Abstract
Childhood attention-deficit/hyperactivity disorder (ADHD) is prospectively linked to substance use and disorder. Depression emerging in adolescence is an understudied risk factor that may explain some of this risk. In the present study, we considered mediating and moderating roles of adolescent depression in explaining this association by using longitudinal data from the prospective 16-year follow-up of the Multimodal Treatment Study of ADHD (MTA). Participants were 547 children diagnosed with DSM-IV ADHD Combined Type, and 258 age- and sex-matched comparison children. In adolescence, depressive symptoms did not exacerbate effects of childhood ADHD on any substance use. For both groups, time-varying and average depressive symptoms were associated with more frequent use of all substances. Prospectively, we found no evidence of depression mediation to adult substance use. However, adolescent depression moderated the association between childhood ADHD and adult marijuana use. Although adults without ADHD histories used marijuana more frequently if they had elevated depressive symptoms in adolescence, marijuana use by adults with ADHD histories was independent of their adolescent depression. In adulthood, depression diagnoses and ADHD persistence continued to operate as independent, additive correlates of substance use risk. Our findings suggest a circumscribed role for depression in substance use risk that adds to, but does not alter or explain, ADHD-related risk.
Keywords: ADHD, depression, substance use, adolescence, early adulthood, longitudinal
Childhood attention-deficit/hyperactivity disorder (ADHD) is prospectively linked to early substance use (King et al. 2004), elevated substance use in adolescence (Molina & Pelham, 2003; Sibley et al., 2014) and adulthood (Molina et al., 2018), and increased risk for substance use disorders by early adulthood (e.g., Breyer et al., 2014). Depression in adolescence is a potentially important factor in explaining the link between childhood ADHD and later substance use. For example, social and emotion regulation deficits typical of ADHD place these children at elevated risk of experiencing depression (e.g., Humphreys et al., 2013). In a recent meta-analysis, childhood ADHD and depression each equally predicted the later development of a substance use disorder (Groenman et al., 2017). What remains unclear is whether adolescent depression plays a role in the developmental course of substance use in children with ADHD. In the present study, we examine two possibilities: (1) that adolescent depression mediates ADHD-related risk for substance use in adulthood by emerging more often in those with ADHD and leading to more frequent later substance use; and (2) that adolescent depression moderates ADHD-related substance use risk, where effects of childhood ADHD depend on adolescent depressive symptom levels.
Depression and ADHD-related risk for substance use
Large-scale epidemiologic studies show that substance use and depression frequently cooccur (e.g., Henry et al., 1993; Kandel et al. 1997), but the evolution of their association across development is less clear. Substance use is strongly linked to externalizing behaviors that predate substance use and correlate with depression, notably ADHD, conduct disorder (CD), and oppositional-defiant disorder (ODD; King et al., 2004). As a result, the depression-substance use association is often subsumed by an externalizing-substance use association. A recent systematic review of longitudinal studies highlights the problem: after adjusting for externalizing symptoms, depression positively predicted substance use later in adolescence in only 32% of tests, with null associations in virtually all others (Hussong et al., 2017). Studies that disentangle the unique contributions of adolescent depression to ADHD-related substance use vulnerability are needed, but depression, ADHD, and substance use are only rarely studied in concert (Truter et al., 2017).
Cross-sectional research in adolescents diagnosed with ADHD and SUD receiving treatment for substance abuse showed that rates of non-nicotine substance use were highest in those with a comorbid diagnosis of major depressive disorder (Warden et al., 2012). Pooled data from individuals receiving substance use treatment also showed that diagnoses of ADHD and depression (both classified from symptom self-reports) were among the most common problems co-occurring with substance dependence in emerging adults (Chan et al., 2008). However, these studies both focused on clinical cases receiving SUD treatment, and the latter did not compare substance dependence rates when ADHD was versus was not comorbid with depression. One recent longitudinal study showed that co-occurring increases in depressive symptoms and alcohol use were stronger in young adults with childhood ADHD versus a non-ADHD comparison group (Wang et al., 2019), but this association was only tested in adulthood.
Young people traveling an internalizing pathway to substance use disorder (SUD) are expected to develop depressive and anxious symptoms followed by tension-reduction expectancies for substance use (Hussong et al., 2011). Perceived substance-related benefits such as mood enhancement develop gradually with accumulated substance experience (Katz, Fromme, & D’Amico, 2000). Depressive symptoms or depression comorbidity assessed in adulthood thus offer few insights into the role of depression as a developmental mechanism that contributes to substance use vulnerability. Tests of prospective effects are needed that link adolescent depression—as a mediator or moderator of ADHD-related risk—to later substance use, before patterns of use and substance-related expectancies are well-established.
Mechanisms linking depression and ADHD to substance use across development.
A key reason to suspect that adolescent depression may be involved in explaining the association between childhood ADHD and later substance use is the robust body of evidence showing that rates of depression are frequently higher for children and adolescents diagnosed with ADHD compared to typically developing children (Meinzer et al., 2014). Findings from longitudinal studies with large samples (Molina et al., 2009), annual follow-up assessments through adolescence or emerging adulthood (e.g., Chronis-Tuscano et al., 2010; Meinzer et al., 2016), and symptom assessments to supplement diagnoses (Hinshaw et al., 2006) clearly show that childhood ADHD prospectively predicts elevated depression. Depression is also more prevalent in young adults whose ADHD symptoms persist into adulthood (e.g., Hechtman et al., 2016; Meinzer et al., 2016). Studies showing no association between childhood ADHD and depression are limited by very low numbers of depression cases (e.g., 3 depressed probands vs. 2 comparison; Mannuzza et al., 1998) and comparisons based on lifetime rates of depression (e.g., Claude & Firestone, 1995).
Although no single explanatory conceptual model has emerged to account for the co-occurrence of ADHD and depression (see Meinzer et al., 2014, for a meta-analysis and review), depression is a plausible mediator between childhood ADHD and substance use. In addition to shared etiology and shared genetic factors, reasons that ADHD might contribute to later depression include inconsistent, controlling, or dysfunctional parent-child interactions observed in families of children with ADHD (Humphreys et al., 2013; Ostrander & Herman, 2006); social impairments associated with peer rejection (Blackman et al., 2005; Humphreys et al., 2013), typical of children with ADHD (Hoza, 2007); and ADHD-related emotion regulation deficits that increase vulnerability to depression (Anastopoulos et al., 2011). Each of these processes has also been implicated in ADHD-related substance use risk (Molina & Pelham, 2014). Consistent with the idea of depression as mediator, one study found that internalizing symptoms assessed in 6th grade mediated the link between childhood ADHD symptoms and onset of tobacco use in 9th grade (but not alcohol or marijuana; Vitulano et al., 2014). Another found that internalizing problems that emerged after childhood hyperactivity predicted social withdrawal and in turn substance use disorder by young adulthood (Tarter et al., 2007). One retrospective analysis of medical records from a large birth cohort (Yoshimasu et al., 2016) suggested that the presence of a depressive disorder partially mediated the association between childhood ADHD and SUD diagnosis by age 19. In the present study, we test whether depressive symptoms based on repeated assessments in adolescence mediate associations between childhood ADHD and heavy drinking, marijuana use, daily smoking, and other illicit drug use in adulthood.
Alternatively, depression may affect the strength of the association between childhood ADHD and later substance use. If so, its role may be more conditional (a moderator) than causal (a mediator). Indeed, many children with ADHD do not go on to develop depression (Chronis-Tuscano et al., 2010). A mediation account implies that more severe ADHD-related impairments and other vulnerabilities lead to depression in adolescence. Yet profiles of ADHD symptoms, clinical impairment, conduct problems (Blackman et al., 2005) and cognitive functioning (Roy et al., 2017) in adolescence are highly similar in community samples of children with ADHD with and without a comorbid depression diagnosis. Rather than depression leading to later substance use, it may be that the mere presence of depression alters how ADHD-related vulnerabilities increase risk for substance use. For example, tension-reduction expectancies and coping motives (e.g., Cooper, Frone, Russell, & Mudar, 1995) are acknowledged as key mechanisms by which depressed affect might invite substance use. Emotion regulation deficits (Anastopoulous et al. 2011) and external attribution biases (Ostrander & Herman, 2006) might limit coping resources available to adolescents with ADHD, increasing the appeal of substances. This may be especially true for impulsivity facets of ADHD that predict acting rashly in response to negative affect (negative urgency; see Marmorstein, 2013).
