Abstract
Background:
Lipopolysaccharide-responsive beige-like anchor (LRBA) deficiency presents with susceptibility to infections, autoimmunity and lymphoproliferation. The long-term efficacy of CTLA4-Ig (abatacept) as targeted therapy for its immune dysregulatory features remains to be established.
Objective:
We sought to determine the clinical and immunological features of LRBA-deficiency and long-term efficacy of abatacept treatment in controlling the different disease manifestations.
Methods:
Twenty-two LRBA-deficient patients were recruited from different immunology centers and followed prospectively. Eighteen patients on abatacept were evaluated every 3 months for long-term clinical and immunological responses. LRBA expression, lymphocyte subpopulations and circulating T follicular helper (cTFH) cells were determined by flow cytometry.
Results:
The mean age of the patients was 13.4±7.9 years and the follow up period was 3.4±2.3 years. Recurrent infections (n:19, 86.4%), immune dysregulation (ID, n:18, 81.8%) and lymphoproliferation (LP, n:16, 72.7%) were common clinical features. The long-term benefits of abatacept in 16 patients were demonstrated by complete control of LP and chronic diarrhea (CD) followed by ID, most notably autoimmune cytopenias. Weekly or every other week administration of abatacept gave better disease control compared to every 4-weeks. There were no serious side effects related to the abatacept therapy. cTFH cell frequencies were found to be a reliable biomarker of disease activity, which decreased on abatacept therapy in the majority of subjects. However, high cTFH cell frequencies persisted in two patients who had a more severe disease phenotype that was relatively resistant to abatacept therapy.
Conclusion:
Long-term Abatacept therapy is effective in the majority of patients with LRBA deficiency.
Keywords: Lipopolysaccharide-responsive beige-like anchor, LRBA, immune dysregulation, natural history, abatacept, T Follicular helper cells, autoimmunity
1. Introduction
Lipopolysaccharide-responsive beige-like anchor (LRBA) deficiency is a primary immunodeficiency characterized by recurrent sinopulmonary infections with hypogammaglobulinemia, lymphoproliferation and immunodysregulation, which presents by enteropathy, cytopenias and autoimmune endocrinopathy. LRBA plays a pivotal role in the intracellular trafficking of cytotoxic T lymphocyte protein-4 (CTLA-4) by re-routing it away from lysosomal degradation and back to the cell surface (1, 2). CTLA-4 is an key immune checkpoint protein that is constitutively expressed on fork-head box P3 (FOXP3)+ regulatory T (Treg) cells and is also induced upon activation of conventional T cells (3). LRBA deficiency results in very low CTLA4 expression, which explains the phenotypic overlap between LRBA and CTLA4 deficient subjects (4, 5). Furthermore, reduced Treg cells number and function have been demonstrated in LRBA-deficient patients (6, 7). Consequent upon this, LRBA deficiency may manifest as an IPEX like disease with early onset autoimmunity (8, 9).
LRBA was originally described as a common variable immune deficiency (CVID)-like disease with autoimmunity (10, 11). Two longitudinal cohorts were subsequently published that dwelt on the clinical and immunological features of LRBA deficiency (7, 12). To date, different agents have been applied in the treatment of LRBA deficiency, including corticosteroids, intravenous immunoglobulin therapy (IVIG), sirolimus, infliximab, rituximab and azathioprine (7, 13). Some patients also benefit from hematopoietic stem cell transplantation (HSCT), which can be curative (14). More recently, studies have suggested the effectiveness of abatacept, a CTLA4-Ig fusion protein, in controlling disease-related immune dysregulatory phenotypes (1, 13). In addition, some biomarkers like soluble CD25 and circulating T Follicular helper (cTFH) cells were described as useful to monitor patients’ disease activity (15). Nevertheless, the long-term effectiveness of abatacept is not well documented. Also, there is no established consensus as to the dose and frequency of abatacept therapy for the treatment of LRBA deficiency and which biomarker is most reliable for follow up of patients. The spectrum of the clinical responses of LRBA deficient patients to abatacept treatment is also obscure.
In this report, we present the findings on a well-defined LRBA-deficient cohort, in which we prospectively evaluated the clinical and immunological responses to abatacept therapy. Our studies establish the efficacy of long-term abatacept therapy in curbing the immune dysregulatory features of this disease in the majority of cases and also highlight the limitations of this therapy in occasional patients with severe disease phenotype.
2. Material and Methods
2.1. Patient and inclusion criteria
The study included 22 patients with proven LRBA mutation. The patients were recruited from 12 different pediatric immunology centers in Turkey. They were enrolled into the study at different time points started from November 2016 and followed up prospectively until December 2018. The study protocol was approved by the local ethics committee of Marmara University (IRB number: IRB00009067) and a written informed consent was obtained from all parents. Due to the young age of our patients, a simple oral description of the study was presented to participating children in the presence of their parent(s) and a verbal assent was requested.
2.2. Study design
The LRBA-deficient patients were enrolled to the study prospectively from related centers. During the study, baseline demographic, clinical and immunological data were collected. Blood samples from all the participating patients from the respective medical centers were sent to the Marmara University Pediatric Allergy and Immunology laboratory (MUPAI) for immunological assessment, including extensive lymphocyte subset analysis, cTFH cell enumeration and intracellular LRBA and CTLA4 staining. The changes in lymphocyte subsets and cTFH cells were evaluated at 6th month and compared to baseline. The detailed methods for flow cytometric and genetic analyses were given in the Online Repository Text.
2.3. Abatecept therapy and clinical benefits
The detailed abatacept treatment courses were mentioned for each patient separately. The physicians were questioned for the effect of abatacept during follow up. The degree of severity of each symptom was recorded as mild, moderate or severe at baseline, 3th, 6th, 9th and 12th months and categorized as complete remission (CR), partial remission (PR) or non-responsive (NR) according to the response to abatecept. Other immunosuppressants, which were used before and after abatacept and abatacept side effects were recorded during the study.
2.4. Statistical analysis
Comparison between groups were carried out using Student’s paired, unpaired and 1-way ANOVA with Bonferroni posttest analysis, as indicated. Categorical variables were compared by chi-square analysis. Receiver operating characteristic (ROC) test was used to determine the sensitivity and specificity. Differences in mean values were considered significant at a p< .05.
