Abstract
Inflammation has an important physiological influence on mood and behavior. Kynurenine metabolism is hypothesized to be a pathway linking inflammation and depressed mood, in part through the impact of kynurenine metabolites on glutamate neurotransmission in the central nervous system. This study evaluated whether the circulating concentrations of kynurenine and related compounds change acutely in response to an inflammatory challenge (endotoxin administration) in a human model of inflammation-induced depressed mood, and whether such metabolite changes relate to mood change. Adults (n=115) were randomized to receive endotoxin or placebo. Mood (Profile of Mood States), plasma cytokine (interleukin-6, tumor necrosis factor-α) and metabolite (kynurenine, tryptophan, kynurenic acid, quinolinic acid) concentrations were repeatedly measured before the intervention, and at 2 and 6 hours post-intervention. Linear mixed models were used to evaluate relationships between mood, kynurenine and related compounds, and cytokines. Kynurenine, kynurenic acid, and tryptophan (but not quinolinic acid) concentrations changed acutely (p’s all <0.001) in response to endotoxin as compared to placebo. Neither kynurenine, kynurenic acid nor tryptophan concentrations were correlated at baseline with cytokine concentrations, but all three were significantly correlated with cytokine concentrations over time in response to endotoxin. Quinolinic acid concentrations were not correlated with cytokine concentrations either before or following endotoxin treatment. In those who received endotoxin, kynurenine (p=0.049) and quinolinic acid (p=0.03) positively correlated with depressed mood, although these findings would not survive correction for multiple testing. Changes in tryptophan and kynurenine pathway metabolites did not mediate the relationship between cytokines and depressed mood. Further work is necessary to clarify the pathways leading from inflammation to depressed mood in humans.
Keywords: kynurenine metabolism, inflammation, depression, sex differences, experimental design
1. INTRODUCTION
Depression is a common and debilitating disorder (Kessler et al., 2005), and current treatments are inadequate (Rush et al., 2006). Novel treatment targets for depression must be identified through improved understanding of the biology of depressive symptoms. Inflammation holds promise as a treatment target, given evidence that depressed patients have higher concentrations of inflammatory markers relative to controls (Dowlati et al., 2010; Kohler et al., 2017); experimental induction of inflammation induces depressive symptoms in humans (Capuron et al., 2002; Eisenberger et al., 2010a; Raison et al., 2006; Reichenberg et al., 2001; Udina et al., 2012); and patients with higher levels of inflammation appear to respond differently to depression treatment interventions (Amitai et al., 2016; Eller et al., 2008; Hashimoto, 2015; Kruse et al., 2018; Lanquillon et al., 2000; Machado-Vieira et al., 2016; Raison et al., 2013; Rapaport et al., 2015; Rethorst et al., 2013; Strawbridge et al., 2015; Uher et al., 2014; Yang et al., 2015). However, the pathways leading from inflammation to depression and the reasons for variable depressive responses to inflammation are not fully understood and require further investigation.
Alteration in glutamate neurotransmission is hypothesized to link inflammation and depression, which may in part be accounted for by a change in the kynurenine pathway of tryptophan metabolism (Lawson et al., 2013; O’Connor et al., 2009; Raison et al., 2010). The kynurenine pathway is regulated by the immune system; in response to an inflammatory stimulus, pro-inflammatory cytokines activate indoleamine 2,3-dioxygenase (IDO) (Bay-Richter et al., 2014; Campbell et al., 2014; Erhardt et al., 2013). In turn, IDO metabolizes tryptophan (Trp) to kynurenine (KYN), thus increasing concentrations of KYN and decreasing concentrations of Trp. The ratio of KYN/Trp is used as a measure of IDO activity. KYN is actively transported across the blood-brain barrier (Fukui et al., 1991). Additional enzymes in both peripheral and central compartments metabolize KYN along various pathways to form the metabolites quinolinic acid (QA) and kynurenic acid (KynA), among others. These metabolites are neuroactive, impacting glutamate neurotransmission at N-methyl-D-aspartate (NMDA) glutamate receptors. For example, QA is an agonist at the glutamate binding site of the NMDA receptor, and KynA is an antagonist at the glycine site of this receptor. It is hypothesized that imbalances between QA and KynA, with a net overstimulation of the NMDA receptor, may contribute to the link between inflammation and depression (Miller, 2013; Schwarcz et al., 2012; Walker et al., 2013).
Animal studies have provided insight into the relationship between inflammation, the kynurenine pathway, NMDA receptor activation, and depressive-like behavior. In mice, peripheral administration of endotoxin (lipopolysaccharide, LPS) leads to higher concentrations of brain KYN and QA, as well as depressive-like behavior (Walker et al., 2013), and this behavior is prevented by blocking IDO activation (O’Connor et al., 2009). Further, administration of the NMDA receptor antagonist ketamine successfully blocks (or reverses) depressive-like behaviors (Walker et al., 2013). Together, these results suggest that endotoxin-induced depressive-like behavior in mice may be mediated by NMDA receptor activation by QA (Walker et al., 2013).
