Abstract
Depressive and anxiety disorders substantially contribute to the global burden of disease, particularly in poor countries. Higher prevalence rates for both disorders among women indicate sex hormones may be integrated in the pathophysiology of these disorders. The Kshetriya Gramin Financial Services study surveyed a random sample of 4,160 households across 876 villages in rural Tamil Nadu, India. An interviewer-administered questionnaire was conducted to quantify depressive (K6-D) and anxiety (K6-A) symptoms. Alongside, hair samples for sex hormone profiling were collected from a subsample of 2,105 women aged 18–85 years. Importantly, 5.9%, 14.8%, and 46.3% of samples contained non-detectable hormone levels for dehydroepiandrosterone, progesterone, and testosterone, respectively. Our primary analysis imputes values for the non-detectable sample and we check robustness of results when non-detectable values are dropped. In this cohort of women from rural India, higher depressive symptomatology is associated with lower levels of dehydroepiandrosterone and higher depressive and anxiety symptoms are associated with higher levels of testosterone. Progesterone shows no clear association with either depressive or anxiety symptoms. These results support a potential protective effect of higher endogenous dehydroepiandrosterone levels. An important caveat on the potential negative effect of hair testosterone levels on women’s mental health is that the testosterone analysis is sensitive to how non-detectable values are treated.
Keywords: depression, anxiety, women, India, sex hormones, androgens
1. Introduction
Depression is a leading cause of disability world-wide and is associated with high personal and societal costs (Hasin et al., 2018; WHO, 2017). For women, depressive and anxiety-related disorders are consistently associated with more than two times higher prevalence and number of years lived with disability than for men (Vos et al., 2017; World Health Organisation, 2017). This underlines the value of a gender specific approach investigating depressive and anxiety disorders (Bekker and van Mens-Verhulst, 2007; Schiller et al., 2016; Walther et al., 2017). Existing research also highlights an urgent need to improve mental health in Indian women, a particularly burdened population with insufficient access to mental health services (Shidhaye et al., 2016; Trivedi and Gupta, 2010). Among Indian women, depression prevalence often exceeds the reported annual global prevalence of 10%, ranging between 9.4% to 16.7% (Chauhan et al., 2017; Shidhaye et al., 2016), while Indian women show particularly high suicide death rates (Dandona et al., 2018). They also fare poorly on anxiety disorders, the second most frequently reported mental disorder after depression (Vos et al., 2017): the prevalence of anxiety disorders among Indian women is reported to be as high as 32.2%, compared to 9.7% for Indian men (Khambaty and Parikh, 2017; Trivedi and Gupta, 2010).
An important step towards identifying appropriate policy responses is to identify correlates of depressive and anxiety disorders among Indian women. Here, an area of interest is sex hormones, which are neuroactive and thereby directly influence mood and behavior (Schiller et al., 2016). Sex steroid specific receptors are distributed throughout the brain and are abundant in all depression and anxiety-relevant brain areas (Mahmoud et al., 2016). Epidemiological studies showing increased prevalence rates of depressive and anxiety disorders in women suggest that differences in sex hormone levels may contribute to gender differences (Kessler, 2003; Weissman and Klerman, 1977).
In this paper, we combine survey data on depressive and anxiety symptoms with hair-sample based data on two sex hormones (progesterone [P], and testosterone [T]) and one hormonal precursor to sex hormones (dehydroepiandrosterone [DHEA]) to examine the correlation between self-reported depression and anxiety symptoms and sex hormones. In addition, we evaluate sensitivity of observed correlations to alternative ways of treating non-detectable hormonal values. Our contributions are twofold. First, we provide evidence from a very large sample of poor Indian women – to the best of our knowledge, this is one of the largest samples with sex hormone data from hair samples in any setting. Second, while it is well-known that hormone levels are more likely to be non-detectable when truly low levels of that hormone are present (Gao et al., 2016), the sensitivity of study results to alternative ways of treating non-detectable values in analysis has received insufficient research attention (Graham, 2009). Given that low levels of sex hormones can directly influence depression and anxiety, we believe such sensitivity analysis is critical and this forms a central part of our analysis.
A large body of laboratory research has investigated the potential role of sex steroids in alleviating depression or anxiety symptoms (Fiacco et al., 2018; Li and Graham, 2017; Walther et al., 2019a). Rodent models show that DHEA, P or T administration is causally related to increased serotonin release (Li and Graham, 2017) and facilitates general and antidepressant-induced neuroplasticity in the hippocampal formation (Walther et al., 2019b). Reduced serotonin release and neuroplasticity are hypothesized as the central mechanisms that predispose individuals to depression and anxiety. This suggests that higher endogenous levels of sex steroids are depression- and anxiety-protective (Li and Graham, 2017).
Research in human populations is less conclusive. Among men, despite conflicting evidence across clinical populations and cohort studies (Asselmann et al., 2019; Heald et al., 2017; Kische et al., 2017; T’sjoen et al., 2005; Wu et al., 2010), increasing evidence suggests that higher sex hormone levels are associated with lower depressive symptoms (Almeida et al., 2008; Barrett-Connor et al., 1999b; Ford et al., 2016; Kische et al., 2018; Korenman et al., 2018; Veronese et al., 2015; Walther et al., 2016; Westley et al., 2015).
For women, study results are more conflicting and report anywhere from no associations (Asselmann et al., 2019; Giltay et al., 2017) to both positive and negative associations with depression. For instance, some studies suggest that higher T levels contribute to higher depressive symptoms during the menopausal transition (Bromberger et al., 2010), or that women with depression generally exhibit elevated levels of T (Matsuzaka et al., 2013; Weber et al., 2000), but other studies suggest that lower levels of T and DHEA are associated with depression (Barrett-Connor et al., 1999a; Giltay et al., 2012; Kische et al., 2017), although these effects fade in some studies when controlling for relevant moderators (Kische et al., 2017). In another study, women with lower T levels report more depressive symptoms (Santoro et al., 2005). Furthermore, some T and DHEA supplementation studies show promising anti-depressive effects in women with and without diagnosed depression (Arlt et al., 1999; Davis et al., 2006; Dias et al., 2006; Goldstat et al., 2003; Miller et al., 2009; Rabkin et al., 2006; Schmidt et al., 2005).
With regard to androgens and anxiety, results are mixed for both genders: a large male cohort study finds a weakly negative association between T levels and anxiety symptoms, with T deficient men showing increased anxiety (Berglund et al., 2011), and men undergoing androgen deprivation therapy showing increased de novo development of anxiety disorders (DiBlasio et al., 2008), but other studies suggest T levels are positively associated with risk for anxiety disorders (Asselmann et al., 2019). The few studies considering women present mixed evidence on how T and DHEA levels relate to anxiety symptoms or disorders (Asselmann et al., 2019; Gerra et al., 2000; Granger et al., 2003; Maner et al., 2008; Oulis et al., 2014).
Lastly, amidst some conflicting literature (Deligiannidis et al., 2016; Fiacco et al., 2018), P has been shown to exhibit anti-depressive and anxiolytic effects (Schüle et al., 2014; Freeman et al., 1993; Gordon et al., 2018; Kanes et al., 2017). Studies on its highly correlated precursor, pregnenolone, and its derivative, allopregnenalone (ALLO) are of further interest. Low ALLO levels during pregnancy are associated with increased risk for post-partum depression (Osborne et al., 2017), and in patients suffering from an acute major depressive disorder, ALLO levels from cerebrospinal fluid are about 60% lower than in controls (Uzunova et al., 1998).