3. Results
3.1. Diagnosis of LRBA deficient patients
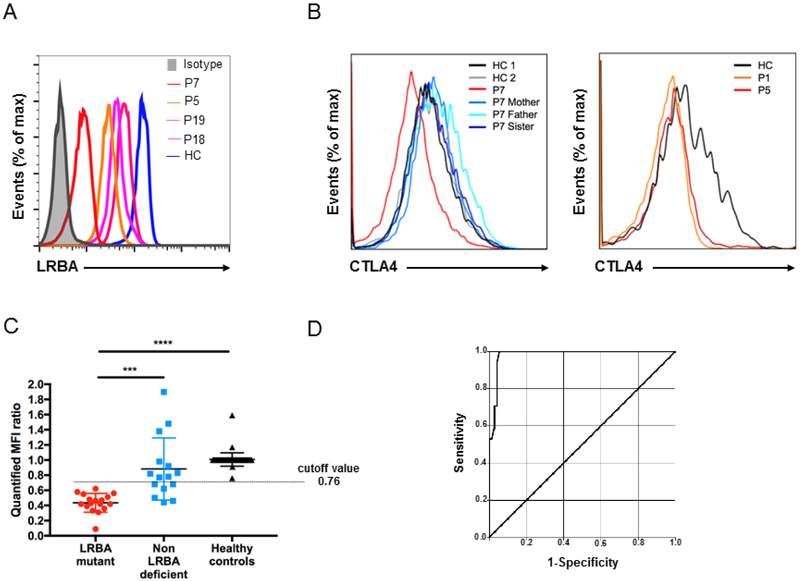
22 genetically confirmed LRBA-deficient patients were included in this study. All patients had homozygous mutations, which were confirmed by Sanger sequencing (Table 1). All analyzed patients (P1-7, P9, P10, P12-19) had low LRBA protein expression (Fig 1, A). P8 and P11 died before flow cytometric evaluation, while samples from P20-22 could not be shipped for assessment. CTLA4 intracellular staining was evaluated in 6 patients and found to be lower than controls, as expected (Fig 1, B). To assess the performance of LRBA intracellular flow staining, we prospectively collected 89 samples referred to our center [Genetically known LRBA-deficient patients (n:17), patients presented as CVID phenotype but had no LRBA mutation (n:15), healthy controls (n:57)]. The quantified MFI ratio, determined by dividing raw mean fluorescence intensity (MFI) to the background staining and then quantified by normalization to control MFI, was statistically lower in LRBA-deficient patients and higher in healthy controls (Fig 1, C). Then, to differentiate LRBA patients from others, ROC analysis was performed and sensitivity and specificity were determined. The ROC analysis yielded an area under curve of 0.98 with a 95% CI (0.96-1.00) (Fig 1, D). Our results revealed higher sensitivity (100%) and specificity (91.7%) by using a cutoff value of 0.76 for the quantified MFI ratio. This cutoff value was able to catch up all LRBA patients and the positive predictive and negative predictive values were calculated as 73.9% and 100%, respectively.
Table 1.
Demographic, clinical features and mutations of patients with LRBA deficiency
| Patient | Family | Current age (yrs)/Sex |
Consanguinity | AOO (mo) | Phenotype | Clinical Dx | Mutation | Outcome |
|---|---|---|---|---|---|---|---|---|
| P1 | F1 | 13/M | + | 18 | RTI, CD, LP, ID | CVID | c.5047C>T, p.R1683* | Alive |
| P2 | F1 | 9/F | + | 48 | CD, ID, LP | ALPS | c.5047C>T, p.R1683* | Alive |
| P3 | F2 | 7/M | + | 8 | RTI, CD, LP | ALPS | c.7885delA, p.R2629fs | Alive |
| P4 | F2 | 13/M | + | 1 | RTI, CD, LP, ID | ALPS | c.7885delA, p.R2629fs | Alive |
| P5 | F3 | 26/M | + | 3 | RTI, CD, LP, ID | CVID | c.767+5_767+8delGTAT, p? | Alive |
| P6 | F4 | 11/M | + | 7 | RTI, CD, LP, ID | CVID | c.2599C>T, p.Q867* | Alive |
| P7 | F5 | 3/F | + | 8 | RTI, ID | IPEX like | c.5172-2A>G, p? | Alive |
| P8 | F6 | 14/M | + | 6 | RTI, CD, LP, ID | ALPS | c.1963C>T, p.R655* | Died |
| P9 | F7 | 18/M | − | 18 | RTI, CD, ID | CVID | c.2836_2839delGAAA, p.E946* | Alive |
| P10 | F8 | 35/F | + | 84 | RTI, CD, LP, ID | CVID | c.7238dupG, p.S2413Rfs*1 | Alive |
| P11 | F8 | 14/M | + | 8 | RTI, CD, LP | ALPS | c.7238dupG, p.S2413Rfs*1 | Died |
| P12 | F9 | 23/M | + | 42 | RTI, CD, ID | ALPS | c.2818dupC, p.Q940fs | Alive |
| P13 | F10 | 18/M | − | 72 | RTI, CD, ID, LP | ALPS | c.1963C>T, p.R655* | Alive |
| P14 | F11 | 16/F | + | 4 | RTI, CD, ID | CVID | c.2735_2738delGGGT, p.T912* | Alive |
| P15 | F12 | 13/M | + | 24 | RTI, ID, LP | ALPS | c.3396-3397delAC, p.D975Yfs*15 | Alive |
| P16 | F13 | 11/M | + | 13 | RTI, ID, LP | IPEX like | c.2496C>A, p.C832* | Alive |
| P17 | F13 | 12/M | + | 60 | RTI, ID, LP | IPEX like | c.2496C>A, p.C832* | Alive |
| P18 | F14 | 16/F | + | 18 | RTI | CVID | c.5537C>T, p.S1846L | Alive |
| P19 | F15 | 21/F | + | 6 | RTI, CD, ID, LP | CVID | c.7976C>G, p.S2659* | Alive |
| P20 | F16 | 3.5//F | + | 9 | CD | IPEX like | c.1496C>A, p.S499* | Alive |
| P21 | F17 | 13/F | + | 9 | CD, ID | IPEX like | c.3549_3550insA, p.A1184Sfs*34 | Alive |
| P22 | F17 | 4/M | + | − | − | Asymptomatic | c.3549_3550insA, p.A1184Sfs*34 | Alive |
Abbreviations: ID: Immune dysregulation, CD: Chronic diarrhea, LP: Lymphoproliferation, RTI: Respiratory tract infections, Unk: Unknown, AOO: Age of onset.
