Abstract
Background:
There is a need to identify and test low cost approaches for cardiovascular disease (CVD) risk reduction that can enable health systems to achieve such a strategy.
Objective:
Community health workers (CHWs) are an integral part of health-care delivery system in lower income countries. Our aim was to assess impact of CHW based interventions in reducing CVD risk factors in rural households in India.
Methods:
We performed an open-label cluster-randomized trial in 28 villages in 3 states of India with the household as a unit of randomization. Households with individuals at intermediate to high CVD risk were randomized to intervention and control groups. In the intervention group, trained CHWs delivered risk-reduction advice and monitored risk factors during 6 household visits over 12 months. Households in the non-intervention group received usual care. Primary outcomes were a reduction in systolic BP (SBP) and adherence to prescribed BP lowering drugs.
Results:
We randomized 2312 households (3261 participants at intermediate or high risk) to intervention (1172 households) and control (1140 households). At baseline prevalence of tobacco use (48.5%) and hypertension (34.7%) were high. At 12 months, there was significant decline in SBP (mmHg) from baseline in both groups-controls 130.3±21 to 128.3±15; intervention 130.3±21 to 127.6±15 (p<0.01 for before and after comparison) but there was no difference between the two groups at 12 months (p=0.18). Adherence to antihypertensive drugs was greater in intervention vs control households (74.9% vs 61.4%, p=0.001).
Conclusion:
A 12-month CHW-led intervention at household level improved adherence to prescribed drugs, but did not impact SBP. To be more impactful, a more comprehensive solution that addresses escalation and access to useful therapies is needed.
Keywords: Primary prevention, cluster randomized trial, cardiovascular risk, hypertension, smokeless tobacco, smoking
Introduction
Cardiovascular diseases (CVD) are leading cause of mortality worldwide, and prevalence of modifiable CVD risk factors (hypertension, diabetes, obesity, hypercholesterolemia and tobacco consumption) is high. (1) CVD risk factors are on the increase in low- and lower-middle-income countries (LMIC), both in urban and rural areas.(2) CVD risk reduction requires health systems that can efficiently screen and stratify at-risk individuals, provide useful lifestyle modification advice, initiate pertinent preventive therapies, and have mechanisms to promote lifelong adherence to lifestyle changes and drug therapies. There is a need to identify and test low cost approaches that can enable health systems to achieve such a strategy. (3,4)
Community Health Workers (CHWs) are members of the community, and in India they are usually high-school educated women who are trained to perform specific health tasks. They form an integral part of health-systems, and have a documented role in reduction of infectious diseases and maternal and childhood morbidities. (5) They could have a role in CVD-risk reduction as they know and identify with the communities, can communicate with various members of the household, and have an ability to repeatedly reinforce behaviors. However lifestyle modification has a complex behavioral construct that has multiple levels of interaction with both negative (smoking and tobacco cessation, reduction in dietary salt and fat intake) and positive (adherence to drugs, more physical activity, healthy diet) components.(6) Barriers in CVD-risk reduction construct include poor acceptance of risk by the participants, reduced adherence to life-long lifestyle modification and drug therapies, and limited access to therapies and subsequent monitoring of targets. (7) It is not known whether CHW-led lifestyle interventions alone can overcome these barriers and reduce CVD risk, without the ability to prescribe simple drugs for BP or lipid lowering. (8,9)
The Primary pREvention strategies at the community level to Promote Adherence of treatments to pREvent cardiovascular diseases, or PREPARE trial was a cluster-randomized trial to evaluate a strategy of CHW based intervention for CVD-risk factor reduction in a rural population in a low-middle income country-India. Our hypothesis was that households one or more individuals at high-CVD risk, that would be visited by trained CHWs will have improved adherence to useful therapies, and hence a lower blood pressure, as compared to those receiving standard clinic based care. The current trial was different from previous studies as it evaluated role of lay CHWs (in contrast to professionally trained nurses and nurse practitioners), used a CVD-risk (rather than disease-specific) approach in three linguistically distinct regions of India. When the trial was conducted, involvement of CHWs for NCD management was being contemplated by Government of India, and has since been launched under National program for chronic diseases, diabetes, cancer and stroke (NPCDCS). However evidence of their impact in achieving intermediate term outcomes remains unknown.
Methods
Ethics statement and funding source
The study design of this multi-centric study was approved by the ethics committees of participating institutes. Additional regulatory approvals were obtained from government of India, and NHLBI. The trial was registered with clinical trial registry of India (CTRI/2012/09/002981). This trial was funded as part of chronic disease initiative by US National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, Department of Health and Human Services, under Contract no. HHSN268200900025C and by the United Health group, USA. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Study design and eligibility
Detailed methods of this study have been described elsewhere. (10) Briefly, PREPARE is a multicenter, household-level, cluster-randomized trial with 1:1 allocation to intervention and control arms. Household was considered as a unit of randomization as a tradeoff between individual and a village level randomization, as it was logistically feasible and to account for clustering of risk factors, and the nature of planned intervention. The study was conducted in 28 villages from 3 rural regions in India. We preferentially sought to identify CHWs from within the communities, who were at least high school educated. Of the 14 CHWs, 11 were women and 3 were men. These CHWs were trained for four weeks in survey methods (mapping households, enumeration of members, administration of questionnaires), measurement techniques (blood pressure measurement using a digital device, anthropometry to estimate body mass index and waist-to-hip circumference), and life style advice to modify CVD-risk factors (promoting adherence to prescribed medication, assisting in optimizing drug therapies by obtaining follow up blood pressure measurements, and promoting tobacco cessation, salt reduction, reducing consumption of oil, promoting intake of fruits and vegetables and physical activity). They were however not allowed to prescribe drugs.
All households with individuals of age ≥35 years living in these villages were invited to participate in a baseline survey that was conducted by CHWs. Baseline survey collected information about CVD risk factors (tobacco smoking, other tobacco use, previous history of hypertension, diabetes mellitus, dietary practices, physical activity, stress levels) and measured blood pressure (using standard digital sphygmomanometers), body mass index (BMI) and waist-to-hip ratio (WHR). CHWs obtained a set of 3-blood pressure values 5-minutes apart, using Omron digital blood pressure apparatus. CHWs were trained in the technique prior to the start of the study, and ensured that participants had rested, and did not use tobacco, or had meals in the previous half an hour. They were instructed to use a suitable furniture in the household or neighborhood so that arm and back could be well supported. In case it was not available, participants were seated on the floor and arm supported on a cot or a stool. We used these variables to estimate INTERHEART risk score (IHRS), which is a summative measure of change in risk factors. We used color-coded charts derived from non–laboratory-based National Health and Nutrition Examination Survey score (or NHANES score) for risk stratification. These charts require information about age, gender, and history of diabetes, systolic blood pressure and BMI. Individuals with greater than 10% risk of CVD in next ten years are classified as moderate risk, and those with greater than 20% risk as high risk for CVD.
