Abstract
Background:
Cadmium and lead are hazardous pollutants.
Objective:
We examined the relation between serum levels of cadmium and lead and current wheeze, current asthma, and lung function in U.S. adults.
Methods:
Cross-sectional study of 13,888 adults aged 20 to 79 years old in 2007-2012 National Health and Nutrition Examination Survey (NHANES). Multivariable logistic or linear regression was used for the analyses of current wheeze, current asthma, and lung function measures (FEV1% predicted, FEV1/FVC% predicted, and fractional exhaled nitric oxide [FeNO]), which were conducted first in all participants, and then separately in never/former smokers and current smokers.
Results:
High levels of serum cadmium were significantly associated with current wheeze in all participants and in current smokers (odds ratio for fourth vs. first quartile = 2.84, 95% confidence interval = 2.07 to 3.90, P for linear trend<0.01), as well as with current asthma in current smokers. Serum lead was not significantly associated with current wheeze or current asthma, regardless of smoking status. Serum cadmium was significantly associated with lower FEV1% predicted, FEV1/FVC% predicted, and fractional exhaled nitric oxide in all participants and in never/former smokers, and serum lead was significantly associated with lower FEV1/FVC% predicted in all participants, with similar findings in never/former smokers and in current smokers.
Conclusions:
Our findings suggest that exposure to cadmium is associated with an increased risk of wheeze and asthma in U.S. adults who currently smoke. Moreover, our results suggest that exposure to cadmium or lead has negative effects on lung function in non-smoking U.S. adults.
Keywords: Serum cadmium, serum lead, asthma, lung functions
INTRODUCTION
Asthma is a major public health issue in the United States, with 8.3% of population are affected by current asthma(1). Environmental risk factors, including pollutants, have been implicated in the pathogenesis of abnormal immune responses, asthma(2-4) and atopy(3, 5, 6). Such pollutants can be present both outdoors (e.g., particulate matter(7), NO2 and SO2(8) and heavy metals(6, 9)) and indoors (e.g., dust mite and mold allergens(10), and second-hand smoke(8, 11)).
Cadmium and lead are among the most common environmental and occupational pollutants. In 2017, the Agency for Toxic Substances and Disease Registry reported that lead and cadmium ranked second and seventh among the top ten environmental hazardous substances(12). Cadmium and lead are highly toxic metals that can be derived from natural resources or as a byproduct from industries such as mining. Cadmium is also a major toxicant in tobacco smoke and thus significantly higher in current smokers than in non-smokers (13). The general population can be exposed through active or passive tobacco smoking exposure, contaminated water, soil, or food, while workers are mostly exposed by inhalation and ingestion of fumes or dusts(14).
Experimental studies have shown toxic effects of cadmium and lead on the immune system. In an in vitro study, low concentrations of cadmium led to diminished activation and proliferation of human B cells, likely through interruption of cell activation and induction of cytotoxic signals(15). Moreover, higher cadmium concentration in bronchoalveolar lavage was association with higher expression of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-6 and interleukin-8(16). In a murine model, lead exposure inhibited Th1 cells and stimulated Th2 cells, thus skewing immune responses toward Th2 predominance(17).
In this study, we hypothesized that serum cadmium and lead levels would be associated with current wheeze, current asthma, and worse lung function in U.S. adults. We further hypothesized that the estimated negative effects of serum cadmium or lead on lung function would be more pronounced among non-smokers. We examined these hypotheses in a large cross-sectional study of the U.S. adults.
METHODS
Study population
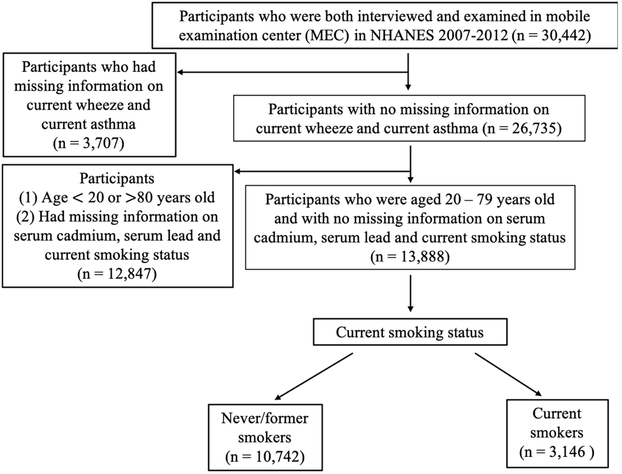
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional nationwide survey of the non-institutionalized U.S. civilian population. Study participants are selected using a stratified multistage probability design and are thus representative of the U.S population. Due to study design, NHANES over-samples persons 60 years and older and ethnic minorities (African Americans and Hispanics), to increase statistical power for data analysis in those subgroups. Figure 1 shows the flow chart for selection of study participants. Adults aged 20 to 79 years who participated in the 2007-2012 NHANES surveys and had complete data on serum cadmium, serum lead, and smoking status were included in the current analysis.
Figure 1.
Flowchart for selection of study participants included in the current analysis
NHANES was approved by the Institutional Review Board of the National Center for Health Statistics of the U.S. Centers for Disease Control and Prevention. Informed consent was obtained from all participants. Details of the methods, protocols, and definitions used in NHANES can be found at https://www.cdc.gov/nchs/nhanes/index.htm.