In human studies, differences in concentrations of KYN pathway metabolites in depressed patients relative to controls have been identified (Bay-Richter et al., 2014; Erhardt et al., 2013; Meier et al., 2016; Savitz et al., 2015; Steiner et al., 2011). The KYN pathway thus may have clinical relevance for understanding mechanisms related to depression. Depressed patients were found to have lower serum ratios of KynA to QA (Savitz et al., 2015), suggesting potential for greater NMDA receptor stimulation in these patients. Suicide attempters were found to have higher cerebrospinal fluid (CSF) concentrations of QA than controls. Among suicide attempters, greater CSF QA concentrations correlated with higher total scores on the suicide intent scale, and lower CSF KynA concentrations were associated with more severe depressive symptoms (Bay-Richter et al., 2014; Erhardt et al., 2013). In depressed patients who died by suicide, microglial QA was increased in the subgenual anterior cingulate cortex and anterior midcingulate cortex compared to controls (Steiner et al., 2011). Thus, a disrupted balance of KYN pathway metabolites, potentially leading to greater NMDA receptor activation, is associated with suicide attempts and more severe depressive symptoms.
Further human evidence of the link between inflammation, the KYN pathway, and depression emerges from the literature on interferon-α treatment for hepatitis C. Interferon-α is a pro-inflammatory cytokine which leads to induction of a new onset major depressive episode in up to 45% of those treated (Musselman et al., 2001). In a small study of hepatitis C patients (n=27), those undergoing interferon-α treatment had higher CSF concentrations of KYN and QA as compared to the no-treatment condition. CSF concentrations of KYN and QA closely correlated with plasma concentrations of the same metabolites, and also with depressive symptoms (Raison et al., 2010).
In humans, no prior experimental study has evaluated whether changes in concentrations of KYN and its metabolites might be related to increases in depressive symptoms in response to an inflammatory challenge. Given substantial evidence that administration of endotoxin to healthy volunteer participants leads to increases in inflammatory cytokines and depressed mood (Moieni et al., 2015b), the present study evaluated the link between inflammation, kynurenine pathway measures, and depression by testing 1) whether an experimentally administered inflammatory challenge (i.e., endotoxin) induced changes in KYN metabolism; and 2) whether these changes in KYN metabolites correlated with inflammation-induced depressed mood.
2. METHODS
2.1. Participants
One hundred and fifteen healthy participants (age range 18–50 years; 69 females and 46 males) were deemed eligible and completed a randomized, placebo-controlled trial of endotoxin administration, which was conducted between March 2011 and 2013, described in detail previously (Moieni et al., 2015b). (Supplemental Figure 1). Sixty-one received endotoxin and 54 received placebo (group comparisons shown in Table 1). Portions of these data have been previously reported (Cho et al., 2019; Irwin et al., 2019; Moieni et al., 2015a; Moieni et al., 2015b).
Table 1.
Baseline Characteristics of the Sample
| Placebo (n=54) | Endotoxin (n=61) | Group Differences | ||
|---|---|---|---|---|
| Age, mean (SD) | 23.3 (6.0) | 24.9 (7.1) | t(113)= 1.32 | p=0.19 |
| Sex, female, % | 57% | 62% | χ2(1, N=115)= 0.29 | p=0.59 |
| Body mass index, mean (SD) | 23.5 (2.6) | 24.2 (2.9) | t(113)= 1.44 | p=0.15 |
| Kynurenine, pmol/μl, mean (SD) | 3.6 (0.3) | 3.5 (0.3) | t(113)= 0.92 | p=0.36 |
| Tryptophan, pmol/μl, mean (SD) | 55.0 (3.0) | 55.0 (2.8) | t(113)= 0.09 | p=0.93 |
| Kynurenic acid, pmol/μl, mean (SD) | 0.26 (0.03) | 0.25 (0.02) | t(113)= 1.87 | p=0.06 |
| Quinolinic acid, pmol/μl, mean (SD) | 1.5 (0.3) | 1.5 (0.3) | t(113)= 0.13 | p=0.90 |
2.2. Procedures
In this double-blind, placebo-controlled, randomized clinical trial, participants were randomly assigned to receive either an infusion of low dose endotoxin (0.8 ng/kg body weight, Escherichia coli group O:113) or placebo (same volume of 0.9% saline). Our prior study involving healthy volunteers used 0.8 ng/kg (Eisenberger et al., 2010b)—the dose used by Reichenberg et al. (Reichenberg et al., 2001)—and had induced a significant depressive response with no major adverse effects. Thus, the same dose 0.8 ng/kg was used in the current study also involving healthy volunteers. Participants provided baseline demographic data and self-reported information regarding mood. At baseline and following the intervention, approximately every hour (for six hours) participants had blood draws and completed self-reports of depressed mood.
2.3. Measures
2.3.1. Depressed mood
Depressed mood was assessed at each time point using the depression subscale of the short-form Profile of Mood States (Baker et al., 2002; McNair et al., 1971). Participants rated the extent to which they felt at the moment (“right now”): “unhappy,” “sad,” “blue,” “hopeless,” “discouraged,” “miserable,” “helpless,” and “worthless,” on a scale from 0 (not at all) to 4 (extremely). Depressed mood was calculated by summing scores from each of these items at each time point. The reliability of the scale (assessed at the time of peak response) was high (Chronbach’s α=0.83).