Figure 1.
LRBA-deficient patients have low or absent LRBA and CTLA4 protein expression. A, LRBA expression in lymphocytes from LRBA-deficient patients, P7 (red line), P5 (orange line), P19 (purple line) and P18 (pink line) compared with a healthy control (blue line). B, Flow cytometric analysis demonstrates low CTLA4 expression on CD4+FOXP3+ Treg cells in LRBA-deficient patients P1, P5, and P7 compared to healthy controls (HC) and unaffected family members. C, LRBA mutant patients (red dots) have significantly decreased mean fluorescence intensity (MFI) ratio of LRBA expression in peripheral blood mononuclear cells compared to non-LRBA-deficient (blue dots) and healthy controls (black dots). D, Receiver operating characteristic (ROC) curve analysis shows the sensitivity and specificity of flow cytometric analysis for the detection of LRBA-deficient patients. The analysis was conducted on 17 mutation-verified LRBA-deficient patients, 15 patients who presented with a LRBA phenotype but had no LRBA mutation and 57 healthy controls. Area under curve was yielded as 0.98 with a 95% CI (0.96-1.00). *** p<.001 and **** p<.0001, Student unpaired 2-tailed t-test.
3.2. Demographics and clinical presentations of LRBA deficient patients
The patients’ demographics and their salient clinical phenotypes are shown in Table 1 and are further detailed in the Online Repository Text. There were 14 (63.6%) males and 8 females (36.3%) in our cohort. The mean age ± S.D. of the patients was 13.4±7.9 years and the follow up period was 3.4±2.3 years. The mean age of the first symptoms was 24±23 months, while the delay time in diagnosis was observed as 9.5±9.0 years. All patients had consanguinity except for P9 and P13. When the LRBA-deficient patients were evaluated according to their first clinical manifestations, 8 (36.3%) presented as common variable immunodeficiency (CVID), 8 (36.3%) as autoimmune lymphoproliferative syndrome (ALPS), and 5 (22.7%) as IPEX-like disease (Table 1). One asymptomatic patient (P22) was diagnosed in family screening. By the end of the study, twenty patients were alive, while 2 patients were deceased (P6 after HSCT, P11 due to severe disease course), with an overall survival of 91.0%.
3.3. Clinical phenotype of LRBA deficient patients
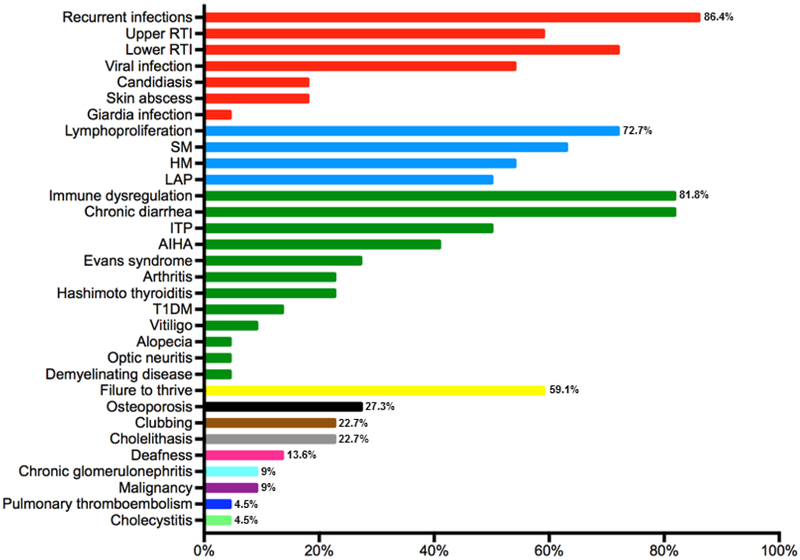
The clinical phenotype of LRBA-deficient patients mainly consisted of recurrent infections (n:19, 86.4%), immune dysregulation (ID) (n: 18, 81.8%) and lymphoproliferation (LP) (n: 16, 72.7%). The other common manifestations related to LRBA deficiency were failure to thrive (n: 13, 59.1%), osteoporosis (n:6, 27.3%), finger clubbing (n: 5, 22.7%), cholelithasis (n: 5, 22.7%), deafness (n:3, 13.6%), malignancy (n:2, 9.1%), chronic glomerulonephritis (n:2, 9.1%), pulmonary thromboembolism (n:1, 4.5%) and cholecystitis (n:1, 4.5%). The clinical presentations of the patients are summarized in Fig 2, Table E1 and in the Online Repository Text.
Figure 2.
Clinical features of LRBA-deficient patients. The bars are depicted as percentages. The disease symptom clusters are indicated with different colors. Red bars show infections, blue bars denote lymphoproliferation, green bars demonstrate immune dysregulation, yellow bar shows failure to thrive, black bar indicates osteoporosis, brown bar represents clubbing, silver bar indicates cholelithasis, pink bar show deafness, light blue, purple, dark blue and light green bars indicate chronic glomerulonephritis, malignancy, pulmonary thromboembolism and cholecystitis, respectively. RTI, respiratory tract infection; SM, splenomegaly; HM, hepatomegaly; LAP, lymphadenopathy; ITP, immune thrombocytopenia; AIHA, autoimmune hemolytic anemia; T1DM, type 1 diabetes mellitus.
Immune dysregulation was the second predominant feature in our cohort. Most patient had chronic diarrhea (CD) (n:18, 81.8%) and enteropathy was proven by biopsy in 14 (63.6%) patients. Villus atrophy, intraepithelial lymphocytosis, lymphoid hyperplasia and aggregates, chronic gastritis and duodenitis, active colitis and eosinophilic infiltration were frequently observed. Autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP) were the most common hematological manifestation and observed in 9 (40.9%) and 11 (50%) patients, respectively. Due to autoimmune AIHA, choledocholithiasis was observed in 5 (22.7%) patients. Patients (P8, P12, P13, P15, P17, P19) developed Evans syndrome, which was usually intractable and resistant to treatment with immunosuppressive drugs. It was controlled after HSCT in P8, splenectomy in P12, abatacept in P17 and P19. Three patients (13.6%, P1, P7 and P21) had type 1 diabetes mellitus (DM) requiring regular insulin injections. During the course of study, 5 (22.7%) patients (P5, P6, P9, P12, P21) had arthritis without a discernable etiology. Other less frequent autoimmune features were alopecia (P3), vitiligo (P6, P9), Hashimoto thyroiditis (P3, P4, P5, P10, P16), optic neuritis and demyelinating disease (P12).