All at-risk individuals at moderate or high risk were invited to attend a physician-led village level clinic that provided a CVD risk reduction prescription and education about target risk factor levels for CVD control. Prescription included medication for some individuals based on standard guidelines. The at-risk households were defined as those with individuals aged ≥35 years and at moderate-to high risk for CVD, who had attended clinic visit. All such at-risk households were eligible for randomization.
Intervention
CHWs visited intervention households every two months (6 household visits over 12 months). Each visit lasted about 30–45 minutes and during these visits, CHWs measured blood pressure, ascertained and reinforced adherence to prescribed therapies. If blood pressure values were above the target 140/90) and the individual had reported poor adherence (as self or spouse reported) CHW would reinforce adherence. If adherence were reported as adequate, CHW would contact the study physician by telephone, who could either advise dose-escalation and to issue a proxy-prescription or provide an appointment to attend the sub-center level clinic. Proxy prescriptions were allowed for dose escalation, but not for addition of a new drug. The CHWs also placed short goal-directed slogans printed on common household objects in the household to promote integration of preventive therapies with activities of daily living. Households randomized to the control arm did not receive CHW visits.
Individuals in both intervention and control households had access to a clinic which was run in the sub-center location (a health sub-center caters to about 5 to 7 villages) throughout the duration of the study on one specified day of the week. Sub-centers are located within 0 to 7 kilometers of the participating villages. Usually participants use their own transportation to travel from village to sub-centers. Providing anti-hypertensive drugs was not part of the study design. These medications were available either as a low cost generic on the day of the clinic. Participants could also access these drugs from public health facilities (primary health center located within a 15kilometer distance from the villages) at no cost. None of the households had any health insurance to cover cost of anti-hypertensive drugs.
Outcomes
The co-primary outcomes of the study were a 3mm Hg reduction in SBP in households with intermediate and high cardiovascular risk and 30% higher adherence to prescribed antihypertensive drugs 12 months after randomization. In this complex intervention, various pathways could contribute to SBP reduction (including drug therapy, tobacco cessation, increased physical activity, consumption of healthy diet). Adherence was ascertained only for prescribed anti-hypertensive drugs, as a proportion of consumed and prescribed number of pills. Secondary outcomes were reduction in INTERHEART risk score (IHRS), body mass index, and waist-to-hip ratios 12 months after randomization. IHRS is a composite score derived from various continuous variables: tobacco related variables (number of cigarettes/beedi use), diet related variables (consumption of deep fried foods/snacks/fast foods weekly, fruits daily, fruits daily, vegetables (leafy/other/cooked) per day, meat/poultry per day), exercise related variables (active during leisure time, active during work), and stress related variables (financial stress. feel stress in the last year, feel blue/sad). Outcomes were recorded again in the post-intervention period (18 months after randomization) to evaluate their sustainability.
Statistical Analysis
Household was considered as a cluster in all analyses, as the risk factors among individuals who are residing in same household are likely to be clustered. Outcome variables were continuous: adherence to prescribed medication, systolic blood pressure, BMI, waist circumference, and IHRS. Other variables such as smoking, non-smoking tobacco use, history of hypertension or diabetes, number of years of education, were categorical. We used mixed linear modeling to examine the differences in continuous outcomes by treatment group. The variance–covariance matrix that gave the best fit was considered. The Akaike Information Criteria was used as a statistic to identify the best fitting model. GEE (generalize estimation equations) was used to examine the treatment differences in binary outcomes by treatment group. The Quasi likelihood under the Independence Model Criterion was used to identify the best fitting model for different variance–covariance matrices. Subgroup analysis for change in SBP (by age, gender, hypertension, diabetes, BMI, tobacco use, and participating region) was also carried out within intervention and control groups to assess in the change in outcomes between times points 0 and 12 months. All analyses were conducted with the use of SAS software (version 9.2).
Results
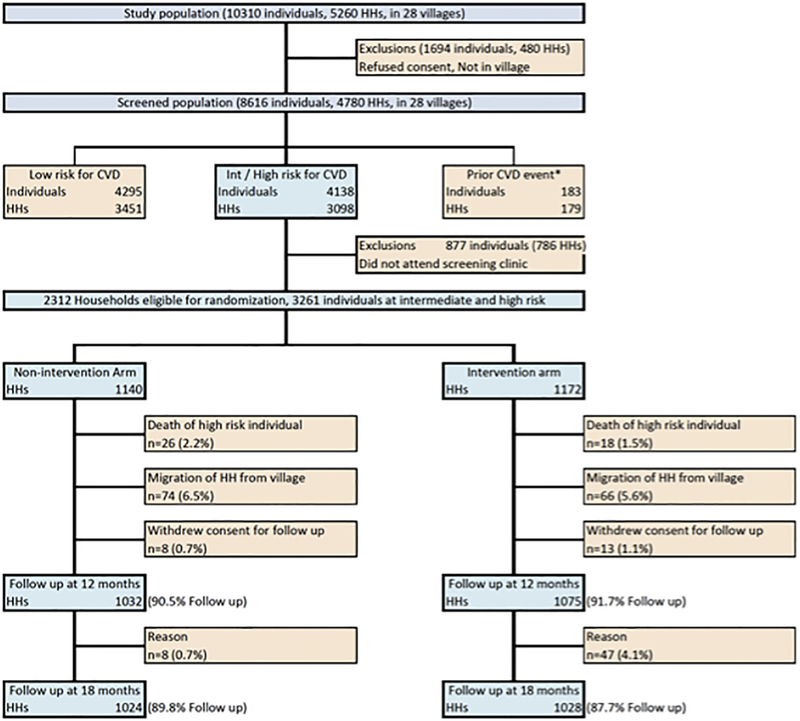
Between August 2011 and February 2012, 4780 households with 8616 individuals were screened for participation in the study. About 51.7% individuals in screened households consumed tobacco, and only 6.4% previously knew that they had hypertension. (Table 1) Of all individuals with hypertension, 51.8% were identified as first time during screening. Of the screened households, 2312 households with 3261 individuals at intermediate or high CVD-risk were eligible for randomization (Figure 1). Between March and May 2012 we randomized 1172 households to the intervention arm and 1140 households to control arm of the study. We were able to follow up 2107 (91.7%) households at 12 months and 2052 (88.7%) households at 18 months. The main reason for non-response was seasonal or permanent migration of households from the villages.