Overall, there is a noticeable lack of large cohort studies of well-characterized female populations examining symptoms of depression and anxiety and their relation to sex hormones. This is especially true in low and middle income settings (Fontanarosa and Bauchner, 2018). This, in part, reflects measurement issues arising from the fact that as sex hormone levels vary over the menstrual cycle, hormonal measurement based on serum, saliva, or urine samples requires collecting multiple samples to correctly assess basal sex hormone levels (Giltay et al., 2012). It is more challenging to obtain these samples for women living in low-income settings. Given this, the relatively new method of hair steroid profiling presents a promising opportunity for large cohort studies examining the association between sex hormone levels and depression and anxiety symptoms in women, especially in lower income settings (Gao et al., 2016). Hair steroid profiling circumvents diurnal and lunar hormonal variation by quantifying the integrated hormone concentration accumulated over several months. Therefore, hair-based measures of DHEA, P, and T concentrations from hair holds the potential of yielding a clearer picture of the association between sex steroids and depression and anxiety symptoms. That said, having larger more representative samples of women also highlights the need for sensitivity analysis regarding non-detectable values of sex hormone, which has been largely absent thus far. Non-detectable values may reflect the sensitivity of the assays to detect very low levels, or truly low levels of these hormones, and hence removing these samples from analysis may lead to a systematic bias in results. Because hair-based assays are still being refined, it is important to approach analyses in multiple ways to understand and interpret correlations. Our paper undertakes such a sensitivity analysis.
2. Methods
2.1. Study population
The Kshetriya Gramin Financial Services (KGFS) study is a cohort study evaluating the impact of village access to financial services in rural Tamil Nadu, India. The study occurred in the context of the expansion by KGFS, a private rural bank, across villages in three districts in Tamil Nadu. Sample flow is shown in Figure 1. The main study sample of 4,160 households is a randomly selected subset of households across 876 villages. In total, 4,575 households were approached for surveying, yielding a high response rate of 91%. In 3,764 households, an eligible female household member was identified as a potential participant in the hair steroid profiling study. Criteria for inclusion in hair steroid profiling study were (in order of priority): (i) the mother of the youngest child in the household (with a resident husband), (ii) the youngest married woman (with a resident husband), and (iii) the youngest single adult woman in the household. All study participants were asked for informed consent, and excluded from hair sampling if informed consent was not provided (N = 485). Between 2011 to 2016, 3,279 women provided hair samples. However, prior to the 2013 examination wave, sex steroids were not assayed reducing the sample to 2,357 observations. Data on hormonal outcome measures (DHEA, P, T) in parallel with the examined psychological measures were available for 2,105 women because, for 252 (10.7%) of the 2,357 samples, biochemical measurements were not possible due to insufficient hair quantity or quality (e.g. the hair strands in the aluminum foil had fallen apart in such a way that no beginning or end could be identified). Of the 2,105 hair samples analyzed for sex steroids, several individual samples failed to reach the limit of quantification especially for T, which is frequently present in only trace amounts in women. For our main analysis the mean between zero and the lower limit of detection of a hormone parameter was imputed for non-detectable values (NDs) and we provide more details below.
Figure 1.
Sample flow. Note: Final sample size for DHEA and T is 2,105 observations and for P 2,097 (due to 8 missing values for P).
2.2. Hair sample collection and biochemical analysis
Hair samples were collected, regardless of participants’ usage of hair products (e.g. hair dying, usage of hair oil, hair washing frequency). Usage of these products or other factors that could affect hair steroid concentration (e.g. regular hormonal cream usage) were assessed by self-report.
Our hair sampling procedure followed the procedure described by Gao et al. (2013). The hair strand was cut as close as possible to the scalp from a posterior vertex position. A minimum of 20 mg of hair was obtained from each participant in order to provide sufficient material for biochemical analysis. The first scalp-near 3 cm hair segment was used for analyses. Based on an average hair growth rate of 1 cm per month (Wennig, 2000), steroid concentrations in this segment are assumed to reflect integrated hormone secretion over the 3 month period prior to hair sampling (Stalder and Kirschbaum, 2012). Hair samples were sent to the Dresden Lab GmbH for biochemical analyses.
Hair steroids were determined via liquid chromatography tandem mass spectrometry (LC-MS/MS) according to the protocol by Gao et al., which is considered the current gold-standard approach for hair steroid analyses (Gao et al., 2016). It achieves good sensitivity, specificity, and reliability for hair steroids (T: intra and inter-assay CVs between 3.1 – 8.8% and a limit of detection ≤ .1 pg/mg, DHEA: intra and inter-assay CVs between 4.5 – 9.1% and a limit of detection of ≤ .9 pg/mg, P: 4.3 – 8.3% and a limit of detection of ≤ .1 pg/mg) (Gao et al., 2013).
In the 2,105 hair samples, three sex steroids - DHEA, P, T - were assayed. Hair DHEA was successfully quantified in 1,981 samples. For 124 samples (5.9%) with NDs due to low concentration in samples, our main analysis uses values imputed with the mean between the lower limit of detection and zero. The analysis sample has 2,105 observations. Hair P and hair T were successfully quantified in 1,787 and 1,130 samples, respectively. For P, 310 samples (14.8%) were classified as NDs, while 8 observations were identified as missing due to no quantification report by the laboratory, and for T, 975 (46.3%) NDs were identified and imputed resulting in 2,097 observations for P and 2,105 observations for T.
2.3. Psychometric measure: K6
An interviewer-administered version of the K6 was used to assess mental health symptoms experienced during the last 30 days by the participant, including feelings of nervousness, restlessness, depressed mood, hopelessness, worthlessness, and decreased initiation (Kessler et al., 2003; Lace et al., 2018). Subjects’ assessment of how often each of these six feelings were experienced were converted to a five-point Likert scale ranging from 0 (“none”) to 4 (“all”). The six scores were summed to obtain a final score of nonspecific psychological distress, ranging from 0–24. Although the K6 was designed as a brief instrument screening for nonspecific psychological distress, the cut point at 12 is often used to identify serious mental illness (SMI) (scores 0–12 meaning no serious mental illness; 13–24 indicating cases of serious mental illness) (Kessler et al., 2010, 2003). More recently, there has been interest in constructing two K6 sub-indices (two-factor model). In particular, the six measures can be used to construct separate indicators of depressive symptoms based on four items (depressed mood, hopelessness, worthlessness, and decreased initiation), and anxious symptoms based on the remaining two items (nervousness, restlessness). The two-factor solution is shown to perform better in a predominantly Caucasian sample of 1,060 individuals (67.6% female) from the general population (Lace et al., 2018). Finally, each woman’s household completed a household survey which collected information on behaviors, socio-economic conditions and health.
2.4. Statistical analysis
Sample characteristics are described in means and standard deviations (SD). Of the 2,105 included participants, hormone parameters from hair (DHEA, P, T) are log-transformed to approximate a normal distribution. We follow a trimming procedure for outliers: values with ±3 standard deviations from the mean are excluded. This leads to an exclusion of 124 (5.9%) cases for DHEA (N = 1,981), of 0 (0%) cases for P, and of 0 (0%) cases for T.