The third common manifestation in LRBA-deficient patients was LP, which was characterized by splenomegaly (n:14, 63.6%), hepatomegaly (n:12, 54,5%) and lymphadenopathy (n: 11, 50%).
3.4. Immunological phenotype of LRBA deficient patients
Immunological data was available on all 22 patients. During the time of evaluation, lymphopenia (n:6, 27.2%), neutropenia (n:1, 4.5%), anemia (n:3, 13.6%), thrombocytopenia (n:8, 36.3%) were recorded. Serum immunoglobulin levels before IVIG were available in 22 patients and showed low IgG in 10 (45.5%), low IgM in 12 (54.5%) and low IgA in 16 (72.7%) patients. The rest of patients had normal or high immunoglobulin levels (Table E2). Extensive flow cytometric analysis including T, B, NK, T cells and B cells subtypes were investigated and summarized in Fig E1 and Table E2. Reduced CD3+ T cells counts were observed in 6 (27.2%) patients, while three had an increased value (13.6%). The CD4+ and CD8+ T cells were low in 7 (31.8%) and 8 (32%) patients, respectively. Double-negative T cells percentages were increased in 7 (33.3%) patients; all of them were considered as ALPS initially. B cells compartment showed abnormalities characterized by reduced total B cells in 45.5%, increased naive (CD27- IgD+) in 27.7%, reduced class switched memory (CD27+IgD-) in 63.1% and increased activated B cells (CD21lowCD38low) in 30.7% patients. NK cells were found to be low in 8 (36.3%) patients.
3.5. Mutational analysis and genotype-phenotype correlations
A schema depicting the LRBA mutation sites is shown in Fig E2, A. All the patients had homozygous mutations and consanguinity was prominent (except P9 and P13) in our cohort. Mutations of P1, P2, P3, P4, P7, P8, P13, P16, P17 were described previously (1, 7, 10, 15-17). The P5, P6, P7, P9, P10-11, P12, P14, P15, P18, P19, P20, P21-22 had novel mutations. Most of the mutations were nonsense and frameshift, but two mutations (P5 and P7) were splice junction type (Table 1). cDNA analysis confirmed that both splice site mutations resulted in exon aberrant splicing, which was associated with low LRBA protein expression as detected by immunoblotting and flow cytometry analyses (Fig 1A, Fig E2, B and C). Further analysis pointed to identical LRBA mutations giving rise to divergent clinical outcomes, even among siblings. Overall, there were no strong genotype- phenotype relationships that governed the manifestations of LRBA deficiency in our patient population.
3.6. Medications and disease course
Due to the recurrent infections, hypoglobulinemia and immune dysregulation, 17 patients (77.2%) received prophylactic antibiotics and all patients were on IG replacement (two of them with subcutaneous preparations). Two out of 17 patients were placed on azithromycin (11.2%), while the rest were on trimethoprim-sulfamethoxazole (88.2%) prophylaxis. Due to candidiasis, fluconazole prophylaxis was given to P1, P5, P16, P17 and P21. Acyclovir prophylaxis was used in P16, P17 and P18 (Table E1). HSCT was performed in P6, P8, P9, P21.
To control immune dysregulatory features observed in the patients, various mono or combination therapies with immunosuppressive agents were started as follows: abatacept (n:18, 81.8%), prednisolone (n:12, 54.5%), mycophenolate mofetil (MMF) (n:5, 22.7%), cyclosporine A (n:4, 18.1%), sirolimus (n:2, 9%), sulfasalazine (n:1, 4.5%), azathioprine (n:1, 4.5%) adalimumab (n:1, 4.5%) and hydroxychloroquine (n:1, 4.5%). Splenectomy was performed in three patients due to uncontrolled LP (P11, P15) and Evans syndrome (P12). Five patients (P5, P6, P8, P9 and P21) were transplanted due to the advanced disease and poor response to treatment. More specifically, the indications for the transplantation were persistent hematological findings (P5, P6, P8), uncontrolled LP (P6), CD (P6, P21), and severe side effects of immunosuppressants (P9). A variety of donors were used, including matched related donors (P5, P8), matched unrelated donors (P6, P9), mismatched unrelated donor (P21). Myeloablative regimens were applied in P5, P8, P9, P21, while reduced intensity conditioning regimen in P6. Decisions about the choices of applied conditioning regimens were made by the respective institutions. P6 was deceased after transplant due to acute graft versus host disease and sepsis, while P5, P8, P9, P21 are still alive with chimerism between 95-98% of donor cells.
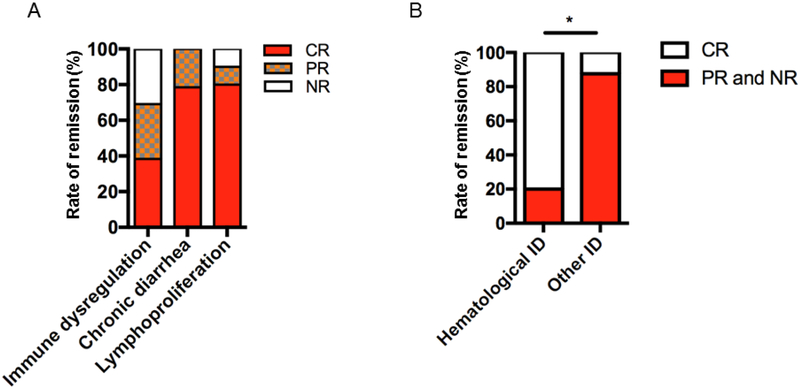
Patients were followed up prospectively during abatacept treatment (P1-7, P9, P10, P12, P14-21). The median duration of abatacept therapy was 12.5 months (range: 5-33 months). The main clinical phenotypes of the 18 patients at the start of abatacept therapy were categorized as ID, CD and LP. Remission after abatacept therapy was calculated for each phenotype (P6 and P15 were not evaluated due to the short-term duration of abatacept therapy in their case). All patients responded to abatacept therapy but to different degrees (Table 2). ID symptoms were present in 13 patients and five (38.4%) patients showed CR, four (30.7%) patients had PR and four were NR (30.7%). CD was observed in 14 patients and CR was observed in 11 (78.5%), while PR in three (21.5%) of these patients. LP was determined radiologically in 10 patients and followed regularly during abatacept treatment. At the end of the study period, CR in eight (80%), PR in one (10%) and NR in one (10%) patient was achieved. As a result, although some patients received abatacept for a short period during the study (P6 and P9-due to HSCT, P14 due to short follow up), the best CR was achieved for LP and followed by CD and ID (Fig 3, A). In all patients at least one of the symptoms was completely or partially controlled and there was no unresponsiveness to abatacept. Among the ID manifestations, AIHA and ITP were statistically the most frequently controlled manifestations (Fig 3, B). Type 1 DM was not reversible after abatacept (P1, P7 and P21).