Table 1.
Prevalence of CVD risk factors in study population (n=8616)
| All screened (n=8616) | Intermediate or high risk (n=4138) | |||||
|---|---|---|---|---|---|---|
| Variable | Men | Women | p-value | Men | Women | p-value |
| Number (proportion) | 4132 (47.9) | 4484 (52.1) | 2398 (57.9) | 1740 (42.1) | ||
| Mean Age (years) | 52.0 (13.04) | 50.8 (12.5) | <0.0001 | 59.8 (10.7) | 62.8 (9.4) | <0.0001 |
| Number of Years of education Mean (SD) | 5.2 (4.4) | 2.9 (3.7) | <0.0001 | 4.3 (4.1) | 1.2 (2.5) | <0.0001 |
| Standard of Living index score Mean (SD) | 25.6 (10.2) | 23.6 (10.04) | <0.0001 | 25.6 (10.5) | 22.9 (10.5) | <0.0001 |
| Previously known Hypertension n(%) | 209 (5.1) | 351 (7.8) | <0.0001 | 158 (6.6) | 240 (13.8) | <0.0001 |
| Previously known Diabetes n(%) | 183 (4.4) | 172 (3.8) | 0.16 | 151 (6.3) | 131 (7.5) | 0.12 |
| Tobacco use | ||||||
| Never used n(%) | 1235 (29.9) | 2672 (59.6) | <0.0001 | 465 (19.4) | 726 (41.7) | <0.0001 |
| Past user n(%) | 143 (3.5) | 110 (2.5) | 100 (4.2) | 73 (4.2) | ||
| Current user n(%) | 2754 (66.7) | 1701 (37.9) | 1833 (76.4) | 941 (54.1) | ||
| Age tobacco use started Mean (SD) | 23.9 (10.6) | 25.7 (12.1) | 0.002 | 24.7 (11.8) | 25.5 (13.5) | 0.085 |
| Type of tobacco use | ||||||
| Cigarettes n(%) | 246 (6.0) | 5 (0.1) | <0.0001 | 167 (7.0) | 2 (0.1) | <0.0001 |
| Beedi n(%) | 968 (23.4) | 10 (0.2) | <0.0001 | 754 (31.4) | 5 (0.3) | <0.0001 |
| Chewable tobacco n(%) | 1616 (39.1) | 1458 (32.5) | <0.0001 | 1059 (44.2) | 896 (51.5) | <0.0001 |
| Pipe n(%) | 12 (0.3) | 0 | 0.006 | 10 (0.4) | 0 | 0.02 |
| Snuff n(%) | 345 (8.3) | 471 (10.5) | <0.0001 | 231 (9.6) | 216 (12.4) | <0.0001 |
| Paan n(%) | 156 (3.8) | 55 (1.2) | <0.0001 | 76 (3.2) | 35 (2.0) | 0.51 |
| Alcohol use n(%) | 1233 (29.8) | 44 (1.0) | <0.0001 | 694 (28.9) | 29 (1.7) | <0.0001 |
| Dietary risk factors | ||||||
| Diet has deep fried foods 3 times or more/week n(%) | 31 (0.8) | 21 (0.5) | 0.09 | 14 (0.6) | 6 (0.3) | 0.27 |
| Diet has fruit 1 times or more /daily n(%) | 5 (0.1) | 6 (0.1) | 3 (0.1) | 2 (0.1) | ||
| Diet has vegetables 1 times or more /daily n(%) | 3484 (84.3) | 3663 (81.7) | 2025 (84.4) | 1426 (82.0) | ||
| Diet has meat/poultry 2 or more times daily n(%) | 0 | 0 | 0 | 0 | ||
| Physical activity | ||||||
| Sedentariness during leisure time n(%) | 3529 (85.4) | 3912 (87.3) | <0.0001 | 2095 (87.4) | 1554 (89.3) | <0.0001 |
| Sedentariness during work n(%) | 235 (5.7) | 375 (8.4) | <0.0001 | 165 (6.9) | 232 (13.3) | <0.0001 |
| Stress | ||||||
| Financial stress Moderate or severe n(%) | 1473 (35.6) | 1627 (36.3) | 0.53 | 889 (37.1) | 563 (32.4) | 0.002 |
| Several periods of stress n(%) | 1482 (35.9) | 1648 (36.8) | 0.38 | 903 (37.7) | 602 (34.6) | 0.043 |
| Felt sad/ blue or depressed n(%) | 1453 (35.2) | 1619 (36.1) | 0.35 | 862 (35.9) | 564 (32.4) | 0.018 |
| Measurements | ||||||
| Systolic blood pressure Mean (SD) | 126 (19.62) | 124.4 (20.47) | 0.007 | 129.3 (21.9) | 135.9 (22.1) | <0.0001 |
| Diastolic blood pressure Mean (SD) | 79.1 (12) | 77.9 (11.07) | <0.0001 | 79.3 (12.77) | 80.2 (12.05) | 0.029 |
| Body mass index Mean (SD) | 20.9 (3.8) | 21.1 (4.5) | 0.002 | 20.5 (3.86) | 21.2 (4.83) | <0.0001 |
| Waist circumference Mean (SD) | 81 (10.99) | 74.12 (11.85) | <0.0001 | 81.0 (11.12) | 75.5 (12.08) | <0.0001 |
| Waist-hip ratio Mean (SD) | 0.9 (0.08) | 0.8 (0.08) | <0.0001 | 0.9 (0.08) | 0.8 (0.08) | <0.0001 |
| INTERHEART risk score Mean (SD) | 10.6 (5.03) | 7.32 (3.65) | <0.0001 | 11 (4.98) | 7.3 (4.06) | <0.0001 |
p-values are for the differences in distribution of variables between men and women.
Figure 1:
Study flow
Mean age of individuals in randomized households was 61.7 years and mean number of years of education was 3.1 years in control and 3.3 years in intervention households. A total of 1582 (48.5%) individuals (800 in intervention and 782 in control arms) used smokeless tobacco and 543 (16.7%) (269 in intervention and 274 in control households) smoked beedis or cigarettes. A total of 1164 individuals (34.7%) had hypertension (blood pressure ≥ 140/90 mm Hg or previously known to have hypertension; 597 in intervention and 567 in control arms). After the clinic visit 709 of these 1164 individuals (60.9%) were receiving drug therapy. Remaining individuals had blood pressure value ≥ 140/90 mm Hg on a single occasion, and only received life-style measures. Amongst dietary risk factors, consumption of fruits was very low, while consumption of vegetables (mostly cooked) was high. Consumption of meat or poultry more than twice a week, and deep fried foods or salty snacks more than three times a week was low. Most individuals were active at work. (Table 2)
Table 2.