To evaluate whether a one-factor K6 “general psychological distress” or a two-factor K6 with factors “depressive symptomatology” and “anxiety symptomatology” better fits the data, an exploratory (EFA) and subsequent confirmatory factor analysis (CFA) is performed. This is conducted using the R 3.4.3 statistical software by the R Core Team (R Core Team, 2017) in combination with the psych package 1.7.8. by Revelle (Revelle, 2017) and Lavaan package version 0.6–2 (Rosseel, 2012). In accordance with Lace et al. (Lace et al., 2018), factor analyses are estimated by unweighted least squares. After Kaiser normalization, a Promax rotation is conducted. A forced one-factor model and a forced two-factor model are estimated. Model fit is determined by Root Mean Square Residuals (RMSR), Tucker Lewis Index (TLI), and Root Mean Square Error of Approximation (RMSEA). A Chi-square difference test is then conducted to indicate which factor solution demonstrates better fit.
To examine potential associations between the K6 scales and steroid hormones, two-tailed bivariate Pearson correlations are conducted. To check for potential influential data points, two-tailed bivariate Spearman correlations are subsequently conducted. Two-tailed hypothesis testing is applied due to the conflicting literature showing positive and negative associations between sex steroids and depressive or anxiety symptoms. Next, two-tailed partial correlations are conducted to control for a priori defined confounders as outlined above. Partial correlations carried out include the following covariates: age, BMI, tobacco consumption, pregnancy status, working status, use of cortisol creams, hair dye use, and when hair samples were collected.
Linear stepwise regression analyses are used to examine associations between steroid hormone levels and depressive and anxious symptomatology. To account for confounding variables, in the first step of the regression analysis, the described set of covariates is included in the regression model, and in a second step, the predictor variable of interest (e.g. DHEA) is entered into the model. This allows us to identify the additional explanatory effect of the predictor variable of interest in addition to the covariates on the investigated criterion (e.g. depressive symptoms). To evaluate potential subgroup effects, moderation analyses following Preacher and Hayes (Hayes, 2013) are conducted examining the effects of age, BMI, tobacco consumption, working and pregnancy status on the associations between sex hormones and anxio-depressive burden (for moderation analyses and supporting information see supplementary material).
Analyses were carried out using SPSS (IBM Statistics, Version 23, Armonk, NY: IBM Corp.) and R 3.4.3. (R Core Team, 2017).
3. Results
3.1. Sample characteristics
Our sample includes 2,105 women from 876 villages in Tamil Nadu, India. Table 1 reports study population demographics. Women are on average 39 years old and almost exclusively Hindu (96.5%). Most women are married (93.4%) with at least one child (94.7%), consistent with the inclusion criteria. About one third (34.8%) report working. The distributions of general psychological distress, depressive symptoms, and anxiety symptoms measured by the K6 scale tend to be normally distributed (see supplementary Figure 1). The distributions of log-transformed and trimmed (±3 SD from mean) sex steroids are significantly non-normally distributed in the sample (all p < .2), but by the central limit theorem, given the large sample size and our visual inspection of the distribution, parametric testing is appropriate (see supplementary Figure 2). Using a K6 cut off score of “above 12 of the total score” to indicate cases of serious mental illness (SMI) (Kessler et al., 2010, 2003) suggests a positive screening for 11.5% of the total sample. This is consistent with large epidemiological studies reporting rates for depressive and anxiety disorders combined that range between 6 – 12% in the global population (World Health Organisation, 2017).
Table 1.
Sample characteristics
| Sociodemographics | Total sample |
|---|---|
| N = 2,105 | |
| Age, M (SD) | 39.3 (10.3) |
| BMI, M (SD) | 22.8 (4.7) |
| Tobacco use, % | 39.2 |
| Hindu, % | 93.2 |
| Married, % | 93.4 |
| Having children, % | 94.9 |
| Pregnant, % | 1.6 |
| Working, % | 34.3 |
| Psychological Distress (K6), M (SD) | 8.90 (3.27) |
| Serious Mental Illness (K6 > 12), % | 11.5 |
| Serious Mental Illness (K6 > 10), % | 29.1 |
| Depressive Symptoms (K6-D), M (SD) | 5.55 (2.48) |
| Anxiety Symptoms (K6-A), M (SD) | 3.35 (1.43) |
| Dehydroepiandrosterone (DHEA) (pg/mg), M (SD) | 5.81 (7.21) |
| Progesterone (P) (pg/mg), M (SD) | 6.03 (10.4) |
| Testosterone (T) (pg/mg), M (SD) | .393 (.98) |
| Hair is dyed, % | 6.5 |
| Use of cortisol cream, % | 1.7 |
| Sample collection time, % |
Note: BMI: body mass index; K6: Kessler-6 Psychological Distress Scale, K6-D: Kessler-6 Depression Subscale, K6-A: Kessler-6 Anxiety Subscale. Numbers of participants vary depending on variable.
3.2. Exploratory (EFA) and confirmatory (CFA) factor analysis for the K6 scale
Before testing the main hypotheses, we conduct factor analyses of the K6 with results shown in supplementary Table 2. A forced one-factor (psychological distress) EFA and a forced two-factor analysis (K6-Depression Scale [K6-D]/K6-Anxiety Scale [K6-A]) are conducted. The one-factor model explains 22% of the variance. Loadings range between .25 to .55. The two-factor model accounts for 29% of the variance (depression = 17%, anxiety = 12%). The extracted factor structure is similar to the one reported in Lace et al. (2018). Both extracted factors correlate at r = .67. All fit parameters display a good to excellent fit. Comparison of the two models confirms a better fit with the two-factor model (Δχ2 = 123.89, Δdf = 5, p < .001). Loadings for three of the four items of the depression factor range between .34 to .66, while for the two items of the anxiety factor they are .52 and .78, respectively. However, the item “decreased initiation”, in Lace et al. loading on the depression factor, shows a contradicting cross-loading on both factors (depression: .09, anxiety: .17). CFA further shows an overall better model fit for the two-factor solution (one-factor: χ2 = 178.2, df = 9, GFI = .984, NIF = .924, SRMR = .036; two-factor: χ2 = 86.7, df = 8, GFI = .992, NIF = .963, SRMR = .026), which is also statistically significant for CFA (Δχ2 = 91.5, Δdf = 1, p < .001). Based on these results, we apply the two-factor model. To ensure comparability between our study and the results obtained by Lace et al. (2018), we compute the two factors as previously described (K6-D/K6-A) (Lace et al., 2018). Importantly, Lace et al. report the two factor model accounts for 85.5% of the total variance in their Western sample, while in our Indian sample 29% of the total variance is explained. Therefore, additional analyses are conducted evaluating a potential exclusion of the item which could not be clearly allocated. In the supplementary material, we provide results based on the two factor solution excluding the item with the contradictory cross-loadings “decreased initiation” for the calculation of K6-D. Our main results are robust to this alternative approach. For the purpose of comparison, results based on the one-factor solution identifying nonspecific psychological distress with sex steroids are reported in the supplementary material.
3.3. Correlation analyses
Next, we examine the association between the K6-D and the K6-A and sex steroids. Bivariate two-tailed Pearson correlations in Table 2a reveal a significant inverse correlation between the K6-D and DHEA levels (r = −.086, p < .001), but a positive association with T levels (r = .109, p < .001). Using Spearman correlation analyses to account for potential influential data points, our results show the same pattern with slightly stronger correlations for DHEA and P identifying an inverse association for both (DHEA: rs = −.100, p < .001; P: rs = −.047, p < .05) and a positive association for T (rs = .088, p < .001). Partial correlation analyses adjusting for confounders shows a significant inverse association between the K6-D and DHEA levels (rp = −.100, p < .001), no association for P levels (rp = −.004, p > .1), and a positive association for T levels (rp = .102, p < .001).
Table 2a.