Depression, then, is also a plausible moderator: if (and when) present in adolescence, depressive symptoms may modify ADHD-related vulnerability to engaging in substance use, concurrently (in adolescence) or prospectively (in adulthood). One prominent finding from a 10-year follow-up of children diagnosed with ADHD and non-ADHD comparison children was that a lifetime mood disorder diagnosis in the ADHD group predicted a lifetime non-alcohol drug use disorder, but not alcohol use disorder or cigarette smoking (Wilens et al., 2011). However, depression and SUDs were assessed concurrently and across a wide range of ages spanning mid-adolescence to the late twenties, precluding inferences about a prospective moderating effect of adolescent depression. Similar limitations apply to findings from a retrospective analysis showing that childhood ADHD and depression each predicted SUD, but only children without ADHD and depression were at reduced risk of SUD (Yoshimasu et al., 2016).
In the present study, we test moderation concurrently and prospectively, isolating between- and within-person moderating effects of depression. If depression is present in adolescence, we anticipate ADHD-related differences in adolescent and adult heavy drinking, marijuana use, daily smoking, and other illicit drug use for those whose depressive symptoms are higher on average (pooling over repeated measures) compared to their peers. When depression is present in adolescence, we anticipate further ADHD-related differences in adolescent substance use at points in time when depressive symptoms are higher than usual for a given person.
The Current Study
Understanding developmental processes linking adolescent depression to ADHD-related substance use risk has been identified as a priority for future research (Molina & Pelham, 2014). Crucially, no studies have prospectively tested childhood ADHD and adolescent depression associations with substance use frequency. Also notably absent are tests of ADHD-related prospective risk for substance use based on repeatedly-assessed depressive symptoms in adolescence. The comprehensive longitudinal follow-up of the Multimodal Treatment Study of Children with ADHD (MTA; Hechtman et al., 2016; Sibley et al., 2017; Swanson et al., 2017) is ideally positioned to rigorously examine mediating and moderating associations between ADHD, depression, and substance use prospectively from late childhood through early adulthood, when rates of substance use reach their peak in the general population (Center for Behavioral Health Statistics and Quality, 2015). We test these associations in three ways.
First, we test whether depressive symptoms in adolescence mediates the effect of childhood ADHD on adult substance use. A mediating role implies that a childhood ADHD diagnosis predicts more severe depressive symptoms in adolescence that, in turn, predict more frequent heavy drinking, marijuana use, other illicit drug use, and daily smoking in adulthood.
Second, we test whether elevated depressive symptoms in adolescence moderate ADHD-related risk for substance use in adolescence and adulthood. A moderating role implies that differences between ADHD and non-ADHD youth in adolescent and adult substance use depend on the severity of adolescent depressive symptoms, between- and within-persons. We test this hypothesis with a series of interactions: (1) whether effects of childhood ADHD on heavy drinking, marijuana use, daily smoking, and other illicit drug use assessed in adolescence depend on average levels of adolescent depressive symptoms (a between-persons effect) and on time-varying depressive symptoms in adolescence (a within-person effect); and (2) whether prospective effects of childhood ADHD on the same substance outcomes assessed in adulthood depend on average levels of depressive symptoms in adolescence.
Third, we take advantage of diagnostic information available in adulthood from the MTA to examine rates of SUD and depressive disorder in young adults whose ADHD symptoms did and did not persist into adulthood. With this supplementary test, we extend the scope of this paper to report clinically meaningful substance use outcomes when depression diagnoses are present versus absent at the young-adult endpoint of the MTA (mean age 25).
Method
Participants were 579 children diagnosed with DSM-IV ADHD Combined Type and a local normative comparison group (LNCG) of 289 children followed to adulthood. Probands were initially recruited into the MTA when they were aged 7.0 to 9.9 (M=8.5, SD=.80). At each of six sites in the U.S. and Canada, between 95 and 98 children were randomly assigned to one of four treatment groups: multicomponent behavioral treatment, systematic medication management, the combination of medication and behavioral treatment, or referral to usual community care. Children with comorbid diagnoses participated; exclusion criteria were limited to conditions requiring study-incompatible treatments (e.g., antipsychotic medication), inability to participate in a lengthy clinical trial (e.g., hospitalization, IQ below 80), cross-arm contamination (children in the same classroom or household), and family-related risks and threats to full participation (e.g., history of abuse, parent stimulant abuse, non-English-speaking primary caregiver). The MTA recruitment strategy, detailed inclusion/exclusion criteria, diagnostic procedures, treatment, and sample demographic characteristics appear in other reports (Arnold et al., 1997; MTA Cooperative Group, 1999). Informed consent (parental permission and child assent) was obtained from all participating families prior to completing study procedures. Once they reached age 18, participants provided their own informed consent. All participating study sites (see Author note for a detailed list) obtained IRB ethics approval. Secondary data analysis procedures were approved by the Carleton University Research Ethics Board.
Participants with ADHD were assessed at baseline prior to treatment randomization, at 3 months, 9 months, the conclusion of the 14-month treatment phase, and 2, 3, 6, 8, 10, 12, 14, and 16 years after treatment randomization. LNCG children were recruited at the 2-year assessment, randomly sampled from the same schools and grades as probands, matched for age and sex. Study inclusion/exclusion criteria, questionnaire administration, and diagnostic procedures for the LNCG were the same as for probands, except they were not required to have ADHD (n=31 LNCG children who met criteria for ADHD were excluded from our analyses). LNCG children were demographically similar to the ADHD group (see Swanson et al., 2017). The LNCG were followed from the 2- through 16-year assessments. All LNCG participants and 95% of ADHD group participants contributed one or more data points from 2 through 16 years and were retained in our analyses (for detailed retention information see Table 1 in Swanson et al., 2017). The final sample size was n=805 (n=547 ADHD, n=258 LNCG without childhood ADHD).
In a previous longitudinal analysis of the same substance use outcomes reported here (Molina et al., 2018), we performed comparisons between participants with complete versus incomplete data to evaluate the potential impact of attrition. Participants with complete substance use data (ADHD n=289/547; LNCG n=176/258) were not significantly different from participants with incomplete data on over 20 baseline sociodemographic and adversity variables tested (e.g., age, parent marital status, child health). However, male sex, racial/ethnic minority, and lower parent incomes and education were more common in participants with incomplete data. As in Molina et al. (2018), we included sex, race/ethnicity, and a composite measure of household (dis)advantage as covariates in all analyses to satisfy the missing at random (MAR) assumption that patterns of missingness in the data were unrelated to our dependent variables after adjusting for relevant measured variables (e.g., see Enders, 2010).
Measures
Substance use.
The self-report Substance Use Questionnaire (SUQ; Molina & Pelham, 2003) was adapted for the MTA (Molina et al., 2013). At all assessments from the 2-year follow-up and thereafter, participants responded to questions about frequency of use of alcohol, marijuana, cigarettes, and a range of illicit and prescription drugs. As in our prior report (Molina et al., 2018), we defined past-year heavy drinking as the higher score of two items assessing frequency of binge drinking and of drunkenness (“How many times did you drink five or more drinks when you were drinking?”, “How many times have you gotten drunk or ‘very, very high’ on alcohol?”). Past-year marijuana use frequency was one question. Responses to each substance were coded to a four-point scale: 0 (none), 1 (less than once per month), 2 (at least monthly but less than weekly), and 3 {once per week or more).
Past-year daily smoking was coded as 0 (no) and 1 (yes) if participants reported smoking at least one cigarette per day or if they responded “yes” to “Are you currently a daily smoker?”. Other illicit drug use was coded by taking the maximum frequency of use across multiple drugs, such as cocaine, heroin, inhalants, hallucinogens, and misused prescription drugs (stimulants, sedatives, and opioids). Frequencies were coded to a three-point scale: 0 (none), 1 (less than once per month), and 2 (once per month or more often).
Depressive symptoms.