Baseline characteristics of randomized households
| Control | Intervention | |
|---|---|---|
| Number of Households | 1140 | 1172 |
| Number of individuals | 1611 | 1650 |
| Mean Age (years) | 61.7(10.38) | 61.7 (10.23) |
| Years of education Mean (SD) | 3.1 (3.6) | 3.3 (3.6) |
| Standard of Living index score Median (IQR) | 24.7 (10.5) | 24.8 (10.3) |
| Individuals with hypertension n(%) | 567 (35.2) | 597(36.2) |
| Individuals with Diabetes n(%) | 97 (6.0) | 114(6.9) |
| Individuals who smoke beedi or cigarettes n(%) | 274(17.0) | 269(16.3) |
| Individuals who used chewable tobacco n(%) | 782(48.5) | 800 (48.5) |
| Mean SBP of included members (mm Hg) | 130.3 (20.8) | 130.3 (20.5) |
| Mean DBP of included members (mm Hg) | 79.4 (11.7) | 78.9 (11.3) |
| Mean Body mass index of included members (Kg/m2) | 20.4 (4.03) | 20.5 (3.61) |
| Mean Waist-hip ratio of included members | 0.9 (0.002) | 0.9 (0.002) |
| INTERHEART risk score for household (Median (IQR)) | 10(6) | 10(6) |
| Total number of individuals prescribed drug therapy n(%) | 342 | 367 |
| Mean number of daily pills prescribed per individual | 1.21(0.02) | 1.22(0.02) |
Standard of Living index score is based on asset ownership is used for comparisons within populations
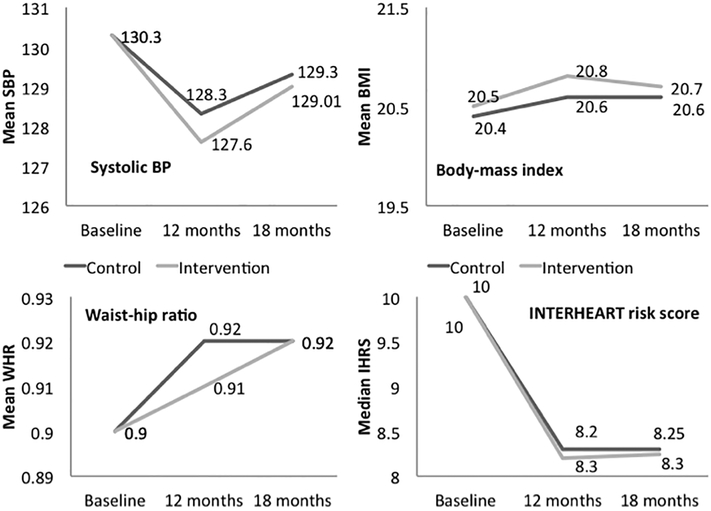
At 12 months, there was a significant decline in systolic BP from baseline in both groups (intervention 130.3±20.5 to 127.6±15.0 mmHg; control 130.3±20.8 to 128.3±14.9 mmHg; p<0.01 for each), a reduction of 2.7 mm Hg (95%CI 1.40 to 3.99) and 2.0 mm Hg (95%CI 0.66 to 3.33) respectively. There was no inter-group difference in SBP (adjusted for the effect of clustering and baseline values) in intervention and control households (mean difference −0.7 mm Hg (95%CI −1.82 to +0.42; p=0.18). Thus, the primary outcome of 3mm lower SBP in the intervention arm compared to the control arm was not achieved. Among those with hypertension at baseline, the change in SBP from baseline to 12 months was +0.40mm Hg (95%CI −1.35 to +2.15; from 150.7 ± 15.4 to 151.1 ± 15.5mm Hg) in the intervention group and −0.5mmHg (95%CI −2.27 to +1.27; from149.8 ±15.3 to 149.3 ±15 mm Hg) in the control group. These differences were not significant. Blood pressure values in intervention arm during each home visit over these twelve months is presented in Supplementary Appendix.
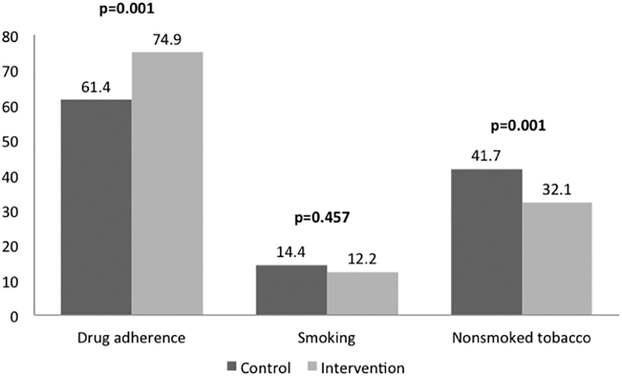
Of the individuals who were on anti-hypertensive drug therapy 74.9% (95% CI 70.2 to 79.0%; 275 of 367) in the intervention arm and 61.4% (95%CI 56.14 to 66.41%; 210 of 342) in the control arm continued to take their medications at 12 months (p=0.001). The mean daily number of pills consumed in these individuals was however similar in both arms (1.21 and 1.22 in intervention and control arms at baseline; 1.0 and 0.98 at 12 months). There was significant decline in median IHRS in intervention (10.0 to 8.2) as well as control (10.0 to 8.3) groups, with no intergroup difference at end of the intervention. (p=0.50), secondary outcomes of reduction in body mass index, waist-to-hip ratio, and IHRS were not achieved. (Table 3, Figures 2 and 3)
Table 3:
Primary and secondary outcome indicators at baseline, 12 months and 18 months
| Baseline | 12 months | 18 months | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | P value | Control | Intervention | P value | |
| Number of Households | 1140 | 1172 | 1032 | 1075 | 1024 | 1028 | ||
| Number of individuals | 1611 | 1650 | 1331 | 1414 | 1268 | 1338 | ||
| Primary outcomes | ||||||||
| Mean SBP (mm Hg) | 130.3 (20.8) | 130.3 (20.5) | 128.3 (14.95) | 127.6 (15.04) | 0.18 | 129.3 (15.31) | 129 (15.36) | 0.98 |
| Individuals on drug therapy n(%) | 342 | 367 | 210 | 275 | 137 | 181 | ||
| Mean daily drug pills consumed | 1.21 (0.80) | 1.22 (0.81) | 1.0 (1.34) | 0.984 (1.20) | 0.73 | 0.97 (1.38) | 0.97 (1.17) | 0.88 |
| Secondary outcomes | ||||||||
| Mean Body mass index (Kg/m2) | 20.4 (4.03) | 20.5 (3.61) | 20.62 (2.18) | 20.84 (2.25) | 0.02 | 20.60 (2.13) | 20.7 (2.19) | 0.18 |
| Mean Waist-hip ratio of included members | 0.9 (0.002) | 0.9 (0.002) | 0.92 (0.002) | 0.91 (0.002) | 0.07 | 0.9167 (0.003) | 0.92 (0.003) | 0.68 |
| INTERHEART risk score for household (Median (IQR)) | 10 (6) | 10 (6) | 8.3 (3.61) | 8.20 (3.64) | 0.50 | 8.28 (3.12) | 8.25 (3.58) | 0.88 |
| Tobacco consumption | ||||||||
| Individual who smoke n(%) | 274 (17.0) | 269 (16.3) | 191 (14.4) | 173 (12.2) | 0.45 | 182 (14.4) | 158 (11.8) | 0.38 |
| Individual who chew n(%) | 782 (48.5) | 800 (48.5) | 555 (41.7) | 454 (32.1) | <0.001 | 513 (40.5) | 476 (35.6) | 0.09 |
All values are adjusted for the effect of clustering and baseline parameters
Figure 2:
Change in mean systolic BP, body mass index, waist hip ratio and median INTERHEART risk score from baseline to 12 months (active intervention) and at 18 months (passive follow-up) in control and intervention groups. Significant declines are observed in mean systolic BP and INTERHEART risk scores and no significant differences in intergroup comparisons.