Correlation table for depression and anxiety symptoms (K6-D/A) and DHEA, P, T including estimated NDs
| Pearson, r | K6-D | K6-A | DHEA | P | T |
| K6-D | 1 | ||||
| K6-A | .350** | 1 | |||
| DHEA | −.086** | −.032 | 1 | ||
| P | −.020 | −.023 | −.062** | 1 | |
| T | .109** | .101** | −.169** | .145** | 1 |
| Spearman, rs | K6-D | K6-A | DHEA | P | T |
| K6-D | 1 | ||||
| K6-A | .289** | 1 | |||
| DHEA | −.100** | −.052* | 1 | ||
| P | −.047* | −.014 | .095** | 1 | |
| T | .088** | .079** | −.098** | .068** | 1 |
| Partial, rp | K6-D | K6-A | DHEA | P | T |
| K6-D | 1 | ||||
| K6-A | .327** | 1 | |||
| DHEA | −.100** | −.036 | 1 | ||
| P | −.004 | −.004 | −.063** | 1 | |
| T | .102** | .105** | −.176** | .145** | 1 |
Note: Partial correlations were conducted controlling for age, bmi, tobacco consumption, pregnancy status, working status, hair dying, usage of cortisol cream, season of sampling. One-tailed correlations are reported. NDs: non-detectable values,tindicates associations below p = .1,
indicates associations below p = .05,
indicates associations below p = .01.
For K6-A, bivariate two-tailed Pearson correlations show no association with DHEA levels (r = −.032, p > .1), or with P levels (r = −.023, p > .1). For T levels a positive association emerges (r = .101, p < .001). Spearman correlations show for DHEA a negative association (rs = −.052, p < .05), but not for P (rs = −.014, p > .1). For T consistently a positive association is identified (rs = .079, p < .001). Partial correlation analyses adjusting for confounders identifies no association between the K6-A and DHEA levels (rp = −.036, p > .1), or P levels (rp = −.004, p > .1), but a positive association with T levels (rp = .105, p < .001) (see Table 2a).
Results examining the sensitivity sample (excluding imputed NDs) are shown in Table 2, and reveal that data imputation for NDs significantly affects results for P and T. P shows in the sensitivity sample a negative association with depressive symptoms. The positive association between T and depression or anxiety symptoms diminishes completely and a negative trend between T and depressive symptoms emerges.
3.4. Regression analyses
Next, regression models are computed. To account for the potential effect of sex steroids in addition to established sociodemographic and confounding variables, models are calculated step-wise with relevant sociodemographic and confounding variables being introduced into the model first.
Sex steroids as predictors for depressive symptoms and anxiety symptoms
As shown in Table 3a, employing K6-D as criterion, a significant age/BMI-adjusted regression model emerges (F = 33.20, p < .001, R2 = .033), while including DHEA in a second step improves the model (F = 28.04, p < .001, R2 = .041). The improved model includes age (standardized β-coefficient [sβ] = .178, p < .001), BMI (sβ = −.055, p = .013) and DHEA (sβ = −.091, p < .001) as predictors. The multivariable-adjusted regression model shows similar results suggesting DHEA (sβ = −.100, p < .001) significantly improves the entire model (F = 19.963, p < .001, R2 = .052). Employing K6-A as criterion, no significant effects for DHEA emerge (see Table 3a). Conducting stepwise regression analyses including P as predictor on K6-D or K6-A as criteria, P is not identified (either in age/BMI-adjusted nor multivariable adjusted regression models) as significant predictor (see Table 3a). By contrast, T is identified as a significant predictor of K6-D, revealing for the entire model a significant effect (F = 29.372, p < .001, R2 = .040) with age (sβ = .164, p < .001), BMI (sβ = −.058, p = .007), and T (sβ = .101, p < .001) as significant predictors. The multivariable-adjusted regression model shows similar results (F = 23.475, p < .001, R2 = .046) with T as a significant predictor (sβ = .101, p < .001). Similarly, T is identified as significant positive predictor of K6-A in age/BMI (sβ = .097, p < .001) and multivariable adjusted (sβ = .100, p < .001) regression models.
Table 3a.
Between subject effects for stepwise linear regression models for steroids as predictors on depression and anxiety symptoms as criterion (including estimated NDs)
| Depression Symptoms (K6-D) | Anxiety Symptoms (K6-A) | |||
|---|---|---|---|---|
| F (p) | β-coefficient (R2) | F (p) | β-coefficient (R2) | |
| Dehydroepiandrosterone (DHEA) | ||||
| Age-/bmi-adjusted | 28.044 (<.001) | −.091** (.041) | 61.892 (<.001) | −.038 (NI) (.030) |
| Multivariable-adjusted | 19.963 (<.001) | −.100** (.052) | 34.334 (.004) | −.034 (NI) (.036) |
| Progesterone (P) | ||||
| Age-/bmi-adjusted | 32.940 (<.001) | −.006 (NI) (.031) | 58.986 (<.001) | −.005 (NI) (.027) |
| Multivariable-adjusted | 24.234 (<.001) | −.002 (NI) (.036) | 31.119 (<.001) | −.001 (NI) (.031) |
| Testosterone (T) | ||||
| Age-/bmi-ad)usted | 29.372 (<.001) | .101** (.040) | 40.026 (<.001) | .097** (.037) |
| Multivariable-adjusted | 23.475 (<.001) | .101** (.046) | 27.830 (<.001) | .100** (.041) |
Note: Data are standardized β-coefficients, with p < 0.05 marked as *, and p < 0.01 marked as **. NDs: non-detectable values, NI: K6-D/A was not included into the step-wise regression model. All models were adjusted for age and bmi, while multivariable models were additionally adjusted for smoking status, pregnancy status, working status, use of hormonal creams, hair being dyed, season of sampling.
Similarly to the correlational analysis, the sensitivity analysis yields markedly different results for P and T (see Table 3b). P emerges as negative predictor of the K6-D, while T is no longer positively associated with either K6-D or K6-A.
Table 3b.
Between subject effects for stepwise linear regression models including sex steroids as predictors on depression and anxiety symptoms as criterion (excluding estimated NDs)
| Depression Symptoms (K6-D) | Anxiety Symptoms (K6-A) | |||||
|---|---|---|---|---|---|---|
| F (p) | β-coefficient (R2) | F (p) | β-coefficient (R2) | |||
| Dehydroepiandrosterone (DHEA) | ||||||
| Age-/bmi-adiusted | 28.154 (<.001) | −.092** (.041) | 61.929 (<.001) | −.038t (NI) (.030) | ||
| Multivariable-adjusted | 20.142 (<.001) | −.092** (.049) | 37.478 (<.001) | −.038t (NI) (.037) | ||
| Progesterone (P) | ||||||
| Age-/bmi-adjusted | 21.742 (<.001) | −.054* (.035) | 49.916 (<.001) | −.008 (NI) (.027) | ||
| Multivariable-adjusted | 16.074 (<.001) | −.048* (.044) | 32.096 (<.001) | −.015 (NI) (.035) | ||
| Testosterone (T) | ||||||
| Age-/bmi-adjusted | 25.695 (<.001) | −.019 (NI) (.044) | 43.948 (<.001) | .028 (NI) (.038) | ||
| Multivariable-adjusted | 23.372 (<.001) | −.023 (NI) (.040) | 21.886 (<.001) | .032 (NI) (.038) | ||
Note: Data are standardized β-coefficients, with p < 0.05 marked as *, and p < 0.01 marked as **. NDs: non-detectable values, NI: K6-D/A was not included into the step-wise regression model. All models were adjusted for age and bmi, while multivariable models were additionally adjusted for smoking status, pregnancy status, working status, use of hormonal creams, hair being dyed, season of sampling.