Study procedures
Levels of serum cadmium and lead were measured using inductively coupled plasma mass spectrometry (PerkinElmer Norwalk, CT). Spirometry was performed according to American Thoracic Society recommendations(18). Participants excluded from testing include those who had been on supplemental oxygen or who had: current chest pain or a physical problem with forceful expiration, recent surgery (eye, chest or the abdomen) or a recent heart attack or stroke, tuberculosis exposure or recent coughed with blood, a personal history of detached retina, or a collapsed lung. The best FEV1 and forced vital capacity (FVC) values were selected for data analysis. Our analyses of lung function measures were performed using percent predicted values calculated based on the Global Lung Function Initiative (GLI)(19). Fractional Exhaled nitric oxide (FeNO) was measured using the Aerocrine NIOX MINO® (Aerocrine AB, Solna, Sweden). The NHANES protocol required two valid FeNO measurements that were reproducible. Participants excluded from FeNO testing include those who had current chest pain or a physical problem with forceful expiration, or those who were using supplemental oxygen. Detailed examination procedures can be found online at (https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Examination&CycleBeginYear=2007
Current wheeze was defined by a positive answer to the question “In the past 12 months, have you had wheeze or whistling in your chest?”. Current asthma was defined by positive answers to the questions: (1) “Has a doctor or other health professional ever told you that you have asthma?” and (2) “Do you still have asthma?”. Subjects who had neither been diagnosed with asthma nor had current wheeze were selected as control subjects for the analysis of current wheeze and current asthma.
Statistical analysis
Primary sampling units and strata for the complex NHANES survey design were considered in data analysis. The R package “survey” was used to account for sampling weights, stratification, and clusters, in order to obtain proper effect estimates and their standard errors.
Chi-square tests and t tests were used for bivariate analyses of binary and continuous variables, respectively. Logistic or linear regression models were used for the multivariable analysis of serum cadmium or lead and current wheeze, current asthma, and lung function measures. All multivariable models were adjusted for age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic and others), annual household income (≥$20,000/year vs. <$20,000/year), body mass index (BMI), family history of asthma, serum cotinine level, smoking status (never/former vs. current), and occupational exposure to mineral dust or exhaust fumes. Models for lung function measures were adjusted for annual household income, BMI, serum cotinine, smoking status, occupational exposure to mineral dust or exhaust fumes (defined as the participant ever having been exposed to mineral dust or exhaust fumes at the kind of work he/she had done the longest), and use of oral or inhaled steroids in the previous two days. All analyses were first conducted in all participants, and then separately in current smokers and never/former smokers.
Our primary analysis of was conducted using quartiles of serum cadmium or lead level. Because of consistently significant linear trends, serum cadmium or lead level was also considered as continuous in the analysis of lung function measures. A two-tailed P value of less than 0.05 was considered statistically significant. R program (Version 3.4.2) was used for all analyses.
Results
The main characteristics of the 13,888 study participants are shown in Table 1. Compared with the 11,767 control subjects, the 2,121 subjects with current wheeze were more likely to be: older, female, non-Hispanic white, current smokers, and users of oral or inhaled steroids in the previous two days; and to have: a household income less than $20,000, a family history of asthma, vitamin D insufficiency, occupational exposure to mineral dusts or exhaust fumes, a higher BMI, a higher serum cotinine level, higher levels of serum cadmium and lead, a higher FeNO, and lower FEV1, FVC and FEV1/FVC. Table E1 (see Table E1 in the Online Repository) shows a comparison of the characteristics of the adults with current asthma (n = 1,217) with those of the control subjects (n = 11,767), which yielded similar results to that of adults with current wheeze and control subjects (see above), except that there were no significant differences in age, vitamin D insufficiency, or occupational exposure to mineral dusts or exhaust fumes and there was significant difference in health insurance coverage between adults with current asthma and control subjects.
Table 1.
Main characteristics of study participants, by current wheeze1
| Characteristics | Control subjects2 (n = 11,767) |
Current wheeze3 (n = 2,121) |
|---|---|---|
| Age, years | 45.7 ± 0.4 | 46.6 ± 0.4 * |
| Female gender | 5912 (50.4) | 1135 (54.3) ** |
| Race | ||
| Non-Hispanic White | 4833 (66.9) | 1056 (70.8) ** |
| Mexican American | 2106 (9.4) | 213 (5.1) |
| Other Hispanic | 1312 (5.8) | 195 (4.7) |
| Non-Hispanic Black | 2414 (10.6) | 519 (13.1) |
| Other | 1102 (7.3) | 138 (6.2) |
| Had health insurance coverage | 8662 (79.1) | 1600 (79.0) |
| Annual household income ≥ $20,000 | 8920 (86.5) | 1380 (77.8) ** |
| Smoking status | ||
| Never/former smoker | 9481 (81.5) | 1261 (59.0) ** |
| Current smoker | 2286 (18.5) | 860 (41.0) |
| Body mass index (BMI) | 28.4 ± 0.1 | 30.5 ± 0.2 ** |
| Serum cotinine level, ng/mL | 50.9 ± 2.6 | 112.4 ± 5.1 ** |
| Family history of asthma | 1796 (15.6) | 738 (34.3) ** |
| Vitamin D insufficiency (<30 ng/mL) | 7887 (62.2) | 1468 (67.6) ** |
| Use oral or inhaled steroid in past 2 days | 121 (1.3) | 339 (19.9) ** |
| Occupation exposure to mineral dusts or exhaust fumes | 4233 (36.7) | 1081 (50.0) ** |
| Serum level of heavy metals | ||
| Cadmium, μg/L | 0.47 ± 0.01 | 0.82 ± 0.04 ** |
| Lead, μg/L | 1.54 ± 0.04 | 1.65 ± 0.05 * |
| Lung function | ||
| Percent predicted FEV1 | 98.8 ± 0.3 | 88.5 ± 0.6 ** |
| Percent predicted FVC | 102.1 ± 0.2 | 96.7 ± 0.5 ** |
| Percent predicted FEV1/FVC | 96.5 ± 0.1 | 90.8 ± 0.4 ** |
| Fractional exhaled nitric oxide (FeNTO), ppb | 16.7 ± 0.3 | 21.1 ± 0.8 ** |
Values are presented as number (%) or mean (mean ± standard deviation)
Control subjects had neither current wheeze nor an asthma diagnosis
Wheeze or whistling in the chest in the previous 12 months.