Depressive symptoms in adolescence were assessed by self-report using the 27-item Child Depression Inventory (CDI; Kovacs, 1992), rated 0 to 2 (e.g., “I feel sad once in a while/many times/all the time”). Between the 2- and 10-year follow-ups, participants rated the CDI at each assessment at which they were under 18 (between 3 and 5 assessments per person). A mean score was calculated by averaging responses to CDI items at each assessment. Omega total (ω) reliability exceeded .83 at each assessment. Across assessments, scores ranged from 0 to 1.78 for ADHD (M=.24, SD=.24) and from 0 to 1.41 for LNCG (M=.18, SD=.21), with higher scores reflecting more severe depressive symptoms. ADHD group symptoms were more severe than LNCG (mixed effects t=5.86, p<.001).
Scores were converted to average (between-persons) and time-varying (within-person) measures. Average depressive symptoms in adolescence were calculated as the mean of each participant’s repeated measures. Time-varying depressive symptoms were calculated by subtracting each participant’s own average symptom score from each repeated measure score (i.e., person-mean centering; see Curran & Bauer, 2011). Crucially, average and time-varying measures are uncorrelated and provide distinct tests of two elements of the symptom-substance use relation: (1) whether more severely depressed people use substances more frequently in adolescence and in adulthood (effects of average adolescent symptoms), and (2) whether specific ages featuring greater-than-usual depressive symptoms for a given person are associated with more frequent substance use at that age (effect of time-varying adolescent symptoms).
ADHD symptoms.
Models also adjusted for adolescent inattention and hyperactivity-impulsivity symptoms, assessed using combined parent and teacher ratings from the Swanson, Nolan, and Pelham Rating Scale (SNAP; Swanson, 1992). The SNAP consists of 9 items corresponding to DSM inattention symptoms and 9 items corresponding to DSM hyperactivity-impulsivity symptoms on a scale ranging from 0 (not at all) to 3 (very much). Between the 2- and 10-year follow-ups, parents and teachers rated symptoms at each assessment at which participants under age 18 (between 3 and 5 assessments/person). For inattention (parent- and teacher-rated), ω exceeded .94 at each assessment. For hyperactivity-impulsivity (parent- and teacher-rated), ω exceeded .91 at each assessment.
As in a prior report (Howard et al., 2015), we combined parent and teacher ratings in two steps. Scores were first rescaled by standardizing scores for each rater in each domain assessed at baseline (M=0, SD=1), then centering raters’ scores at all subsequent assessments around their respective raw baseline means and standard deviations. Next, centered scores were averaged across raters at each assessment. Final scores were back-transformed to approximate the original metric of the 0-to-3 response scale. We then used the strategy described above for depressive symptoms to obtain distinct measures of average and time-varying symptoms in adolescence.
ADHD symptom persistence and adult diagnoses.
For young adults in the ADHD group, ADHD symptom persistence in adulthood was defined using self- and parent-reported symptom ratings from the Conners’ Adult ADHD Rating Scale (CAARS; Conners et al., 1999), administered when participants were aged 18 and older. Participants with childhood ADHD were symptom-persistent in adulthood if five or more symptoms were self- or parent-reported as 2 or 3 on the 0-3 scale within either the inattention or hyperactive-impulsive domains at the latest available assessment point in adulthood. Participants were symptom-desistent in adulthood if neither domain met the five-symptom threshold (Hechtman et al., 2016; Sibley et al., 2017).
The Diagnostic Interview Schedule for Children-Young Adult version (DISC-YA;Shaffer et al., 2000) was used to determine whether ADHD group participants also met DSM-IV criteria for adult diagnoses of depression (major depressive disorder or dysthymia), alcohol use disorder (abuse or dependence), marijuana use disorder (abuse or dependence), nicotine dependence, and other SUD (abuse of or dependence on other substances) at the latest available assessment in adulthood (12, 14, or 16 years after baseline; see Hechtman et al., 2016). Participants were coded as having an adult diagnosis of depression if they met criteria at any assessment in adulthood (9.1%; n=42 of 461 assessed by self-report in adulthood).
Covariates.
Three baseline sociodemographic variables were primary covariates in our analyses, as defined in other reports (Mitchell et al., in press; Molina et al., 2018). Sex was coded as Male=0, Female=1. Race ethnicity was coded using three weighted effects codes for Black, Hispanic, and Mixed/other racial/ethnic groups. Household (dis)advantage was coded similarly, with codes for children from two-parent households with at least one college-educated parent (“household advantage”) and for children from single-parent households with no college-educated parents (“household disadvantage”). Children meeting neither of these criteria and White children were assigned negative reference weights in their respective coding schemes. Regression coefficients for effects codes represent the difference between the coded group and the sample mean, and are particularly suited to nominal variable coding (versus dummy or contrast coding) when one group does not stand out as a meaningful “reference” category (see Cohen, Cohen, West, & Aiken, 2003; pp. 320-321).
Within the ADHD group, we adjusted for differences between participants with and without a childhood diagnosis of CD or ODD. Comorbid diagnoses were taken from the baseline assessment, when presence of ADHD was confirmed for study enrollment. Of n=547 cases with childhood ADHD included in the present study, 15.0% (n=82) also had a CD diagnosis and 39.7% (n=217) also had an ODD diagnosis. Only n=6 LNCG met criteria for CD or ODD at their initial assessment—mean age 10—precluding further comparisons within this group. We tested differences between LNCG and ADHD groups, and between cases with childhood ADHD plus CD/ODD versus ADHD-only using planned contrast codes (see Cohen et al., 2003 pp. 332-335).
Analysis Plan
Longitudinal data were collected at eight assessments, but the CDI was administered only when participants were under 18. All participants were under 18 in years 2 and 3 (Mage=11.04, SD=1.15); all but one were under 18 in year 6 (M age=14.80, SD= 1.06); 84.9% were under 18 in year 8 (M age=16.43, SD=.84); 32.0% were under 18 in year 10 (M age=17.50, SD=.50); none were under 18 at subsequent waves. We structured our longitudinal data for analysis purposes by age rather than by wave (see Mehta & West, 2000), with assessments occurring as young as age 8 (1.1% of observations) and the majority occurring at ages 11 and up (76.0% of observations). For simplicity, we designated assessments occurring before age 18 as “adolescence”, while those at ages 18 and older were designated “adulthood”.
To test depressive symptom mediation, we performed multilevel mediation analyses in which childhood ADHD diagnosis was a predictor of average adolescent depressive symptoms (measured before age 18; up to five repeated measures), which was in turn a predictor of substance use in adulthood (age 18 and later), measured up to six times. We used the Monte Carlo method to construct confidence intervals for indirect effects (see Preacher & Selig, 2012).
To test depressive symptom moderation, we adapted a multivariate multilevel modeling technique (see the Supplement for technical details). We tested effects of childhood ADHD, adolescent depressive symptoms, and their interactions on adult substance use using the GLIMMIX procedure in SAS for categorical outcomes (sample syntax also provided in the Supplement). Our models included separate tests of concurrent associations linking childhood ADHD and average and time-varying adolescent depressive symptoms to substance use in adolescence, and prospective associations linking childhood ADHD and average adolescent depressive symptoms to substance use in adulthood. Two-way interactions between depressive symptoms and our ADHD contrast codes tested our moderation hypotheses. All models adjusted for ADHD symptom severity and baseline covariates. Additional exploratory tests included two-way interactions between depressive symptoms and rates of change in substance use (adolescent and adult), and between depressive symptoms and ADHD symptom severity. We trimmed nonsignificant interactions one at a time, in order of largest to smallest p-values, repeating analyses after each exclusion (see recommendations of Aiken & West, 1991, pp. 111-113). Interaction terms were retained ifp < .10 to control for Type II error.
To examine differences in adult SUD diagnoses, we performed chi-square tests and logistic regression analyses to compare rates of different SUDs in adulthood among participants with childhood ADHD for whom a depression diagnosis was either present or absent in adulthood. We tested whether ADHD symptom persistence in adulthood (as defined in Hechtman et al., 2016) moderated the effect of a depression diagnosis on SUD diagnoses by including a depression × ADHD symptom persistence interaction term. Logistic regression analyses adjusted for participant age at the assessment when substance use diagnoses were recorded.