2.3.2. Tryptophan and kynurenine metabolites
Plasma was collected at baseline (T0), approximately two hours (T2), and approximately six hours (T6) following administration of endotoxin or placebo, and were assayed for Trp, KYN, KynA, and QA, by high-performance liquid chromatography coupled to tandem mass spectrometry using an adaptation of a previously described method.(Midttun et al., 2009)
Solutions of internal standards (500 pmol of 2H5-KYN and 2 nmol of 2H3-Trp), both in 10 μL of water, were added to plasma aliquots (100 μL). The samples were mixed vigorously then treated with neat trifluoroacetic acid (20 μL) and mixed vigorously again. After incubation [30 min, room temperature (RT)] followed by centrifugation (16,060 × g, 5 min, RT) the supernatants were transferred to clean microcentrifuge tubes and dried in a vacuum centrifuge. Dilute hydrochloric acid (0.1 N, 100 μL) was added to the dried residues and the samples were vigorously agitated, centrifuged again (16,060 × g, 5 min, RT) and the supernatants transferred to liquid chromatography injector vials. Aliquots of the supernatants were injected (5 μl) onto a reversed phase column (Scherzo C18 100 × 2.1mm, 1.7 μ particle size and 100 Å) equilibrated in solvent A (water/acetonitrile/formic acid, 100/3/0.1, all by vol) and eluted (200 μL/min) with an increasing concentration of solvent B (450 mM ammonium formate/acetonitrile, 6.5/93.5, vol/vol: min/%B; 0/0, 5/0, 30/100, 40/100, 50/0, 60/0). The effluent from the column was directed to an electrospray ion source connected to a triple quadrupole mass spectrometer (Agilent 6460) operating in the positive ion multiple reaction monitoring (MRM) mode. The intensities of peaks in selected MRM transitions were recorded at previously determined retention times and optimized instrumental settings (KYN m/z 209→192 and 174 at retention time (rt) 16.2 min; 2H5-KYN m/z 214→96 and 122 at rt 16.2 min; W m/z 205→188 and 146 at rt 17.5 min; 2H3-Trp 208→147 and 119 at rt 17.5 min; KynA m/z 190→144 and 172 at rt 20.8 min; QA 168→150 at rt 33.5).
The 345 samples (three per subject) were divided into seven batches (48 samples in each of four batches, and 51 samples in each of 3 batches). Batches were arranged so that each was similar with regard to group assignment and sex. Samples from the same subject were assayed in the same batch. Each batch included ten standards (five dilutions, each in duplicate), prepared as above with pH 7.2 phosphate-buffered saline substituting for plasma, with the same amount of internal standards and increasing amounts of KYN, KynA and QA (0, 50, 100, 200, and 400 pmol) and Trp (0, 1.25, 2.5, 5, and 10 nmol). The data from the standards was used to construct standard curves in which the ratio of peak intensities using the most intense transition for each compound (transitions in bold font above; ordinate; KYN/2H5-KYN, Trp/2H3-Trp, KynA/2H5-KYN and QA/2H5-KYN) was plotted against amount of analyte (abscissa); the analyte content of each sample was interpolated from the respective standard curves. Samples in Batch 1 were prepared and run in duplicate, and the intra-assay Coefficients of Variation for the entire batch were 10.3%, 17.5%, 16.6%, and 3.5% for KYN, KynA, QA and Trp, respectively. Quality control (QC) samples were also included in each batch to monitor for significant differences between batches. The inter-assay CV for the same compounds calculated from the data for the QC samples was 2.7%, 4.1%, 4.9% and 0.8%, respectively. The limit of detection (LOD) for all metabolites was around 50 fmol injected (signal 3-fold greater than background), equivalent to 1 pmol/sample when injecting 5 μL out of 100 μL. Signals greater than that obtained from water injections, made periodically during the analysis of every batch, were detected in all samples for all compounds. The Lower Limit of Quantitation (LLOQ) for all compounds was around 20 pmol/sample (estimated as 20-fold greater than the LOD) and this was significantly lower than the amount of KYA, QA and Trp in the samples, and the amount of KynA in the samples was at about the LLOQ. All four metabolites were detectable and quantifiable in 100% of samples.
2.3.3. Cytokines
Plasma levels of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α) were quantified by high sensitivity bead-based multiplex (Luminex) immunoassays (Performance High Sensitivity Human Cytokine, R&D Systems, Minneapolis, MN, USA), as previously described (Moieni et al., 2015a; Moieni et al., 2015b). The lower limits of detection for IL-6 and TNF-α were 0.2 and 0.8 pg/ml, respectively. TNF-α was detectable in 100% of samples. For the small proportion of samples with IL-6 below the lower limit of detection (<1%), the values were treated as missing. The mean intra-assay CV% of the standards was <8% for IL-6 and TNF-a; the interassay CV% of an internal laboratory quality control sample was <3% for both analytes. In the current study, analyses focused only T0, T2, and T6 time points, given that these are the time points for which KYN pathway data were available for comparison.
2.4. Data analysis
All data were examined for distributional qualities. Because the cytokine concentrations and kynurenine pathway metabolite concentrations were not normally distributed at any time point, we performed a base 10 logarithmic transformation on the data prior to statistical analyses. For the same reason, and also to minimize the impact of outliers, we also performed a base 10 logarithmic transformation on the POMS depression scores.
To establish between-group differences in the effect of endotoxin versus placebo on kynurenine metabolite concentrations, we used linear mixed models examining condition by time interactions. Linear mixed models use all available data, account for correlations between repeated measurements on the same subject, have greater flexibility to model time effects, and can handle missing data more appropriately than traditional models such as repeated-measures analysis of variance.(Gueorguieva and Krystal, 2004) Compound symmetry with heterogeneous variances was selected as the covariance structure in all models, as this covariance structure was determined to best fit the data, using information criteria scores. These models were not adjusted for covariates, as the independent variable in these analyses was a randomly assigned intervention (i.e., endotoxin versus placebo).