* P< 0.05 and ** P< 0.01 for the comparison of subjects with current wheeze with control subjects.
Serum cotinine was significantly but weakly correlated with serum cadmium among never or former smokers (weighted Pearson correlation coefficient=0.11, P <0.01). Among current smokers, serum cotinine was more strongly correlated with serum cadmium, but the correlation was below 0.50 (weighted Pearson coefficient=0.35, P<0.01). Table 2 shows the results of multivariable analyses of serum cadmium and current wheeze. In the analysis of all participants, subjects whose serum cadmium was in the highest (fourth) quartile had 1.5 times higher odds of current wheeze than those whose serum cadmium level was in the lowest (first) quartile (95% confidence interval [CI] for the odds ratio [OR] = 1.20 to 1.86, P for linear trend <0.01). After stratification by smoking status, there was no significant association between serum cadmium and current wheeze among never or former smokers. Among current smokers, subjects whose serum cadmium level was above the first quartile had 1.91 to 2.84 times significantly higher odds of current wheeze than those whose serum cadmium level was in the first (lowest) quartile (P for linear trend <0.01).
Table 2.
Serum cadmium and current wheeze in study participants, NHANES 2007-20121
| Serum cadmium (μg/L) |
|
|---|---|
| Exposure | Odds ratio (95% confidence interval) |
| All participants (n = 13,888) | |
| Quartile 1 (<0.22 μg/L) | 1.00 |
| Quartile 2 (0.22 – 0.34 μg/L) | 0.92 (0.75, 1.13) |
| Quartile 3 (0.34 – 0.62 μg/L) | 1.01 (0.82, 1.25) |
| Quartile 4 (≥0.62 μg/L) | 1.50 (1.20, 1.86) ** P for linear trend < 0.01 |
| Never/former smokers (n = 10,742) | |
| Quartile 1 (<0.19 μg/L) | 1.0 |
| Quartile 2 (0.19 – 0.28 μg/L) | 0.83 (0.63, 1.08) |
| Quartile 3 (0.28 – 0.43 μg/L) | 1.10 (0.88, 1.37) |
| Quartile 4 (≥0.43 μg/L) | 1.19 (0.92, 1.54) P for linear trend = 0.08 |
| Current smokers (n = 3,146) | |
| Quartile 1 (<0.61 μg/L) | 1.0 |
| Quartile 2 (0.61 – 0.99 μg/L) | 1.91 (1.39, 2.63) ** |
| Quartile 3 (0.99 – 1.50 μg/L) | 2.40 (1.70, 3.39) ** |
| Quartile 4 (≥1.50 μg/L) | 2.84 (2.07, 3.90) ** P for linear trend < 0.01 |
All models were adjusted for age, gender, race/ethnicity, annual household income, BMI, family history of asthma, serum cotinine, and occupational exposure to mineral dusts or exhaust fumes. The model for all participants was additionally adjusted for smoking status.
* P< 0.05 and ** P< 0.01
Serum cotinine was significantly but weakly correlated with serum lead in either never/former smokers (weighted Pearson correlation coefficient=0.07, P<0.01) or in current smokers (weighted Pearson correlation coefficient=0.10, P<0.01). Table 3 shows the results of the multivariable analysis of serum lead and current wheeze. In this analysis, serum lead was not significantly associated with current wheeze, either in all participants or in current smokers. We found no significant modification of the estimated effect of serum cadmium or serum lead on current wheeze by FeNO.
Table 3.
Serum lead and current wheeze in study participants1
| Serum lead (μg/L) |
|
|---|---|
| Exposure | Odds ratio (95% confidence interval) |
| All participants (n = 13,888) | |
| Quartile 1 (<0.83 μg/L) | 1.00 |
| Quartile 2 (0.83 – 1.27 μg/L) | 0.98 (0.81, 1.19) |
| Quartile 3 (1.27 – 2.00 μg/L) | 0.96 (0.78, 1.18) |
| Quartile 4 (≥2.00 μg/L) | 1.15 (0.91, 1.45) P for linear trend = 0.34 |
| Never/former smokers (n = 10,742) | |
| Quartile 1 (<0.78 μg/L) | 1.0 |
| Quartile 2 (0.78 – 1.20 μg/L) | 1.00 (0.81, 1.24) |
| Quartile 3 (1.20 – 1.83 μg/L) | 0.95 (0.76, 1.19) |
| Quartile 4 (≥1.83 μg/L) | 1.10 (0.82, 1.46) P for linear trend = 0.63 |
| Current smokers (n = 3,146) | |
| Quartile 1 (1.04 μg/L) | 1.0 |
| Quartile 2 (1.04 – 1.60 μg/L) | 0.94 (0.67, 1.31) |
| Quartile 3 (1.60 – 2.48 μg/L) | 0.91 (0.59, 1.41) |
| Quartile 4 (≥ 2.48 μg/L) | 1.24 (0.78, 1.96) P for linear trend = 0.43 |
All models were adjusted for age, gender, race/ethnicity, annual household income, BMI, family history of asthma, serum cotinine, and occupational exposure to mineral dust or exhaust fumes. The model for all participants was additionally adjusted for smoking status.