Results
Mediating influences of depressive symptoms on substance use risk in adulthood
Table 1 summarizes tests of mediation linking childhood ADHD history to adult substance use through adolescent depressive symptoms, adjusted for baseline sociodemographic covariates and adolescent inattention and hyperactivity symptoms. Adolescent depressive symptoms did not mediate any associations between childhood ADHD (versus LNCG) or childhood ADHD plus comorbid CD/ODD (versus ADHD-only) and any adult substance use outcomes. Accordingly, nonsignificant mediated effects were small in magnitude; at most, our mediated effects predicted less than a 4% increase in the odds of more frequent adult substance use as a result of elevated depressive symptoms in adolescence1 that our analysis attributed to childhood ADHD.
Table 1.
Transmission of ADHD-Related Risk for Adult Substance Use via Adolescent Depressive Symptoms: Tests of Mediation
| Depression Est (95% CI) |
Heavy Drinking Est (95% CI) | Marijuana Use Est (95% CI) | Daily Smoking Est (95% CI) | Illicit Drugs Est (95% CI) | |
|---|---|---|---|---|---|
| Direct effects |
|||||
| a ADHD v. LNCG → Depression | .06** (.03, .08) | - | - | - | - |
| a ADHD+CD/ODD v. ADHD-only → Depression | .04** (.01, .07) | - | - | - | - |
| b Depression → Adult SU | - | −.34 (−1.41, .74) | .79 (−.60, 2.17) | 2.62 (−.93, 5.74) | 1.17 (−.26, 2.60) |
| c ADHD v. LNCG → Adult SU | - | −.25 (−.63, .12) | .26 (−.23, .74) | 2.28** (1.20, 3.37) | −.10 (−.61, .41) |
| c ADHD+CD/ODD v. ADHD-only → Adult SU | - | .09 (−.33, .52) | −.09 (−.65, .47) | .52 (−.70, 1.74) | .56 (−.03, 1.15) |
| Indirect (mediated) effects |
|||||
| ADHD v. LNCG → depression → Adult SU | - | −.02 (−.09, .04) | .04 (−.03, .13) | .15 (−.03, .35) | .07 (−.01, .16) |
| ADHD+CD/ODD v. ADHD-only → depression → Adult SU | - | −.01 (−.06, .03) | .03 (−.02, .11) | .10 (−.02, .28) | .05 (−.01, .13) |
Note. Est = regression coefficient. CI = confidence interval around the estimate. For indirect (mediated) effects, Est = product coefficient representing the indirect effect testing mediation via depressive symptoms. Positive values suggest depression transmits increased ADHD-related risk for adult substance use; negative values indicate depression transmits reduced ADHD-related risk for adult substance use.
“a” paths in the mediation model.
“b” paths in the mediation model.
“c-prime” (direct effect) paths in the mediation model
p<.006
Moderation of the ADHD-substance use association by depressive symptoms
Adolescent substance use.
Table 2 (Part 1: Concurrent effects on adolescent use) shows that there were no moderating effects for either time-varying or average depressive symptoms on any ADHD-SU links in adolescence. Main effects of childhood ADHD on adolescent substance use in the MTA also appear in prior reports using these same data and similar analyses (Molina et al., 2013; Molina et al., 2018). Time-varying effects of depressive symptoms, equivalent for ADHD and LNCG, showed that all substances were used more frequently at specific times when adolescents experienced depressive symptoms that were more severe than usual, regardless of ADHD history. To illustrate effect size, we selected age 17 as an adolescent end-point at which to compare model-implied rates of substance use for relatively lower versus higher depressive symptoms (see Table 3). At age 17, higher-than-usual depressive symptoms predicted rates of use 14% to 65% more frequent compared to lower-than-usual depressive symptoms.
Table 2.
Effects of Depressive Symptoms in Adolescence on Adolescent Substance Use (Concurrent Effects) and on Adult Substance Use (Prospective Effects): Nonlinear Multivariate Multilevel Model Results Testing Moderation
| Heavy Drinking B(SE) | Marijuana Use B(SE) | Daily Smoking B(SE) | Illicit Drugs B(SE) | |
|---|---|---|---|---|
| Part 1: Concurrent effects on adolescent use (age <18) | ||||
| LNCG vs. ADHDa | .11 (.28) | −.25 (.30) | −1.53** (.46) | −.71* (.36) |
| ADHD + CD/ODD vs. ADHD-only | .19 (.33) | .27 (.33) | −.03 (.44) | −.04 (.36) |
| Effects of time-varying symptoms | ||||
| Depression | .97* (.47) | .96* (.48) | 1.30* (.66) | 1.41* (.60) |
| Inattention | .46* (.24) | .22 (.25) | .50 (.35) | .25 (.32) |
| Hyperactivity-impulsivity | .24 (.28) | .59* (.28) | .50 (.40) | .51 (.38) |
| Effects of average adolescent symptoms | ||||
| Avg depression | .65 (.85) | 3.05** (.77) | 3.29* (1.22) | 1.77* (.78) |
| Avg inattention | .47 (.32) | .16 (.34) | .86 (.46) | −.07 (.34) |
| Avg hyperactivity-impulsivity | .38 (.35) | .82* (.36) | .55 (.48) | .59 (.36) |
| Interactions | ||||
| Avg adolescent depression x Age | −.91** (.27) | - | −.59 (.35) | - |
| Part 2: Prospective effects on adult use (age 18+) | ||||
| LNCG vs. ADHDa | .16 (.22) | −.43 (.30) | −2.48** (.52) | .13 (.32) |
| ADHD + CD/ODD vs. ADHD-only | −.13 (.25) | .20 (.36) | .21 (.54) | .51 (.37) |
| Effects of average adolescent symptoms | ||||
| Avg depression | −.34 (.54) | 1.52* (.73) | 1.87 (1.25) | .94 (.75) |
| Avg inattention | −.09 (.23) | .86* (.31) | 1.78** (.53) | .27 (.33) |
| Avg hyperactivity-impulsivity | .45 (.25) | .26 (.37) | .70 (.57) | .73* (.35) |
| Interactions | ||||
| Avg adol depress x LNCG v. ADHD | - | 3.25* (1.52) | - | - |
| Avg adol hyper x LNCG v. ADHD | - | −1.49* (.74) | - | - |
Note. Coefficients represent the log odds of using each type of substance. Effects not shown include model intercepts, trajectories of change in use over age and by group (LNCG, ADHD, ADHD+CD/ODD), baseline sociodemographic covariates, and study site. These were presented in Molina et al. (2018) and are not repeated here.
Regression coefficients represent LNCG-ADHD differences unadjusted for ADHD symptom severity. Dashes in some cells ( - ) refer to interaction terms not present in the given model due to trimming, as described in the analysis plan.
p<.05
p<.005.
Table 3.
Model-implied marginal probabilities of substance use at age 17 given relatively lower versus higher time-varying and average depressive symptoms in adolescence
| Monthly Heavy Drinking % | Monthly Marijuana Use % | Daily Smoking % | Any Illicit Drug Use % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LoDe | HiDe | %H | LoDe | HiDe | %H | LoDe | HiDe | %H | LoDe | HiDe | %H | |
| p | p | L | p | p | L | p | p | L | p | p | L | |
| ADHD | ||||||||||||
| Time-varying depressive symptoms | 9.1 | 10.4 | 14.3 | 11.8 | 13.4 | 13.6 | 11.9 | 14.0 | 17.6 | 5.5 | 7.3 | 32.7 |
| Average depressive symptoms | 9.1 | 10.3 | 13.2 | 9.4 | 16.7 | 77.7 | 9.6 | 17.1 | 78.1 | 4.9 | 8.1 | 65.3 |
| LNCG | ||||||||||||
| Time-varying depressive symptoms | 9.6 | 11.0 | 14.6 | 10.2 | 11.7 | 14.7 | 5.3 | 6.3 | 18.9 | 4.9 | 8.1 | 65.3 |
| Average depressive symptoms | 9.7 | 11.0 | 13.4 | 8.1 | 14.6 | 80.2 | 4.2 | 7.8 | 85.7 | 2.7 | 4.5 | 66.7 |
Note. Table entries are estimated marginal probabilities of each behavior given as percentages. “LoDep” = One standard deviation below mean for the measure of depressive symptoms. “HiDep” = One standard deviation above mean for the measure of depressive symptoms. “%HL” = Percent by which substance use frequency in the HiDep column exceeds substance use frequency in the LoDep column.