Further analyses for specific subpopulations (e.g. age above vs. below 51 years, excluding women with serious mental illness) and moderator analyses for different potential influential variables (e.g. age, BMI, work status) are presented in the supplementary material.
3.5. Sensitivity analysis
As shown in supplementary Figure 2, the distribution, mean and SD of hormone parameters vary depending on the inclusion or exclusion of NDs. We hypothesize that ND values do not occur at random, but systematically reflect too low hormone concentrations in the samples. Therefore, we impute NDs uniformly as the mean between the respective lower limit of detection of a hormone and zero, described in detail in the supplement.
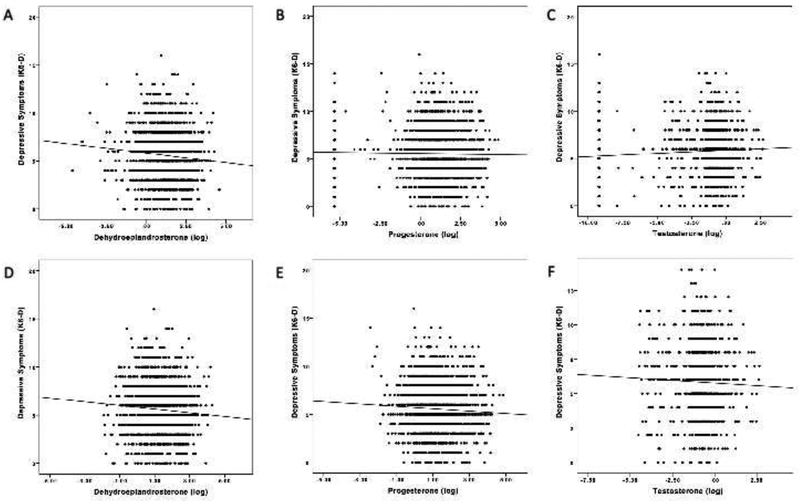
Our approach of imputing differs from the common practice of excluding ND values. Figure 2 and Tables 2b and 3b report how the results differ across the two approaches. Bivariate two-tailed Pearson correlations excluding NDs show significant inverse correlations between the K6-D and DHEA levels (r = −.078, p < .001) and P levels (r = −.058, p = .015), but not T levels (r = −.032, p = .281). Effect size interpretation of the identified associations suggests small effects (Cohen, 1992). Using Spearman correlation analyses to account for potential influential data point results shows the same pattern with slightly stronger correlations, and for T identifies an association on trend level (DHEA: rs = −.096, p < .001; P: rs = −.066, p < .001; T: rs = −.053, p = .075). Partial correlation analyses adjusting for confounders show significant inverse associations between the K6-D and DHEA levels (rp = −.099, p < .001) as well as for T levels (rp = −.065, p = .038), and a negative trend for P levels (rp = −.047, p = .058). Supplementary Table 4 shows correlations using the three-item K6-D-r scale; results did not differ from the correlation analyses with the original K6-D scale. For the K6-A, bivariate two-tailed correlations show a significant negative association with DHEA levels, but no association with P or T levels (DHEA: r = −.045, p = .045; P: r = −.003, p > . 1; T: r = .00, p > .1). Spearman and partial correlations further support these findings (see Table 2b). A detailed description of the sensitivity analysis for regression analyses is provided in the supplementary materials. Similarly to the correlational analysis, P emerges as negative predictor of the K6-D, while T is no longer associated with either the K6-D or the K6-A.
Figure 2.
Linear associations between depressive symptoms (K6-D) and sex steroids (DHEA, P, T) for the analyses including NDs (panel A-C) and excluding NDs (panel D-F).
Table 2b.
Correlation table for depression and anxiety symptoms (K6-D/A) and DHEA, P, T excluding estimated NDs
| Pearson, r | K6-D | K6-A | DHEA | P | T |
| K6-D | 1 | ||||
| K6-A | .402** | 1 | |||
| DHEA | −.078** | −.045* | 1 | ||
| P | −.058* | −.003 | .276** | 1 | |
| T | −.032 | .00 | .359** | .155** | 1 |
| Spearman, rs | K6-D | K6-A | DHEA | P | T |
| K6-D | 1 | ||||
| K6-A | .331** | 1 | |||
| DHEA | −.096** | −.059** | 1 | ||
| P | −.066** | −.003 | .305** | 1 | |
| T | −.053t | −.015 | .409** | .196** | 1 |
| Partial, rp | K6-D | K6-A | DHEA | P | T |
| K6-D | 1 | ||||
| K6-A | .375** | 1 | |||
| DHEA | −.099** | −.053* | 1 | ||
| P | −.047t | .002 | .280** | 1 | |
| T | −.065* | −.006 | 393** | .152** | 1 |
Note: Partial correlations were conducted controlling for age, bmi, tobacco consumption, pregnancy status, working status, hair dying, usage of cortisol cream, season of sampling. One-tailed correlations are reported. NDs: non-detectable values,
indicates associations below p = .1,
indicates associations below p = .05,
indicates associations below p = .01.
To summarize, analyses of DHEA are robust to either imputing or excluding ND values, which is unsurprising given that so few observations are classified as NDs (a difference of 124 [5.9%] observations). The choice of approach to ND values is more relevant for the cases of P and T, which contain 310 (14.8%) and 975 (46.3%) NDs, respectively. In the case of P, when NDs are imputed, there is a consistent negative association with K6-D but is only statistically significant using Spearman correlations, while if NDs are excluded, there is a consistent significant negative association between K6-D and P across nearly all analyses. For T, a significant positive association emerges for T and K6-D as well as K6-A when imputing for NDs, a pattern that is entirely absent and even in some cases reversed when NDs are excluded. Excluding NDs for variables that are present in trace amounts for a large fraction of the population can lead to misleading conclusions about the underlying associations between variables in the dataset. Hence, for measures such as T in the female population, which are anticipated to have many ND values ex-ante, it is important not to rely on protocols that have been developed to deal with variables and populations that contain a very small number of ND values.
4. Discussion
4.1. Summary of results
This is the first study of a large cohort of 2,105 women from a developing country to report an inverse association between depressive symptoms and long-term integrated DHEA concentrations quantified from hair samples. Further, a positive correlation between hair concentrations of T and both depressive and anxiety symptoms emerges when using imputed values. Based on these results, lower levels of DHEA and higher levels of testosterone are associated with increased psychological distress in women, and these findings emerge more clearly for depressive versus anxiety symptoms. The present study cannot state conclusively the causal direction of this association due to its cross-sectional nature, but research suggests a bidirectional relationship with interdependent adaptations on the psychological and hormonal level. The magnitudes of the associations are overall modest, potentially due to large heterogeneity in the sample and relevant moderators influencing these relations, meaning DHEA, P, or T levels explain little of the overall variance in psychological distress. When NDs are not considered (i.e. when excluding imputed NDs), the negative relation between depressive symptoms and DHEA remains robust, but for T, its positive association with psychological distress fades and trends negative in relation to depressive symptoms. The previously null association between P and depressive symptoms also becomes significantly negative. Moderation analyses (see supplementary material), though not statistically significant after correction for multiple testing, indicate that the negative association between psychological distress and DHEA may be especially relevant for rural Indian women who are daily wage workers, while associations between psychological distress and T are moderated by BMI, and less consistently by tobacco consumption and work status. Taken together, our primary analysis suggests that higher endogenous levels of DHEA may be regarded as protective against depressive symptoms, while higher levels of T are associated with increased psychological distress. Further analysis would be needed to understand which subgroups of women are particularly affected.