Figure 3:
Participants adherent to on anti-hypertensive drug therapies, smoking cessation and nonsmoked tobacco cessation at 12 months in control and intervention groups
CHW led interventions stopped at 12 months, and between 12 and 18 months all households had access to sub-center level clinics. Proportion of individuals on drug therapy declined to 49.3% (95%CI 44.2 to 54.4%) in intervention and to 40% (95%CI 35.0 to 45.3%) in control arms (p=0.01 for the difference between the two arms). Mean SBP also increased by 1.4mm Hg (95%CI 0.26 to 2.53) and 1.0 mm Hg (95%CI −0.16 to +2.16) in intervention and control arms respectively.
While reduction in tobacco consumption was not a-priori outcome, tobacco cessation was one of the important messages CHWs delivered as part of their interventions. Proportion of individuals who use smokeless tobacco significantly declined between baseline and 12 months by 16.4% (95%CI 12.92 to 19.77) in intervention arm, and by 6.8%(95%CI 3.23 to 10.43) in control arm (mean difference 9.59 (95%CI 5.98 to 13.17; p=0.001). Proportion of individuals who smoked declined by 4.1% (95%CI 1.58 to 6.53)) in intervention arm and by 2.6% (95%CI 0.01 to 5.27)) in control arm but the difference between the two groups was not statistically significant. (p=0.33). (Table 3, Figure 3)
Discussion
The present study did not meet its primary outcomes of reduction in SBP of 3mm Hg, or 30% higher adherence to drug therapy in intervention vs control arms. The decline in SBP was similar across two arms (2.7 and 2 mm Hg). Although the endpoint target of 30 % improvement in adherence to antihypertensive medications was not met there was significantly higher medication adherence in the intervention arm by an absolute 13.5%. If we assume that a single antihypertensive drug reduces SBP by 5 to 6 mm Hg, then this would translate into about a 0.5 to 0.6 mm Hg greater reduction in SBP, which is consistent with our results. This indicates that to reduce BP to greater degree, there has to be a greater difference in the percent of participants prescribed antihypertensive medication (either a new drug as an add on or a new drug in those not receiving any drugs previously). In the post-intervention period, there was a sharp decline in adherence to prescribed medication in which group, and marginal rise in mean SBP. This post-intervention change observed just 6-months later, emphasizes that life-style change and promotion of drug adherence is difficult and would need a longer reinforcement.
The current study is the largest cluster randomized trial that evaluated multiple-risk factor reduction strategies using CHWs as “change-agents”. It tested intervention with household as a unit of randomization, across three linguistic and culturally diverse rural populations in India. Risk factor reduction for primary prevention of cardiovascular events is challenging. It requires screening for risk factors, initiating useful therapies and most importantly engaging those positively screened in a behavior change process.(11) Our cluster randomized trial required community engagement, motivational and advocacy skills as in program implementation.(12) A major effort in the current study was to convince individuals and households that some prevalent cultural practices such as tobacco consumption are a cardiovascular risk, and that despite being asymptomatic individuals with risk factors may benefit by taking medications everyday for life.(12) This is a real challenge in prevention especially in primary prevention. It contrasts with primary prevention strategies for infectious diseases such as an annual or a 5 year vaccination.
We found that about half of all screened individuals in rural India are at intermediate or high risk for cardiovascular events, as a result of tobacco use, and elevated blood pressure. This is a huge burden, among asymptomatic individuals living in communities where consumption of tobacco is a cultural practice-prevalent in three-fourths of men and more than half the women at intermediate or high risk. More than a third of all at-risk individuals had hypertension. Only one-third of all individuals with hypertension knew they had the condition. The remaining two-thirds were detected on screening. While, all positively screened individuals were initiated on anti-hypertensive drug therapies, subsequent escalation and intensification of drug therapies was sub-optimal. This remains an important limitation of our approach.
CHWs in our study could deliver the desired intervention, and did achieve improved adherence to prescribed medication. While this was appreciable ie a 13% absolute improvement in adherence, it would be expected to translate into a 0.5 mm Hg greater reduction in SBP in the entire study population. Therefore to reduce SBP by a greater and more meaningful degree (e.g. 3 mm Hg which would correspond to a 6 to 10 percent greater relative risk reduction in CVD or strokes) requires additional measures. These could include systematic approaches to increase the percent of those with hypertension receiving any hypertensive medication as well as simultaneously increasing the proportion receiving 2 or more BP lowering medications. While our results on a lack of significant reduction in SBP between groups by the use of CHW are disappointing, they illustrate that lifestyle advice alone is unlikely to lead to sufficient reductions in BP to lead to a meaningful reduction on BP in the community that can reduce the risk of clinical events by even 5 %. Our data suggest that strategies to increase the use of BP lowering medications are likely to be key to improving BP control in the community. Most participants in our study were elderly men with less than five years of education, and a low standards of living score. Despite these constraints, the proportion of individuals on drug therapy always remained modestly but not significantly higher in the intervention households. Attrition in number of individuals on drug therapy occurred in both intervention and non-intervention groups, which was not entirely unexpected given the barriers of cost, and negative attitudes towards indefinite duration of drug therapy.(13) Our interventions did not tackle these challenges directly.