Next, relationships between kynurenine pathway measure concentrations and cytokine concentrations were evaluated. First, correlations between baseline concentrations were evaluated among all participants. Then, to evaluate relationships in response to endotoxin, linear mixed models were used only in the endotoxin group (n=61), with individual cytokines and kynurenine pathway measures entered, respectively, as independent and dependent variables. These models were adjusted for age, sex, and BMI, given possible impact of these covariates.
Relationships between kynurenine pathway measures and depressed mood among the endotoxin-exposed participants were also examined using linear mixed models, with individual kynurenine pathway measures entered as the independent variables, and depressed mood entered as the dependent variable. These models were adjusted for age, sex, and BMI, given possible impact of these covariates, as the independent variables in these models were not randomly assigned and thus there is a potential for confounding effect.
The Sobel-Goodman test was used for mediation analysis (MacKinnon et al., 2002) to evaluate whether kynurenine pathway measures mediated the effect of cytokines on depression.
Lastly, since we previously identified sex differences in depressive response following endotoxin administration (Moieni et al., 2015b), sex was tested as a moderator (i.e., effect modifier) of: 1) the changes in kynurenine metabolites in response to endotoxin; and 2) the associations between kynurenine pathway measures and endotoxin-induced depressed mood using linear mixed models with an interaction term for sex.
All analyses, except the Sobel-Goodman test, were conducted using IBM SPSS (Version 25), and statistical significance was established at p≤0.05 (two-sided). As this was primarily an exploratory effort to identify possible mechanisms for future study, corrections for multiple testing were not applied. The Sobel-Goodman test was conducted using Stata (Version 14).
3. RESULTS
3.1. Baseline Characteristics
Table 1 summarizes participant demographic information and baseline concentrations of tryptophan, kynurenine, quinolinic acid, and kynurenic acid. No demographic characteristics or baseline concentrations were different between the endotoxin and placebo groups. Of note, at the peak depressive response time (T2), the mean score of the POMS depression subscale in the endotoxin group was 2.02 (standard deviation 4.30), indicating a mild subclinical level of depressed mood. Comparatively, the psychometric evaluation of the POMS depression subscale in cancer patients had shown a mean score of 7.04 (Baker et al., 2002).
3.2. Biomarker Responses to Endotoxin
3.2.1. Kynurenine, Tryptophan, and the Kynurenine/Tryptophan Ratio (IDO Activity)
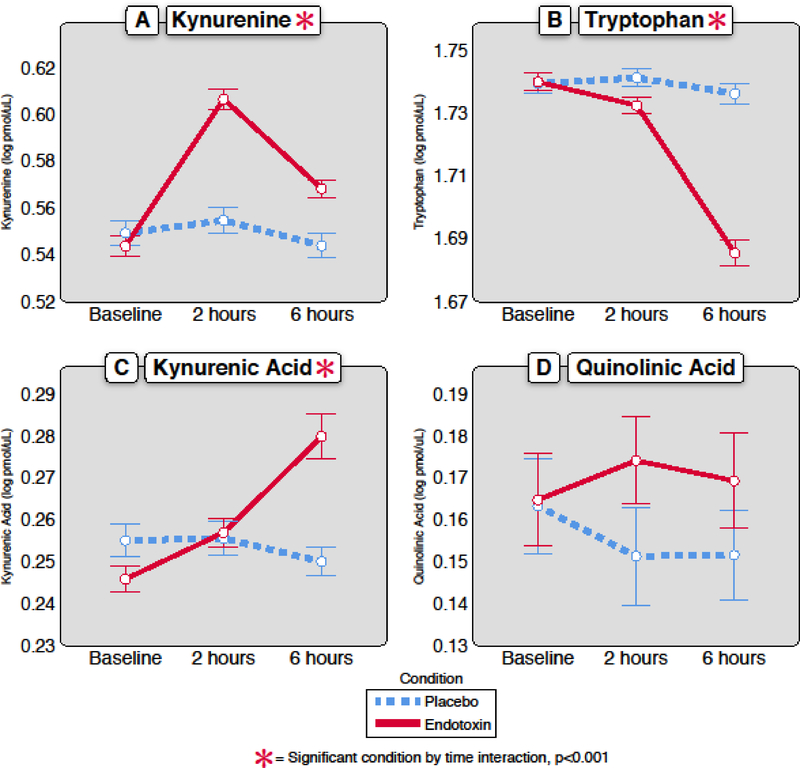
Linear mixed models demonstrated that KYN [F(2,182.36)=26.50; p <0.001] and Trp [F(2,153.35)=50.70; p <0.001] both changed in response to acute administration of endotoxin versus placebo (Table 2, Figure 1). As expected, the experimental inflammatory challenge robustly increased the KYN/Trp ratio [F(2,167.99)=44.56; p<0.001], an indicator of IDO activity, which is the rate limiting enzymatic step of the KYN pathway.
Table 2.