4.2. Depression and anxiety disorders and androgens in women: Integration of findings
Depressive symptoms and androgens in women
Our results contribute to a growing body of evidence suggesting a role of androgens in the pathophysiology of depressive and anxiety disorders in women. However, the direction of this relation varies from negative to positive between studies depending on the examined sample of women. In the present study the observed negative association between long-term integrated DHEA levels extracted from hair samples and depressive symptoms is comparable with a previous report of one large US sample comprising of 699 non-estrogen using postmenopausal women aged between 50 and 90 years. In the US study, of plasma T, DHEA, and its sulfated conjugated metabolite (DHEA-S), only plasma DHEA-S is inversely associated with depressive symptoms showing a correlation coefficient of r = .14 (Barrett-Connor et al., 1999a). This has been further confirmed by other studies examining DHEA-S in elderly women aged between 70 and 79 years (Morsink et al., 2007) or in patients with chronic fatigue syndrome or depression (Scott et al., 1999). Yet, there have also been reports of increased or unaltered DHEA/-S levels in relation to increased depressive symptoms (Maninger et al., 2009).
Our observed positive association between T and psychological distress based on using imputed values differs from several previous studies showing a negative association (Giltay et al., 2012; Kische et al., 2017). Giltay et al. (2012) finds, using pooled saliva samples from 1,380 women from Netherland (aged 18 to 64), that lower salivary T levels are present among women who exhibit a current depressive or anxiety disorder, in comparison to healthy controls. Furthermore, women using antidepressants show significantly higher T levels compared to non-users. In another large multi-site US study, women with a lower calculated free androgen index – based on plasma T, DHEA-S, and sex hormone binding globulin (SHBG) levels – report more depressive symptoms (Santoro et al., 2005). While the above studies suggest some association between depressive symptoms and sex steroids, not all studies find these associations: while Kische et al. (2017) first identify inverse associations between overnight fasting one-time point morning serum androgens with depressive symptomatology in a sample of 2,148 women from Germany, multivariable adjustment renders these identified associations insignificant. Similarly, Giltay et al. (2017) find that no clear association between serum T and depressive symptoms emerges in a sample of 303 older women aged 60 years or above. Finally, although only few, there do exist studies reporting a positive association between depressive symptoms and T in female populations (Matsuzaka et al., 2013; Weber et al., 2000). Bromberger et al. (2010) identify that during the menopausal transition, higher T levels are cross-sectionally and longitudinally positively associated with increased depressive burden in 3,302 women aged between 42 and 52 years.
The current study, thus, provides new insights into this relation and also demonstrates the importance of accounting for ND values, especially in studies of hair T. We note that since the hair androgens in this study reflect integrated hormone concentrations accumulated over a three months period, these measures are only comparable to a limited extent with the above mentioned studies using single or pooled saliva or serum samples, and therefore provide new insight into the temporal dynamics between hair sex steroids and psychological distress in women.
Anxiety symptoms and androgens in women
Given the dearth of studies focusing on women and the association between their androgens and anxiety, our study contributes on multiple fronts. First, we report results based on one of the largest samples of women drawn from a low income setting. Second, our results are mixed, with a rather negative but not entirely consistent relation between anxiety symptoms and DHEA, and a positive association with T which is sensitive to treatment of ND values.
The modest negative relation between hair DHEA levels and the K6-A scale finds some support in the literature: for instance, a previous study comparing plasma T and DHEA levels in women with diagnosed major depressive disorder, high anxiety symptoms, and low anxiety symptoms with healthy controls reports that the women with depression and high levels of anxiety symptoms exhibit the lowest serum T and DHEA levels (Oulis et al., 2014).
The negative association between the K6-A and hair DHEA and the positive association between the K6-A and hair T levels are in keeping with the mixed findings in the literature. Some studies find that low salivary T levels are not associated with anxiety symptoms in adolescent females (Granger et al., 2003). A small sample study of college students finds no significant correlation between social anxiety levels and salivary T levels in women, although we cannot rule out the possibility that this reflected power limitations related to small sample size (Maner et al., 2008). Turning to T, Giltay et al. (2012) shows a large cross-sectional study that lower pooled salivary T levels are associated with diagnosed general anxiety disorder, social phobia, and agoraphobia in women. In contrast, a recent prospective analysis finds no association between elevated serum T and increased risk for anxiety disorders in women (Asselmann et al., 2019). We note here two reasons that may limit the applicability of these studies to our context: our study uses hair samples rather than saliva or serum samples, and is drawn from a distinct population of rural women in a low-income setting.
It is also important to note that our study employed the K6-A, which is based on two items covering general nervousness and restlessness. This may limit our ability to identify smaller effects, in comparison, for example, to the large study of 1,380 women by Giltay et al. (2012). The latter reports clear differences between anxiety patients and healthy controls based on DSM-IV diagnostics by Composite International Diagnostic Interview. That said, we argue that hair samples are particularly suited for large scale analysis due to simple sampling procedure and the minimization of bias compared to other specimen, where circadian and menstrual cycle variations in sex hormones constrain accurate quantification (Stalder and Kirschbaum, 2012). The combination of sex steroid hormone quantification from hair and diagnostics with Composite International Diagnostic Interview in longitudinal designs holds great promise for future studies on the relation between anxiety (disorders) and sex steroids in women.
P and anxio-depressive symptomatology in women
Our study found no association between P levels from hair and depressive or anxiety symptoms. Existing studies examining the role of P in women with depressive disorders report mixed results on how P levels are associated with depression and anxiety (for a review, see Schüle et al., 2011). In contrast, ALLO – a metabolite of P and not P itself – has been found to be inversely related to different forms of depressive disorders in women. One pioneering study shows markedly reduced cerebrospinal fluid ALLO levels in acutely depressed patients (Uzunova et al., 1998). Women suffering from premenstrual dysphoric disorder are found to have lower circulating ALLO levels compared to controls, for whom P levels are not different (Rapkin et al., 1997). Administration studies using P or its metabolites such as ALLO in women further reveal beneficial effects on depressive symptoms (Gordon et al., 2018; Kanes et al., 2017). Future studies on hair sex steroid levels in women and depressive burden might include ALLO quantification in parallel to P quantification to further confirm these findings.
Given the existing evidence, it is unsurprising that we did not identify an association between hair P levels and anxiety symptoms.
4.3. Strengths and Limitations
While our study has many strengths, including state of the art data collection of hormones from hair samples of a large cohort of understudied women from rural India, and detailed information on critical covariates related to depressive and anxiety symptoms and the hair sample quality, some limitations must be taken into account when interpreting the results. First, no men were included which prevents us from examining gender-differences in the associations and examining the sensitivity of T analysis to ND values among men. Second, while steroid profiling from hair is a fast growing and promising field, we find that it can yield a relatively large amount of NDs for P and T. The field, clearly, requires common standards on how to handle ND values (criteria for imputation vs. exclusion), especially for T. Third, the K6 - while commonly used as a measure of psychological distress in large-scale cross-country studies of mental health - is not a clinical measure for depressive disorders or anxiety disorders. The previously mentioned cross-loading for the item “decreased initiation” with regard to the two-factor structure may have introduced imprecision in the factor structure. Questionnaire data on psychosocial stress would have been useful in better understanding the relation between hair sex steroids and anxio-depressive symptoms.