Tobacco consumption, particularly the smokeless form was most prevalent risk factor in our study. It was not a pre-specified outcome in the design phase, as it was considered as the most difficult to intervene upon. Further, IHRS a secondary outcome measure in our study (a cumulative score of smoking, physical activity, healthy diet, stress, waist-hip-ratios, and systolic blood pressure) does not capture chewable tobacco. The reduction in reported lower use of chewable tobacco was significantly higher in the intervention arm; a likely consequence of CHW induced behavior-change. It is likely that some of this change is also attributable to social desirability bias (14) that differentially affected intervention arm, as CHWs were delivering tobacco cessation messages at every visit.
What can we learn from this trial? First, blood pressure control at a community level needs a more intensive approach. Once individuals with elevated blood pressure are identified, they must be initiated on drug therapy, adhere to prescribed therapies and receive appropriate escalations if targets are not met. We need a systems approach that incorporates education of patients regarding the long-term benefits of continual drug therapy, and putting in place support systems to reinforce the importance of adhering to these measures. (15)
Second, decision to escalate therapy is usually made by a physician at a health facility and this is the only place in rural areas where drugs for refill are available. Thus both escalation and drug-refill incur direct travel and indirect time cost towards lost wages. Availability and cost of drug therapy are additional barriers to successful treatment. Recently PURE study reported on global availability and affordability of antihypertensive drugs, and found that availability of anti-hypertensive drugs was high in local pharmacies of India, but about one-fourth of population does not afford even a single drug. (16) These barriers can be overcome if there is a mechanism for CHW to visit participants homes, check BP and also dispense a limited range of antihypertensive drugs at low doses (which are generally very safe) directly to the patient in their homes. The latter is not permitted because of legal restrictions in most LMIC and will require a change in the health systems as well as training of CHW to enable them to learn about simple pharmacotherapy for limited range of drugs.
Our study population was heterogeneous. Key driver of high CVD risk was age and tobacco use. Two-thirds of our trial population was non-hypertensive with a mean SBP of 130.3 mm Hg and so only a modest further BP lowering can be expected in this population, as compared to a populations that comprises largely of high-risk individuals. Another study by our group in those early after an ACS, which represents a much higher risk population (SPREAD trial in survivors of an acute CVD event) achieved a larger and statistically significant 3.9mm Hg difference in SBP in the intervention vs. control arm. (17) This trial had adherence promotion interventions similar to the present study but was based around tertiary care health-care facilities and in a population with a recent ACS. The expected decline of 2.7mm Hg within 12 months in the study population is relatively large, considering that the PREPARE population was at a lower risk. In a Cochrane meta-analysis by Ebrahim S and colleagues (18) of a total of 52 multiple risk factor interventions in primary prevention setting that reported change in SBP, only seven were large in size (1000 or more participants in each arm). Of these large studies only the WHO Factories Study had a SBP reduction of 2.7mm Hg in the intervention arm, while all others (i.e. 51 studies) had lower reductions in BP or even an increase from the baseline. While our before and after effect matches this effect size, the between group difference in our study was relatively modest.
Similar cluster randomized trials (CRTs) using multiple risk factor approaches have had mixed results. A study from rural China and India that used a village as a unit of randomization, for a year-long CHW and smart-phone based intervention reported significantly higher proportion of individuals adhering to treatment of hypertension. The study also reports a 2.7mm Hg reduction in SBP in the intervention arm as compared to the control arm. (19) However this was not a primary prevention trial as about 50% of those in intervention arm and 40% in control arm had a manifest vascular event such as coronary artery disease or stroke. Differences in population characteristics are likely to be responsible for a much higher acceptance and uptake of drug therapies. Further, participants had much higher mean SBP at baseline (161 mm Hg) versus 130mm Hg in our study allowing greater blood pressure difference in intervention and non-intervention arms to be detected (2.7 mm Hg vs 0.7 mm Hg in our study). (19) A recently reported CRT from Nepal that randomized 14 wards of a single municipality to CHW-led blood pressure lowering intervention (three home visits in a year) achieved a reduction of 4mm Hg among individuals with hypertension in intervention as compared to control clusters. On the other hand, in this resource intensive intervention (43 CHWs for 1638 participants) blood pressure values among normotensive individuals and those with pre-hypertension increased. This study also emphasizes the complexities of multiple-risk factor control. (20) While drug therapy was integral part in both these trials from China and Nepal; study physicians provided drug prescriptions. A CRT from Ghana performed a CHW-led salt reduction intervention, and achieved a 2.5mmHg reduction in SBP. This simple community based intervention in a high-salt consumption setting did not involve use of anti-hypertensive medications. (21) Another CRT from Australia, with urban general practice as a unit of randomization, tried a six month long intervention using patient education as well as facility improvement strategies. (22) While risk recording of risk factors improved, the proportion of individuals with optimal BP control did not improve.
As opposed to population level approaches, researchers have also evaluated facility level interventions to improve cardiovascular risk factors. A trial involving 3443 patients in 87 general practices in Germany compared a simple (provision of health education advice) and a complex (health education advice, phone-calls and peer-visits) intervention among individuals with cardiovascular risk. The mean SBP at baseline in two groups were 138.7 and 137.8 mm Hg. After nine months of intervention, there was no statistically significant difference in cardiovascular risk or SBP values, while there was a higher uptake of statin therapy in both groups. The change in blood pressure in both groups was most in high CV risk individuals (0.7mm Hg lower in those who received a simple intervention). (23) In contrast another small facility level Dutch cluster randomized trial (four practices, 146 participants) that included hypertensive immigrants who were provided with culturally appropriate health education reported large reductions in SBP. Baseline mean SBP in intervention clusters was 156.7 mm Hg and 155.2 mm Hg in non-intervention arm. SBP reduction of 9.95 mm Hg and 6.26 mm Hg was observed in the two arms, but the difference in BP between the two arms was not statistically significant (mean change −1.69 mmHg (95% CI: −6.01 to 2.62; p = 0.44)). (24) Other completed facility level cluster randomized trials from developed world had only a limited success. Measures used in these CRTs include periodic quality improvement initiatives,(25) improved cardiovascular preventive care, (26) increased participant information, (27) and enhanced decision-making tools for drug escalation. (28) A 2× 2 factorial design cluster randomized trial was conducted in Pakistan that tested both provider level and individual level health education for blood-pressure control. This trial was different from ours as it only included participants with confirmed hypertension, and mean-SBP of participants at baseline was 21mmHg higher (151.7 vs 130.3 mm Hg in current study). This trial reported a 10.8mm Hg reduction in systolic blood pressure in a group where both individual level and facility level interventions were performed, as compared to none of these interventions. (29)
Various ongoing CRTs are either evaluating different information dissemination approaches (electronic decision support systems,(30) phone-calls,(31) or community gatherings at pharmacies(32)), or are testing performance incentive based systems,(33) at multiple levels of care(34) or for a longer intervention duration. (35–37) A CRT involving 30 communities in Malaysia and Columbia in currently underway (HOPE 4) which combines many of these approaches including allowing CHWs to prescribe anti-hypertensive prescriptions using m-health tools, provision of free drugs, and using a network of treatment supporters in the community. (38) While we await the results of these ongoing studies to know if these different approaches have a better outcome, we also need to improve complex programs and their implementation.