* P< 0.05 and ** P< 0.01
Table E2 and Table E3 in the Online Repository show the results of the multivariable analysis of serum cadmium or serum lead and current asthma. In this analysis, serum cadmium was not significantly associated in all participants or in never/former smokers. Among current smokers, subjects whose serum cadmium was above the second quartile had 1.86 to 3.09 significantly higher odds of current asthma than those whose serum cadmium was in the first quartile (P for linear trend <0.01, Table E2). Serum lead was not significantly associated with current asthma, either in all participants or in current smokers (Table E3). We found no significant modification of the estimated effect of serum cadmium on current asthma by FeNO among all participants.
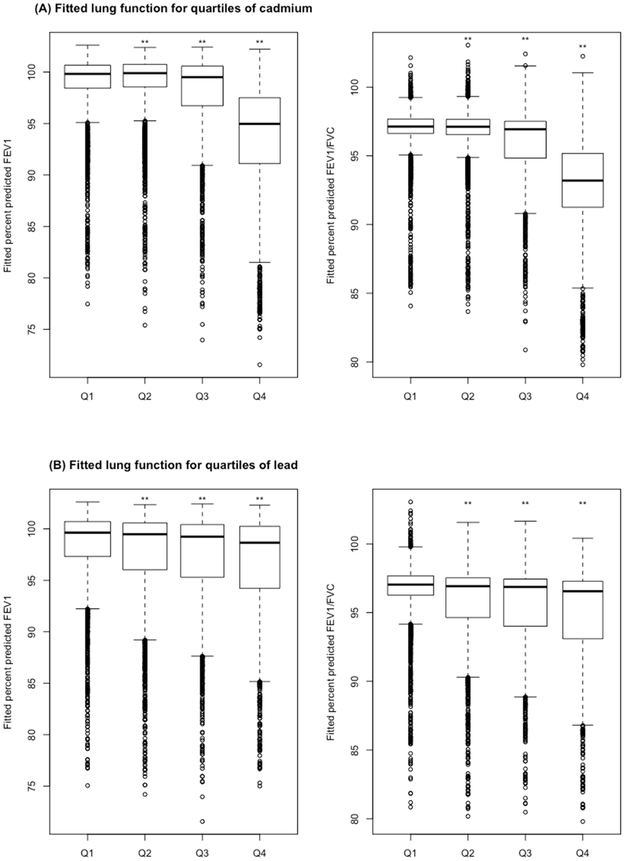
Figure 2 shows the results of the multivariable analysis of serum cadmium or serum lead (as quartiles) and percent predicted FEV1 or percent predicted FEV1/FVC. In this analysis, percent predicted FEV1 and percent predicted FEV1/FVC were significantly lower in subjects whose serum cadmium or serum lead was above the first (lowest) quartile than in subjects whose serum cadmium or serum lead level was in the first quartile (P for linear trend <0.01 in all instances).
Figure 2.
((A) and (B)) Boxplots of lung function measures for quartiles of serum cadmium and quartiles of serum lead in all adult participants. (A) Fitted lung function measures for quartiles of serum cadmium, (B) Fitted lung function measures for quartiles of serum lead. All models were adjusted for annual household income, body mass index, current wheeze status, serum cotinine, occupational exposure to mineral dust or exhaust fumes, use of oral or inhaled steroid in the past 2 days, and smoking status. Q1, the first quartile (lowest); Q2, the second quartile; Q3, the third quartile; Q4, the fourth quartile (highest). The widths of the boxes are proportional to the sample size in every quartile. * P< 0.05 and ** P< 0.01 for comparison with the first quartile (Q1).
Table 4 shows the results of the multivariable analysis of serum cadmium or serum lead and lung function measures, before and after stratification by smoking status. Among all participants, each μg/L increment in serum cadmium was significantly associated with decrements in percent predicted FEV1, percent predicted FEV1/FVC, and FeNO. After stratification by smoking status, serum cadmium was significantly associated with decrements in percent predicted in FEV1 and FEV1/FVC in both never/former smokers and current smokers, but the magnitude of the observed associations was greater in never/former smokers than in current smokers. Moreover, serum cadmium was significantly associated with decreased FeNO in never/former smokers but not in current smokers. Serum lead was significantly associated with decrements in percent predicted FEV1/FVC in all participants, with similar results in never/former smokers and current smokers. Moreover, serum lead was significantly associated with increments in percent predicted FVC in all participants and in current smokers. We found no significant association between serum lead and FEV1 or FeNO, before or after stratification by smoking status.
Table 4.