Analyte Concentrations Over Time (Endotoxin group, n=61)
| Baseline | 2 hours | 6 hours | Group (endotoxin vs. placebo) x time interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Q1 | Median | Q3 | Q1 | Median | Q3 | Q1 | Median | Q3 | F statistic | p value |
| Kynurenine (pmol/μL)* | 3.30 | 3.55 | 3.70 | 3.92 | 4.12 | 4.23 | 3.56 | 3.79 | 3.91 | F(2,182.36)=26.50 | <0.001 |
| Tryptophan (pmol/μL)* | 52.57 | 55.28 | 57.40 | 52.19 | 53.84 | 56.00 | 47.20 | 49.34 | 50.11 | F(2,153.35)=50.70 | <0.001 |
| Kynurenic Acid (pmol/μL)* | 0.228 | 0.244 | 0.266 | 0.235 | 0.257 | 0.277 | 0.250 | 0.278 | 0.311 | F(2,157.62)=12.55 | <0.001 |
| Quinolinic Acid (pmol/μL)* | 1.26 | 1.43 | 1.69 | 1.35 | 1.58 | 1.69 | 1.24 | 1.56 | 1.77 | F(2,170.10)=0.55 | 0.58 |
| IL-6, pg/mL* | 1.03 | 1.71 | 2.69 | 50.41 | 98.78 | 153.55 | 3.46 | 4.97 | 8.0 | F(2,180.78)=226.57 | <0.001 |
| TNF-α, pg/mL* | 5.19 | 6.21 | 7.82 | 82.95 | 136.99 | 201.36 | 14.20 | 19.13 | 27.61 | F(2,211.93)=475.71 | <0.001 |
Values were transformed by base 10 logarithm before all statistical analyses, but original scale medians and IQR are presented here.
Figure 1. Change in kynurenine pathway metabolites over time in response to endotoxin versus placebo.
In response to endotoxin (n=61), there was a significant change in the plasma concentration of A) kynurenine [F(2,182.36)=26.50; p <0.001], B) tryptophan [F(2,153.35)=50.70; p <0.001], and C) kynurenic acid [F(2,157.62)=12.55; p <0.001], but not D) quinolinic acid [F(2,170.10)=0.55; p= 0.58]. All units for the analytes are in log pmol/μL. Error bars are +/− 1 SE.
3.2.2. Kynurenic Acid, Quinolinic Acid
In addition to evaluating KYN and Trp concentrations in response to endotoxin, changes in the downstream metabolites KynA and QA were examined. Linear mixed model regression analyses demonstrated that KynA increased significantly in response to endotoxin versus placebo [F(2,157.62)=12.55; p <0.001], but concentrations of QA did not acutely change in response to endotoxin [F(2,170.10)=0.55; p= 0.58] (Figure 1).
3.2.3. Cytokines
As previously reported by our group, concentrations of the pro-inflammatory cytokines IL-6 and TNF-α in this sample increased significantly in response to endotoxin versus placebo (p’s <0.001), without moderation by sex.(Moieni et al., 2015b) For consistency with the methods used to analyze the kynurenine metabolites, prior analyses of cytokine change were repeated with linear mixed models, utilizing only T0, T2, and T6 time points. The results of these analyses were highly significant for both IL-6 [F(2,180.78)=226.57; p <0.001] and TNF-α [F(2,211.93)=475.71; p <0.001], consistent with prior results. Concentrations of cytokines over time are reported in Table 2.
3.2.4. Relationships between Cytokines, Tryptophan and Kynurenine Pathway Measures
There were no significant correlations at baseline between Trp and any KYN pathway measure and IL-6 or TNF-α. To better understand and describe relationships over time between cytokines and kynurenine pathway measures in response to endotoxin (N=61), linear mixed models were used, with individual cytokines and kynurenine pathway measures entered, respectively, as independent and dependent variables in the models. Within the group who received endotoxin, there were significant positive relationships identified over time between KYN and cytokines [IL-6: F(1,97.54)=110.97; p <0.001; TNF-α: F(1,98.85)=124.58; p <0.001], as well as between KynA and cytokines [IL-6: F(1,81.37)=7.45; p= 0.008; TNF-α: F(1,80.93)=5.53; p=0.02]. Negative relationships were identified between Trp and cytokines [IL-6: F(1,80.00)=9.07; p=0.003; TNF-α: F(1,80.53)=11.00; p=0.001]. No significant relationships were identified between QA and cytokines [IL-6: F(1,113.41)=1.17; p=0.28; TNF-α: F(1,114.86)=1.11; p=0.3]. See Supplemental Figure 2 for graphical temporal relationships between cytokine and kynurenine measure change over time.
3.3. Affective Responses to Endotoxin
As previously reported (Moieni et al., 2015b), participants exposed to endotoxin versus placebo had a significant increase in depressed mood at T2. This finding did not change when controlling for physical sickness symptoms at T2 (Moieni et al., 2015b). Depressed mood increased from baseline to T2, and then decreased from T2 to T6.
3.3.1. Relationships between Cytokines and Depressive Response
As previously reported (Moieni et al., 2015b), there were significant relationships identified between cytokine change and affective response to endotoxin. These analyses of cytokine change in relation to depressed mood were repeated with linear mixed models, utilizing only T0, T2, and T6 time points. Results were highly significant, with correlations identified between depressed mood and both IL-6 [F(1,99.15)=18.01, p<0.001] and TNF-α [F(1,106.45)=9.74, p=0.002].