CHWs in our study were successful in adherence promotion and reducing smokeless tobacco use in predominantly elderly, low education, and low-income setting. In such populations where access to healthcare is an additional barrier and management of hypertension remains a challenge, CHWs have a potential to bridge the gap. However to be even more effective, greater task shifting such as empowering CHWs to prescribe simple low doses of BP lowering drugs, using decision support systems to act on need for escalation of drug therapy will be required.
Limitations
Cluster randomized design in our study minimized selection and ascertainment bias. However contamination is likely in our study as household was the unit of randomization. This was a trade-off between greater perceived contamination of individual-randomization, and requirement of much larger sample size with villages as units of randomization. This tradeoff is likely to have led to percolation of information and similar adoption of behaviors and practices by both intervention and non-intervention households in proximity to each other.
Intervention period of 12 months in our study may have been too short to enable adequate uptake of desired behaviors. During the 12 months of intervention period when CHWs were reinforcing adherence in home visits, 68% continued to be adherent to medications. This number dropped to 44% in the subsequent six months after the intervention was over. This suggests that additional strategies are needed which include an efficient tracking system for those initiated on therapy, feedback loops from dispensing pharmacies, longer periods of reinforcement, or need for a continual intervention from the CHW. Our study design did not directly address access to study medications, and measures for escalation of drug therapy in the design were sub-optimal.
Supplementary Material
Funding:
This trial was conceived as part of chronic disease initiative funded by US National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, Department of Health and Human Services, under Contract no. HHSN268200900025C and by the UnitedHealth group, USA
Disclosures: There are no disclosures regarding industry support for this study.
Abbreviations:
- CVD
Cardiovascular disease
- CHW
Community health worker
- LMIC
Low and Middle income country
- BMI
Body mass index
- WHR
Waist to hip ratio
- IHRS
Interheart risk score
- BP
Blood-pressure
- SBP
Systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beaglehole R, Bonita R, Horton R, et al. Priority actions for the non-communicable disease crisis. Lancet. 2011;377(9775):1438–1447. doi: 10.1016/S0140-6736(11)60393-0. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo-Larco RM, Miranda JJ, Li X, et al. Prevalence of Pragmatically Defined High CV Risk and its Correlates in LMIC A Report from 10 LMIC Areas in Africa, Asia, and South America. Glob Heart. 2016;11(1):27–36. doi: 10.1016/j.gheart.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma K Burden of non communicable diseases in India: Setting priority for action. Int J Med Sci Public Heal. 2013;2(1):7. doi: 10.5455/ijmsph.2013.2.7-11. [DOI] [Google Scholar]
- 4.Ebrahim S, Smeeth L. Non-communicable diseases in low and middle-income countries: A priority or a distraction? Int J Epidemiol. 2005;34(5):961–966. doi: 10.1093/ije/dyi188. [DOI] [PubMed] [Google Scholar]
- 5.Viswanathan M, Kraschnewski J, Nishikawa B, et al. Outcomes of community health worker interventions. Evid Rep Technol Assess (Full Rep). 2009;(181):1–144, A1–A2, B1–B14, passim. http://www.ncbi.nlm.nih.gov/books/NBK44601/. [PMC free article] [PubMed] [Google Scholar]
- 6.Alageel S, Gulliford MC, McDermott L, Wright AJ. Implementing multiple health behaviour change interventions for cardiovascular risk reduction in primary care: a qualitative study. BMC Fam Pract. 2018;19(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatib R, Schwalm JD, Yusuf S, et al. Patient and healthcare provider barriers to hypertension awareness, treatment and follow up: a systematic review and meta-analysis of qualitative and quantitative studies. PLoS One. 2014;9(1):e84238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi R, Alim M, Kengne AP, et al. Task shifting for non-communicable disease management in low and middle income countries--a systematic review. PLoS One. 2014;9(8):e103754. doi: 10.1371/journal.pone.0103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katende G, Donnelly M. Shining a light on task-shifting policy: Exploring opportunities for adaptability in non-communicable disease management programmes in Uganda. Sultan Qaboos Univ Med J. 2016;16(2):e161–e167. doi: 10.18295/squmj.2016.16.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fathima FN, Joshi R, Agrawal T, et al. Rationale and design of the Primary pREvention strategies at the community level to Promote Adherence of treatments to pREvent cardiovascular diseases trial number (CTRI/2012/09/002981). Am Heart J. 2013;166(1):4–12. doi: 10.1016/j.ahj.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liddy C, Hogg W, Singh J, et al. A real-world stepped wedge cluster randomized trial of practice facilitation to improve cardiovascular care. Implement Sci. 2015;10:150. doi: 10.1186/s13012-015-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal T, Fathima FN, Hegde SKB, Joshi R, Srinivasan N, Misquith D. Challenges in conducting communitybased trials of primary prevention of cardiovascular diseases in resource constrained rural settings. WHO South-East Asia J Public Heal. 2015;4:98–103. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwlaat R, Schwalm JD, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013. May;34(17):1262–9. [DOI] [PubMed] [Google Scholar]
- 14.Spector PE. Social Desirability Bias. In: The SAGE Encyclopedia of Social Science Research Methods.; 2004:1045–1046. doi: 10.1002/9781444316568. [DOI] [Google Scholar]
- 15.Maimaris W, Paty J, Perel P, Legido-Quigley H, Balabanova D, Nieuwlaat R, McKee M. The influence of health systems on hypertension awareness, treatment, and control: a systematic literature review. PLoS Med. 2013;10(7):e1001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attaei MW, Khatib R, McKee M, Lear S, Dagenais G, Igumbor EU et al. Availability and affordability of blood pressure-lowering medicines and the effect on blood pressure control in high-income, middle-income, and low-income countries: an analysis of the PURE study data. Lancet Public Health. 2017. September;2(9):e411–e419. [DOI] [PubMed] [Google Scholar]