Serum cadmium or lead and lung function measures in study participants1
| Serum cadmium (μg/L) | Serum lead (μg/L) | |
|---|---|---|
| Measures | β(95% confidence interval) | |
| All participants (N = 13,888) | ||
| Percent predicted FEV1 | −2.20 (−3.51, −0.88) ** | −0.21 (−0.45, 0.03) |
| Percent predicted FVC | −0.67 (−1.54, 0.20) | 0.19 (0.02, 0.35)* |
| Percent predicted FEV1/FVC | −1.68 (−2.36, −1.00) ** | −0.37 (−0.59, −0.14) ** |
| FeNO2, ppb | −0.95 (−1.46, −0.44) ** | −0.07 (−0.21, 0.07) |
| Never/former smokers (N = 10,742) | ||
| Percent predicted FEV1 | −4.51 (−6.78, −2.25) ** | −0.26 (−0.55, 0.03) |
| Percent predicted FVC | −0.82 (−2.53, 0.89) | 0.13 (−0.09, 0.36) |
| Percent predicted FEV1/FVC | −4.04 (−5.16, −2.91) ** | −0.35 (−0.61, −0.10) ** |
| FeNO2, ppb | −2.66 (−4.21, −1.11) ** | −0.07 (−0.25, 0.12) |
| Current smokers (N = 3,146) | ||
| Percent predicted FEV1 | −1.47 (−2.81, −0.13) * | −0.10 (−0.43, 0.22) |
| Percent predicted FVC | −0.49 (−1.45, 0.47) | 0.29 (0.06, 0.51) * |
| Percent predicted FEV1/FVC | −1.12 (−1.81, −0.42) ** | −0.38 (−0.63, −0.12) ** |
| FeNO2, ppb | −0.24 (−0.62, 0.14) | −0.07 (−0.21, 0.06) |
All models adjusted for annual household income, BMI, serum cotinine, occupational exposure to mineral dust or exhaust fumes, use of oral or inhaled steroid in the past 2 days, current wheeze status. The model for all participants was additionally adjusted for smoking status.
In additional adjusted for age, gender, race/ethnicity
* P< 0.05 and ** P< 0.01
We conducted a secondary analysis to examine potential joint effects of elevated serum levels of cadmium and lead on current wheeze or lung function measures (Figures E1 and E2, see Figures E1 and E2 in the Online Repository). Compared to subjects with low serum cadmium and low serum lead levels, those who had both a high serum cadmium level and a high serum lead level had 1.8 times significantly higher odds of current wheeze, and those who had a high serum cadmium level but a low lead level had 1.5 times significantly higher odds of current wheeze. In contrast, subjects who had a high lead level but a low cadmium level had no significantly higher odds of current wheeze than those who had low levels of both serum cadmium and serum lead (Figure E1). Similar results were obtained for the analysis of serum cadmium and serum lead and percent predicted FEV1 or percent predicted FEV1/FVC (Figure E2).
DISCUSSION
In a representative sample of 13,888 adults in the U.S., a high level of serum cadmium was significantly associated with current wheeze and current asthma in current smokers. Serum cadmium was also significantly associated with decreased FEV1% predicted, FEV1/FVC% predicted, and FeNO in all participants, and this inverse association was more pronounced in never or former smokers than in current smokers. Moreover, serum lead was significantly associated with decreased FEV1/FVC% predicted in all participants, with similar findings in never/former smokers and in current smokers.
Two studies of children in Egypt (20) and the U.S.(21), limited by small sample size(20) or lead exposure assessment(21), reported no association(20) or a borderline significant association(21) between serum lead(20) or self-reported exposure(21) and asthma. A case-control study of 1,102 adults 18 to 78 years old who attended outpatient clinics in a hospital in Wuhan (China) reported that urinary cadmium was significantly associated with increased odds of asthma and lower FEV1 and FEV1/FVC, but that urinary lead was significantly associated with reduced odds of asthma and increased FEV1/FVC(4). In a population-based study of 5,912 Korean adults, subjects who had a serum cadmium level in the highest quintile (range = 1.60–6.42 μg/L, similar to the range of the highest quartile for current smokers but higher than that in all participants in our study), had 1.55 times significantly higher odds of lifetime physician-diagnosed asthma than those whose serum cadmium was in the lowest quintile (95% CI for OR = 1.03–2.33, P for linear trend = 0.02), with similar findings for serum lead and lifetime asthma (OR for highest quintile [range = 3.01–17.71 μg/L, higher than the range of the fourth quartile in our study participants] vs. lowest quintile = 1.67, 95% CI = 1.10–2.55, P for linear trend = 0.02)(5). In that study, a high serum lead level (but not a high serum cadmium level) was significantly associated with total serum IgE, suggesting that serum cadmium may affect asthma pathogenesis through non-allergic mechanisms. Limitations of prior studies in East Asian adults include selection bias(4), residual confounding by tobacco use(4, 5), and misclassification of chronic obstructive pulmonary disease (COPD) as asthma(4, 5).