Average depressive symptom effects, also statistically equivalent for ADHD and LNCG, showed that adolescents whose depressive symptoms were more severe on average across repeated measures tended to use all substances other than alcohol more frequently in adolescence. The sizes of these effects were considerably larger than for time-varying depressive symptoms. Again illustrating effect size (Table 3), predicted rates of use at age 17 for adolescents with higher average depressive symptoms were 65% to 86% more frequent for substances other than alcohol. For heavy drinking, whose effect diminished with age, the predicted rate of use at age 17 was just 13% more frequent for adolescents with higher average depressive symptoms.
Exploratory tests yielded no depression × inattention or depression × hyperactivity symptom interactions, and main effects of ADHD symptom severity were sparse: heavy drinking was more frequent at times when inattention was more severe than usual, and time-varying and average hyperactivity-impulsivity symptoms were only associated with more frequent marijuana use. No other time-varying effects or average effects of ADHD symptom severity were found.
Adult substance use.
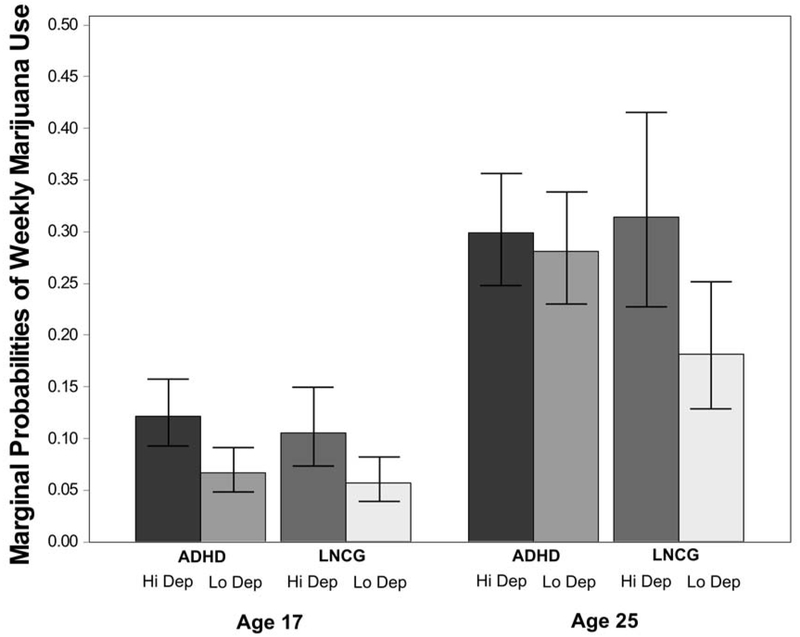
Table 2 (Part 2: Prospective effects on adult use) shows that adolescent depressive symptoms moderated the effect of childhood ADHD only on adult marijuana use. The Figure contrasts the previously-discussed concurrent effect of average adolescent depression on adolescent marijuana use (illustrated at age 17) against the prospective effect of average adolescent depression on adult marijuana use (illustrated at age 25) in the LNCG and ADHD groups. In adolescence, people with and without childhood ADHD used marijuana at similar rates, though rates in both groups were higher at more severe depressive symptom levels. In contrast, rates of weekly marijuana use were high in young adults with childhood ADHD histories regardless of their adolescent depressive symptoms, and in young adults without childhood ADHD whose adolescent depressive symptoms were more severe. Young adults with no history of childhood ADHD and few adolescent depressive symptoms were the least likely to use marijuana. Aside from this finding, average depressive symptoms in adolescence did not prospectively predict use of any other substances measured in adulthood.
Figure.
Estimated marginal probabilities of weekly or greater marijuana use at ages 17 and 25 for cases with childhood ADHD (ADHD) and without childhood ADHD (LNCG), as a function of average depressive symptoms reported in adolescence. Hi Dep = depressive symptoms 1 standard deviation above average. Lo Dep = depressive symptoms 1 standard deviation below average. Lines show 95% confidence intervals around each estimate.
Exploratory tests also ruled out interactions between adolescent depressive symptoms and inattention or hyperactivity symptoms in predicting adult substance use. Main effects were that average adolescent inattention symptoms predicted more frequent adult marijuana use and daily smoking, and average adolescent hyperactivity-impulsivity symptoms predicted more frequent illicit drug use. We probed the significant interaction between average adolescent hyperactivity-impulsivity symptoms and diagnostic status (i.e., ADHD versus LNCG), and clarified that adolescent hyperactivity-impulsivity symptoms only prospectively predicted marijuana use frequency in adults with childhood ADHD histories (B=.75, SE=.34, p=.027). For adults without childhood ADHD, adolescent hyperactivity-impulsivity symptoms were unrelated to adult marijuana use frequency (B=−.71, SE=.73, p=.33).
Major depression and SUDs in adulthood among ADHD cases
Table 4 shows results of chi-square tests comparing rates of alcohol use disorder, marijuana use disorder, nicotine dependence, and other SUD for participants with childhood ADHD with versus without a depression diagnosis in young adulthood (n=42/461; 9.1% had depression). Logistic regression analyses showed no significant interactions between depression diagnosis and ADHD persistence for any SUD. Models containing only main effects of depression diagnosis, persistent ADHD symptoms in adulthood, and age showed that depression was significantly associated with higher rates of nicotine dependence (OR=3.02, CI95%= 1.22 – 7.51, p=.017), but not alcohol use disorder (OR=1.67, CI95%=.78 – 3.56, p=.183), marijuana use disorder (OR=1.02, CI95%=.44 – 2.38, p=.964), or other SUD (OR=.80, CI95%=.67 – 7.42, p=.190). Persistent ADHD symptoms were unrelated to nicotine dependence (OR=1.95, CI95%=.93 – 4.06, p=.075), and Hechtman et al. (2016) previously reported that ADHD persistence predicted marijuana use disorder and other SUD, but not alcohol use disorder.
Table 4.
Presence of Substance Use Disorder at Mean Age 25 (last available adult assessment) for ADHD Group Cases with and without a Depression Diagnosis in Adulthood
| Percentage with SUD | |||||
|---|---|---|---|---|---|
| n | Depression Present | Depression Absent | χ2 | p | |
| Alcohol use disorder | 96 | 12/40 (30.0%) | 84/416 (20.2%) | 2.11 | .15 |
| Marijuana use disorder | 89 | 8/39 (20.5%) | 81/416 (19.5%) | .02 | .88 |
| Nicotine dependence | 39 | 9/36 (25.0%) | 30/398 (7.5%) | 12.31 | <.001 |
| Other SUD | 21 | 4/40 (10.0%) | 17/416 (4.1%) | 2.90 | .09 |
Discussion
We examined several ways that adolescent depressive symptoms might play a role in ADHD-related risk for both adolescent and young adult substance use in a 16-year longitudinal study of children with and without ADHD. Adolescent depression, both time-varying and averaged over time, were associated with use of all substances in adolescence, but there was no support for its role as mediator and there was limited support for its role as moderator, of the association between childhood ADHD and later substance use. With one exception (adolescent depression predicted more adult marijuana use for the LNCG only), depression did not prospectively mediate or moderate ADHD-related risk for any other substance. By young adulthood, nicotine dependence (but no other SUD) at age 25 was more prevalent in the ADHD group when a depression diagnosis was also present. Persistent adult ADHD symptoms did not interact with depression diagnosis to further increase risk of any SUD at age 25. Together, findings suggest depression and ADHD are independent, additive sources of substance use risk.
Adolescent depressive symptoms are consistently associated with adolescent substance use in ADHD and non-ADHD groups alike.