Table 2.
Abatacept treatment courses and clinical responses of LRBA-deficient patients
| Pts | Time of usage (mo) |
Clinical indication for abatacept |
Initial dose | Maintenance dose during follow up or transplantation |
Remission rate and time (mo) | Final status |
|---|---|---|---|---|---|---|
| P1 | 15 | Lymphoproliferation Diarrhea DM |
15 mg/kg/per 1w. | Switched to per 2w. (at 6th mo of therapy) |
Lymphoproliferation (CR) - 6th mo. Diarrhea (CR) - 3th mo. DM (NR) |
Insulin dependent DM continued |
| P2 | 7 | Lymphoproliferation Diarrhea ITP |
15 mg/kg/per 1w. | Switched to per 2w. (at 2th mo of therapy) |
Lymphoproliferation (CR) - 3th mo. Diarrhea (CR) - 3th mo. ITP (CR) - 3th mo. |
Symptoms are controlled |
| P3 | 33 | Lymphoproliferation Diarrhea |
10 mg/kg/per 4w. | Switched to 20 mg/kg/per 2w. (at 4th mo of therapy) |
Lymphoproliferation (CR) - 4th mo. Diarrhea (PR) - 10th mo. |
CR of Diarrhea was achieved at 3th mo of maintenance therapy |
| P4 | 33 | Lymphoproliferation Diarrhea Alopecia |
10 mg/kg/per 4w. | 10 mg/kg/per 4w. | Lymphoproliferation (CR) - 6tn mo. Diarrhea (CR) - 6th mo. Alopecia (PR) - 12th mo. |
Alopecia is partially controlled |
| P5 | 9 | Lymphoproliferation Diarrhea ITP |
20 mg/kg/per 2w. | HSCT (at 6th mo of therapy) |
Lymphoproliferation (CR) - 3th mo. Diarrhea (CR) - 3th mo. ITP (PR) - 6th mo. |
Alive with 98% chimerism |
| P7 | 25 | Respiratory tract infections DM |
20 mg/kg/per 4w. | 20 mg/kg/per 4w. | DM (NR) | Insulin dependent DM continued |
| P9 | 5 | Lymphoproliferation Diarrhea AIHA |
10 mg/kg/per 1w. | HSCT (at 5th mo of therapy) |
Lymphoproliferation (CR) - 2nd mo. Diarrhea (CR) - 2nd mo. AIHA (CR) - 2nd mo. |
Alive with 98% chimerism |
| P10 | 12 | Lymphoproliferation Diarrhea |
10 mg/kg/per 4w. | 10 mg/kg/per 2w. | Lymphoproliferation (CR) - 6th mo. Diarrhea (PR) - 6th mo. |
CR of Diarrhea was achieved at 1st mo of maintenance therapy |
| P12 | 12 | Lymphoproliferation Diarrhea Arthritis Demyelinating disease |
15 mg/kg/per 2w. | 15 mg/kg/per 2w. | Lymphoproliferation (CR) - 2nd mo. Diarrhea (CR) - 2nd mo. Arthritis (CR) - 2nd mo. Demyelinating disease (NR) |
Symptoms are controlled except for demyelinating disease |
| P14 | 7 | Diarrhea | 10 mg/kg/per 2w. | Switched to per 4w. (after 3 doses) |
Diarrhea (CR) - 4th mo. | Symptoms are controlled |
| P16 | 11 | Diarrhea | 10 mg/kg/per 2w. | 10 mg/kg/per 2w. | Diarrhea (CR) - 5th mo. | Symptoms are controlled |
| P17 | 18 | Evans syndrome Lymphoproliferation Diarrhea GILID |
10 mg/kg/per 2w. | 10 mg/kg/per 2w. | Evans syndrome (CR) - 3th mo. Lymphoproliferation (PR) - 6th mo. Diarrhea (PR) - 4th mo. GILID (PR) - 6th mo. |
Symptoms are partially controlled for lymphoproliferation, diarrhea and GILID |
| P18 | 5 | Respiratory tract infections | 10 mg/kg/per 2w. | Switched to per 4w. (after 3 doses) |
No autoimmunity at the beginning and during follow up | |
| P19 | 18 | Evans syndrome Diarrhea Lymphoproliferation |
10 mg/kg/per 2w. | Switched to per 4w. (after 3 doses) |
Evans syndrome (CR) - 6th mo. Diarrhea (PR) - 4th mo. Lymphoproliferation (NR) |
Diarrhea is partially controlled, lymphoproliferation is not respond to therapy |
| P20 | 21 | Diarrhea | 10 mg/kg/per 2w. | Switched to per 4w. (after 3 doses) |
Diarrhea (CR) - 6th mo. | Diarrhea is controlled |
| P21 | 12 | Diarrhea | 10 mg/kg/per 2w. | Switched to per 4w. (after 3 doses), HSCT (at 12th mo of therapy) |
Diarrhea (CR) - 3th mo. Arthritis (PR) - 6th mo. DM (NR) |
Alive with 98% chimerism |
Abbreviations: CR: Complete remission, PR: Partial remission, NR: Non-response, HSCT: Hematopoietic stem cell transplantation, Mo: Month, W: Week.
Figure 3.
LRBA disease symptoms display different responses to abatacept treatment. A, The remission rates for immune dysregulation (ID), chronic diarrhea and lymphoproliferation. B, The comparison of the remission rates of hematological ID (autoimmune hemolytic anemia, immune thrombocytopenia) versus other immune dysregulatory symptomatologies (Diabetes, alopecia, arthritis, demyelinating disease, granulomatous-lymphocytic interstitial lung disease). The remissions are indicated as complete (CR), partial (PR) or non-responsive (NR). The bars are presented as percentages. * p<.01, Chi-square test.
