolerance, Oral tolerance, Gut, Regulatory T cells
1. Introduction
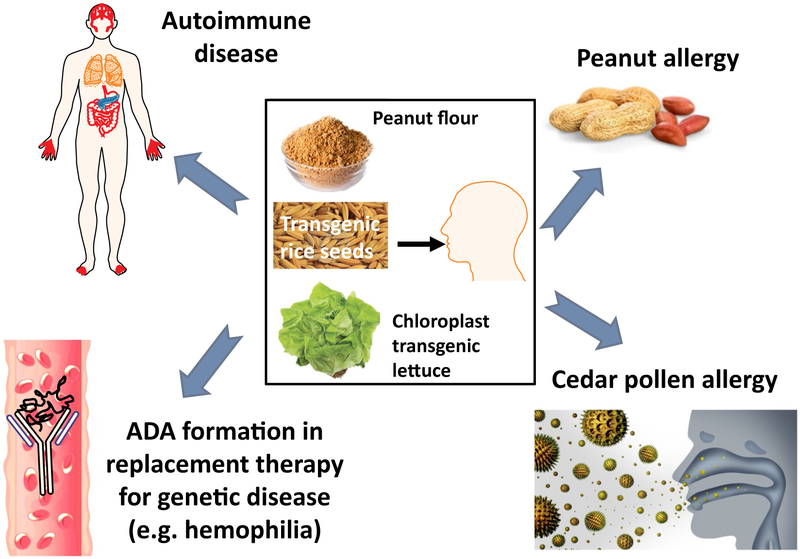
The toolkit of suppression immunotherapy has been substantially expanded with the development of monoclonal antibodies, fusion proteins, and regulatory T cell therapies, among others, for the treatment of autoimmune diseases and prevention of transplant rejection. While these biological drugs enable more targeted treatments than traditional immune suppressive drugs, they are extraordinarily expensive, and may even require specialized centers to perform the required manipulations. Antigen-specific induction of immune tolerance in humans largely remains an unmet challenge. Treatment of autoimmune diseases, for which at least some of the target antigens are known, could greatly benefit from antigen-specific regimens. Such protocols are also lacking for the prevention of antibody-mediated rejection of therapeutic proteins. Formation of anti-drug antibodies (ADA) is a widespread problem in protein replacement therapies for genetic diseases. Antigen-specific tolerance induction would be far more desirable than general immune suppression, avoiding risks of infection and side effects of immune suppressive drugs. A potential pathway toward such a protocol is the introduction of the target antigen via a route that naturally leads to tolerogenic antigen presentation. From a wealth of pre-clinical data, it has long been known that oral delivery of protein antigens may induce immune tolerance (Kuhn and Weiner, 2016; Mowat, 2018; Pabst and Mowat, 2012; Rezende and Weiner, 2017; Wang et al., 2013). This approach has only recently been successfully used in the prevention of peanut allergy through regular ingestion of peanut-containing foods in infants (Du Toit et al., 2015). Additionally, oral immunotherapy (OIT) for life-threatening food allergies has demonstrated high rates of success in desensitization of the allergic response and in some cases long lasting tolerance, termed sustained unresponsiveness (Vickery et al., 2017; Vickery et al., 2014; Wood, 2016). The front-runner for first FDA approval of an orally delivered drug based on a plant product, currently is in the final stages of FDA approval for peanut allergy (Tilles and Petroni, 2018; Vickery et al., 2014). In order to be able to orally deliver specific auto-, allo-, and therapeutic antigens, a next generation of drugs, based on transgenic plants, is currently in advanced pre-clinical development (Fig. 1 and Table 1).
Fig. 1.
Examples of oral immune modulatory therapy using plant cells. Antigens bioencapsulated in plant cells from leaves, nuts, or seeds can be orally delivered to promote tolerance to autoantigens, allergens, and therapeutic proteins used to treat genetic disease. If the antigens are not naturally present in the plants, they can be expressed in transgenic plants.
Table 1.
Recent studies on Antigen Production in Plant Cells for Oral Tolerance Induction.
| Disease | Antigen | Plant species | Expression system |
Expression levels | Tolerized species |
Functional evaluation | Reference |
|---|---|---|---|---|---|---|---|
| Hemophilia A | CTB-hFVIII | Lettuce | Chloroplast | Up to 3622 μg/g lyophilized leaf cells | Mouse | Oral delivery of a mixture of bioencapsulated hFVIII heavy chain (HC) and C2 domain or HC and light chain (LC) antigens suppressed inhibitor formation in hemophilia A mice. LAP surface expression on Treg described as biomarker. | Kwon et al., 2018, Plant Biotechnol J 16:1148–1160, |
| Peanut Allergy | Peanut proteins (AR101 Drug) | Peanut | N/A | 300 mg protein daily dose | Human | In this Phase 2 trial, peanut allergic subjects were randomized to daily oral dosing of pharmaceutical-grade peanut flour (AR101) or placebo. 62% of subjects taking AR101 tolerated > 1043 mg of peanut during the final food challenge whereas 0% of subject on placebo met this endpoint. | Bird et al., 2018, J Allergy Clin Immunol Pract. 6:476–485 |
| Peanut Allergy | Peanut proteins in flour | Peanut | N/A | 300 mg or 3000 mg protein daily dose | Human | Peanut allergic subjects aged 9 to 36 months were enrolled and randomized to 300 mg or 3000 mg peanut protein/day. After a median of 29 months of dosing, 78% of subjects achieved sustained unresponsiveness to peanut four weeks after stopping oral immunotherapy dosing. | Vickery et al., 2017, J Allergy Clin Immunol. 139:173–181 |
| Hemophilia B | CTB-hFIX | Lettuce | Chloroplast | 1 mg/g lyophilized leaf cells | Dog, Mouse | Prevention of inhibitor formation and of anaphylaxis to hFIX; lack of toxicity. | Herzog et al., 2017, Mol Ther. 25:512–522; Su et al., 2015b, Biomaterials 70:84–93 |
| Peanut Allergy | Peanut proteins in Bamba | Peanut | N/A | 6 g of peanut protein per week | Human | In this prevention trial, infants aged 4–11 months deemed at-risk of developing peanut allergy were assigned to ingest peanut or avoid peanut until 60 months of age. 17% of the avoidance group ended up with peanut allergy compared to 3.2% of the group consuming peanuts. This provides strong evidence of oral tolerance induction in humans. | Du Toit et al., 2015, N Engl J Med. 372:803–13 |
| Pompe | CTB-GAA | Tobacco | Chloroplast | 190 μg per g of dry leaves | Mouse | Substantially blocked inhibitor formation against GAA in Pompe mice by oral administration of the chloroplast-made GAA epitopes. | Su et al., 2015a, Plant Biotechnol J. 13:1023–32 |
| Rheumatoid arthritis | CTA1 (R7K)-COL-DD fusion | Arabido-psis | Nuclear | 2.5% TSP | Mouse | Showed either no collagen-induced arthritis (CIA) symptoms or substantially reduced CIA severity. Decreased effector T-cell activity with suppressed IFNγ, IL-13 and IL-17A and up-regulation of IL-10 in the CIA mice. | Hansson et al., 2016, Plant Biotechnol J. 14:1106–15 |
| Hemophilia A | CTB-hFVIII | Lettuce | Chloroplast | 4.2% TP (FVIII-C2) and 0.8% TP (FVIII-HC) | Mouse | Oral delivery of a mixture of bioencapsulated hFVIII heavy chain (HC) and C2 domain antigens suppressed T helper cell responses and inhibitor formation against FVIII in hemophilia A mice. Pre-immune inhibitors were reversed by feeding plant-made HC and C2 mix. | Sherman et al., 2014, Blood. 124:1659–68, |
| Rheumatoid arthritis | Peptide ligands of type II collagen (CII) | Rice | Seeds | 7–24 mg/g seeds | Mice | Significantly inhibited the development of arthritis and delayed disease onset during the early phase of arthritis mediated by the induction of IL-10 from CD4+ CD25− T cells against CII antigen. | Iizuka et al., 2014, Plant Biotechnol J. 12:1143–52, |
| Pollen allergy | Japanese cedar pollen allergens, destructed Cry j 1 and Cry j 2 | Rice | Seeds | NA | Mice | Marked suppression of allergen-specific CD4+ T-cell proliferation, IgE and IgG levels. As clinical symptoms of pollinosis, sneezing frequency and infiltration of inflammatory cells such as eosinophils and neutrophils were also significantly reduced in the nasal tissue. | Wakasa et al., 2013, Plant Biotechnol J. 11:66–76 |
| Allergic asthma | mite allergen (Der p 1) | Rice | Seeds | ~7.5% total seed protein | Mice | Prophylactic oral vaccination with the transgenic rice seeds clearly reduced the serum levels of allergen-specific IgE and IgG. | Suzuki et al., 2011, Plant Biotechnol J. 9:982–90 |
| Allergic asthma | Allergen, Dermatopha-goides pteronyssin-us 2 (Der p2) | Tobacco | Nuclear | 0.5% TP | Mice | Reduced Der p2-specific IgE and IgG1 titers in serum, decreased IL-5 and eotaxin levels in bronchial alveolar lavage fluid, and eosinophil infiltration in the airway. | Lee et al., 2011, Cell Mol Immunol. 8:404–14 |
| Hemophilia B | CTB-hFIX | Tobacco | Chloroplast | 3.8% TSP | Mice | Eliminated fatal anaphylactic reactions; blocked formation of inhibitory antibodies undetectable or up to 100-fold less than controls. | Verma et al., 2010, Proc Natl Acad Sci U S A. 107:7101–6 |
Notes: h, human antigen; NA, not available; TP, total protein; TSP, total soluble protein.
The oral tolerance method does not require custom design of antigens for specific major histocompatibility complex (MHC) molecules (as in the case of peptide-based methods), nor are genetic manipulations of host cells required (as in gene therapy). Nonetheless, translation of this method is limited by the costs of production of large amounts of antigen for oral delivery and by inefficiency of delivery to the gut immune system due to antigen digestion in the stomach by acids/enzymes and blockage of antigen absorption by gut epithelium. Recent breakthrough inventions have addressed these limitations by expression of antigens or protein drugs in plant cells that protect them from stomach acids or enzymes through bioencapsulation. Commensal bacteria degrade plant cell wall and release antigens in the gut lumen. Transmucosal carriers fused to antigens deliver them across the gut epithelium and to the immune system through ubiquitous binding sites (Daniell et al., 2016a; Daniell et al., 2016b; Kwon and Daniell, 2016).
Use of plant cells as “Green Bioreactors” is now becoming a promising approach for production and delivery of biopharmaceutical proteins. US Food and Drug Administration (FDA) approved the first plant-made biopharmaceutical for human use. “Taliglucerase alfa” is produced for enzyme-replacement therapy for the rare genetic disorder Gaucher's disease. This was developed by Protalix Biotherapeutics (Israel) via expression in carrot cells, grown in contained bioreactors and is now marketed by Pfizer (Grabowski et al., 2014; Maxmen, 2012). While most recombinant protein drugs in the clinic are made in E. coli, yeast or CHO cells, this is the first FDA approved drug made in plant cells. Plant cells offer unique advantages for production and delivery of protein drugs by significantly reducing the cost of goods through elimination of prohibitively expensive fermentation, purification, sterile injections, cold storage/transportation and short shelf life of current purified protein drugs. Indeed, lyophilized plant cells used for oral drug delivery maintain folding, assembly and disulfide bonds and therapeutic efficacy for 2–3 years when stored at ambient temperature (Herzog et al., 2017; Su et al., 2015b).
Oral tolerance approaches to prevent peanut allergy have been reported and treatment with orally-delivered peanut proteins has been demonstrated to increase the amount of peanut an allergic patient can consume without symptoms. Moving toward tolerance in treatment of genetic disease, several plant-based oral tolerance protocols have been developed (Ruhlman et al., 2007; Sherman et al., 2014; Verma et al., 2010). Efficacy of immune tolerance has been tested in materials produced in FDA approved cGMP facilities (Su et al., 2015b), and human doses have been tested in hemophilic dogs (Herzog et al., 2017). IND enabling toxicology, pharmacokinetic and pharmacodynamics studies have been completed, and Phase I study design is in progress (Daniell, presented at World Hemophilia Federation, 2018). These recent advances augur well for launching this new platform for affordable protein drug delivery concept. In this review, we discuss delivery of disease-specific antigens to induce oral tolerance in a diverse set of genetic, autoimmune, and allergic diseases. These include peanut allergy, hemophilia, rheumatoid arthritis, pollen and allergic asthma. Furthermore, mechanisms of antigen-delivery and the immune regulatory pathways that promote oral tolerance to the plant-derived antigens are discussed.