Another limitation is the lack of information about intake of medication or oral contraception, which are confounders of sex steroid concentrations in women; in Tamil Nadu, the National Family Health Survey 2015–2016 found 53% of women between the ages of 15–49 use any form of family planning methods, but we lack sample-specific knowledge on oral contraception and other medication use. Future studies should also consider impact of controlling for the stage of the reproductive phase, given their central influence on the levels of sex steroids. That said, the onset of menopause in women is worldwide on average around 51 years of age and lasts on average about 10 years until the status of post-menopause is reached, such that the vast majority of women in our sample are too young to be at risk of menopausal influences on sex steroid levels. Moreover, our results are robust given we control for subjects’ age in the regression estimates. Effect sizes are generally small and indicate modest associations with many other interdependent influencing variables. Longitudinal associations can typically improve on cross-sectional estimates in multiple ways, which makes it important to examine these identified associations in longitudinal designs. Repeated measurements of depression and anxiety symptoms with paralleled hair steroid concentrations could enable causal analyses. As an additional point, it seems important to consider that depending on women’s sexual orientation, sex hormones and stress-physiological profiles differ (Juster et al., 2016), so that higher T and P levels are associated with increased cortisol secretion after stress in sexual minority women (Juster et al., 2015). Inclusion of the women’s sexual orientation and its relation to steroid profiles in depression and anxiety is also an interesting avenue for future analysis.
4.4. Conclusion
This is the first study to use data from a large cohort of rural Indian women and show that higher depressive symptomatology is associated with lower levels of hair DHEA. Higher levels of T are associated with increased depressive burden and anxiety symptoms; however, this relationship is sensitive to imputation of ND values.
Higher DHEA levels reflected by long term integrated concentrations from hair are potentially protective against depressive disorders in women. Findings for T in women are difficult to interpret and future work needs to further probe the implications of ND values for analysis. Specific subgroups of Indian women (e.g. obese and working women) demonstrate particularly strong associations. That said, the magnitude of the identified associations remain small, pointing to the value of replication studies. To fully understand the biological underpinnings related to the increased prevalence of depressive and anxiety disorders in women, adequate basal levels and dynamic changes in sex steroid levels need to be considered. Only by doing so can we advance the development of sex-specific biopsychic informed screening instruments for early detection of serious psychological distress. Assessing these biomarkers will then enable us to identify social, behavioral, and clinical prevention programs for depression and anxiety at much earlier stages of disease development, as well as more effective therapies for treatment.
Supplementary Material
Highlights.
In a sample of 2,105 women from rural India, hair samples were obtained for steroid hormone quantification.
Higher depressive burden is associated with lower levels of dehydroepiandrosterone.
A positive association between testosterone and both depressive and anxiety symptoms emerges.
The association between psychological distress and testosterone is sensitive to how non-detectable values are treated.
Progesterone shows no clear association with either depressive or anxiety symptoms.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health under award number R01HD069546. The total project costs of $2,579,000 were wholly financed by Federal funds. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L, 2008. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch. Gen. Psychiatry 65, 283–289. doi: 10.1001/archgenpsychiatry.2007.33 [DOI] [PubMed] [Google Scholar]
- Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, Ron de Graaf WV, Dragomirecka E, Kohn R, Keller M, Kessler R, Kawakami N, Kilic C, Offord D, Ustun T, Wittchen H, 2003. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int. J. Methods Psychiatr. Res 12, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, others, 1999. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N. Engl. J. Med 341, 1013–1020. [DOI] [PubMed] [Google Scholar]
- Asselmann E, Kische H, Haring R, Hertel J, Schmidt C, Nauck M, Beesdo-baum K, Grabe H, Pané-farré CA, 2019. Prospective associations of androgens and sex hormone-binding globulin with 12-month, lifetime and incident anxiety and depressive disorders in men and women from the general population. J. Affect. Disord 245, 905–911. doi: 10.1016/j.jad.2018.11.052 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Von Mühlen D, Laughlin GA, Kripke A, 1999a. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: The Rancho Bernardo study. J. Am. Geriatr. Soc 47, 685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D, 1999b. Bioavailable testosterone and depressed mood in older men: The Rancho Bernardo study. J. Clin. Endocrinol. Metab 84, 573–577. doi: 10.1210/jc.84.2.573 [DOI] [PubMed] [Google Scholar]
- Bekker MHJ, van Mens-Verhulst J, 2007. Anxiety Disorders: Sex Differences in Prevalence, Degree, and Background, But Gender-Neutral Treatment. Gend. Med 21, 178–193. doi: 10.1016/S1550-8579(07)80057-X [DOI] [PubMed] [Google Scholar]
- Berglund LH, Prytz HS, Perski A, Svartberg J, 2011. Testosterone levels and psychological health status in men from a general population: the Tromsø study. Aging Male 14, 37–41. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Schott LL, Kravits HM, Sowers M, Avis NE, Gold EB, Randolph JF, Matthews KA, 2010. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition. Arch. Gen. Psychiatry 67, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P, Kokiwar PR, Shridevi K, Katkuri S, 2017. A study on prevalence and correlates of depression among elderly population of rural South India. Int. J. Community Med. Public Heal 3, 236–239. [Google Scholar]
- Cohen J, 1992. A power primer. Psychol. Bull 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Dandona R, Kumar GA, Dhaliwal RS, Naghavi M, Vos T, Shukla DK, Vijayakumar L, Gururaj G, Thakur JS, Ambekar A, Sagar R, Arora M, Bhardwaj D, Chakma JK, Dutta E, Furtado M, Glenn S, Hawley C, Johnson SC, Khanna T, Kutz M, Mountjoy-Venning WC, Muraleedharan P, Rangaswamy T, Varghese CM, Varghese M, Reddy KS, Murray CJL, Swaminathan S, Dandona L, 2018. Gender differentials and state variations in suicide deaths in India: the Global Burden of Disease Study 1990–2016. Lancet Public Heal 3, e478–e489. doi: 10.1016/S2468-2667(18)30138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Van Der Mooren MJ, Van Lunsen RHW, Lopes P, Ribot J, Rees M, Moufarege A, Rodenberg C, Buch A, Purdie DW, 2006. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: A randomized, placebo-controlled trial. Menopause 13, 387–396. doi: 10.1097/01.gme.0000179049.08371.c7 [DOI] [PubMed] [Google Scholar]
- Deligiannidis KM, Kroll-Desrosiers AR, Mo S, Nguyen HP, Svenson A, Jaitly N, Hall JE, Barton BA, Rothschild AJ, Shaffer SA, 2016. Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology 70, 98–107. doi: 10.1016/j.psyneuen.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RS, Kerr-Corrêa F, Moreno RA, Trinca LA, Pontes A, Halbe HW, Gianfaldoni A, Dalben IS, 2006. Efficacy of hormone therapy with and without methyltestosterone augmentation of venlafaxine in the treatment of postmenopausal depression: a double-blind controlled pilot study. Menopause 13, 202–211. [DOI] [PubMed] [Google Scholar]
- DiBlasio CJ, Hammett J, Malcolm JB, Judge BA, Womack JH, Kincade MC, Ogles ML, Mancini JG, Patterson AL, Wake RW, 2008. Prevalence and predictive factors for the development of de novo psychiatric illness in patients receiving androgen deprivation therapy for prostate cancer. Can. J. Urol 15, 4249–4256. [PubMed] [Google Scholar]