Elevated adolescent depressive symptoms were associated with more frequent substance use in adolescence across LNCG and ADHD groups. Adolescent depressive symptoms that were more severe on average, and depressive symptoms in adolescence that were more severe at a given age than usual for a given person, were associated with more frequent adolescent use of all substances. Crucially, it is not just adolescents experiencing persistent depressive symptoms who are at risk for substance use. Time-limited periods of heightened depressive symptoms are also associated with adolescent substance use risk, though not especially so for teens with childhood ADHD histories. Depressive symptoms were more severe, on average, for adolescents from the ADHD group compared to the LNCG, consistent with prior cross-sectional findings (Meinzer et al., 2014) and with a prior MTA analysis of adolescent depressive symptoms and disorder (Molina et al., 2009). Yet the absence of adolescent moderating effects suggests that depression strictly adds to, but does not modify, ADHD-related risk for substance use in adolescence. Whether these associations are reciprocal or transactional over time merits further study.
Overall, results are consistent with other research showing that depressive symptoms are an important correlate of elevated substance use in a developmental period when young people are beginning to experiment with substances, largely in social settings, and when persistent patterns of use are not yet established (Katz, Fromme, & D’Amico, 2000). Drinking for tension-reduction or to self-medicate against negative mood is unlikely to be the principal force behind depression-substance use associations at this age (Molina & Pelham, 2014), but substance use may co-occur with other problems in adolescence related to negative affect, such as academic, family, and behavioral difficulties (Chassin, Colder, Hussong, & Sher, 2013).
We did not find a parallel pattern of associations between adolescent depression and adult substance use. This is not entirely surprising given that longitudinal connections between substance use and depression (disorder or symptom severity) have emerged in only a minority of studies (Hussong et al., 2017; Lev-Ran et al., 2014). For many people, depressive symptoms come and go (Judd & Akiskal, 2000), raising the possibility that its influence on substance use may not accumulate for people whose symptoms are time-limited or who do not experience recurring depressive episodes. In the present sample just 5.4% of the LNCG (n=14/258) and 9.7% of the ADHD group (n=53/547) reported mean CDI scores in adolescence at or above threshold for clinical concern (i.e., ≥13; Kovacs, 1992). Depression in another study was not longitudinally associated with alcohol use until the late twenties (age 25-29; Wang et al., 2019), perhaps reflecting an evolution of chronic problematic substance use associated with negative affect that reciprocally reinforces continued use. In adolescence, however, adolescent depression did not prospectively predict young adult substance use (when occasional use is normative and regular use is not unusual). The notable exception is our finding for marijuana use.
Adolescent depressive symptoms prospectively predict adult marijuana use.
Marijuana was the only substance for which average depressive symptoms in adolescence prospectively predicted more frequent use in young adulthood, in line with prior evidence that depression-related prospective risk was heightened for drug use but not alcohol or cigarette smoking (Wilens et al., 2011). However, we interpret this finding with caution because we repeated our tests of depression effects across four distinct substance classes, increasing risk of Type I error. We found that young adults with ADHD histories were at increased risk for regular marijuana use regardless of their depressive symptom severity in adolescence—consistent with a similar antagonistic moderating effect from a birth cohort study in which low adolescent depressive symptoms appeared to protect against frequent marijuana use in young adults without childhood ADHD (Yoshimasu et al., 2016). Unlike alcohol, for which we observed no prospective prediction, marijuana is commonly used while alone (Buckner et al., 2015), perhaps increasing its appeal for managing affective states in non-ADHD populations.
Vulnerabilities such as maladaptive coping (e.g., Harty et al., 2017) may contribute to frequent marijuana use by young adults with ADHD histories, many of whom believe marijuana to be therapeutic in managing ADHD symptoms and impairment (Cooper et al., 2017; Mitchell et al., 2017). Adults with ADHD routinely report benefits such as feeling calmer, less restless, and improved focus (Cooper et al., 2017), and online forums also feature comments promoting marijuana as therapeutic for ADHD (Mitchell et al., 2016). Qualitative interviews in the MTA showed that beliefs about ADHD-related therapeutic effects of marijuana were held by half of substance-using young adults with childhood ADHD (Mitchell et al., 2017). For young adults without ADHD histories, expectations of marijuana-related tension-reduction that become more established toward the end of adolescence and may account for its frequent use in young adulthood by those with elevated adolescent depression. For young adults with ADHD histories, such expectations may be overshadowed by the belief that marijuana has ADHD-specific benefits, accounting for its frequent use by those with high and low adolescent depression.
ADHD symptom severity predicts but does not compound adult substance use risk.
Adolescent ADHD symptoms were prospectively associated with more frequent adult use of every substance except alcohol. In addition, ADHD symptom persistence in young adulthood predicted marijuana and other drug use disorders but not alcohol use disorder. Prospective links between ADHD symptoms and frequent use of a broad range of substances in adulthood is consistent with the strong longitudinal associations typically found between externalizing problems and later substance use (e.g., Hussong et al., 2011). Importantly, none of our exploratory depression × ADHD symptom interactions nor depression diagnosis × ADHD symptom persistence interactions were significant, indicating that these two potential vulnerabilities do not appear to exacerbate each other’s effects in early adulthood. An adult depression diagnosis was associated with nicotine dependence at mean age 25, whereas ADHD symptom persistence was associated with marijuana and other SUD.
Study Limitations
We considered the potential impact of ADHD symptoms on depression mediation and moderation, and we also adjusted for differences between children whose ADHD was or was not comorbid with CD or ODD. However, our analyses did not account for associations with other relevant diagnoses, particularly anxiety. Anxiety disorders have been linked to SUDs (e.g., Grant et al., 2004) but less consistently to substance use. A recent systematic review found that anxiety predicted both increases and decreases in later substance use (Hussong et al., 2017), likely owing to differences in how specific forms of anxiety convey risk (e.g., Marmorstein et al., 2010). Future studies devoted specifically to untangling these complexities are needed.
Depressive symptoms were measured using self-reports at ages 8 through 17, generally preferred assessing youth internalizing symptoms when multiple reports are not collected (e.g., De Los Reyes et al., 2015). However, the difference in depressive symptom severity between ADHD and LNCG was small, suggesting low power to detect mediated and moderated effects. Similarly, by mean age 25, rates of past-year major depressive disorder in the ADHD group were modest and similar to those in the LNCG (<5% in both groups; Hechtman et al., 2016). The relatively few ADHD cases with young adult depression (n=42) suggests that we also had low power to test differences in rates of SUDs for those with and without a depressive disorder.
Conclusion
In a 16-year longitudinal study of children with and without ADHD, we found no evidence that depressive symptoms mediated ADHD-related substance use risk. In adolescence, depression did not exacerbate effects of childhood ADHD on any substance use. Average and time-varying depressive symptoms in adolescence were concurrently associated with more frequent use of all substances in both ADHD and non-ADHD groups. Prospectively, our finding that adolescent depression moderated ADHD-related risk for adult marijuana use invites further exploration. Beliefs about potential symptom-relieving benefits of marijuana may help to explain elevated rates of marijuana use in young adults with ADHD histories. Adult depression diagnoses and ADHD persistence into adulthood provided separate but additive risks for substance use disorder. Our findings did not show depression playing a role in explaining or altering the developmental course of substance use in children with ADHD. However, given their additive risks, relatively modest depression in adolescence (along with other underlying risks such as academic and behavioral impairments) may signal an early opportunity for intervention during a developmental period of escalating substance experimentation.
Supplementary Material
Acknowledgments
J.T.M. consulted with Avanir. L.E.A. received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, Supernus, and YoungLiving and consulted with or was on advisory boards for Gowlings, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Sea-side Therapeutics, Sigma Tau, Shire, and Tris Pharma and received travel support from Noven. J.M.S. acknowledges research support, advisory board/ speaker’s bureau and/or consulting for Alza, Richwood, Shire, Celgene, Novartis, Celltech, Gliatech, Cephalon, Watson, CIBA, UCB, Janssen, McNeil, Noven, NLS, Medice, and Lilly. The other remaining authors have declared that they have no competing or potential conflicts of interest.