2. Concept of tolerance induction via delivery to the gut
The mammalian digestive system is constantly exposed to a large number of diverse foreign antigens derived from food and intestinal microbes. Hence, the gut immune system needs to balance the ability to provide protective immunity against ingested pathogens with a need to prevent inflammatory responses to antigens derived from food and commensal bacteria. As a result, a complex immune regulatory network has evolved. The gut-associated lymphoid tissue (GALT) is the largest immune organ in the human body, consisting of numerous lymphoid cells distributed throughout the entire intestinal surface (~1012 cells per meter). GALT represents 70% of the entire immune system and 80% of IgA-bearing cells (Vighi et al., 2008). It is also a major source of regulatory T cells (Treg) that control unwanted immune responses. It is thought that in the large intestine, the role of Treg is primarily to control responses against microbiota, which provide a major source of natural antigenic stimulation (Atarashi et al., 2011). In contrast, oral tolerance induction to food antigens occurs in the small intestine (Rezende and Weiner, 2017). In general, the intestine is enclosed by the musosa, which is comprised of an epithelium and the lamina propria (LP, a thin layer of connective tissue). The middle section of the small intestine, called ileum, contains a large number of lymphoid nodules called Peyer's patches (PP) that are located in the LP and extend into the submucosa. The epithelium of PP is comprised of epithelial cells and microfold or M cells that are specialized in transporting antigen from the gut lumen to specialized antigen presenting cells (APCs). These include dendritic cells (DCs) and macrophages. While the epithelium of the LP contains fewer M cells, protrusions of DC and of macrophage-like CX3CR1+ cells can sample antigens directly from the gut lumen. In addition, epithelial cells can take up antigens (Xiao et al., 2016).
CD103+ DCs represent the major subset of conventional DC in the LP and play a critical role in tolerance induction. They transport antigen to the mesenteric lymph nodes (MLNs), where they present to T cells and convert them to FoxP3 expressing Treg by production TGF-β and the vitamin A metabolite all-trans retinoic acid (RA) (Ruane and Lavelle, 2011). RA also promotes induction of IL-10 and gut homing rector expression by Treg (Bakdash et al., 2015). The induction not only of CD4+CD25+FoxP3+ Treg, but also of LAP+CD4+ Treg (expressing latency associated peptide of TGF-β on the cell surface) is TGF-β dependent. In addition, TGF-β is required for class switching in B cells to IgA, an immunoglobulin that is secreted into the intestinal lumen and that is critical for mucosal immunity. Other subsets of Treg that can be induced in the GALT include regulatory CD8+ T cells and type 1 regulatory T cells (CD49b+LAG-3+CD4+ Tr1 cells that overexpress IL-10). IL-10 production by Treg is critically required to prevent inflammation of mucosal interphases (Rubtsov et al., 2008). Thus, both TGF-β and IL-10 are key cytokines in immune regulation and tolerance induction in the gut.
While PPs contribute to oral tolerance induction, the currently prevailing opinion is that MLNs are strictly required (Pabst and Mowat, 2012). It has been proposed that Treg are initially induced in the MLNs upon antigen presentation by CD103+ DC, followed by migration to the LP, where Treg are further expanded (Hadis et al., 2011). FoxP3+ Treg peripherally induced in this environment express aryl hydrocarbon receptor, which is important for their gut homing and function (Ye et al., 2017). CX3CR1+ cells can pass on antigen to CD103+ DC, induce CD8+ Treg, and also take up blood-borne antigens, therefore representing an important link between the GALT and the systemic circulation (Chang et al., 2013; Mazzini et al., 2014; Shakhar and Kolesnikov, 2014). Finally, it should be pointed out that antigen doses influence oral tolerance (high doses may promote T cell anergy or deletion, while low doses may be sufficient for Treg induction), and that the microbiome plays an important role, as commensal bacteria promote intestinal homeostasis (Aychek and Jung, 2014; Mortha et al., 2014).
3. Oral tolerance in human food allergy
The prevalence of food allergy has increased over the past two decades, now affecting an estimated 8% of children in the US (Baker et al., 2000). Peanut allergy is of particular concern because it is often a life-long allergy and is the leading cause of fatal food-induced anaphylaxis. Interestingly, clinical practice guidelines in the late 1990s recommended nursing mothers avoid allergenic foods, such as peanut, egg, and milk and delay introduction of these foods to infants from at-risk families possibly exacerbating the food allergy problem (Baker et al., 2000). The observation was later made that delayed introduction, typically after the first year of life, was associated with significantly higher rates of peanut allergy compared to early introduction in two distinct populations (Du Toit et al., 2008). This observation led researchers to theorize that there was a critical window of opportunity in early life wherein introduction of peanuts would lead to oral tolerance rather than allergy.
The landmark clinical trial, LEAP (Learning Early About Peanut allergy) demonstrated that early introduction of peanuts suppressed allergy (Du Toit et al., 2015). Infants (n = 640) with severe eczema and/or egg allergy between the ages of 4 to 11 months were enrolled as this population reflects patients with a heightened risk of developing peanut allergy. Subjects were assigned to a peanut avoidance or peanut consumption group. The consumption group was instructed to consume Bamba, a peanut butter and corn snack, until the age of 60 months and the avoidance group avoided all forms of peanut. Each participant underwent a double-blind, placebo-controlled peanut challenge when they turned 60 months old. The outcome was striking as 17.2% of the avoidance group developed peanut allergy, whereas only 3.2% of the consumption group was peanut allergic at 60 months of age. Furthermore, study participants who were consuming peanuts were then put on an avoidance diet for 12 months without any significant increase in the rate of peanut allergy at age 72 months (Du Toit et al., 2015). These data were used to orchestrate changes in clinical guidelines for early introduction of peanut as a method to prevent peanut allergy (Togias et al., 2017).
Currently, an oral immunotherapy drug (AR 101) for peanut allergy treatment has completed a Phase III clinical trial and may receive FDA approval in 2019 (Tilles and Petroni, 2018). Developed by Aimmune Therapeutics, this plant-based oral immunotherapy is a regimen of daily intake of peanut antigens, starting from 1 mg and ending on a maintenance dose of 300 mg. In the Phase III trial, 550 subjects (4–55 years old) were enrolled in the U.S., Canada, and Europe (Palisade Group of Investigators et al., 2018). This large study follows prior clinical investigations that supported safety and efficacy of this approach, which is based on oral administration of peanut flour (Bird et al., 2018; Hofmann et al., 2009; Varshney et al., 2011; Vickery et al., 2014). Desensitization with this approach was definitively demonstrated in several small, Phase II trials as subjects on oral immunotherapy could safely ingest large doses of peanut protein whereas the placebo group could not (Anagnostou et al., 2014; Bird et al., 2018; Varshney et al., 2011). The therapy was well tolerated in most subjects over the course of dose accumulation (Bird et al., 2018; Hofmann et al., 2009). Following initial close monitoring, oral administration could be safely continued as home therapy with very rare allergic reactions. Impressively, unresponsiveness to peanut was sustained for up to 4–6 weeks when tested in children as young as 1 year old at the onset of therapy (Vickery et al., 2017). However, this form of tolerance after oral immunotherapy is stopped is only seen in a subset of subjects (Vickery et al., 2014). Immune modulatory effects were similar over a ten-fold dose range (Kulis et al., 2019). Production of pro-allergenic cytokines IL-5, IL-13, and IL-9 (typically associated with Th2 and Th9 responses) was suppressed, and the proportion of FoxP3hi cells was increased among CD4+CD25+FoxP3+ regulatory T cells (Treg), suggesting improved immune regulation (Kulis et al., 2019; Varshney et al., 2011). Allergen-specific IgE formation was initially reduced, and changes in epitope binding of allergen-specific IgE and IgG4 were documented in patients receiving OIT (Vickery et al., 2013). Allergens in stored peanut flour were stable for at least 1 year (Berglund et al., 2017). E. coli or Salmonella were not detected in the produce, while other microbes were within established US Pharmacopeia guidelines (Berglund et al., 2017).
Importantly, it is unclear whether immunologic tolerance mechanisms underlying the efficacy in LEAP and OIT are the same. It is clear that overlapping immune modulation occurs, as both modalities yield increased peanut-specific IgG4 and decreased mast cell activity.(Kulis et al., 2018) However, limited immunologic data has been published from the LEAP trial, so immune changes in T cells are unknown. The LEAP-ON trial showed long lasting tolerance in nearly all subjects, whereas OIT leads to tolerance in a large portion of young subjects but fewer older subjects and has only been assessed for several weeks after stopping therapy (Vickery et al., 2017; Vickery et al., 2014). Oral tolerance induction with other foods has been studied and the results are less clear whether all food allergies can be prevented by early introduction (Perkin et al., 2016). It is apparent that there is room to improve both the prevention and treatment approaches surrounding peanut, and other food allergies to induce robust tolerance faster with longer durability, possibly through use of approaches described below.
4. Production of protein antigens in plants for oral tolerance induction
Plant-made therapeutic proteins refer to protein drugs with clinical or veterinary applications produced in transgenic plant systems (Chan and Daniell, 2015; Daniell et al., 2016a; Daniell et al., 2016b; Ma et al., 2013; Ma et al., 2015; Shahid and Daniell, 2016). There are three major plant systems for manufacturing therapeutic proteins, stable nuclear transgenic and stable chloroplast transplastomic production as well as the plant virus vector-mediated transient expression system. Stable production systems involve permanently inserting genes into the plant genomes. Thus, the transgenes expressing therapeutic proteins are inherited in subsequent generations of transgenic plants. Nuclear transgenic plants are generated by inserting the transgenes into the nuclear genome. A number of recombinant proteins produced by this method in plants or in plant cell culture have advanced to clinical trials. Some are approved for use in human or veterinary medicine or as cosmetic ingredients (Sack et al., 2015).
The first vaccine antigen expressed in the chloroplast genome was the cholera non-toxic B subunit (CTB) (Daniell et al., 2001). Transplastomic plants are generated via chloroplast transformation by inserting transgenes into chloroplast genome through homologous recombination. This transplastomic technology has several unique advantages, including exceptionally high levels of expression (up to 72% of proinsulin in the total leaf protein), for smaller human blood proteins (Ruhlman et al., 2010). While larger blood proteins like blood clotting factors or angiotensin converting enzyme expression from native human genes is very poor, a new software has been developed for codon optimization and enhancing their expression 50–100 fold in chloroplasts (Kwon et al., 2016). The transplastomic plants maintained normal growth and reproduction despite hyper-expression of the foreign protein (Kwon et al., 2018; Ruhlman et al., 2010; Su et al1., 2015b). Most importantly, transgene integration into the chloroplast genome offers containment from pollen transmission because chloroplast genomes are maternally inherited in most crops (Daniell, 2002, 2007). Furthermore, harvesting therapeutic proteins expressed in leaves before appearance of any reproductive structures facilitates transgene containment through pollen or seeds. Other advantages of chloroplast genetic engineering include multi-gene engineering in a single transformation event, lack of gene silencing or position effect via site-specific transgene integration, and minimal or complete lack of pleiotropic effects by compartmentalization of toxic transgene products within chloroplasts (Daniell et al., 2016a; Daniell et al., 2016b).
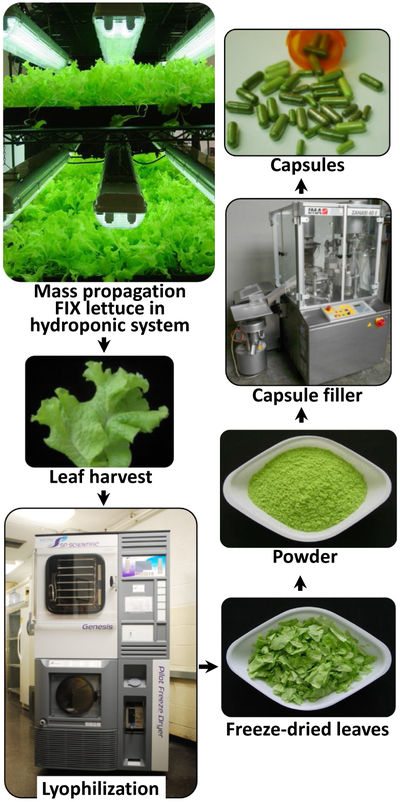
Plants expressing therapeutic proteins in chloroplasts have been grown in the field, with USDA-APHIS approval (Arlen et al., 2007). However, a recent USDA-APHIS notice states that “transplastomic lines do not fit the definition of a regulated article under USDA-APHIS regulations 7 CFR part 340, because there are no plant pest components, which should further help in advancing this technology” (Kwon and Daniell, 2015). Oral delivery of therapeutic proteins using lyophilized plant cells eliminates prohibitively expensive fermentation, purification, cold storage/transportation, sterile injectable delivery and short shelf life of current protein drugs (Fig. 2). Lyophilized plant cells can be stored at ambient temperature up to 30 months without losing their efficacy (Herzog et al., 2017; Su et al., 2015b).