A previous study of 9,575 adults in NHANES 2007-2010 reported that increasing concentrations of serum cadmium and serum lead were each associated with significantly increased odds of obstructive lung disease (OLD, defined as an FEV1/FVC <0.70) and worsening severity of OLD in current smokers, but not in former or never smokers(22). In that study, serum cadmium and serum lead were significantly associated with reduced FEV1% predicted in current smokers, but only serum lead was significantly associated with reduced FEV1% predicted in never or former smokers. In contrast to those results in U.S. adults, a study of 1,974 Korean men (40 years and older) showed that serum cadmium was significantly associated with OLD (defined as an FEV1/FVC <0.70), in both never/former smokers and in current smokers(23). However, those findings were not confirmed in a subsequent larger study of 5,972 Korean men and women (20 years and older), which showed that serum cadmium was significantly associated with OLD in current smokers but not in never or former smokers(24). In that study, serum lead was not significantly associated with OLD, regardless of smoking status. Moreover, serum cadmium was significantly associated with lower FEV1/FVC in current smokers but not in never smokers, while serum lead was significantly associated with lower FEV1/FVC in never smokers but not in current smokers(24).
While serum cadmium is correlated with recent exposure, urine cadmium level generally reflects longer duration of exposure. However, serum cadmium has been shown to have overlap (25) and significant correlation (rs = 0.5, P < 0.01)(26) with urinary cadmium. In a study of 96 men followed for up to 8 years, a single 24-hour urinary cadmium level at baseline was associated with lower FEV1 and FEV1/FVC, with stronger estimated negative effects of urinary cadmium on FEV1/FVC in current smokers than in never smokers (27). Similar findings were obtained in a study of 16,024 adults, which found a significant association between urinary cadmium and lower FEV1 and FEV1/FVC in current and former smokers but not in never smokers (28). In another study of U.S. adults, urinary cadmium was correlated with smoking status and pack-years of smoking (29). In that study, the estimated effects of former and current smoking on OLD were reduced by ~23%-43% after adjustment for urinary cadmium, suggesting that cadmium exposure has independent effects on OLD or that urinary cadmium is a better marker of cumulative smoking exposure than self-reported smoking status.
Our results in 13,888 U.S. adults expand or differ from previous studies of OLD or lung function (22-24, 26-28) in that we demonstrate that serum cadmium is significantly associated with lower FEV1% predicted, lower FEV1/FVC% predicted, and lower FeNO in never or former smokers, and that serum lead is significantly associated with lower FEV1/FVC% predicted in never or former smokers. Our findings thus suggest that exposure to lower levels of heavy metals (particularly cadmium) may have detrimental (subclinical) effects on lung function in non-smokers. The observed association between serum cadmium and current wheeze or current asthma among current smokers in our study may be explained by residual confounding by longterm effects of smoking (not captured by serum cotinine), synergistic effects of serum cadmium and current smoking, or misclassification of COPD (or other respiratory disorders causing dyspnea, including asthma/COPD overlap syndrome) as current wheeze or current asthma in current smokers.
Our finding of an inverse association between serum cadmium and FeNO in never or former smokers is partially consistent with the results of a previous analysis in 7,813 adult participants in NHANES 2007-2010(30), which showed that serum cadmium was significantly associated with lower FeNO, regardless of smoking status. Since FeNO is a marker of eosinophilic airway inflammation(31), our results suggest that cadmium exposure may affect lung function, current wheeze, and current asthma through non-eosinophilic inflammatory mechanisms, including down-regulation of nitric oxide (NO) synthase and increased oxidative stress(32-35).
Although we found no independent association between serum lead and current wheeze or current asthma, subjects with high serum levels of both cadmium and lead had slightly higher odds of current wheeze and slightly stronger estimated negative effects on lung function than those with high serum cadmium but a low serum lead. Experimental and observational human studies have shown that co-exposure to cadmium and lead is linked to metabolic syndrome(36) and kidney disease(37), but this is the first report of potential joint effects of cadmium and lead on current wheeze and lung function.
We acknowledge additional study limitations. We cannot determine temporal relationships between levels of serum cadmium or lead and wheeze, asthma, or lung function in this crosssectional study. Moreover, we lack data on potential confounders or modifiers of the relation between cadmium or lead exposure and the outcomes of interest, such as air pollutants such as particulate matter and diesel exhaust.
In summary, our results suggest that U.S. adults who are current smokers have a higher risk of wheeze and asthma after exposure to high levels of cadmium. Our findings also suggest that exposure to cadmium or lead has negative effects on lung function in non-smoking adults in the U.S.
Supplementary Material
Highlights box.
What is already known about this topic? Cadmium and lead are hazardous heavy metals that are linked to negative effects on respiratory health.
What does this article add to our knowledge? This study shows that serum cadmium is associated with current wheeze and current asthma in current smokers, as well as with lower lung function measures in never or former smokers. Serum lead was associated with lower FEV1/FVC, regardless of smoking status.
How does this study impact current management guidelines? Exposure to relatively low levels of heavy metals (particularly cadmium) may negatively affect lung function in non-smoking U.S. adults.
Acknowledgments
G.Y., T.S., W.C. and J.C.C. participated in study design, data analysis, manuscript writing, and interpretation of the study results. Y-Y.H., F.R. and E.F. participated in data analysis. All authors reviewed and approved the final version of the submitted manuscript. J.C.C. is the guarantor of this work and takes responsibility for the integrity of the manuscript.
Funding
Dr. Celedon’s contribution was supported by grants HL117191, HL119952 and MD011764 from the U.S. National Institutes of Health (NIH). Dr. Forno’s contribution was supported by grant HD052892 from the U.S. NIH. Dr. Yang’s contribution was supported by the China Scholarship Council and the Third Xiangya Hospital, Central South University.