The Multimodal Treatment Study of Children with ADHD (MTA) was a National Institute of Mental Health (NIMH) cooperative agreement randomized clinical trial with the observational phase funded by NIMH and the National Institute on Drug Abuse (NIDA) to the following: University of Califomia–Berkeley*: U01MH50461, N01MH12009, N01DA-8-5550; Duke University*: U01MH50477, N01MH12012, N01DA-8-5554; University of California, Irvine*: U01MH50440, N01MH12011, N01DA-8-5551; Research Foundation for Mental Hygiene (New York State Psychiatric Institute*/Columbia University): U01 MH50454, N01 MH12007, N01DA-8-5552; Long Island–Jewish Medical Center*: U01 MH50453; New York University: N01MH 12004, N01DA-8-5549; University of Pittsburgh*: U01 MH50467, N01MH 12010, N01DA-8-5553; McGill University N01MH12008, N01DA-8-5548. A data analysis grant awarded to the University of Pittsburgh (DA039881) followed. IRB ethics approvals for primary data collection at the RCT and observational phases were obtained from all above sites marked with an asterisk* as well as at Mount Sinai Medical Center and Montreal Children’s Hospital. The opinions and assertions contained in this report are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health or the National Institute of Mental Health.
Collaborators formerly at NIMH: Benedetto Vitiello, (University of Turin); Joanne B. Severe; Peter S. Jensen (currently University of Arkansas); L. Eugene Arnold (currently Ohio State University); Kimberly Hoagwood, (currently Columbia); early-phase NIMH contributors: John Richters; Donald Vereen. Site PIs/Co-Is: University of California, Berkeley/San Francisco: Stephen P. Hinshaw (Berkeley), Glen R. Elliott (San Francisco); Duke University: Karen C. Wells; Jeffery N. Epstein (currently Cincinnati Children’s Hospital); Desiree W. Murray (currently UNC Chapel Hill); early-phase Duke contributors: C. Keith Conners; John March; University of California, Irvine: James Swanson, Timothy Wigal; UCLA contributor: Dennis P. Cantwell; NYU School of Medicine: Howard B. Abikoff; Montréal Children’s Hospital/McGill University: Lily Hechtman; NY State Psychiatric Institute/ Columbia University/Mount Sinai Medical Center: Laurence L. Greenhill; Jeffrey H. Newcorn; University of Pittsburgh: Brooke Molina; Betsy Hoza (University of Vermont); William E. Pelham (currently Florida International University). Follow-up statistical collaborators: Robert D. Gibbons (University of Illinois, Chicago); Sue Marcus (Mt. Sinai College of Medicine); Kwan Hur (University of Illinois, Chicago). Original study statistical/design consultant: Helena C. Kraemer (Stanford University). Collaborators from US Department of Education, Thomas Hanley and Karen Stem.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Defined as one standard deviation above average depressive symptoms (.24) in adolescence. Odds ratio associated with the largest mediated effect in Table 1 = exp(.15*.24)/[1 + exp(.15*.24)] = 1.037 (i.e., odds are 3.7% higher).
References
- Aiken LS & West SG (1991). Multiple Regression: Testing and Interpreting Interactions. Sage Publications. [Google Scholar]
- Anastopoulos AD, Smith TF, Garrett ME, Morrissey-Kane E, Schatz NK, Sommer JL, Kollins SH, & Ashley-Koch A. (2011). Self-regulation of emotion, functional impairment, and comorbidity among children with AD/HD. Journal of Attention Disorders, 75(7), 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold LE, Abikoff HB, Cantwell DP, Conners CK, Elliott G, Greenhill LL, …, Wells KC. (1997). National Institute of Mental Health Collaborative Multimodal Treatment Study of Children with ADHD (the MTA). Design challenges and choices. Archives of General Psychiatry, 54(9), 865–70. [DOI] [PubMed] [Google Scholar]
- Blackman GL, Ostrander R & Herman KC (2005). Children With ADHD and Depression: A Multisource, Multimethod Assessment of Clinical, Social, and Academic Functioning. Journal of Attention Disorders, 5(4), 195–207 [DOI] [PubMed] [Google Scholar]
- Breyer JL, Lee S, Winters K, August G, & Realmuto G A Longitudinal Study of Childhood ADHD and Substance Dependence Disorders in Early Adulthood. (2014). Psychology of Addictive Behaviors, 28(1), 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Crosby RD, Wonderlich SA, Ecker AH, & Richter A (2015). Antecedents and consequences of cannabis use among racially diverse cannabis users: An analysis from Ecological Momentary Assessment. Drug and Alc Dependence, 147, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Retrieved from samhsa.gov/data [Google Scholar]
- Chan YF, Dennis ML, & Funk RR (2008). Prevalence and comorbidity of major internalizing and externalizing problems among adolescents and adults presenting to substance abuse treatment. J Subst Abuse Treat, 34( 1), 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Colder C, Hussong A, & Sher KJ (2013). Substance use and substance use disorders InD Cicchetti & D. J Cohen (Eds.) Developmental Psychopathology. Hoboken, NJ: Wiley; 3rd ed. [Google Scholar]
- Chronis-Tuscano A, Molina BS, Pelham WE, Applegate B, Dahlke A, Overmyer M, & Lahey BB (2010). Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry, (67(10), 1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude D & Firestone P (1995). The Development of ADHD boys: a 12-year follow-up. Canadian Journal of Behavioural Science, 27(2), 226–249. [Google Scholar]
- Cohen J, Cohen P, West SG, & Aiken LS (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ, US: Lawrence Erlbaum. [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Conners’ Adult ADHD Rating Scales (CAARS) technical manual. North Tonawanda, NY: Multi-Health Systems, Inc; 1999. [Google Scholar]
- Cooper ML, Frone MR, Russell M, & Mudar P (1995). Drinking to regulate positive and negative emotions: a motivational model of alcohol use. Journal of Personality and Social Psychology, 69(5), 990–1005. [DOI] [PubMed] [Google Scholar]
- Cooper RE, Williams E, Seegobin S, Tye C, Kuntsi J, & Asherson P (2017). Cannabinoids in attention-deficit/hyperactivity disorder: A randomised-controlled trial. Eur Neuropsychopharmacol, 27(8), 795–808. [DOI] [PubMed] [Google Scholar]
- Curran PJ & Bauer DJ (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol, 62, 583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Reyes A, Augenstein TM, Wang M, Thomas SA, Drabick DA, Burgers DE, & Rabinowitz J (2015). The validity of the multi-informant approach to assessing child and adolescent mental health. Psychol Bull, 141(4), 858–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK (2010). Applied missing data analysis. New York, NY, US: Guilford Press. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, …, Kaplan K (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry, 61(8), 807–16. [DOI] [PubMed] [Google Scholar]
- Groenman AP, Janssen TWP, & Oosterlaan J (2017). Childhood Psychiatric Disorders as Risk Factor for Subsequent Substance Abuse: A Meta-Analysis. J Am Acad Child Adolesc Psychiatry, 56(7), 556–569. [DOI] [PubMed] [Google Scholar]
- Harty SC, Gnagy EM, Pelham WE Jr., & Molina BSG (2017). Anger-irritability as a mediator of attention deficit hyperactivity disorder risk for adolescent alcohol use and the contribution of coping skills. Journal of Child Psychology and Psychiatry, 58(5), 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman L, Swanson JM, Sibley MH, Stehli A, Owens EB, Mitchell JT, … & Nichols JQ (2016). Functional Adult Outcomes 16 Years After Childhood Diagnosis of Attention-Deficit/Hyperactivity Disorder: MTA Results. Journal of the American Academy of Child & Adolescent Psychiatry, 55(11), 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B, Feehan M, McGee R, Stanton W, Moffitt TE, & Silva P (1993). The importance of conduct problems and depressive symptoms in predicting adolescent substance use. Journal of Abnormal Child Psychology, 21, 469–480. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Owens EB, Sami N, & Fargeon S (2006). Prospective Follow-Up of Girls With Attention-Deficit/Hyperactivity Disorder Into Adolescence: Evidence for Continuing Cross-Domain Impairment. Journal of Consulting and Clinical Psychology, 74(3,) 489–499. [DOI] [PubMed] [Google Scholar]