Fig. 2.
Production scheme of clinical-grade, lyophilized lettuce cells for oral tolerance induction. Lettuce plants are grown in a hydroponic (soil free) system. Harvested fresh leaves are freeze-dried and assures antigen stability during storage at ambient temperature. Lyophilized leaves are powdered while preserving bioencapsulation. Depending upon application, the powder may be filled into capsules or added to liquid formulations. Figure modified from author's original publication (Su et al., 2015b).
FDA approved genetically modified (GM) edible crops corn and soybean have been consumed for three decades by a very large global population, even though they contain antibiotic resistance genes. After three decades, there is no GM crop free of antibiotic resistance genes in the marketplace and no regulatory agency, including FDA, has mandated removal of antibiotic resistance genes from food or feed crops. However, the retention of the antibiotic resistance gene in transplastomic lines could pose problems because of larger copy numbers in each cell. In addition, removal of the antibiotic resistance genes could reduce unnecessary metabolic load of the transplastomic crops because they are no longer needed after screening of transformation events. Furthermore, the same selection marker gene could be reused for subsequent transformation of additional genes. Therefore, in two recent studies (Daniell et al., 2019; Kumari et al., 2019) antibiotic resistance gene (aadA) was removed from lettuce transplastomic lines expressing pectate lyase, lipase and exoglucanase enzymes, for their use in various food/feed applications. Removal of the aadA gene was accomplished by flanking this gene with two identical sequences (649 bp of two atpB promoter regions) in the expression cassettes (PelB, PelD, LipY, Cbh1, Cbh2), following the method developed for the tobacco chloroplast genome (Iamtham and Day, 2000; Kode et al., 2006). After site-specific integration of complete transgene cassettes PelB, PelD, LipY, Cbh1, Cbh2 into lettuce chloroplast genome, the aadA gene was excised in subsequent rounds of selection. Site-specific integration of transgene cassettes without the antibiotic resistance gene was observed. Development of marker free edible crop plants offers various food/feed or therapeutic applications of chloroplast genetic engineering technology.
5. Fusion proteins containing transmucosal carriers enhance delivery
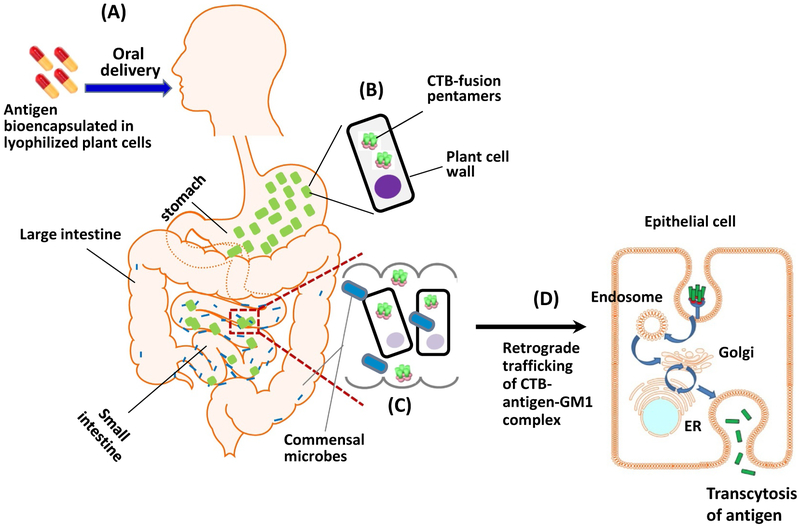
Gut microbiome also plays an important role in release of vitamins and nutrients from plant cells. Human digestive enzymes do not breakdown glycosidic bonds in the plant cell wall. Cellulases secreted by anaerobic gut bacteria cleave plant cell wall glycosidic bonds and release nutrients or protein drugs bioencapsulated within plant cells (Kwon and Daniell, 2016). Strategies are needed to assure efficient transmucosal delivery of the antigen once it is released from the plant cells (Xiao et al., 2016). For this purpose, expression of fusion proteins containing an effective transmucosal carrier has proven to be effective. For example, CTB is known to be a highly efficient carrier molecule for targeting of the gut epithelium. It is composed of five identical 11.5-kDa polypeptide monomers that assemble into a stable 57.5 kDa pentamer that binds to the GM1 ganglioside receptor. The GM1 receptor is highly expressed in the gut epithelial cell membranes and in DCs, which extend their pseudopodia (temporary and reversible structures with expanding and contracting capability) between enterocytes (small intestinal absorptive cells) to sample antigens in the intestinal lumen (Kraehenbuhl et al., 1997; Rescigno et al., 2001). Of importance for the plant system, CTB-fused proteins can form pentameric structures within chloroplasts (Daniell et al., 2001; Limaye et al., 2006; Ruhlman et al., 2007; Sherman et al., 2014; Su et al., 2015b). Upon oral delivery, CTB fusion proteins are released from plant cells in the small intestine via the activity of commensal microbes (Fig. 3A-C). Pentameric CTB fusions bound to GM1 receptors are transported into the gut epithelial cells through retrograde trafficking and transcytosis. Through the retrograde trafficking pathway, the complex traffics to the trans-Golgi Network (TGN) and endoplasmic reticulum (ER) via recycling endosomes (Fig. 3D), (Wernick et al., 2010). Once transported to the ER, unfolding and retro-translocation of the CTB-fused protein occur. Inclusion of a proteolytic cleavage site (e.g. for the ubiquitous protease Furin) assures that CTB is retained in epithelial cells after uptake, while the antigen of interest is released (Thomas, 2002). CTB moves to the basolateral membrane by trafficking back out the secretory pathway by indirect transcytosis. Release of antigens results in delivery to the immune system and to the systemic circulation. A fraction of CTB-protein-drug-GM1 complex may also cross the polarized epithelial cells by transcytosis, which depends on GM1 receptors with ceramide domains containing short or unsaturated fatty acid chains.
Fig. 3.
Mechanism of oral delivery of bioencapsulated CTB fusion antigens. A. Plant cells containing the antigen are taken up orally in form of capsules or mixed into a liquid or food. B. The plant cell wall provides bioencapsulation and thus protects the protein antigens from degradation in the stomach. C. After delivery of plant cells to the small intestine, antigens are released upon enzymatic degradation of cell wall by the action of commensal microbes. D. Transmucosal delivery of antigen: Pentameric form of CTB binds to GM1 receptors on the apical membrane of intestinal epithelial cells, followed by retrograde trafficking through early and recycling endosomes to the trans-Golgi Network and subsequently to the endoplasmic reticulum (ER). When a protease cleavage site is incorporated between the N-terminal CTB and the antigen, the antigen is released, and CTB traffics to the basolateral membrane (not shown). Ultimately, transcytosis is complete when the antigen is released and can thus be taken up by immune cells or be systemically delivered.
First direct visible evidence of oral delivery of a CTB fusion protein produced in plant chloroplasts was provided by Limaye et al. (Limaye et al., 2006). CTB-GFP fusion was highly expressed in chloroplasts; the monomeric and pentameric forms of CTB-GFP were confirmed by immunoblot analysis and GM1 binding assay (Daniell et al., 2001; Limaye et al., 2006). When mice are fed with plant cells expressing CTB-GFP and examined by fluorescence microscopy, GFP was observed in endothelial cells, M cells in the intestinal mucosa, submucosa, hepatocytes and spleen cells, indicating efficacy of protein delivery across the intestinal lumen (Limaye et al., 2006; Xiao et al., 2016). Without the CTB tag, GFP delivery was highly inefficient, suggesting that oral tolerance induction may be feasible at low antigen doses when the protein antigen is expressed as a CTB fusion. Indeed, the fusion of CTB to insulin generated an up to 5000-fold reduction in the amount of autoantigen compared with the amount normally required for immune-tolerization (i.e. without CTB fusion). This concept has been successfully applied to several antigens/therapeutic proteins (Daniell et al., 2016a; Daniell et al., 2016b; Kwon and Daniell, 2016). Proinsulin and extendin-4 could be detected in the circulation of mice within 30 min after oral delivery (Boyhan and Daniell, 2011; Kwon et al., 2013). In toxicology studies using CTB fusion proteins, no drug-related effects were observed for clinical observations, body weights, clinical pathology, and gross necropsy observations (Daniell et al., 2019, unpublished). Dose dependent drug delivery was observed in pharmacokinetic and pharmacodynamic studies of CTB fusion proteins. More recently, Daniell laboratory developed different tags to deliver foreign proteins bioencapsulated in plant cells to immune cells or non-immune cells or both types of cells (Xiao et al., 2016).
One important consideration in the selection of transmucosal carriers is their potential toxicity or other hurdles in the regulatory approval process. Oral administration of CTB was approved for human use few decades ago and is used by hundreds of millions of people around the globe (Hill et al., 2006). Because of repetitive cholera infection and outbreak, oral CTB has been repetitively used in the same patient population. No negative effect for CTB has been reported in the published scientific literature or in the popular press and therefore this product is used regularly for prevention or during outbreaks. Prevention of relapse of autoimmune uveitis in patients with Behçet's disease was tested by oral delivery of a CTB-autoantigen fusion protein, in human clinical trials (Phipps et al., 2003; Stanford et al., 2004). Relapse and recurrence of uveitis was blocked for 10–18 months after cessation of treatment with CTB-BD peptide (Stanford et al., 2004). Therefore, several examples listed below use CTB fusion proteins to induce oral tolerance.
6. Prevention of inhibitor formation in hemophilia
The most extensive clinical experience with ADA development, coupled with a need for antigen-specific tolerance induction, has likely been with protein replacement therapy for the X-linked bleeding disorder hemophilia, which is caused by mutations in coagulation factor IX (FIX, hemophilia B) or its co-factor, factor VIII (FVIII, hemophilia A). As the serine protease FIX has very low activity without FVIII, mutations in either protein can cause the bleeding phenotype. Hemophilia A occurs in 1 in 5000 male birth worldwide, while 1 in 30,000 boys is born with hemophilia B (Berntorp and Shapiro, 2012; Graw et al., 2005; Jayandharan and Srivastava, 2011). In first world countries, treatment for hemophilia patients is based on intravenous (i.v.) infusion with plasma-derived or recombinant factor concentrate. Currently, the major adverse effect of this therapy is the formation of inhibitory antibodies (“inhibitors”), which form in 20–30% of patients with severe hemophilia A and in ~5% of severe hemophilia B patients (Berntorp and Shapiro, 2012; DiMichele, 2012; Ehrenforth et al., 1992; Scott et al., 2013). Factor replacement therapy is seriously complicated by inhibitors and increases morbidity and mortality of the disease. Their formation typically occurs within the first 50 days of exposure to factor product, is dependent on CD4+ T helper cells and can be reversed via immune tolerance induction (ITI) protocols. These ITI protocols require frequent high-dose factor administrations for a long period of time (months to > 1 year), their costs are extremely high (often exceeding one million dollars), and ~30% of FVIII inhibitor patients fail to respond (DiMichele, 2012). Moreover, there are no prophylactic ITI protocols. Preclinical data have shown that one can, for example, prevent inhibitor formation in hemophilia mice by co-administration of rapamycin and FVIII or FIX antigen (Maldonado et al., 2015; Moghimi et al., 2011; Nayak et al., 2009). However, there concern about use of immune suppressive drugs in pediatric patients with hemophilia, which is not an autoimmune disease. In addition, the likelihood of inhibitor formation for an individual patient cannot yet be predicted with sufficient accuracy. An antigen-specific, non-immune suppressive protocol would be more readily acceptable for translation. Toward this goal, a cost-effective system was developed for high-level production of coagulation factor antigens in chloroplasts of transplastomic plant cells, which provide bioencapsulation of the antigen through the cellulose containing cell wall and effective delivery to the gut immune system (Daniell et al., 2016a; Daniell et al., 2016b). According to these results, antigens are not biologically active but they are effective in suppressing the pathogenic antibody responses to i.v. delivered coagulation factors.