Abbreviation list:
- BMI
body mass index
- CI
confidence interval
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
Dr. Celedón has received research materials from Merck and GSK (inhaled steroids), and Pharmavite (vitamin D and placebo capsules), to provide medications free of cost to participants in NIH-funded studies, unrelated to the current work. The other authors report no conflicts of interest.
References
- 1.National Current Asthma * Prevalence (2016): Centers for Disease Control and Prevention; 2016 [updated May 2018]. Available from: https://www.cdc.gov/asthma/most_recent_data.htm.
- 2.Yang S-N, Hsieh C-C, Kuo H-F, Lee M-S, Huang M-Y, Kuo C-H, et al. The Effects of Environmental Toxins on Allergic Inflammation. Allergy, Asthma & Immunology Research. 2014. October/15 03/27/received 04/16/accepted;6(6):478–84. PubMed PMID: PMC4214967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min KB, Min JY. Environmental lead exposure and increased risk for total and allergen-specific IgE in US adults. The Journal of allergy and clinical immunology. 2015. January;135(1):275–7. PubMed PMID: 25441290. Epub 2014/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Xie J, Cui X, Zhou Y, Wu X, Lu W, et al. Association between Concentrations of Metals in Urine and Adult Asthma: A Case-Control Study in Wuhan, China. PLOS ONE. 2016;11(5):e0155818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S, Lee E-H, Kho Y. The association of asthma, total IgE, and blood lead and cadmium levels. Journal of Allergy and Clinical Immunology. 2016. 2016/December/01/;138(6):1701–3.e6. [DOI] [PubMed] [Google Scholar]
- 6.Shiue I Association of urinary arsenic, heavy metal, and phthalate concentrations with food allergy in adults: National Health and Nutrition Examination Survey, 2005–2006. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013. November;111(5):421–3. PubMed PMID: 24125153. Epub 2013/10/16. eng. [DOI] [PubMed] [Google Scholar]
- 7.Chen BY, Chan CC, Lee CT, Cheng TJ, Huang WC, Jhou JC, et al. The association of ambient air pollution with airway inflammation in schoolchildren. American journal of epidemiology. 2012. April 15;175(8):764–74. PubMed PMID: 22408045. Epub 2012/03/13. eng. [DOI] [PubMed] [Google Scholar]
- 8.Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How Exposure to Environmental Tobacco Smoke, Outdoor Air Pollutants, and Increased Pollen Burdens Influences the Incidence of Asthma. Environmental Health Perspectives. 2006. January/26 06/03/received 01/26/accepted;114(4):627–33. PubMed PMID: PMC1440792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, Xu X, Zheng X, Reponen T, Chen A, Huo X. Heavy metals in PM2.5 and in blood, and children's respiratory symptoms and asthma from an e-waste recycling area. Environmental Pollution. 2016. 2016/March/01/;210:346–53. [DOI] [PubMed] [Google Scholar]
- 10.Shiue I Indoor mildew odour in old housing was associated with adult allergic symptoms, asthma, chronic bronchitis, vision, sleep and self-rated health: USA NHANES, 2005–2006. Environmental science and pollution research international. 2015. September;22(18):14234–40. PubMed PMID: 25971810. Epub 2015/05/15. eng. [DOI] [PubMed] [Google Scholar]
- 11.Lajunen TK, Jaakkola JJ, Jaakkola MS. The synergistic effect of heredity and exposure to second-hand smoke on adult-onset asthma. American journal of respiratory and critical care medicine. 2013. October 1;188(7):776–82. PubMed PMID: 23981189. Epub 2013/08/29. eng. [DOI] [PubMed] [Google Scholar]
- 12.ATSDR’s Substance Priority List: Agency for Toxic Substances and Disease Registry 2017. Available from: https://www.atsdr.cdc.gov/spl/.