- Howard AL, Molina BSG, Swanson JM, Hinshaw SP, Belendiuk KA, Harty SC, … Wigal T. (2015). Developmental progression to early adult binge drinking and marijuana use from worsening versus stable trajectories of adolescent attention deficit/hyperactivity disorder and delinquency. Addiction, 110, 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoza B (2007). Peer functioning in children with ADHD. Ambul Pediatr, 7(1), 01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Katz SJ, Lee SS, Hammen C, Brennan PA, & Najman JM (2013). The association of ADHD and depression: Mediation by peer problems and parent-child difficulties in two complementary samples. Journal of Abnorm Psychology, 122(3), 854–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Ennett ST, Cox MJ, & Haroon M (2017). A systematic review of the unique prospective association of negative affect symptoms and adolescent substance use controlling for externalizing symptoms. Psychology of Addictive Behaviors, 31, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, & Boeding S (2011). An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors, 25, 390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, & Akiskal HS (2000). Delineating the Longitudinal Structure of Depressive Illness: Beyond Clinical Subtypes and Duration Thresholds. Pharmacopsychiatry, 33, 3–7. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey BB, …,Schwab-Stone M. (1997). Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. J Abnorm Child Psychol, 25(2), 121–32. [DOI] [PubMed] [Google Scholar]
- Katz EC, Fromme K, & D’Amico EJ (2000). Effects of outcome expectancies and personality on young adults’ illicit drug use, heavy drinking, and risky sexual behavior. Cognitive Therapy and Research, 24 (1), 1–22. [Google Scholar]
- King SM, Iacono WG, & McGue M (2004). Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction, 99, 1548–1559. [DOI] [PubMed] [Google Scholar]
- Kovacs M (1992). The Children’s Depression, Inventory (CDI). Psychopharmacol Bull, 21, 995–998. [PubMed] [Google Scholar]
- Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, & Rehm J (2014). The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med, 44(4), 797–810. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Bessler A, Malloy P, & LaPadula M (1998). Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry, 755(4), 493–8. [DOI] [PubMed] [Google Scholar]
- Marmorstein NR (2013). Associations between dispositions to rash action and internalizing and externalizing symptoms in children. J Clin Child Adolesc Psychol, 42(1), 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein NR, White HR, Loeber R, & Stouthamer-Loeber M (2010). Anxiety as a predictor of age at first use of substances and progression to substance use problems among boys. J Abnorm Child Psychol, 38, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PD, & West SG (2000). Putting the individual back into individual growth curves. Psychological Methods, 5(1), 23–43. [DOI] [PubMed] [Google Scholar]
- Meinzer MC, Pettit JW, & Viswesvaran C (2014). The co-occurrence of attention-deficit/hyperactivity disorder and unipolar depression in children and adolescents: a meta-analytic review. Clin Psychol Rev, 34(8), 595–607. [DOI] [PubMed] [Google Scholar]
- Meinzer MC, Pettit JW, Waxmonsky JG, Gnagy E, Molina BS, & Pelham WE (2016). Does childhood attention-deficit/hyperactivity disorder (ADHD) predict levels of depressive symptoms during emerging adulthood? Journal of abnormal child psychology, 44(4), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, Howard AL, Belendiuk KA, Kennedy TM, Stehli A, Swanson JM, Hechtman L, …, Molina BSG. (in press). Cigarette smoking progression among young adults diagnosed with ADHD in childhood: A 16-year longitudinal study of children with and without ADHD. Nicotine & Tobacco Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, McIntyre EM, English JS, Dennis MF, Beckham JC, & Kollins SHA (2017). Pilot Trial of Mindfulness Meditation Training for ADHD in Adulthood: Impact on Core Symptoms, Executive Functioning, and Emotion Dysregulation. Journal of Attention Disorders, 21(13), 1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JT, Sweitzer MM, Tunno AM, Kollins SH, & McClernon FJ (2016). “I Use Weed for My ADHD”: A Qualitative Analysis of Online Forum Discussions on Cannabis Use and ADHD. PLOS ONE, 11(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, & Pelham WE (2003). Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnorm Psychology, 112(3), 497–507. [DOI] [PubMed] [Google Scholar]
- Molina B, Hinshaw SP, Arnold LE, Swanson JM, Pelham WE, Hechtman L et al. (2013). Adolescent substance use in the Multimodal Treatment Study of Attention-Deficit/Hyperactivity Disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry, 52(3), 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, … & MTA Cooperative Group. (2009). The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry, 48(5), 484–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, & Pelham WE Jr. (2014). Attention-deficit/hyperactivity disorder and risk of substance use disorder: developmental considerations, potential pathways, and opportunities for research. Amm Rev Clin Psychol, 10, 607–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Howard AL, Swanson JM, Stehli A, Mitchell JT, Kennedy TM, et al. (2018). Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: findings from the MTA longitudinal study. Journal of Child Psychology and Psychiatry, 29, 546–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander R & Herman KC (2006). Potential cognitive, parenting, and developmental mediators of the relationship between ADHD and depression. J Consult Clin Psychol, 74(1), 89–98. [DOI] [PubMed] [Google Scholar]
- Preacher KJ & Selig JP 2012. Advantages of Monte Carlo Confidence Intervals for Indirect Effects. Communication Methods and Measures 6(2), 77–98. [Google Scholar]
- Roy A, Oldehinkel AJ, & Hartman CA (2017). Cognitive Functioning in Adolescents with Self-Reported ADHD and Depression: Results from a Population-Based Study. Journal of Abnormal Child Psychology, 45(1), 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone M (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Altszuler AR, Ross JM, Sanchez F, Pelham WE Jr., & Gnagy EM (2014). A parent-teen collaborative treatment model for academically impaired high school students with ADHD. Cognitive and Behavioral Practice, 21, 32–42. [Google Scholar]
- Sibley MH, Swanson JM, Arnold LE, Hechtman LT, Owens EB, Stehli A, …, Pelham WE. (2017). Defining ADHD symptom persistence in adulthood: Optimizing sensitivity and specificity. Journal of Child Psychology and Psychiatry, 55(6), 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM (1992). School-based assessments and interventions for ADD students. Irvine, CA: KC Publications. [Google Scholar]
- Swanson JM, Arnold LE, Molina BSG, Sibley MH, Hechtman LT, Hinshaw SP, … & Kraemer HC. (2017). Young adult outcomes in the follow-up of the multimodal treatment study of attention-deficit/hyperactivity disorder: symptom persistence, source discrepancy, and height suppression. Journal of Child Psychology and Psychiatry, 55(6), 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Feske U, & Vanyukov M (2007). Modeling the pathways linking childhood hyperactivity and substance use disorder in young adulthood. Psychology of Addictive Behaviors, 27(2), 266–271. [DOI] [PubMed] [Google Scholar]
- Truter I, Regnart J, & Meyer A (2017). Critical exploration of co-occurring Attention-Deficit/Hyperactivity Disorder, mood disorder and Substance Use Disorder. Expert Review of Pharmacoeconomics & Outcomes Research, 17(3), 275–282 [DOI] [PubMed] [Google Scholar]
- Vitulano L, Fite PJ, Derek R Hopko, Lochman J., Wells K., & Asif I. (2014). Evaluation of underlying mechanisms in the link between childhood ADHD symptoms and risk for early initiation of substance use. Psychol Addict Behav, 25(3), 816–27. [DOI] [PubMed] [Google Scholar]
- Wang FL, Pedersen SL, Joseph H, Gnagy EM, Curran P, Pelham WE, & Molina BSG (2019). Role of ADHD in the co-occurrence between heavy alcohol use and depression trajectories in adulthood. Alcoholism: Clinical and Experimental Research, 43(2), 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D, Riggs PD, Min SJ, Mikulich-Gilbertson SK, Tamm L, Trello-Rishel K, & Winhusen T (2012). Major depression and treatment response in adolescents with ADHD and substance use disorder. Drug Alcohol Depend, 120(1-3), 214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C et al. (2011). Does ADHD predict substance use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry, 50(6), 543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasu K, Barbaresi WJ, Colligan RC, Voigt RG, Weaver AL, & Katusic SK (2016). Mediating and Moderating Role of Depression, Conduct Disorder or Attention-Deficit/Hyperactivity Disorder in Developing Adolescent Substance Use Disorders: A Population-Based Study. PLOS ONE 11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.