6.1. Suppression of FVIII inhibitors in hemophilia A
FVIII is widely considered a highly immunogenic molecule as it can elicit potent antibody responses at very low i.v. doses. Inhibitor formation occurs in 20–30% of severe patients, with even higher incidence in African American and Latino patients (Scott et al., 2013; Wroblewska et al., 2013). FVIII contains 6 distinct domains, which are structurally organized as A1-A2-B-A3-C1-C2.(Vehar et al., 1984). The large and highly glycosylated central B domain functions in secretion of the FVIII protein (Dorner et al., 1987; Pipe, 2009; Roberts et al., 2011). However, recombinant B domain deleted (BDD) FVIII is biologically active and is currently used in the clinic. A heterodimer form of FVIII is secreted following at least two intracellular cleavages within the B domain. Consequently, circulating FVIII consists of a heavy chain (A1-A2-B domains) and a light chain (A3-C1-C2 domains), which are non-covalently linked. The majority of inhibitors are found to bind to A2, A3, or C2 domain (Markovitz et al., 2013; Meeks et al., 2007; Pratt, 2012; Scott et al., 2013). These highly immunogenic sequences also contain several CD4+ T cell epitopes, although the C1 domain also contains T cell epitopes (Pratt and Thompson, 2009; Scott et al., 2013; Steinitz et al., 2012; Wroblewska et al., 2013). Oral tolerance was first induced using the native FVIII HC and C2 domains expressed in tobacco chloroplasts. The pentameric forms of the CTB-HC and CTB-C2 bioencapsulated in plant cells were delivered to hemophilia A mice via oral gavage of frozen plant cells. Delivery of FVIII antigen to the GALT was evident from presence of chloroplast-derived FVIII antigen in epithelial cells and in DC located in LP and Peyer's patches of the small intestine. The T helper cell responses and inhibitor formation (primarily reflecting IgG1 production) against IV injected FVIII were substantially suppressed in hemophilia A mice (FVIII exon 16 knockout mice of two different strain backgrounds) after oral administration of the plant-made HC/C2 mixture. Importantly, prolonged feeding was effective in controlling inhibitor formation long-term. Oral delivery of chloroplast-derived FVIII also significantly reduced inhibitor titers in pre-immune mice injected with recombinant FVIII, demonstrating that the oral protocol could also reverse inhibitor formation (Sherman et al., 2014). To cover the entire patient population and meet the clinical need, the full-length human clotting FVIII was generated in an edible lettuce plant, and plant cells were produced at cGMP hydroponic facility. Expression level of FVIII heavy chain and light chain were dramatically enhanced by codon optimization (Kwon et al., 2016; Kwon et al., 2018), expressing the largest human blood protein in chloroplasts. Oral delivery of lyophilized lettuce cells expressing FVIII HC and LC to hemophilia A mice robustly suppressed anti-FVIII antibody formation, in a broad dose range (Kwon et al., 2018). Production of the cytokine IL-10 in response to FVIII antigen and surface expression of latency associated peptide (LAP) on Treg have emerged as potential biomarkers to monitor plant-based oral tolerance induction (see below sections on tolerance mechanism for further details).
6.2. Prevention and reversal of the pathogenic antibody response in hemophilia B
Although inhibitor formation is comparatively rare in hemophilia B, with an elevated risk in patients with F9 gene deletions or similarly severe mutations, FIX inhibitors are often more difficult to eliminate because of anaphylactic reactions against FIX or development of nephrotic syndrome. In either case, traditional ITI has to be stopped. In order to develop an oral tolerance protocol for hemophilia B, a CTB-fused human FIX (CTB-FIX) was initially produced in tobacco chloroplasts and frozen plant cells were orally gavaged to hemophilia B mice (Verma et al., 2010). Oral delivery of bioencapsulated CTB-FIX effectively blocked formation of pathogenic antibodies, including IgE and IgG1 (undetectable or up to 100-fold lower than controls) in hemophilia B mice. Consistent with suppression of IgE formation, this treatment also prevented fatal anaphylactic reactions that occurred after four to six exposures to i.v. FIX. Approximately 90% of plant-derived FIX-fed mice survived 12 injections without signs of allergy or anaphylaxis. In contrast, only 20–25% of control animals survived after injecting six to eight recombinant FIX doses. Immunostaining demonstrated delivery of FIX to Peyer's patches in the ileum of small intestine. The oral administration protocol was found effective over a range of oral antigen doses (5–80 μg recombinant FIX/kg), and controlled inhibitor formation and anaphylactic reaction up to 7 months (~ 40% life span of the male mouse strain C3H/HeJ) (Verma et al., 2010). In summary, repeated oral delivery of FIX bioencapsulated in plant cells suppressed inhibitor development and prevented life-threatening anaphylactic reactions to FIX replacement therapy in a murine model of hemophilia B.
In order to translate this oral tolerance approach to treatment of hemophilia B patients, transplastomic edible plants expressing pharmaceutical proteins need to be developed. The CTB-FIX expressing lettuce plants have been generated using a commercial variety (Simpson Elite) (Su et al., 2015b). Lyophilization of the fresh CTB-FIX lettuce leaves increased the CTB-FIX concentration by ~20 fold, reaching up to ~1.5 mg CTB-FIX protein per gram of dry leaves. Furthermore, the lettuce chloroplast-made CTB-FIX was stable with proper folding, disulfide bonds and pentamer assembly in lyophilized cells for up to ~2 years at ambient temperature. Approximately 43.5 kg (dry weight) of CTB-FIX expressing lettuce leaves can be produced using Fraunhofer (Newark, Delaware) cGMP (current good manufacturing practices) hydroponic system per thousand square feet per annum, yielding up to 36,000 doses for treatment of 20-kg pediatric patients (Fig. 2). Dose escalation studies were performed using the lyophilized lettuce leaves, and successful oral tolerance induction was reported in all tested doses (1.5–15 μg CTB-FIX antigen per dose), indicating that plant cell-mediated oral tolerance induction can be applied to patients at different ages or with diverse genetic background (Su et al., 2015b). A wide range of effective doses will further facilitate translation. Lettuce-based oral tolerance effectively induced LAP+ Treg, resulting in suppression of inhibitor formation and of IgE, thereby preventing anaphylaxis to i.v. delivered FIX.
6.3. Oral tolerance in non-rodent model of canine hemophilia B
The vast majority of pre-clinical oral tolerance studies have been performed in rodent models. Recent clinical successes with oral tolerance induction to prevent severe food allergies, such as peanut allergy, show that this concept is not limited to rodents and in fact applies to the treatment of patients (Hamad and Burks, 2017; Yanagida et al., 2017). Further support of oral tolerance in prevention of anti-drug antibody formation comes from a recent translational study in a large, non-rodent model of hemophilia B (dogs with a natural F9 mutation). Here, suppression of antibody formation against FIX replacement therapy was demonstrated (Herzog et al., 2017). Therefore, oral tolerance can be achieved in diverse species. The study initially determined a dose of weekly i.v. injections of recombinant human FIX that reliably induced antibody formation of a certain titer in dogs with severe hemophilia B. IgG formation against FIX was highly reproducible in this model. As a result, inhibitors formed, albeit at titers that were more variable than binding antibodies. In addition, the dogs also developed severe allergic reactions that correlated with IgE formation and could be prevented with anti-histamine. Importantly, inclusion of lyophilized lettuce cells, expressing CTB-FIX, in dog chow (given twice per week, at an antigen dose of 0.3 μg/kg) prevented these responses to i.v. FIX. Oral immune modulation robustly suppressed IgG and IgE formation against FIX, resulting in prevention of inhibitor formation and of anaphylaxis in 3 of 4 dogs. As a result, these animals showed complete correction of coagulation times after each of 8 weekly i.v. FIX injections, while control dogs failed to show correction after 3–4 injections because of antibody formation. Careful monitoring of general health, comprehensive serum chemistry, and hematological evaluations showed lack of toxicity from oral delivery of bionecapsulated CTB-FIX that was performed for up to 6 months. Lettuce-made antigen had been produced in the hydroponic system discussed above. Given that the weight of these animals is comparable to that of pediatric patients that are likely candidates for this immune tolerance regimen, these outcomes illustrate the scalability to human treatment.
7. Treatment of rheumatoid arthritis (RA)
Rheumatoid arthritis (RA) is the most common form of arthritis and constitutes a disabling autoimmune disease that mainly attacks flexible joints, characterized by synovial inflammation, hyperplasia (“swelling”) and autoantibody production. Inflammation may also spread more systemically, affecting cardiovascular, pulmonary, psychological, and skeletal functions (McInnes and Schett, 2011).
Type II collagen (CII) is an extracellular matrix protein of joint cartilage, and it is required for smooth movement of joints, and autoimmune antibodies against CII have been detected at high level in some RA patients (Banerjee et al., 1988). Immunization of mice against CII is a commonly used pre-clinical model of RA (collagen-induced arthritis, CIA, mice). To test oral tolerance in this model, a glutelin–fused CII with tandem four repeats of CII250–270 peptide, containing a human T-cell epitope, was produced in transgenic rice seeds at a level of about 1 μg per seed (Hashizume et al., 2008). Feeding RA DBA/1 mice with the transgenic rice seeds at 25 μg per dose for 2 weeks showed reduction and delay in IgG2a response against subsequent and repeated injection of type II collagen (Hashizume et al., 2008). More recently, transgenic rice seeds expressing CII256-271 (wild-type sequence or as altered peptide ligands to increase binding to the T cell receptor) at 7-24 mg/g seeds were orally delivered to CIA mice (Iizuka et al., 2014). In this model, CII256-271 is presented by murine MHC II molecule I-Aq. Significant inhibition of the initial onset of arthritis was observed and appeared to be mediated by IL-10 producing CD4+CD25− T cells, albeit that these regulatory cells were not further defined. However, there was no effect on the production of IFN-γ, IL-17, or IL-2. In an alternative approach, Arabidopsis thaliana plants expressing tolerogenic CTA1 (R7K)-COL-DD protein at 2.5% TSP were generated by nuclear transformation technology (Hansson et al., 2016). CIA mice fed with fresh Arabidopsis plants expressing CTA1 (R7K)-COL-DD proteins were either disease free or showed arthritis with substantially less severity. Tolerized mice had improved arthritis scores, reduced joint inflammation, and reduced systemic biomarkers for inflammation. Suppression of effector T-cell activity (Th1, Th2, and Th17 as reflected by reduced IFN-γ, IL-13 and IL-17A production) and up-regulation of IL-10 was observed in the tolerized (Hansson et al., 2016). While these models provide proof of principle that plant-based oral tolerance is applicable to inflammatory joint disease, the target antigens in RA are less well defined and may be more diverse in humans, who also have much more diversity in T cell epitope usage. Therefore, translation of these approaches is less straightforward than for other disease targets described in this review.
8. Desensitization from pollen allergy
Approximately 30% of the population of industrial countries suffers IgE-mediated type I allergies due to pollen, house dust and food allergies. In such individuals, the allergen-induced IgE binds to mast cells and basophils, inducing the release of inflammatory mediators such as histamine (May and Smith, 2008). These allergic diseases are usually treated with medicines (e.g., H1-antihistamines, anti-leukotrienes and corticosteroids) via blocking the release of chemical mediators. Although such medications can alleviate allergic symptoms, they are not curative. Allergen-specific immunotherapy is the only curative treatment that can result in a lasting clinical effect (Bousquet and Demoly, 2006; Frew, 2003). Antigen-specific immunotherapy can direct suppression of allergen-specific IgE, an increase in blocking IgG, suppression of effector Th1, Th2, and Th17 cells, and a reduction in allergen-specific T-cell responsiveness (Akdis and Akdis, 2011). While there have been multiple success in recent years with oral immune modulatory therapy using crude materials such as peanut flour or ground peanuts (Vickery et al., 2014), transgenic plants would be favorable for those allergies with well-defined allergens.