3.3.2. Relationships between Tryptophan, Kynurenine Pathway Metabolites and Depressive Response
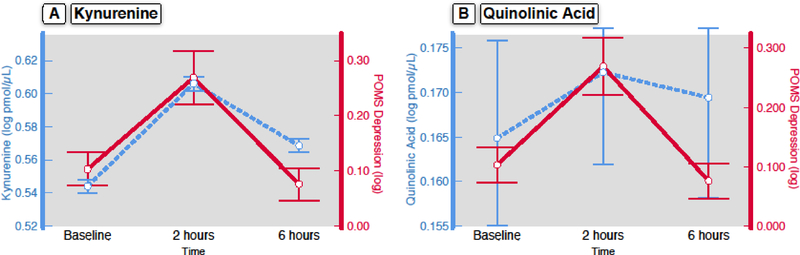
In participants exposed to endotoxin (N=61), we examined the relationships between kynurenine pathway measures and depressed mood over time using linear mixed models, with individual kynurenine pathway measures and depressed mood entered, respectively, as independent and dependent variables in the models. We found significant positive relationships between KYN and depressed mood [F(1,159.47)=3.93, p=0.049], and between QA and depressed mood [F(1,120.99)=4.91, p=0.029], but not between depressed mood and Trp [F(1,105.66)=1.80; p=0.18] or KynA [F(1,100.13)=0.00; p = 0.99], nor between depressed mood and the ratio of KYN/Trp [F(1,107.29)=0.06; p= 0.80]. See Figure 2 for a graphical representation of the joint change in depressed mood, KYN, and QA.
Figure 2. Depressed Mood Associates with Kynurenine and Quinolinic Acid Change in Response to Endotoxin.
In response to endotoxin (n=61), linear mixed effect models identified significant relationships between POMS depression score and both kynurenine [panel A; F(1,159.47)=3.93, p=0.049] and quinolinic acid [panel B; F(1,120.99)=4.91, p=0.029]. All units for the analytes are in log pmol/μL. Error bars are +/− 1 SE.
3.3.3. Mediation Analysis
To evaluate whether kynurenine pathway measures mediated the effect of cytokines on depressed mood, the Sobel-Goodman mediation test was performed, using cytokines as the independent variables, depressed mood as the dependent variable, kynurenine pathway measures as mediating variables, and sex, age, and BMI as covariates. No significant mediation was identified with less than 5% of the total effect of cytokines on depressed mood being mediated by kynurenine pathway measures (p’s all >0.10), indicating that plasma kynurenine pathway measures did not mediate the relationship between pro-inflammatory cytokines and depressed mood.
3.4. Sex Differences
Sex did not moderate changes of kynurenine metabolites after endotoxin challenge or the associations between kynurenine pathway measures and depressive response.
4. DISCUSSION
This study demonstrates that experimentally-induced inflammation in humans leads to rapid changes in plasma concentrations of kynurenine, tryptophan, and kynurenic acid. As expected, endotoxin challenge robustly increased IDO activity as indexed by the KYN/Trp ratio. Whereas baseline levels of cytokines and kynurenine pathway measures did not correlate with one another, inflammatory challenge was followed by positive correlations over time between cytokines and KYN and KynA, and negative correlations between cytokines and Trp. Further, among endotoxin-exposed participants, changes in KYN (p=0.049) and QA (p=0.03) positively correlated with changes in depressed mood. However, these latter findings are exploratory and would not survive correction for multiple testing. Nevertheless, a recent pre-clinical study has shown that the blockade of KYN transport across the blood-brain barrier by leucine effectively abrogates inflammation-induced depression-like behavior in mice. (Walker et al., 2018)
KYN metabolism is increasingly recognized as a key neurochemical pathway in the link between inflammation and depression (Reus et al., 2015; Savitz, 2019). Inflammatory mediators activate IDO, an enzyme that converts Trp to KYN. Circulating KYN is then transported into the brain by the large amino acid transporter LAT1, which is expressed in endothelial cells of the blood-brain barrier. (Dantzer, 2016) To our knowledge, this is the first human experimental study to demonstrate acute changes (i.e. within hours) in KYN metabolism in response to an inflammatory challenge.
Concentrations of kynurenine pathway measures and cytokines did not correlate at baseline. This is in contrast to previously reported correlations between IL-6 and the KYN/Trp ratio in elderly adults (Capuron et al., 2011) and depressed patients (Quak et al., 2014). However, this finding has not been consistently identified across studies (Chiappelli et al., 2018), and may depend upon the group being studied. Here, we have evaluated a group of relatively healthy, young individuals. In the absence of substantial variation in baseline concentrations of inflammatory markers in this population (see Table 2), it may be difficult to detect baseline correlations between these measures.
While concentrations of KYN, Trp, and KynA all changed significantly in response to endotoxin, the time course of this change varied. KYN increased by the 2 hour time point, and by the six hour time point KYN concentrations were decreasing back toward baseline levels. This is similar to our findings regarding cytokine concentrations, which peaked at the 2 hour time point following endotoxin administration (Eisenberger et al., 2010a; Inagaki et al., 2012; Moieni et al., 2019). However, changes in concentrations of KynA and Trp were largely driven by later change, occurring between the 2 hour and the 6 hour time points (Figure 1). Though QA did not change at the time points measured in the current study, it is possible that QA may have changed significantly at unmeasured time points between baseline and six hours, or that a longer time course may be required to observe effects of endotoxin on plasma QA concentrations. However, if QA changed at a later time point, this would be of unclear significance to our model of depression, given that transient induction of depressed mood in response to endotoxin had resolved by the six hour time point.