- 13.Grasseschi RM, Ramaswamy RB, Levine DJ, Klaassen CD, Wesselius LJ. Cadmium Accumulation and Detoxification by Alveolar Macrophages of Cigarette Smokers*. Chest. 2003. 2003/November/01/;124(5):1924–8. [DOI] [PubMed] [Google Scholar]
- 14.Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. International Agency for Research on Cancer, World Health Organization, IARC Working Group on the Evaluation of Carcinogenic Risks to Humans., 1993. [PMC free article] [PubMed] [Google Scholar]
- 15.Jelovcan S, Gutschi A, Kleinhappl B, Sedlmayr P, Barth S, Marth E. Effects of low concentrations of cadmium on immunoglobulin E production by human B lymphocytes in vitro. Toxicology. 2003. 2003/June/03/;188(1):35–48. [DOI] [PubMed] [Google Scholar]
- 16.Sundblad BM, Ji J, Levanen B, Midander K, Julander A, Larsson K, et al. Extracellular cadmium in the bronchoalveolar space of long-term tobacco smokers with and without COPD and its association with inflammation. International journal of chronic obstructive pulmonary disease. 2016;11:1005–13. PubMed PMID: 27274222. Pubmed Central PMCID: PMC4869628. Epub 2016/06/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo Y, Lee WT, Lawrence DA. Differential Effects of Lead and cAMP on Development and Activities of Th1- and Th2-Lymphocytes. Toxicological Sciences. 1998. 1998/June/01/;43(2):172–85. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26(2):319. [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. MULTI-ETHNIC REFERENCE VALUES FOR SPIROMETRY FOR THE 3–95 YEAR AGE RANGE: THE GLOBAL LUNG FUNCTION 2012 EQUATIONS: Report of the Global Lung Function Initiative (GLI), ERS Task Force to establish improved Lung Function Reference Values. The European respiratory journal. 2012. June/27;40(6):1324–43. PubMed PMID: PMC3786581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed AA, Mohamed FY, El-Okda el S, Ahmed AB. Blood lead levels and childhood asthma. Indian pediatrics. 2015. April;52(4):303–6. PubMed PMID: 25929627. Epub 2015/05/02. eng. [DOI] [PubMed] [Google Scholar]
- 21.Motosue AM, Petronella S, Sullivan J, Castillo S, Garcia T, Murillo M, et al. Lead Exposure Risk is Associated with Asthma in a Low-income Urban Hispanic Population: Results of the Communities Organized against Asthma and Lead (COAL) Project. Journal of Allergy and Clinical Immunology. 2009;123(2):S20. [Google Scholar]
- 22.Rokadia HK, Agarwal S. Serum Heavy Metals and Obstructive Lung Disease: Results From the National Health and Nutrition Examination Survey. Chest. 2013. 2013/February/01/;143(2):388–97. [DOI] [PubMed] [Google Scholar]
- 23.Yoon J-H, Kim I, Kim H-R, Won J-U, Bae K-J, Jung P-K, et al. The association between blood cadmium level and airflow obstruction in Korean men. Annals of Human Biology. 2015. 2015/November/02;42(6):569–75. [DOI] [PubMed] [Google Scholar]
- 24.Leem AY, Kim SK, Chang J, Kang YA, Kim YS, Park MS, et al. Relationship between blood levels of heavy metals and lung function based on the Korean National Health and Nutrition Examination Survey IV-V. International journal of chronic obstructive pulmonary disease. 2015;10:1559–70. PubMed PMID: 26345298. Pubmed Central PMCID: PMC4531039. Epub 2015/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams SV, Newcomb PA. Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. Journal Of Exposure Science And Environmental Epidemiology. 2013. September/04/online;24:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birgisdottir BE, Knutsen HK, Haugen M, Gjelstad IM, Jenssen MT, Ellingsen DG, et al. Essential and toxic element concentrations in blood and urine and their associations with diet: results from a Norwegian population study including high-consumers of seafood and game. The Science of the total environment. 2013. October 1;463–464:836–44. PubMed PMID: 23867847. Epub 2013/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 27.Lampe BJ, Park SK, Robins T, Mukherjee B, Litonjua AA, Amarasiriwardena C, et al. Association between 24-hour urinary cadmium and pulmonary function among community-exposed men: the VA Normative Aging Study. Environmental health perspectives. 2008;116(9):1226–30. PubMed PMID: 18795167. Epub 2008/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannino DM, Holguin F, Greves HM, Savage-Brown A, Stock AL, Jones RL. Urinary cadmium levels predict lower lung function in current and former smokers: data from the Third National Health and Nutrition Examination Survey. 2004;59(3):194–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y-S, Caffrey JL, Chang M-H, Dowling N, Lin J-W. Cigarette smoking, cadmium exposure, and zinc intake on obstructive lung disorder. Respiratory research. 2010;11(1):53. PubMed PMID: 20459696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min J-y, Min K-b. Cadmium, smoking, and reduced levels of exhaled nitric oxide among US adults. International Journal of Hygiene and Environmental Health. 2014. 2014/March/01/;217(2):323–7. [DOI] [PubMed] [Google Scholar]
- 31.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. American journal of respiratory and critical care medicine. 2011. September 1;184(5):602–15. PubMed PMID: 21885636. Pubmed Central PMCID: PMC4408724. Epub 2011/09/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majumder S, Gupta R, Reddy H, Sinha S, Muley A, Kolluru GK, et al. Cadmium attenuates bradykinin-driven nitric oxide production by interplaying with the localization pattern of endothelial nitric oxide synthase. Biochemistry and Cell Biology. 2009. 2009/August/01;87(4):605–20. [DOI] [PubMed] [Google Scholar]
- 33.RodrÍGuez-Serrano M, Romero-Puertas MC, Zabalza ANA, Corpas FJ, GÓMez M, Del RÍo LA, et al. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant, Cell & Environment. 2006. 2006/August/01;29(8):1532–44. [DOI] [PubMed] [Google Scholar]
- 34.Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. American journal of respiratory and critical care medicine. 1994. 1994/February/01;149(2):538–51. [DOI] [PubMed] [Google Scholar]
- 35.Ricciardolo FLM, Sterk PJ, Gaston B, Folkerts G. Nitric Oxide in Health and Disease of the Respiratory System. Physiological Reviews. 2004. 2004/July/01;84(3):731–65. [DOI] [PubMed] [Google Scholar]
- 36.Moon S-S. Additive effect of heavy metals on metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocrine. 2014. 2014/June/01;46(2):263–71. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Wang H, Li J, Chen D, Liu Z. Simultaneous Effects of Lead and Cadmium on Primary Cultures of Rat Proximal Tubular Cells: Interaction of Apoptosis and Oxidative Stress. Archives of Environmental Contamination and Toxicology. 2011. 2011/October/01;61(3):500–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.