It has been documented that ~15–25% of the Japanese population, approximately 33 million patients, are afflicted with a seasonal allergy, showing clinical symptoms of allergic rhinitis, conjunctivitis, and asthma during February to April. IgE is formed in 90% of affected people to both allergens Cryptomeria japonica 1 (Cry j 1) and Cryptomeria japonica 2 (Cry j 2), with the remaining 10% having IgE against one of these (Okamoto et al., 2009). To target T cells specific to these antigens, CTB- fused Cry j 1 and 2 were engineered to accumulate in ER-derived protein bodies (PB-I) of transgenic rice seeds (Takagi et al., 2005). The production of IL-4, IL-5, and IL-13 (allergen-specific CD4+ T cell-derived allergy-associated Th 2 cytokines) and histamine release in serum were significantly reduced in Cry j 1 and Cry j 2 fed mice after systemic challenge with total protein of cedar pollen (Takagi et al., 2005). In order to further refine this approach and reduce the allergenicity while retaining the ability of the expressed allergen peptides to tolerize CD4+ T cells, both Cry j 1 and Cry j 2 were reconstructed using a molecular shuffling method to disrupt the tertiary structure (Wakasa et al., 2013). The modified Cry j 1 allergen showed decreased IgE-binding capacity and thus reduced its allergenic activity. The engineered Cry j 2 was also demonstrated to have reduced binding capacity to the Cry j 2-specific IgE from mouse sera. Suppression of allergen-specific CD4+ T-cell proliferation and IgE and IgG formation was observed in mice fed with transgenic rice seeds. Clinical symptoms such as sneezing frequency, infiltration of inflammatory cells in the nasal tissue, and allergen-specific cytokine responses were also significantly reduced (Wakasa et al., 2013). Similarly, oral administration of rice seeds containing three CD4+ T cell epitopes (derived from of Cry j 1 and Cry j 2) fused to CTB suppressed allergen-specific IgE responses and cedar pollen-induced clinical symptoms in mice at 50-fold lower doses when compared with the analogous glutelin fusion (Takagi et al., 2008).
More recently, efficacy of oral immunotherapy with the Cry transgenic rice by conjunctival antigen challenge of allergic conjunctivitis in mice was investigated (Fukuda et al., 2018). BALB/c mice were injected with Japanese cedar pollen, then challenged with pollen in eyedrops, and then fed with Cry transgenic rice seeds or with non-transgenic rice seeds as a control for 16 days. These mice were then challenged twice with pollen in eyedrops. After the first challenge, the clinical signs were evaluated at 15 min; and the eyes, blood, spleen, and lymph nodes were isolated at 24 h after the second challenge. In mice fed the transgenic rice seeds, the clinical score was significantly lower than those fed non-transgenic rice.
9. Treatment of allergic asthma
Asthma is a chronic inflammatory disease of the airways, which causes reversible airway obstruction, bronchospasm, and multiple clinical symptoms, including shortness of breath. Over 200 million people are affected globally by this potentially life-threatening condition. The disease may be triggered by allergens. In allergic asthma, CD4+ T helper (Th) 2 cells are thought as the major effector cells in airway inflammation, and these Th 2 cells are associated with lung dysfunction in their recruitment and activation of eosinophils (Corrigan and Kay, 1992; Robinson et al., 1992; Watanabe et al., 1995).
Suzuki et al. generated transgenic rice seeds expressing a fragment (residues 45–145) of mite Dermatophagoides pteronyssinus allergen Der p 1 containing the major mouse and human T cell epitopes (Suzuki et al., 2011). Oral administration of the transgenic rice seeds to mice before systemic challenge with Der p 1 induced oral tolerance, as shown by inhibition of allergen-specific Th 2 cytokine synthesis (IL-4, IL-5, and IL-13) and IgE formation. The induction of oral tolerance inhibited bronchial hyper-responsiveness following exposure to Der p 1 (Suzuki et al., 2011). Transgenic tobacco plants expressing Der P2 antigen were also developed and tested in oral tolerance studies in mice (Lee et al., 2011). Animals receiving total protein extract from Der P2 transgenic plant cells orally once per day for 6 days showed reduced sensitivity to recombinant Der p2 (rDer p2). When compared to untreated controls, orally treated animals had reduced Der p2-specific IgE and IgG1 titers, IL-5 and eotaxin levels in bronchial alveolar lavage fluid. Eosinophil infiltration in the airways was also reduced. CD4+CD25+Foxp3+ regulatory T cells were significantly increased in mediastinal and mesenteric lymph nodes when fed with tobacco plant cells and splenocytes exhibited decreased proliferation and increased IL-10 secretion, after stimulation with rDer p2 (Lee et al., 2011).
10. Potential for treatment of inflammatory bowel disease (IBD)
Inflammatory bowel disease (IBD) is a chronic autoimmune disorder characterized by mucosal inflammation and injury of the colon and small intestine, which affects more than one million North Americans. For unclear reasons, the incidence of this disease has been increasing (Hanauer, 2006). The two major forms of IBD, Crohn's disease (CD) and ulcerative colitis (UC), are diagnosed based on different gut location and clinical presentation. Although UC is typically observed in the large intestine and CD occurs in the entire gastrointestinal tract, immunological mucosal injury is a central feature for both disorders. Symptoms include nausea, diarrhea, abdominal pain and weight loss, even death resulting from severe dehydration, blood loss and malnutrition. The pathogenesis of the disease is complex and not well understood, in part due to the diversity of bacterial, viral and environmental factors in genetically susceptible individuals (Menassa et al., 2007). While it is possible to treat IBD with systemic administration of recombinant IL-10, side effects make this approach unattractive. Local introduction of the anti-inflammatory cytokine IL-10 in the gut environment would solve this conundrum. Feasibility can be tested in certain strains of IL-10 deficient mice, a commonly used pre-clinical model of colitis. Oral delivery of tobacco cells expressing human interleukin-10 (hIL-10) reduced the severity of colitis at the sites of inflammation in IBD-susceptible IL-10−/− mice (Menassa et al., 2007). Dietary supplementation of plant cells hIL-10 was well tolerated by treated mice. Gut histology revealed reduced inflammation, which was correlated with lower expression levels TNF-α mRNA in the small bowel and an increase in IL-2 and IL-1 β mRNA levels (Menassa et al., 2007).
11. Mechanisms of oral tolerance induction using plant cells
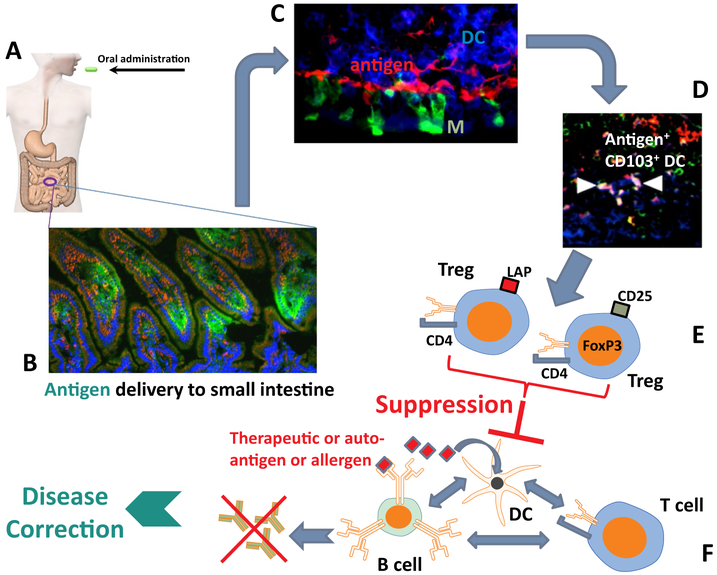
In order to prevent inflammatory responses to food antigens and commensal bacteria, the immune system of the small intestine has evolved an anti-inflammatory environment, characterized by the presence of specialized APCs, production of suppressive cytokines, and specialized lymphoid structures such as the PPs of the ileum. DCs, CX3CR1+ macrophages/DCs, and endothelial cells are capable of taking up antigen from the gut lumen. In addition, the epithelium of PPs is rich in M cells that channel antigen from the gut lumen to areas with abundance of DCs. Enhancing delivery to immune system of the small intestine may improve tolerance induction and reduce the required antigen dose. For example, CTB fusions released from cells of chloroplast transgenic leaves efficiently target epithelial and M cells (Limaye et al., 2006; Ruhlman et al., 2010; Sherman et al., 2014; Wang et al., 2015; Xiao et al., 2016). As a result, the antigens are delivered to CD11c+ DCs of the LP of all sections of the small intestine and to DC in PP (Fig. 4A–D) (Wang et al., 2015). These include CD103+CX3CR1− DC, which are known to transport antigen to the MLN and promote TGF-β dependent Treg induction through generation of RA or expression of IDO (Fig. 5) (Matteoli et al., 2010). In fact, the MLN is generally required for oral tolerance induction. While pDC are rare in the LP, this subset of DC is present in the MLN and PP and has been shown to contribute to oral tolerance (Goubier et al., 2008). While pDC have been known to play an important role in innate immunity against pathogens (for example producing high levels of IFN-α/β), it has recently become clear that these cells also have immune regulatory functions and aid in FoxP3+ Treg induction, which may involve up-regulation of IDO (Biswas et al., 2015; Park et al., 2008). Oral administration of transplastomic leaf cells expressing FIX into hemophilia B mice followed by systemic challenge with the therapeutic protein antigen resulted in an increase in total numbers and in frequencies of CD103+ DC and pDC in MLN and PP (and for pDC also in the spleen) (Wang et al., 2015). Therefore, interactions between the responses to the orally delivered and the systemic antigen can enrich for tolerogenic DCs, thereby increasing systemic immune regulation. In the jejunum, i.e. the section proximal to the stomach, delivery of the ingested antigen to LP macrophages may be more prevalent. Interestingly, CX3CR1+ DC/macrophages in the LP acquire systemic antigens and also pass on antigen to CD103+ DC via gap junction transfer (Fig. 5) (Chang et al., 2013; Mazzini et al., 2014). These examples illustrate the connections between the gut and systemic immune systems.
Fig. 4.
Concept of oral tolerance induction using plant cells. A. Transgenic plant cells expressing the specific antigen are orally delivered. B. Upon release in the small intestine, the antigen (shown in green) is translocated to the gut-associated immune system (which is facilitated by use of transmucosal carrier polypeptides fused to the antigen). C. Antigen (here shown in red) accumulates in areas rich in to dendritic cells (DCs, blue). Also shown are M cells (green). D. Some of the antigen is taken up by tolerogenic CD103+ DCs (arrows point to antigen-loaded CD103+ DCs, which are shown in white, representing triple stain for antigen, CD11c, and CD103). E. As a result, antigen-specific regulatory T cells (CD4+CD25+FoxP3+ and CD4+CD25−FoxP3−LAP+ T cells) are induced. F. Induced Treg suppress B and T cell responses against the antigen, resulting in elimination of autoimmune or allergic responses. In the case of treatment for genetic disease, oral tolerance induction to the therapeutic protein suppresses formation of antidrug antibodies, so that administration of replacement therapy can correct the disease. (For inter-pretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
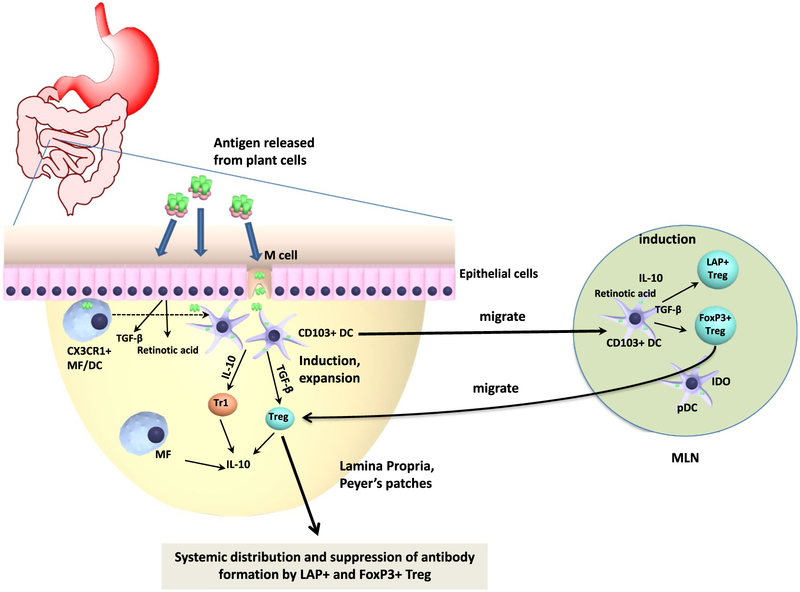
Fig. 5.