Interestingly, though QA concentrations did not change significantly in response to endotoxin at the time points measured in the current study, nor did they correlate with cytokine change, plasma QA concentrations correlated with depressed mood. Similar to our lack of finding regarding QA change in response to an inflammatory challenge, a study of hepatitis C patients, who were either treated with interferon-α (IFN-α) or not treated, found significant differences in plasma KYN concentrations between groups (p<0.01), yet no difference in plasma concentrations of QA (Raison et al., 2010). However, in the current study, despite lack of change in QA concentrations in endotoxin-exposed participants, we have found an intriguing correlation between plasma QA concentration and mood following endotoxin exposure (p=0.03). Given that the statistical significance of this correlation would not survive correction for multiple comparisons, this finding should be interpreted with caution. However, the IFN-α study provides an interesting context within which to consider this finding. Despite lack of plasma QA change, there was a highly significant difference in cerebrospinal fluid (CSF) QA concentrations (p<0.001) in IFN-α treated patients that positively correlated with depression scale score (p=0.019). Additionally, plasma and CSF QA concentrations following IFN-α treatment were highly correlated (p<0.001), but relationships between plasma QA and mood were not reported. In the current study, we do not have central measures of Trp, KYN and its metabolites, which is a limitation.
The current study may have implications for understanding the mechanisms of inflammation-associated depression in humans. This study demonstrates that in a human experimental model of inflammation-induced depressed mood, there is rapid change in plasma concentrations of KYN pathway measures (in tandem with previously demonstrated change in pro-inflammatory cytokines), with positive correlations between depressed mood and KYN pathway measures (KYN, QA). However, KYN pathway measures did not mediate the relationship between cytokine change and depressive response in this sample.
There are several strengths of this study, including the randomized placebo-controlled design and the repeated paired monitoring of mood, cytokines, and kynurenine metabolites over several hours, which allowed for the evaluation and tracking of acute effects of inflammation on multiple variables in an otherwise healthy population. This is in comparison to the existing influential and important quasi-experimental studies in patients with hepatitis C virus (HCV). In studies of HCV patients, the impact of cytokine therapy (IFN-α) on mood, cytokines, and kynurenine metabolites has been evaluated over longer time frames. However, due to the population studied and the chronicity of IFN-α therapy in HCV patients, these studies do not closely map the ebb and flow of acute mood symptoms onto acute patterns of cytokine and KYN metabolite change, as we have done in the current study.
The absence of central measures of inflammation (e.g. CSF concentrations of analytes) is a limitation of the current study. Future studies of inflammation-associated depression would benefit from analysis of CSF concentrations of Trp, KYN and its metabolites, and/or neuroimaging measures of central inflammation. Our mediation analysis approach was limited by the number of available time points for kynurenine pathway measures, and we were thus unable to complete time-lagged mediation analysis, which would be a strength of further research. Another limitation is that the participants were all healthy and free of psychiatric illness. It is unclear the extent to which inflammation-induced depressive symptoms in a nonpsychiatric population is relevant to clinical psychiatric populations. In this regard, this research informs current efforts to better identify and treat the “inflammatory subtype” of depression, by evaluating mechanisms related to the acute induction of depressive symptoms in response to inflammation, without the confounding influence of other illnesses. Finally, though the vast majority of significant results would remain so with a correction for multiple testing, the relationships of KYN and QA to depressed mood would not.
5. CONCLUSION
To our knowledge, this study provides novel evidence testing the link between KYN, QA, and inflammation-induced depression in humans. An inflammatory challenge rapidly increased concentrations of KYN, which in turn was correlated with inflammation-induced depressed mood, as was QA. However, KYN pathway metabolites did not mediate the relationship between inflammation and depressed mood. To the extent that these experimental findings are relevant to depression, it remains unclear whether the KYN pathway serves as mediator between inflammation and depression in humans.
Supplementary Material
HIGHLIGHTS.
Experimental inflammatory challenge acutely increases plasma levels of kynurenine pathway measures in humans.
Changes in plasma kynurenine and quinolinic acid positively correlate with inflammation-induced depressed mood.
Kynurenine metabolism may be a pathway linking inflammation and depressed mood.
Acknowledgments
Funding: This work was supported by NCATS Grant #UL1TR000124, K23MH116127 K23AG049085, KL2TR000122, NARSAD Young Investigator Grant, R01MH091352, and the Norman Cousins Center for Psychoneuroimmunology. The funders had no roles in the conduct of the research or preparation of the article.