- 17.Xavier D, Gupta R, Kamath D, Sigamani A, Devereaux PJ, George N, Joshi R, Pogue J, Pais P, Yusuf S. Community health worker-based intervention for adherence to drugs and lifestyle change after acute coronary syndrome: a multicentre, open, randomised controlled trial. Lancet Diabetes Endocrinol. 2016. March;4(3):244–53. doi: 10.1016/S2213-8587(15)00480-5. [DOI] [PubMed] [Google Scholar]
- 18.Ebrahim S, Beswick A, Burke M, Davey Smith G, 2006. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev 18: CD001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian M, Ajay VS, Dunzhu D, et al. A Cluster-Randomized, Controlled Trial of a Simplified Multifaceted Management Program for Individuals at High Cardiovascular Risk (SimCard Trial) in Rural Tibet, China, and Haryana, India. Circulation. 2015;132(9):815–824. doi: 10.1161/CIRCULATIONAHA.115.015373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neupane D, McLachlan CS, Mishra SR, Olsen MH, Perry HB, Karki A, Kallestrup P. Effectiveness of a lifestyle intervention led by female community health volunteers versus usual care in blood pressure reduction (COBIN): an open-label, cluster-randomised trial. Lancet Glob Health. 2018. January;6(1):e66–e73. [DOI] [PubMed] [Google Scholar]
- 21.Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health. 2006. January 24;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris MF, Parker SM, Litt J, et al. Implementing guidelines to routinely prevent chronic vascular disease in primary care: the Preventive Evidence into Practice cluster randomised controlled trial. BMJ Open. 2015;5(12):e009397. doi: 10.1136/bmjopen-2015-009397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortsiefer A, Meysen T, Schumacher M, Abholz HH, Wegscheider K, In der Schmitten J. From hypertension control to global cardiovascular risk management: an educational intervention in a cluster-randomised controlled trial. BMC FamPract. 2015. May 7;16:56. doi: 10.1186/s12875-015-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beune EJ, Moll van Charante EP, Beem L, Mohrs J, Agyemang CO, Ogedegbe G, Haafkens JA. Culturally adapted hypertension education (CAHE) to improve blood pressure control and treatment adherence in patients of African origin with uncontrolled hypertension: cluster-randomized trial. PLoS One. 2014. March 5;9(3):e90103. doi: 10.1371/journal.pone.0090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ornstein S, Jenkins RG, Nietert PJ, et al. A multimethod quality improvement intervention to improve preventive cardiovascular care: a cluster randomized trial. Ann Intern Med. 2004;141(7):523–532. [DOI] [PubMed] [Google Scholar]
- 26.Liddy C, Hogg W, Russell G, et al. Improved delivery of cardiovascular care (IDOCC) through outreach facilitation: study protocol and implementation details of a cluster randomized controlled trial in primary care. Implement Sci. 2011;6:110. doi: 10.1186/1748-5908-6-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selby JV, Schmittdiel JA, Fireman B, et al. Improving treatment intensification to reduce cardiovascular disease risk: a cluster randomized trial. BMC Health Serv Res. 2012;12:183. doi: 10.1186/1472-6963-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vagholkar S, Zwar N, Jayasinghe UW, et al. Influence of cardiovascular absolute risk assessment on prescribing of antihypertensive and lipid-lowering medications: a cluster randomized controlled trial. Am Heart J. 2014;167(1):28–35. doi: 10.1016/j.ahj.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Jafar TH, Hatcher J, Poulter N, Islam M, Hashmi S, Qadri Z, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Annals of internal medicine. 2009;151(9):593–601 [DOI] [PubMed] [Google Scholar]
- 30.Anchala R, Pant H, Prabhakaran D, Franco OH. “Decision support system (DSS) for prevention of cardiovascular disease (CVD) among hypertensive (HTN) patients in Andhra Pradesh, India”--a cluster randomised community intervention trial. BMC Public Health. 2012;12:393. doi: 10.1186/1471-2458-12-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eakin EG, Reeves MM, Lawler SP, et al. The Logan Healthy Living Program: a cluster randomized trial of a telephone-delivered physical activity and dietary behavior intervention for primary care patients with type 2 diabetes or hypertension from a socially disadvantaged community--rationale, de. Contemp Clin Trials. 2008;29(3):439–454. doi: 10.1016/j.cct.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Kaczorowski J, Chambers LW, Karwalajtys T, et al. Cardiovascular Health Awareness Program (CHAP): a community cluster-randomised trial among elderly Canadians. Prev Med (Baltim). 2008;46(6):537–544. doi: 10.1016/j.ypmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Yan LL, Fang W, Delong E, et al. Population impact of a high cardiovascular risk management program delivered by village doctors in rural China: design and rationale of a large, cluster-randomized controlled trial. BMC Public Health. 2014;14:345. doi: 10.1186/1471-2458-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiner BJ, Pignone MP, DuBard CA, et al. Advancing heart health in North Carolina primary care: the Heart Health NOW study protocol. Implement Sci. 2015;10:160. doi: 10.1186/s13012-015-0348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb DR, Khunti K, Srinivasan B, et al. Rationale and design of the ADDITION-Leicester study, a systematic screening programme and randomised controlled trial of multi-factorial cardiovascular risk intervention in people with type 2 diabetes mellitus detected by screening. Trials. 2010;11:16. doi: 10.1186/1745-6215-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei X, Zou G, Gong W, et al. Cardiovascular disease risk reduction in rural China: a clustered randomized controlled trial in Zhejiang. Trials. 2013;14:354. doi: 10.1186/1745-6215-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills KT, Rubinstein A, Irazola V, et al. Comprehensive approach for hypertension control in low-income populations: rationale and study design for the hypertension control program in Argentina. Am J Med Sci. 2014;348(2):139–145. doi: 10.1097/MAJ.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heart Outcomes Prevention and Evaluation 4 (HOPE-4), registered at ClinicalTrials.gov Identifier: NCT01826019. Available at https://clinicaltrials.gov/ct2/show/NCT01826019, Accessed 14th February 2018
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.