Detailed immunological mechanism of oral tolerance induction. Antigens released from plant cells in the small intestine upon oral administration are taken up by epithelial and M cells, followed by transmucosal delivery. Dendritic cells (DCs) may take up transcytosed antigen, sample antigen directly from the gut lumen, or acquire antigen initially taken up by CX3CR1+ macrophages (MF). Ultimately, antigen-loaded CD103+ DCs migrate to mesenteric lymph nodes (MLN) and present the acquired antigen to CD4+ T cells while producing TGF-β and retinoic acid, resulting in induction of Treg. Plasmacytoid DCs (pDCs) may enhance Treg induction. Upon migration back to the lamina propria, induced Treg further expand. The cytokines TGF-β and IL-10 are critical to Treg induction. Peripheral induction of FoxP3+ and of LAP+ Treg is TGF-β dependent, while IL-10 may enhance induction of Tr1 cells. IL-10 is crucial in preventing chronic inflammation in the gut and for the immune suppressive function of Treg on mucosal interfaces. Induced FoxP3+ and LAP+ Treg systemically distribute to lymphoid organs outside the gut immune system, such as the spleen, and suppress T cell responses and antibody formation.
Another potentially important although less well understood feature of oral antigen delivery is the uptake of antigen by the liver. In this gut-liver-axis, food antigens primarily enter the liver via the portal vein (albeit a lymphatic route has also been described) (Pabst and Mowat, 2012). Interestingly, CTB and other fusion proteins expressed in orally delivered transplastomic cells are routinely found to accumulate to some extent in the liver within hours after oral delivery (Limaye et al., 2006; Ruhlman et al., 2007; Xiao et al., 2016). The liver provides another tolerogenic environment, characterized by local antigen presentation and expression of anti-inflammatory cytokines such as IL-10. Liver transplants graft more easily and require less immune suppression than that is the case for other organs, and antigens ectopically expressed in liver via gene therapy can induce immune tolerance by promoting programmed cell death of effector CD4+ T cells and inducing FoxP3+ Treg (Herzog, 2017; Perrin et al., 2016). To what extent the liver contributes to tolerance induced by oral delivery of plant cells and which cell type in the liver capture and present the antigen remains to be defined.
11.1. Induction of regulatory T cells
Early on in the experience with oral tolerance based on feeding of transgenic plants it was recognized that active suppression is part of the tolerance mechanism as suppression of autoimmunity could be transferred from tolerized mice by adoptive transfer of splenocytes (Ma et al., 1997). Although high doses of orally delivered antigen can also result in specific deletion of T cells, the amounts of antigen released from plant cells upon digestion by commensal bacteria more likely induce tolerance via active suppression mediated by induced Treg. In more recent studies on oral delivery of plant cell-made antigens in animal models of autoimmune diseases and allergies, a recurring observation has been the induction of T cells, including CD4+CD25− T cell, that express IL-10, a key anti-inflammatory cytokine in environmental interphases such as the gut or the airways (Hansson et al., 2016; Iizuka et al., 2014; Lee et al., 2011; Ruhlman et al., 2007). Using oral tolerance to FVIII and FIX in hemophilic mice as a model, a very recent and more thorough set of investigations identified induction of two subsets of Treg in response to oral delivery of antigen bioencapuslated in plant cells: CD4+CD25+FoxP3+ Treg and CD4+CD25−FoxP3−LAP+ Treg (Sherman et al., 2014; Wang et al., 2015). Both Treg subsets suppressed antibody formation upon adoptive transfer, while CD4− cells or CD4+CD25−LAP− T cells failed to suppress. CD4+CD25+FoxP3+ Treg were originally discovered by Sakaguchi and colleagues and are required in both humans and mice to maintain tolerance to self-antigens (Morikawa and Sakaguchi, 2014). These Treg undergo thymic development but may also be peripherally induced by TGF-β dependent conversion of conventional CD4+ T cells. CD4+CD25+FoxP3+ Treg constitutively express the receptor for IL-2, a cytokine that they require as a growth factor, and can suppress DC, B and T cells through a variety of mechanisms, depending on the context. These include cell contact dependent and independent mechanisms, ranging from suppression of co-stimulation, disruption of metabolism, cytokine-mediated suppression to cytotoxicity, among others. FoxP3+ Treg are intricately involved in many tolerance mechanisms and are also effectively induced by gut CD103+ DC. Their induction in the small intestine is critical for unresponsiveness to dietary antigens (Kim et al., 2016). They are one of the mediators of oral tolerance to antigens transgenically expressed in plant cells (Fig. 4D, Fig. 5). During oral tolerance induction with CTB fusion proteins, B cells promote further expansion of FoxP3+ Treg (Sun et al., 2008).
Weiner and colleagues found that mucosal antigen administration induces CD4+ T cells that overexpress TGF-β, and initially referred to these as “Th3 cells” (Weiner et al., 2011). Subsequently, these immune regulatory cells were better defined as CD4+CD25−FoxP3−LAP+ Treg that suppress in a TGF-β dependent manner and show cell surface expression of latency-associated peptide (LAP), which forms a latent complex with TGF-β. Induction of LAP+ Treg is dependent on TGF-β, IL-10, and DCs (Sun et al., 2008; Weiner et al., 2011). In the hemophilia model, antigen-specific induction of LAP+ Treg induction was sufficiently robust for the total frequency of CD25−FoxP3−LAP+ cells among CD4+ T cells to increase (Sherman et al., 2014; Su et al., 2015b; Wang et al., 2015). Although the overall frequency of FoxP3+ Treg did not significantly increase, suppression of antibody formation was equally efficient after adoptive transfer as for LAP+ Treg, indicating that antigen-specific FoxP3+ Treg are highly suppressive at low frequencies. LAP expression in circulating Treg (both FoxP3+ and FoxP3−) could potentially serve as a biomarker for oral tolerance induction (Kwon et al., 2018). LAP+ Treg were identified as the main source of IL-10 expression in splenocytes in response to FIX antigen (Wang et al., 2015). Moreover, the entire immune regulatory response, including increase in DC subsets and LAP+ Treg induction, was IL-10 dependent. Hence, IL-10 and TGF-β are critical for the induction and execution of oral tolerance to plant cell-derived antigens (Fig. 4E, Fig. 5). LAP+ and FoxP3+ Treg may provide both synergy and redundancy in systemic suppression of immune responses to the antigen. In order to connect gut and systemic immune responses, and for orally induced Treg to home back to the GALT for antigen-stimulation and expansion, imprinting of gut homing receptor expression onto CD4+ T cells is required. Indeed, plant-based oral tolerance to FIX was associated with significant increases in CCR9 and α4β7 expressing CD4+ T cells in PP, MLN, and spleens (Wang et al., 2015). Locally in the LP, another subset of Treg, namely IL-10 expressing Tr1 cells may aid in maintaining an anti-inflammatory environment that favors Treg induction. Induction of Tr1 cells, as evidenced by increase in frequency of CD4+LAG-3+CD49b+ T cells, was observed in the LP lymphocyte fraction (Wang et al., 2015). Tr1 cells are known to suppress through IL-10 secretion and cytotoxicity against conventional DCs (Roncarolo et al., 2014). Future studies may better define the local role of immunoglobulins in oral tolerance to plant-made antigens. For example, formation of systemic and secreted antigen-specific IgA, a TGF-β dependent immunoglobulin subclass produced by B cells of mucosal immune systems, was observed in response to orally delivered transplastomic cells expressing CTB-Pins in NOD mice or CTB-FIX in hemophilia B mice, albeit without impeding long-term tolerogenic oral delivery of plant cells (Ruhlman et al., 2007; Ruhlman et al., 2010; Wang et al., 2015). Oral immmunotherapy to suppress allergic IgE formation is dependent on FcγRIIB, which has also been implemented in oral tolerance based on CTB fusions and is the only Fc receptor expressed by B cells (Burton et al., 2014; Sun et al., 2013).
12. Conclusions and future prospects
Oral tolerance is an attractive immunotherapy to prevent or reverse autoimmune, allergic, or anti-protein drug responses. This approach is non-invasive and antigen-specific, and a number of successes have recently been documented for food allergies albeit utilizing crude forms of peanut antigens. However, translation of oral tolerance for treatment of autoimmune diseases is more challenging, but could combined with other immune modulatory strategies, such as monoclonal antibody therapy. In blocking antibody formation in replacement therapies for inherited protein deficiencies, the plant-based method appears superior to conventional approaches using purified proteins and is substantially more cost effective. Besides hemophilia, lysosomal storage disorders are another example for diseases that could benefit (Su et al., 2015a). Expressing the target antigen in plant cells represents an elegant way to tap into the naturally existing immune regulatory pathways that are in place to control immune responses to food antigens. In addition, plant cells naturally provide bioencapsulation, eliminating the need for expensive protein purification and formulation. Antigens stored in freeze-dried plant cells are stable at ambient temperature for many years. Moreover, recent advances in plant biotechnology have overcome two major hurdles for this technology: limitations in the levels of antigen expression from the transgene per cell and lack of a suitable transgenic platform based on edible crop plants. Consequently, the numbers of plant-based research papers demonstrating oral tolerance induction and the scope of the diseases that are targeted have substantially increased in recent years. The transgenic chloroplast technology is particularly attractive, because it can achieve substantially higher antigen levels compared to nuclear transformation systems and is now available for lettuce, allowing the harvest and processing of edible leaves under GMP conditions. Expression of the antigen as a fusion with a transmucosal carrier enhances uptake by the gut immune system, resulting in tolerance induction at low antigen doses. A better understanding of the immunological mechanisms of plant-based oral tolerance is also being obtained. Therefore, the field is now ready to go beyond proof-of-principle studies in animal models to clinical studies using the cost-effective approach of delivering plant cells.
Acknowledgements
This work was supported by NIH grants R01 HL133191 and R01 HL107904 to HD and RWH. Authors thank Dr. Jin Su and Ms. Wan Jung Chang for updating Table 1 and organizing text or references.
Footnotes
Conflict of interests
Drs. Henry Daniell and Roland Herzog are inventors on published or awarded patents on induction of oral tolerance in hemophilia. Both authors are currently funded by Takeda (formerly Shire) for research on hemophilia and therefore declare financial conflict of interest. However, none of the research reported in this review was supported by Shire/Takeda.