Footnotes
Declarations of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration: ClinicalTrials.gov
REFERENCES
- Amitai M, Taler M, Carmel M, Michaelovsky E, Eilat T, Yablonski M, Orpaz N, Chen A, Apter A, Weizman A, Fennig S, 2016. The Relationship Between Plasma Cytokine Levels and Response to Selective Serotonin Reuptake Inhibitor Treatment in Children and Adolescents with Depression and/or Anxiety Disorders. Journal of child and adolescent psychopharmacology 26, 727–732. [DOI] [PubMed] [Google Scholar]
- Baker F, Denniston M, Zabora J, Polland A, Dudley WN, 2002. A POMS short form for cancer patients: psychometric and structural evaluation. Psychooncology 11, 273–281. [DOI] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L, 2014. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain, behavior, and immunity. [DOI] [PubMed] [Google Scholar]
- Campbell BM, Charych E, Lee AW, Möller T, 2014. Kynurenines in CNS disease: regulation by inflammatory cytokines. Frontiers in neuroscience 8, 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH, 2002. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26, 643–652. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D, 2011. Chronic Low-Grade Inflammation in Elderly Persons Is Associated with Altered Tryptophan and Tyrosine Metabolism: Role in Neuropsychiatric Symptoms. Biological psychiatry 70, 175–182. [DOI] [PubMed] [Google Scholar]
- Chiappelli J, Notarangelo FM, Pocivavsek A, Thomas MAR, Rowland LM, Schwarcz R, Hong LE, 2018. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43, 1675–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Irwin MR, Eisenberger NI, Lamkin DM, Cole SW, 2019. Transcriptomic predictors of inflammation-induced depressed mood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 44, 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, 2016. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. Current topics in behavioral neurosciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL, 2010. A Meta-Analysis of Cytokines in Major Depression. Biological psychiatry 67, 446–457. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010a. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR, 2010b. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68, 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E, 2008. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 32, 445–450. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L, 2013. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 38, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR, 1991. Blood–Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. 56, 2007–2017. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH, 2004. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 61, 310–317. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, 2015. Inflammatory biomarkers as differential predictors of antidepressant response. International journal of molecular sciences 16, 7796–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI, 2012. Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage 59, 3222–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Cole S, Olmstead R, Breen EC, Cho JJ, Moieni M, Eisenberger NI, 2019. Moderators for depressed mood and systemic and transcriptional inflammatory responses: a randomized controlled trial of endotoxin. Neuropsychopharmacology 44, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF, 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta psychiatrica Scandinavica. [DOI] [PubMed] [Google Scholar]
- Kruse JL, Congdon E, Olmstead R, Njau S, Breen EC, Narr KL, Espinoza R, Irwin MR, 2018. Inflammation and Improvement of Depression Following Electroconvulsive Therapy in Treatment-Resistant Depression. The Journal of clinical psychiatry 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H, 2000. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 22, 370–379. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O’Connor JC, 2013. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. Journal of neuroinflammation 10, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Gold PW, Luckenbaugh DA, Ballard ED, Richards EM, Henter ID, De Sousa RT, Niciu MJ, Yuan P, Zarate CA Jr., 2016. The role of adipokines in the rapid antidepressant effects of ketamine. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V, 2002. A Comparison of Methods to Test Mediation and Other Intervening Variable Effects. Psychological Methods 7, 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, 1971. EITS manual for the Profile of Mood States. Educational and Industrial Testing Service, San Diego, CA. [Google Scholar]
- Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, Bodurka J, Teague TK, Dantzer R, Savitz J, 2016. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain, behavior, and immunity 53, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midttun Ø, Hustad S, Ueland PM, 2009. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry 23, 1371–1379. [DOI] [PubMed] [Google Scholar]
- Miller AH, 2013. Conceptual confluence: the kynurenine pathway as a common target for ketamine and the convergence of the inflammation and glutamate hypotheses of depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38, 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Breen EC, Cho HJ, Arevalo JM, Ma J, Cole SW, Eisenberger NI, 2015a. Trait sensitivity to social disconnection enhances pro-inflammatory responses to a randomized controlled trial of endotoxin. Psychoneuroendocrinology 62, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI, 2015b. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 40, 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Tan KM, Inagaki TK, Muscatell KA, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI, 2019. Sex Differences in the Relationship Between Inflammation and Reward Sensitivity: A Randomized Controlled Trial of Endotoxin. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH, 2001. Paroxetine for the prevention of depression induced by high-dose interferon alfa. The New England journal of medicine 344, 961–966. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R, 2009. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14, 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, Penninx BWJH, Kema IP, de Jonge P, 2014. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology 45, 202–210. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH, 2006. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 27, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH, 2010. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-[alpha]: relationship to CNS immune responses and depression. Molecular psychiatry 15, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH, 2013. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D, 2015. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T, 2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58, 445–452. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Toups MS, Greer TL, Nakonezny PA, Carmody TJ, Grannemann BD, Huebinger RM, Barber RC, Trivedi MH, 2013. Pro-Inflammatory Cytokines as Predictors of Antidepressant Effects of Exercise in Major Depressive Disorder. Molecular psychiatry 18, 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J, 2015. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: Evidences from animal and human studies. J Psychiatr Res 68, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry 163, 1905–1917. [DOI] [PubMed] [Google Scholar]
- Savitz J, 2019. The kynurenine pathway: a finger in every pie. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R, 2015. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ, 2012. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM, 2011. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. European Neuropsychopharmacology 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Udina M, Castellvi P, Moreno-Espana J, Navines R, Valdes M, Forns X, Langohr K, Sola R, Vieta E, Martin-Santos R, 2012. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 73, 1128–1138. [DOI] [PubMed] [Google Scholar]
- Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, McGuffin P, 2014. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment With Escitalopram and Nortriptyline. The American journal of psychiatry. [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R, 2013. NMDA receptor blockade by ketamine abrogates lipopolysaccharideinduced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38, 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Wing EE, Banks WA, Dantzer R, 2018. Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. j., Wang N, Yang C, Shi J. y., Yu H. y., Hashimoto K, 2015. Serum Interleukin-6 Is a Predictive Biomarker for Ketamine’s Antidepressant Effect in Treatment-Resistant Patients With Major Depression. Biological psychiatry 77, e19–e20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.