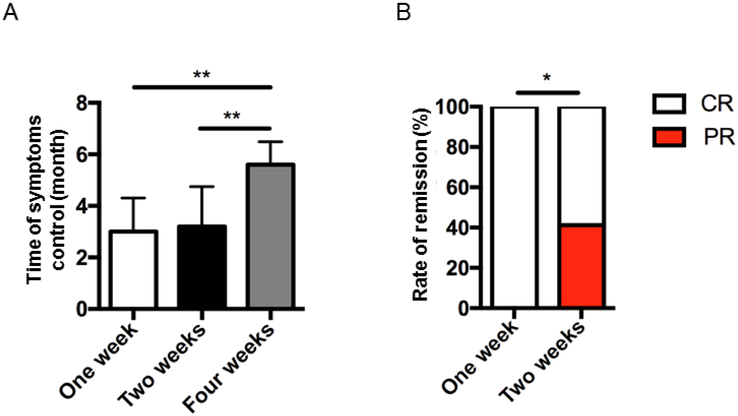
Different centers were used various abatacept therapy regimens for the patients (Table 2), and these were compared with each other to determine which therapy frequency was the most efficient in controlling disease symptoms. Patients on abatacept every week or every other week attained CR in shorter time compared to patients on abatacept every four-weeks (Fig 4, A). When every week and every other week regiments were compared, all symptoms were completely controlled in one-week therapy option, while 2-weeks regimen resulted in partial control of some symptoms (Fig 4, B, Table 2).
Figure 4.
Various complete remission rates in LRBA-deficient patients after treatment with abatacept according to dosing interval. A, The complete remission (CR) rates in patients received abatacept with one-week or two-weeks interval in comparison to four-weeks. C, The percentages of CR or partial remission (PR) rate in LRBA-deficient patients in terms of dose interval. ** p<.01, 1-way ANOVA test, * p<.05, Chi-square test.
At the end of study, only two patients were still on other immunosuppressive drugs (steroids and MMF in P16, steroids in P19) with tapered dosing. Notably, six patients (P1, P5, P9, P12, P20, P21) were able to stop their steroids. P1 and P12 stopped their sirolimus therapy, while P12 also stopped adalimumab and sulfasalazine therapy. P2 and P19 were on MMF and cyclosporine, but discontinued both drugs during treatment with abatacept. P5 was able to stop local budesonide, which was used for colitis. IVIG therapy was not discontinued in any patients during the study. Two patients (P2 and P3) suffered from side effects such as mild eczema and dermatophyte infection, which were tolerated well and managed without cessation of abatacept therapy. P16 had severe oral and esophageal candidiasis and fungal pneumonia after the first dose of abatacept, leading to cessation of treatment. However, it was reinitiated after 9 months without side effects.
3.6. Abatacept and immunological changes
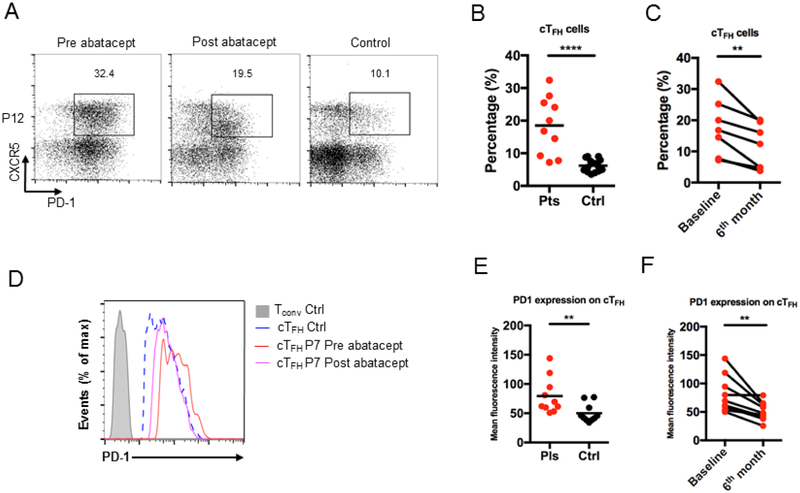
The cellular immunological changes associated with abatacept therapy were serially evaluated by flow cytometry. Abatacept most prominently impacted the patients’ naïve T cells (CD4+CD45RA+ and CD8+CD45RA+), which significantly increased on therapy, while no difference was observed in memory T cells (Fig E3, A - D). There were no changes after abatacept therapy in T, B cell subtypes and NK cells (Fig E4, A - D). The baseline CD4+PD1+CXCR5+ cTFH cells were evaluated in 10 (45.4%) patients and were significantly higher compared to healthy matched controls (Fig 5, A and B). As previously described (15), LRBA-deficient patients had more activated cTFH cells as demonstrated by increased expression of PD-1 (Fig 5, C and D). Baseline PD-1+ cTFH cell profile was compared to cTFH counts at 6th month post therapy initiation (P1, P5, P7, P9, P10, P12, P14, P18, P19). There was a decrease in the frequencies and activation profile of CD4+PD1+CXCR5+ cTFH cells on abatacept therapy , with normalization of cTFH cells was observed in most LRBA-deficient patients (P1, P7, P9, P10, P12, P14, P18) (Fig 5, E and F).
Figure 5.
LRBA-deficient patients have increased activated cTFH cells at baseline, which are normalized after abatacept treatment. Flow cytometric analysis of CXCR5 and PD-1 expression in CD4+ T cells in LRBA-deficient patients before (A, B) and after (A, C) treatment with abatacept. PD-1 expression on patients’ cTFH cells before (D, E) and after (D, F) abatacept treatment. **** p<.0001, ** p<.01, Student unpaired and paired 2-tailed t-test.