References
- Akdis CA, Akdis M, 2011. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 127 (1), 18–27. [DOI] [PubMed] [Google Scholar]
- Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, Palmer C, Deighton J, Ewan P, Clark A, 2014. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet 383 (9925), 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H, 2007. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol. J. 5 (4), 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K, 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331 (6015), 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aychek T, Jung S, 2014. The axis of tolerance. Science 343 (6178), 1439–1440. [DOI] [PubMed] [Google Scholar]
- Bakdash G, Vogelpoel LT, Van Capel TM, Kapsenberg ML, de Jong EC, 2015. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 8 (2), 265–278. [DOI] [PubMed] [Google Scholar]
- Baker SS, Cochran WJ, Greer FR, Heyman MB, Jacobson MS, Jaksic T, Krebs NF, 2000. American Academy of Pediatrics. Committee on nutrition. Hypoallergenic infant formulas. Pediatrics 106 (2 Pt 1), 346–349. [PubMed] [Google Scholar]
- Banerjee S, Luthra H, Moore S, O'Fallon W, 1988. Serum IgG anti-native type II collagen antibodies in rheumatoid arthritis: association with HLA DR4 and lack of clinical correlation. Clin. Exp. Rheumatol. 6 (4), 373–380. [PubMed] [Google Scholar]
- Berglund JP, Szczepanski N, Penumarti A, Beavers A, Kesselring J, Orgel K, Burnett B, Burks AW, Kulis M, 2017. Preparation and analysis of peanut flour used in oral immunotherapy clinical trials. J Allergy Clin Immunol Pract 5 (4), 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntorp E, Shapiro AD, 2012. Modern haemophilia care. Lancet 379 (9824), 1447–1456. [DOI] [PubMed] [Google Scholar]
- Bird JA, Spergel JM, Jones SM, Rachid R, Assa'ad AH, Wang J, Leonard SA, Laubach SS, Kim EH, Vickery BP, Davis BP, Heimall J, Cianferoni A, MacGinnitie AJ, Crestani E, Burks AW, Group, A.R.C.S, 2018. Efficacy and safety of AR101 in Oral immunotherapy for Peanut allergy: results of ARC001, a randomized, double-blind, placebo-controlled phase 2 clinical trial. J Allergy Clin Immunol Pract 6 (2), 476–485 (e473). [DOI] [PubMed] [Google Scholar]
- Biswas M, Sarkar D, Kumar SR, Nayak S, Rogers GL, Markusic DM, Liao G, Terhorst C, Herzog RW, 2015. Synergy between rapamycin and FLT3 ligand enhances plasmacytoid dendritic cell-dependent induction of CD4+ CD25+ FoxP3+ Treg. Blood 125 (19), 2937–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Demoly P, 2006. Specific immunotherapy–an optimistic future. Allergy 61 (10), 1155–1158. [DOI] [PubMed] [Google Scholar]
- Boyhan D, Daniell H, 2011. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol. J. 9 (5), 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Rivas MN, Koleoglou KJ, Chatila TA, Schneider LC, Rachid R, 2014. Oral immunotherapy induces IgG antibodies that act through FcγRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. 134 (6), 1310–1317 (e1316). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HT, Daniell H, 2015. Plant-made oral vaccines against human infectious diseases—are we there yet? Plant Biotechnol. J. 13 (8), 1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-Y, Song J-H, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang H-S, O'Keeffe M, Liao G, Karp CL, 2013. Circulatory antigen processing by mucosal dendritic cells controls CD8+ T cell activation. Immunity 38 (1), 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan C, Kay A, 1992. T cells and eosinophils in the pathogenesis of asthma. Immunol. Today 13 (12), 501–507. [DOI] [PubMed] [Google Scholar]
- Daniell H, 2002. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 20 (6), 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, 2007. Transgene containment by maternal inheritance: effective or elusive? Proc. Natl. Acad. Sci. U. S. A. 104 (17), 6879–6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Lee S-B, Panchal T, Wiebe PO, 2001. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 311 (5), 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Chan H-T, Pasoreck EK, 2016a. Vaccination via chloroplast genetics: affordable protein drugs for the prevention and treatment of inherited or infectious human diseases. Annu. Rev. Genet. 50, 595–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Lin C-S, Yu M, Chang W-J, 2016b. Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol. 17 (1), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, 2018. World Hemophilia Federation presentation. Glasgow, Scotland. [Google Scholar]
- DiMichele DM, 2012. Immune tolerance in haemophilia: the long journey to the fork in the road. Br. J. Haematol. 159 (2), 123–134. [DOI] [PubMed] [Google Scholar]
- Dorner AJ, Bole DG, Kaufman RJ, 1987. The relationship of N-linked glycosylation and heavy chain-binding protein association with the secretion of glycoproteins. J. Cell Biol. 105 (6), 2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, Fox AT, Turcanu V, Amir T, Zadik-Mnuhin G, Cohen A, Livne I, Lack G, 2008. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 122 (5), 984–991. [DOI] [PubMed] [Google Scholar]
- Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G, Team, L.S., 2015. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 372 (9), 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenforth S, Kreuz W, Linde R, Funk M, Güngor T, Krackhardt B, Kornhuber B, Scharrer I, 1992. Incidence of development of factor VIII and factor IX inhibitors in haemophiliacs. Lancet 339 (8793), 594–598. [DOI] [PubMed] [Google Scholar]
- Frew AJ, 2003. Immunotherapy of allergic disease. J. Allergy Clin. Immunol. 111, S712–S719. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Ishida W, Harada Y, Wakasa Y, Takagi H, Takaiwa F, Fukushima A, 2018. Efficacy of oral immunotherapy with a rice-based edible vaccine containing hypoallergenic Japanese cedar pollen allergens for treatment of established allergic conjunctivitis in mice. Allergol. Int. 67 (1), 119–123. [DOI] [PubMed] [Google Scholar]
- Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D, 2008. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29 (3), 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski GA, Golembo M, Shaaltiel Y, 2014. Taliglucerase alfa: an enzyme replacement therapy using plant cell expression technology. Mol. Genet. Metab. 112 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- Graw J, Brackmann H-H, Oldenburg J, Schneppenheim R, Spannagl M, Schwaab R, 2005. Haemophilia a: from mutation analysis to new therapies. Nat. Rev. Genet. 6 (6), 488–501. [DOI] [PubMed] [Google Scholar]
- Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Müller W, Sparwasser T, Förster R, Pabst O, 2011. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34 (2), 237–246. [DOI] [PubMed] [Google Scholar]
- Hamad A, Burks W, 2017. Oral tolerance and allergy. Semin. Immunol. 30, 28–35. [DOI] [PubMed] [Google Scholar]
- Hanauer SB, 2006. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel. Dis. 12 (suppl_1), S3–S9. [DOI] [PubMed] [Google Scholar]
- Hansson C, Schön K, Kalbina I, Strid Å, Andersson S, Bokarewa MI, Lycke NY, 2016. Feeding transgenic plants that express a tolerogenic fusion protein effectively protects against arthritis. Plant Biotechnol. J. 14 (4), 1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume F, Hino S, Kakehashi M, Okajima T, Nadano D, Aoki N, Matsuda T, 2008. Development and evaluation of transgenic rice seeds accumulating a type II-collagen tolerogenic peptide. Transgenic Res. 17 (6), 1117–1129. [DOI] [PubMed] [Google Scholar]
- Herzog RW, 2017. Complexity of immune responses to AAV transgene products–example of factor IX. Cell. Immunol. May 29, 2017 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW, Nichols TC, Su J, Zhang B, Sherman A, Merricks EP, Raymer R, Perrin GQ, Häger M, Wiinberg B, Daniell H, 2017. Oral tolerance induction in hemophilia B dogs fed with transplastomic lettuce. Mol. Ther. 25 (2), 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DR, Ford L, Lalloo DG, 2006. Oral cholera vaccines: use in clinical practice. Lancet Infect. Dis. 6 (6), 361–373. [DOI] [PubMed] [Google Scholar]
- Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, Kamilaris J, Burks AW, 2009. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J. Allergy.Clin. Immunol. 124 (2), 286–291 (291 e281– 286). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iamtham S, Day A, 2000. Removal of antibiotic resistance genes from transgenic tobacco plastids. Nat. Biotechnol. 18 (11), 1172–1176. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Wakasa Y, Tsuboi H, Asashima H, Hirota T, Kondo Y, Matsumoto I, Takaiwa F, Sumida T, 2014. Suppression of collagen-induced arthritis by oral administration of transgenic rice seeds expressing altered peptide ligands of type II collagen. Plant Biotechnol. J. 12 (8), 1143–1152. [DOI] [PubMed] [Google Scholar]
- Jayandharan G, Srivastava A, 2011. Hemophilia: disease, diagnosis and treatment. J. Genet. Syndr. Gene. Ther. 1, 005. [Google Scholar]
- Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, Surh CD, 2016. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351 (6275), 858–863. [DOI] [PubMed] [Google Scholar]
- Kode V, Mudd EA, Iamtham S, Day A, 2006. Isolation of precise plastid deletion mutants by homology-based excision: a resource for site-directed mutagenesis, multigene changes and high-throughput plastid transformation. Plant J. 46 (5), 901–909. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J, Hopkins S, Kerneis S, Pringault E, 1997. Antigen sampling by epithelial tissues: implication for vaccine design. Behring Inst. Mitt. 98, 24–32. [PubMed] [Google Scholar]
- Kuhn C, Weiner HL, 2016. Immunology. How does the immune system tolerate food? Science 351 (6275), 810–811. [DOI] [PubMed] [Google Scholar]
- Kulis MD, Patil SU, Wambre E, Vickery BP, 2018. Immune mechanisms of oral immunotherapy. J. Allergy Clin. Immunol. 141 (2), 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulis M, Yue X, Guo R, Zhang H, Orgel K, Ye P, Li Q, Liu Y, Kim E, Burks AW, Vickery BP, 2019. High- and low-dose oral immunotherapy similarly suppress proallergic cytokines and basophil activation in young children. Clin. Exp. Allergy 49 (2), 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari U, Singh R, Ray T, Rana S, Saha P, Malhotra K, Daniell H, 2019. Validation of leaf enzymes in the detergent and textile industries: launching of a new platform technology. Plant Biotechnol. J. 17 (6), 1167–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Daniell H, 2015. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol. J. 13 (8), 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Daniell H, 2016. Oral delivery of protein drugs bioencapsulated in plant cells. Mol. Ther. 24 (8), 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Nityanandam R, New JS, Daniell H, 2013. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol. J. 11 (1), 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Chan HT, León IR, Williams-Carrier R, Barkan A, Daniell H, 2016. Codon-optimization to enhance expression yields insights into chloroplast translation. Plant Physiol. 172 (1), 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Sherman A, Chang WJ, Kamesh A, Biswas M, Herzog RW, Daniell H, 2018. Expression and assembly of largest foreign protein in chloroplasts: oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia a mice. Plant Biotechnol. J. 16 (6), 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Ho H, Lee K, Jeng S, Chiang B, 2011. Construction of a Der p2-transgenic plant for the alleviation of airway inflammation. Cell. Mol. Immunol. 8 (5), 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye A, Koya V, Samsam M, Daniell H, 2006. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 20 (7), 959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SW, Zhao DL, Yin ZQ, Mukherjee R, Singh B, Qin HY, Stiller CR, Jevnikar AM, 1997. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat. Med. 3 (7), 793–796. [DOI] [PubMed] [Google Scholar]
- Ma JKC, Christou P, Chikwamba R, Haydon H, Paul M, Ferrer MP, Ramalingam S, Rech E, Rybicki E, Wigdorowitz A, 2013. Realising the value of plant molecular pharming to benefit the poor in developing countries and emerging economies. Plant Biotechnol. J. 11 (9), 1029–1033. [DOI] [PubMed] [Google Scholar]
- Ma JKC, Drossard J, Lewis D, Altmann F, Boyle J, Christou P, Cole T, Dale P, van Dolleweerd CJ, Isitt V, 2015. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 13 (8), 1106–1120. [DOI] [PubMed] [Google Scholar]
- Maldonado RA, LaMothe RA, Ferrari JD, Zhang A-H, Rossi RJ, Kolte PN, Griset AP, O'Neil C, Altreuter DH, Browning E, 2015. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. U. S. A. 112 (2), E156–E165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz RC, Healey JF, Parker ET, Meeks SL, Lollar P, 2013. The diversity of the immune response to the A2 domain of human factor VIII. Blood 121 (14), 2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M, 2010. Gut CD103+ dendritic cells express indoleamine 2, 3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 59 (5), 595–604. [DOI] [PubMed] [Google Scholar]
- Maxmen A, 2012. Drug-making plant blooms. Nature 485 (7397), 160. [DOI] [PubMed] [Google Scholar]
- May R, Smith PH, 2008. Pharmacotherapy: A Pathophysiologic Approach. McGraw-Hill, New York. [Google Scholar]
- Mazzini E, Massimiliano L, Penna G, Rescigno M, 2014. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 40 (2), 248–261. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G, 2011. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365 (23), 2205–2219. [DOI] [PubMed] [Google Scholar]
- Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P, 2007. Antihuman factor VIII C2 domain antibodies in hemophilia a mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood 110 (13), 4234–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menassa R, Du C, Yin ZQ, Ma S, Poussier P, Brandle J, Jevnikar AM, 2007. Therapeutic effectiveness of orally administered transgenic low-alkaloid tobacco expressing human interleukin-10 in a mouse model of colitis. Plant Biotechnol. J. 5 (1), 50–59. [DOI] [PubMed] [Google Scholar]
- Moghimi B, Sack BK, Nayak S, Markusic DM, Mah CS, Herzog RW, 2011. Induction of tolerance to factor VIII by transient co-administration with rapamycin. J. Thromb. Haemost. 9 (8), 1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Sakaguchi S, 2014. Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 259 (1), 192–205. [DOI] [PubMed] [Google Scholar]
- Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M, 2014. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343 (6178), 1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, 2018. To respond or not to respond - a personal perspective of intestinal tolerance. Nat. Rev. Immunol. 18 (6), 405–415. [DOI] [PubMed] [Google Scholar]
- Nayak S, Cao O, Hoffman B, Cooper M, Zhou S, Atkinson M, Herzog R, 2009. Prophylactic immune tolerance induced by changing the ratio of antigen-specific effector to regulatory T cells. J. Thromb. Haemost. 7 (9), 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Horiguchi S, Yamamoto H, Yonekura S, Hanazawa T, 2009. Present situation of cedar pollinosis in Japan and its immune responses. Allergol. Int. 58 (2), 155–162. [DOI] [PubMed] [Google Scholar]
- Pabst O, Mowat A, 2012. Oral tolerance to food protein. Mucosal Immunol. 5 (3), 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisade Group of Investigators, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, Hourihane JO, Jones SM, Shreffler WG, Marcantonio A, Zawadzki R, Sher L, Carr WW, Fineman S, Greos L, Rachid R, Ibanez MD, Tilles S, Assa'ad AH, Nilsson C, Rupp N, Welch MJ, Sussman G, Chinthrajah S, Blumchen K, Sher E, Spergel JM, Leickly FE, Zielen S, Wang J, Sanders GM, Wood RA, Cheema A, Bindslev-Jensen C, Leonard S, Kachru R, Johnston DT, Hampel FC Jr., Kim EH, Anagnostou A, Pongracic JA, Ben-Shoshan M, Sharma HP, Stillerman A, Windom HH, Yang WH, Muraro A, Zubeldia JM, Sharma V, Dorsey MJ, Chong HJ, Ohayon J, Bird JA, Carr TF, Siri D, Fernandez-Rivas M, Jeong DK, Fleischer DM, Lieberman JA, Dubois AEJ, Tsoumani M, Ciaccio CE, Portnoy JM, Mansfield LE, Fritz SB, Lanser BJ, Matz J, Oude Elberink HNG, Varshney P, Dilly SG, Adelman DC, Burks AW, 2018. AR101 Oral Immunotherapy for Peanut allergy. N. Engl. J. Med. 379 (21), 1991–2001. [DOI] [PubMed] [Google Scholar]
- Park M-J, Min S-Y, Park K-S, Cho Y-G, Cho M-L, Jung Y-O, Park H-S, Chang S-H, Cho SG, Min J-K, 2008. Indoleamine 2, 3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+ CD25+ regulatory T cells in Peyer's patches in an orally tolerized, collagen-induced arthritis mouse model. Arthritis. Res. Ther. 10 (1), R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, Brough H, Marrs T, Radulovic S, Craven J, Flohr C, Lack G, Team, E.A.T.S., 2016. Randomized trial of introduction of allergenic foods in breast-fed infants. N. Engl. J. Med. 374 (18), 1733–1743. [DOI] [PubMed] [Google Scholar]
- Perrin GQ, Zolotukhin I, Sherman A, Biswas M, De Jong YP, Terhorst C, Davidoff AM, Herzog RW, 2016. Dynamics of antigen presentation to transgene product-specific CD4+ T cells and of Treg induction upon hepatic AAV gene transfer. Mol. Ther. Methods Clin. Dev. 3, 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps PA, Stanford MR, Sun JB, Xiao BG, Holmgren J, Shinnick T, Hasan A, Mizushima Y, Lehner T, 2003. Prevention of mucosally induced uveitis with a HSP60-derived peptide linked to cholera toxin B subunit. Eur. J. Immunol. 33 (1), 224–232. [DOI] [PubMed] [Google Scholar]
- Pipe SW, 2009. Functional roles of the factor VIII B domain. Haemophilia 15 (6), 1187–1196. [DOI] [PubMed] [Google Scholar]
- Pratt KP, 2012. Inhibitory antibodies in hemophilia a. Curr. Opin. Hematol. 19 (5), 399–405. [DOI] [PubMed] [Google Scholar]
- Pratt KP, Thompson AR, 2009. B-cell and T-cell epitopes in anti-factor VIII immune responses. Clin. Rev. Allergy Immunol. 37 (2), 80–95. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J-P, Ricciardi-Castagnoli P, 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2 (4), 361. [DOI] [PubMed] [Google Scholar]
- Rezende RM, Weiner HL, 2017. History and mechanisms of oral tolerance. Semin. Immunol. 30, 3–11. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Dong B, Firrman JA, Moore AR, Sang N, Xiao W, 2011. Engineering factor Viii for hemophilia gene therapy. J. Genet. Syndr. Gene. Ther. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB, 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326 (5), 298–304. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, 2014. Tr1 Cells and the Counter-Regulation of Immunity: natural Mechanisms and Therapeutic Applications, Interleukin-10 in Health and Disease. Springer, pp. 39–68. [DOI] [PubMed] [Google Scholar]
- Ruane DT, Lavelle EC, 2011. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front. Immunol. 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr., 2008. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28 (4), 546–558. [DOI] [PubMed] [Google Scholar]
- Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H, 2007. Expression of cholera toxin B–proinsulin fusion protein in lettuce and tobacco chloroplasts–oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol. J. 5 (4), 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Verma D, Samson N, Daniell H, 2010. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 152 (4), 2088–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack M, Hofbauer A, Fischer R, Stoger E, 2015. The increasing value of plant-made proteins. Curr. Opin. Biotechnol. 32, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Pratt KP, Miao CH, 2013. Progress towards inducing immunologic tolerance to factor VIII. Blood 121 (22), 4449–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid N, Daniell H, 2016. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnol. J. 14 (11), 2079–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar G, Kolesnikov M, 2014. Intestinal macrophages and DCs close the gap on tolerance. Immunity 40 (2), 171–173. [DOI] [PubMed] [Google Scholar]
- Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H, 2014. Suppression of inhibitor formation against FVIII in a murine model of hemophilia a by oral delivery of antigens bioencapsulated in plant cells. Blood 124 (10), 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford M, Whittall T, Bergmeier LA, Lindblad M, Lundin S, Shinnick T, Mizushima Y, Holmgren J, Lehner T, 2004. Oral tolerization with peptide 336– 351 linked to cholera toxin B subunit in preventing relapses of uveitis in Behcet's disease. Clin. Exp. Immunol. 137 (1), 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinitz KN, van Helden PM, Binder B, Wraith DC, Unterthurner S, Hermann C, Schuster M, Ahmad RU, Weiller M, Lubich C, 2012. CD4+ T-cell epitopes associated with antibody responses after intravenously and subcutaneously applied human FVIII in humanized hemophilic E17 HLA-DRB1* 1501 mice. Blood 119 (17), 4073–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Sherman A, Doerfler PA, Byrne BJ, Herzog RW, Daniell H, 2015a. Oral delivery of acid alpha glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant Biotechnol. J. 13 (8), 1023–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Zhu L, Sherman A, Wang X, Lin S, Kamesh A, Norikane JH, Streatfield SJ, Herzog RW, Daniell H, 2015b. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials 70, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J-B, Flach C-F, Czerkinsky C, Holmgren J, 2008. B lymphocytes promote expansion of regulatory T cells in oral tolerance: powerful induction by antigen coupled to cholera toxin B subunit. J. Immunol. 181 (12), 8278–8287. [DOI] [PubMed] [Google Scholar]
- Sun J-B, Xiang Z, Smith KG, Holmgren J, 2013. Important role for FcγRIIB on B lymphocytes for mucosal antigen-induced tolerance and Foxp3+ regulatory T cells. J. Immunol. 191 (8), 4412–4422. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kaminuma O, Yang L, Takai T, Mori A, Umezu-Goto M, Ohtomo T, Ohmachi Y, Noda Y, Hirose S, 2011. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol. J. 9 (9), 982–990. [DOI] [PubMed] [Google Scholar]
- Takagi H, Hiroi T, Yang L, Tada Y, Yuki Y, Takamura K, Ishimitsu R, Kawauchi H, Kiyono H, Takaiwa F, 2005. A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc. Natl. Acad. Sci. U. S. A. 102 (48), 17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Hiroi T, Yang L, Takamura K, Ishimitsu R, Kawauchi H, Takaiwa F, 2008. Efficient induction of oral tolerance by fusing cholera toxin B subunit with allergen-specific T-cell epitopes accumulated in rice seed. Vaccine 26 (48), 6027–6030. [DOI] [PubMed] [Google Scholar]
- Thomas G, 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 3 (10), 753–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilles SA, Petroni D, 2018. FDA-approved peanut allergy treatment - the first wave is about to crest. Ann. Allergy Asthma Immunol. 121, 145–149. [DOI] [PubMed] [Google Scholar]
- Togias A, Cooper SF, Acebal ML, Assa'ad A, Baker JR Jr., Beck LA, Block J, Byrd-Bredbenner C, Chan ES, Eichenfield LF, Fleischer DM, Fuchs GJ 3rd, Furuta GT, Greenhawt MJ, Gupta RS, Habich M, Jones SM, Keaton K, Muraro A, Plaut M, Rosenwasser LJ, Rotrosen D, Sampson HA, Schneider LC, Sicherer SH, Sidbury R, Spergel J, Stukus DR, Venter C, Boyce JA, 2017. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Allergy. Clin. Immunol. 139 (1), 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, Hiegel A, Kamilaris J, Carlisle S, Yue X, Kulis M, Pons L, Vickery B, Burks AW, 2011. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J. Allergy Clin. Immunol. 127 (3), 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehar GA, Keyt B, Eaton D, Rodriguez H, O'Brien DP, Rotblat F, Oppermann H, Keck R, Wood WI, Harkins RN, 1984. Structure of human factor VIII. Nature 312 (5992), 337. [DOI] [PubMed] [Google Scholar]
- Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H, 2010. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. U. S. A. 107 (15), 7101–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, Scurlock AM, Gimenez G, Bardina L, Sampson HA, Burks AW, 2013. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J. Allergy. Clin. Immunol. 131 (1), 128–134 (e121-123). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, Burk C, Hiegel A, Carlisle S, Christie L, Perry TT, Pesek RD, Sheikh S, Virkud Y, Smith PB, Shamji MH, Durham SR, Jones SM, Burks AW, 2014. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J. Allergy. Clin. Immunol. 133 (2), 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, Keet CA, Kulis M, Orgel KG, Guo R, Steele PH, Virkud YV, Ye P, Wright BL, Wood RA, Burks AW, 2017. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J. Allergy Clin. Immunol. 139 (1), 173–181 (e178). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F, 2008. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 153, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasa Y, Takagi H, Hirose S, Yang L, Saeki M, Nishimura T, Kaminuma O, Hiroi T, Takaiwa F, 2013. Oral Immunotherapy with transgenic rice seed containing destructed Japanese cedar pollen allergens, cry j 1 and cry j 2, against Japanese cedar pollinosis. Plant Biotechnol. J. 11 (1), 66–76. [DOI] [PubMed] [Google Scholar]
- Wang X, Sherman A, Liao G, Leong KW, Daniell H, Terhorst C, Herzog RW, 2013. Mechanism of oral tolerance induction to therapeutic proteins. Adv. Drug Deliv. Rev. 65 (6), 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su J, Sherman A, Rogers GL, Liao G, Hoffman BE, Leong KW, Terhorst C, Daniell H, Herzog RW, 2015. Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+ CD4+ T cells. Blood 125 (15), 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Mishima H, Renzi PM, Xu L-J, Hamid Q, Martin JG, 1995. Transfer of allergic airway responses with antigen-primed CD4+ but not CD8+ T cells in brown Norway rats. J. Clin. Invest. 96 (3), 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, da Cunha AP, Quintana F, Wu H, 2011. Oral tolerance. Immunol. Rev. 241 (1), 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernick NL, Chinnapen DJ-F, Cho JA, Lencer WI, 2010. Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins 2 (3), 310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RA, 2016. Food allergen immunotherapy: current status and prospects for the future. J. Allergy Clin. Immunol. 137 (4), 973–982. [DOI] [PubMed] [Google Scholar]
- Wroblewska A, Reipert BM, Pratt KP, Voorberg J, 2013. Dangerous liaisons: how the immune system deals with factor VIII. J. Thromb. Haemost. 11 (1), 47–55. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Kwon K-C, Hoffman BE, Kamesh A, Jones NT, Herzog RW, Daniell H, 2016. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials 80, 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida N, Sato S, Ebisawa M, 2017. Clinical aspects of oral immunotherapy for the treatment of allergies. Semin. Immunol. 30, 45–51. [DOI] [PubMed] [Google Scholar]
- Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, Jobin C, Zong-ming EC, Zhou L, 2017. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep. 21 (8), 2277–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]