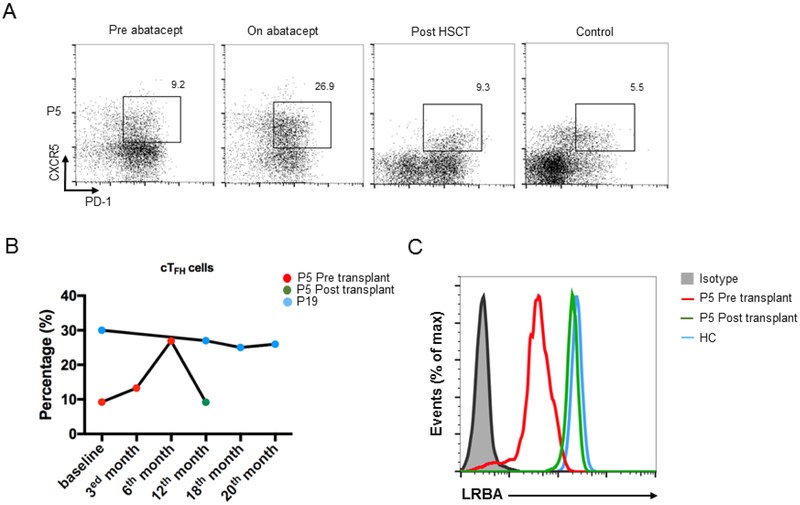
Two patients persisted in having elevated cTFH cells despite abatacept therapy given every other week (P5 and P19) (Fig 6, A and B). Therefore, we investigated the distinguishing clinical and immunological features of those two patients. Both had statistically longer disease course (4.2±2.1 years) compared to others (2.1±1.1 years). Interestingly, P5 and P19 also had a more severe disease course as compared to other patients, although they responded to some extent to the abatacept treatment (Table 2, Table E3). P5 had gastric adenocarcinoma, failure to thrive, LP, diarrhea and pancytopenia. After 9 months of therapy, while the LP and CD were controlled, thrombocytopenia was persistent and his cTFH number increased during the therapy (Fig 6, A and B). Therefore, he was transplanted from his fully matched healthy sibling as a donor, and is now doing well at 6th month of transplantation with 96% donor chimerism. His cTFH number and LRBA expression were normalized after HSCT (Fig 6, B and C). P19 had early onset Evans syndrome, LP and CD. Although her hemolysis was controlled and diarrhea decreased over time, lymphoproliferation and inflammatory bowel disease did not respond well to abatacept. Currently, she is at 20th month of therapy. Immunologically, these two patients with high cTFH numbers exhibited significantly low baseline lymphocyte, CD3+, CD19+ and NK cell counts compared to patients who had decreased cTFH cells after abatacept (P1, P7, P9, P10, P12, P14, P18). They also demonstrated more dysregulated phenotype characterized by inverted naive to memory CD4+ cells (Table E3).
Figure 6.
The cTFH cells guide the disease activity in LRBA patients. A. Flow cytometric analysis of CXCR5 and PD-1 expression in CD4+ T cells in patient (P5) at baseline, on abatacept and after HSCT compared to the healthy control. B. The change of cTFH cells percentages in LRBA-deficient patients on abatacept (P5, P19) and after transplantation (P5). C. The LRBA protein expression in P5 after transplant compared to the baseline level and healthy control.
Discussion
In this report, we prospectively evaluated 22 LRBA-deficient patients from different immunology centers in Turkey. Our results provided a comprehensive long-term evaluation of clinical and immunological characteristics of LRBA-deficient patients. Patients presented with various phenotypes such as early onset RTIs, ID and LP. Our results showed for the first time the long-term benefits of targeted CTLA4-Ig therapy in LRBA deficiency. Abatacept showed the best CR for LP followed by CD and ID symptoms. Interestingly, more favorable responses were achieved for hematological ID symptoms compared to other ID symptoms, while type 1 DM was not controlled well with abatacept. Our results also demonstrated the efficacy of dosing intervals were used for patients. Receiving abatacept at one-week or two-weeks interval exhibited more disease control compared to 4-weeks regimen. Using the CD4+PD1+CXCR5+ cTFH cells as a biomarker for the disease control over time, we showed for the first time that few patients did not responded well to abatacept and found to have persistently high cTFH cells during the study. Those patients were noted to clinically have more disease burden, which was characterized immunologically by lymphopenia, with low total T, B, NK cells.
Flow cytometric analysis revealed that in our cohort, mutations that decreased or abolished LRBA protein expression were present in 17/22 patients. Notably, near normal expression of LRBA protein could be detected by flow cytometry even in patients with early stop gained mutation that can lead to the underestimation of the diagnosis (7, 18). Therefore, as described previously using a MFI threshold would be supportive for determination of all LRBA-deficient patients (18). The calculated MFI ratio cutoff point to determine our normative reference data for LRBA expression was 0.76. This ratio was able to detect all LRBA-deficient patients with high sensitivity (100%) and specificity (91.7%). In this assay, we determined the LRBA protein expression in PBMCs without stimulation, in contrast to Gamez-Diaz et al. study which detected LRBA protein expression in stimulated PBMCs (18). Similar to the aforementioned study, we also found six patients with low protein expression but without identifiable mutations in LRBA. Therefore, we suggest that when evaluating LRBA protein expression by flow cytometry that each laboratory involved in such assays derive its own MFI ratio cutoff point to aid in the identification of LRBA deficiency, rather than use raw MFI values for such purposes.
Recently, abatacept, a T cell modulator, has been proposed as a targeted precision therapy for LRBA-deficient patients. Abatacept mimics the function of the cellular CTLA4 pool, rendered missing by LRBA deficiency, in negatively regulating the immune responses by blockading or capturing CD80/86 molecules found on antigen-presenting cells (1, 13, 15). Patients have been reported to generally respond well to abatacept therapy, with decreased disease symptomatology related to the lung infiltrations, lymphoproliferation, chronic diarrhea and autoimmune features (1, 15). In this study, the relatively large patient number prospectively treated with abatacept allowed for better resolution of the responses of the different phenotypes of the disease to therapy. Thus, the best clinical benefit was achieved for LP, followed by CD and ID. Of note, in all patients at least one disease feature responded to the therapy. Interestingly, the hematological autoimmune features responded better compared to other ID symptoms. Abatacept therapy did not reverse type 1 DM, possibly due to terminal damage of the pancreatic beta islets inflicted by the autoimmunity.
One important question about abatacept therapy in LRBA deficiency that we addressed in our cohort involved its optimal dosing frequency. In previous reports, different dosing intervals, ranging from every 2 to 4 weeks were employed (1, 13). Importantly the response of individual disease attributes, including ID, CD and LP to abatacept therapy was not differentially analyzed. We therefore compared different treatment regimens offered to the patients, and found that abatacept given every 1- or 2-weeks intervals provided better disease control and faster achievement of CR as compared to every 4- week regimen. Interestingly, the one-week regimen came out with CR for all symptoms described. Our results also support the utility of abatacept as a bridge therapy in preparation of HSCT, with the potential for improved transplant outcomes as observed for P5 and P9.
In our study, we used cTFH cell frequencies as a biomarker for monitoring disease activity. As demonstrated previously by our group, the strikingly increased CD4+PD1+CXCR5+ cTFH cells found in LRBA-deficient patients sharply declined after abatacept therapy in the majority of patients (15). Nevertheless, they continued to be persistently high in two patients, both of whom had a more severe disease course compared to those patients whose cTFH cell frequencies normalized. While some of their disease parameters responded to the abatacept treatment, those two patients had a more protracted overall disease activity and demonstrated significant lymphopenia accompanied by low CD3+, CD19+ and NK cells. They also had a more dysregulated immune phenotype characterized by inverted naive to memory CD4+ cells. Thus, persistently high cTFH cells may suggest relative resistance to abatacept therapy, requiring a modified therapeutic approach.
In summary, the presented large cohort provided a prospective evaluation of LRBA-deficient patients during abatacept treatment. The targeted therapy was able to effectively control the different immune dysregulatory disease manifestations in most patients, and more favorable responses were achieved in patients who received abatacept at weekly intervals without serious side effects. Monitoring cTFH cells during abatacept therapy provides a useful measure of disease activity, and may uncover cases of relative therapy resistance that require alternative treatment approaches.
Supplementary Material
What is already known about this topic?
Lipopolysaccharide-responsive beige-like anchor (LRBA) deficiency presents with susceptibility to infections, autoimmunity and lymphoproliferation. Abatacept treatment can control immune dysregulatory disease manifestations.
What does this article add to our knowledge?
Long-term treatment with abatacept is effective in controlling disease activity. Superior clinical responses are achieved with a weekly or biweekly drug dosing regimen. Lymphoproliferation and chronic diarrhea demonstrated the best responses to abatacept therapy, followed by other immune dysregulatory manifestations. The circulating T follicular helper cells are a reliable biomarker for monitoring disease activity.
How does this study impact current management guidelines?
The results of this study may be helpful in the management, follow up and prediction of the response rate to the abatacept as a tailored therapy for LRBA deficiency.
Acknowledgement of funding
This work was supported by grants from the Scientific and Technological Research Council of Turkey (217S847) to S.B. and National Institutes of Health (2R01AI065617) to T.A.C.
Abbreviations
- AIHA
autoimmune hemolytic anemia
- ALPS
autoimmune lymphoproliferative syndrome
- CD
chronic diarrhea
- CR
complete remission
- CTLA4
cytotoxic T lymphocyte associated antigen 4
- CVID
common variable immune deficiency
- DM
diabetes mellitus
- FACS
fluorescent activated cell sorting
- FOXP3
fork-head box P3
- ITP
Immune thrombocytopenia
- HSCT
hematopoietic stem cell transplantation
- IVIG
intravenous immunoglobulin therapy
- ID
immune dysregulation
- LP
lymphoproliferation
- MFI
mean fluorescence intensity
- MMF
mycophenolate mofetil
- NR
non-responsive
- PBMC
peripheral blood mononuclear cells
- PD-1
programmed cell death-1
- PR
partial remission
- RTI
respiratory tract infections
- TFH
T Follicular helper
- Treg
Regulatory T cells.
Footnotes
Conflict of interest: Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349(6246):436–40. [DOI] [PubMed] [Google Scholar]
- 2.Lo B, Fritz JM, Su HC, Uzel G, Jordan MB, Lenardo MJ. CHAI and LATAIE: new genetic diseases of CTLA-4 checkpoint insufficiency. Blood. 2016;128(8):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LSK. EFIS Lecture: Understanding the CTLA-4 checkpoint in the maintenance of immune homeostasis. Immunol Lett. 2017;184:43–50. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135(1):217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamez-Diaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137(1):223–30. [DOI] [PubMed] [Google Scholar]
- 8.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25(6):708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baris S, Schulze I, Ozen A, Karakoc Aydiner E, Altuncu E, Karasu GT, et al. Clinical heterogeneity of immunodysregulation, polyendocrinopathy, enteropathy, X-linked: pulmonary involvement as a non-classical disease manifestation. J Clin Immunol. 2014;34(6):601–6. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, Herholz P, Trujillo-Vargas CM, Phadwal K, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90(6):986–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alangari A, Alsultan A, Adly N, Massaad MJ, Kiani IS, Aljebreen A, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130(2):481–8 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhairy OK, Abolhassani H, Rezaei N, Fang M, Andersen KK, Chavoshzadeh Z, et al. Spectrum of Phenotypes Associated with Mutations in LRBA. J Clin Immunol. 2016;36(1):33–45. [DOI] [PubMed] [Google Scholar]
- 13.Kostel Bal S, Haskologlu S, Serwas NK, Islamoglu C, Aytekin C, Kendirli T, et al. Multiple Presentations of LRBA Deficiency: a Single-Center Experience. J Clin Immunol. 2017;37(8):790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidel MG, Bohm K, Dogu F, Worth A, Thrasher A, Florkin B, et al. Treatment of severe forms of LPS-responsive beige-like anchor protein deficiency with allogeneic hematopoietic stem cell transplantation. J Allergy Clin Immunol. 2018;141(2):770–5 e1. [DOI] [PubMed] [Google Scholar]
- 15.Alroqi FJ, Charbonnier LM, Baris S, Kiykim A, Chou J, Platt CD, et al. Exaggerated follicular helper T-cell responses in patients with LRBA deficiency caused by failure of CTLA4-mediated regulation. J Allergy Clin Immunol. 2018;141(3):1050–9 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eren Akarcan S, Edeer Karaca N, Aksu G, Aykut A, Yilmaz Karapinar D, Cetin F, et al. Two male siblings with a novel LRBA mutation presenting with different findings of IPEX syndrome. JMM Case Rep. 2018;5(10):e005167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson MB, De Franco E, Lango Allen H, Al Senani A, Elbarbary N, Siklar Z, et al. Recessively Inherited LRBA Mutations Cause Autoimmunity Presenting as Neonatal Diabetes. Diabetes. 2017;66(8):2316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamez-Diaz L, Sigmund EC, Reiser V, Vach W, Jung S, Grimbacher B. Rapid Flow Cytometry-Based Test for the Diagnosis of Lipopolysaccharide Responsive Beige-Like Anchor (LRBA) Deficiency. Front Immunol. 2018;9:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.