- Fiacco S, Walther A, Ehlert U, 2018. Steroid secretion in healthy aging. Psychoneuroendocrinology doi: 10.1016/j.psyneuen.2018.09.035 [DOI] [PubMed] [Google Scholar]
- Fontanarosa P, Bauchner H, 2018. Race, ancestry, and medical research. JAMA doi: 10.1001/jama.2018.14438 [DOI] [PubMed] [Google Scholar]
- Ford AH, Yeap BB, Flicker L, Hankey GJ, Chubb SAP, Handelsman DJ, Golledge J, Almeida OP, 2016. Prospective longitudinal study of testosterone and incident depression in older men : The Health In Men Study. Psychoneuroendocrinology 64, 57–65. doi: 10.1016/j.psyneuen.2015.11.012 [DOI] [PubMed] [Google Scholar]
- Freeman EW, Purdy RH, Coutifaris C, Rickels K, Paul SM, 1993. Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendocrinology 58, 478–484. [DOI] [PubMed] [Google Scholar]
- Gao W, Kirschbaum C, Grass J, Stalder T, 2016. LC – MS based analysis of endogenous steroid hormones in human hair. J. Steroid Biochem. Mol. Biol 162, 92–99. doi: 10.1016/j.jsbmb.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C, 2013. Quantitative analysis of steroid hormones in human hair using a column-switching LC – APCI – MS / MS assay. J. Chromatogr. B 928, 1–8. doi: 10.1016/j.jchromb.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Zambelli U, Timpano M, Reali N, Bernasconi S, Brambilla F, 2000. Neuroendocrine responses to psychological stress in adolescents with anxiety disorder. Neuropsychobiology 42, 82–92. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Enter D, Zitman FG, Penninx BWJH, van Pelt J, Spinhoven P, Roelofs K, 2012. Salivary testosterone: Associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J. Psychosom. Res 72, 205–213. doi: 10.1016/j.jpsychores.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Giltay EJ, vn der Mast R, Lauwen E, Heijboer AC, Waal MWM De, Comijs HC, 2017. Plasma Testosterone and the Course of Major Depressive Disorder in Older Men and Women. Am. J. Geriatr. Psychiatry 25, 425–437. doi: 10.1016/j.jagp.2016.12.014 [DOI] [PubMed] [Google Scholar]
- Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR, 2003. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 10, 390–398. doi: 10.1097/01.GME.0000060256.03945.20 [DOI] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS, 2018. Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: A randomized clinical trial. JAMA Psychiatry 75, 149–157. doi: 10.1001/jamapsychiatry.2017.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW, 2009. Missing data analysis: making it work in the real world. Annu. Rev. Psychol 60, 549–76. doi: 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, ZAHN–WAXLER C, Usher B, KLIMES–DOUGAN B, Hastings P, 2003. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: individual differences and developmental effects. Dev. Psychopathol 15, 431–449. [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF, 2018. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75, 336–346. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A, 2013. Introduction to mediation, moderation, and conditional process analysis, New York, NY: Guilford. doi:978-1-60918-230-4 [Google Scholar]
- Heald A, Walther A, Davis J, Moreno GYC, Kane J, Livingston M, Fowler H, 2017. No difference in mood and quality of life in DHEA-S deficient adults with Addison’s disease vs. Type 2 diabetes patients with normal DHEA-S levels: implications for management of these conditions. Front. Psychol 8. doi: 10.3389/fpsyg.2017.00764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Almeida D, Cardoso C, Raymond C, Johnson PJ, Pfaus JG, Mendrek A, Duchesne A, Pruessner JC, Lupien SJ, 2016. Gonads and strife: Sex hormones vary according to sexual orientation for women and stress indices for both sexes. Psychoneuroendocrinology 72, 119–130. doi: 10.1016/j.psyneuen.2016.06.011 [DOI] [PubMed] [Google Scholar]
- Juster RP, Hatzenbuehler ML, Mendrek A, Pfaus JG, Smith NG, Johnson PJ, Lefebvre-Louis JP, Raymond C, Marin MF, Sindi S, Lupien SJ, Pruessner JC, 2015. Sexual orientation modulates endocrine stress reactivity. Biol. Psychiatry 77, 668–676. doi: 10.1016/j.biopsych.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, Doherty J, Epperson CN, Deligiannidis KM, Riesenberg R, Hoffmann E, Rubinow D, Jonas J, Paul S, Meltzer-Brody S, 2017. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet 390, 480–489. doi: 10.1016/S0140-6736(17)31264-3 [DOI] [PubMed] [Google Scholar]
- Kessler RC, 2003. Epidemiology of women and depression. J. Affect. Disord 74, 5–13. doi: 10.1016/S0165-0327(02)00426-3 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Howes MJ, Normand S-LT, Manderscheid RW, Walters EE, 2003. Screening for serious mental illness in the general population. Arch. Gen. Psychiatry 60, 184–189. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Green JG, Gruber MJ, Sampson NA, Bromet E, Cuitan M, Furukawa TA, Gureje O, Hinkov H, Hu C, 2010. Screening for serious mental illness in the general population with the K6 screening scale: results from the WHO World Mental Health (WMH) survey initiative. Int. J. Methods Psychiatr. Res 19, 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambaty M, Parikh RM, 2017. Cultural aspects of anxiety disorders in India. Dialogues Clin. Neurosci 19, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kische H, Gross S, Wallaschofski H, Grabe HJ, Völzke H, Nauck M, Haring R, 2017. Associations of androgens with depressive symptoms and cognitive status in the general population. PLoS One 12, 1–13. doi: 10.1371/journal.pone.0177272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kische H, Pieper L, Venz J, Klotsche J, März W, Koch-gromus U, Pittrow D, Lehnert H, Silber S, Stalla GK, Zeiher AM, Wittchen H, Haring R, 2018. Longitudinal change instead of baseline testosterone predicts depressive symptoms. Psychoneuroendocrinology 89, 7–12. doi: 10.1016/j.psyneuen.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Korenman S, Grotts J, Bell D, Elashoff D, 2018. Depression in Non-Classical Hypogonadism in Young men. J. Endocr. Soc js.2018–00137-js.2018–00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace JW, Merz ZC, Grant AF, Emmert NA, Zane KL, Handal PJ, 2018. Validation of the K6 and its depression and anxiety subscales for detecting nonspecific psychological distress and need for treatment. Curr. Psychol 1–10. doi: 10.1007/s12144-018-9846-2 [DOI] [Google Scholar]
- Li SH, Graham BM, 2017. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. The Lancet Psychiatry 4, 73–82. doi: 10.1016/S2215-0366(16)30358-3 [DOI] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Galea LAM, 2016. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocrinol 41, 129–152. doi: 10.1016/j.yfrne.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Maner JK, Miller SL, Schmidt NB, Eckel LA, 2008. Submitting to defeat: Social anxiety, dominance threat, and decrements in testosterone. Psychol. Sci 19, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol 30, 65–91. doi: 10.1016/j.yfrne.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaka H, Maeshima H, Kida S, Kurita H, Shimano T, Nakano Y, Baba H, Suzuki T, Arai H, 2013. Gender differences in serum testosterone and cortisol in patients with major depressive disorder compared with controls. Int. J. Psychiatry Med 46, 203–221. [DOI] [PubMed] [Google Scholar]
- Miller KK, Perlis RH, Papakostas GI, Mischoulon D, Iosifescu DV, Brick DJ, Fava M, 2009. Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS Spectr 14, 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink LFJ, Vogelzangs N, Nicklas BJ, Beekman ATF, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BWJH, 2007. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: Results from the Health ABC study. Psychoneuroendocrinology 32, 874–883. doi: 10.1016/j.psyneuen.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL, 2017. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology 79, 116–121. doi: 10.1016/j.psyneuen.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulis P, Masdrakis VG, Markianos M, 2014. Testosterone and dehydroepiandrosterone sulfate in female anxious and non-anxious major depression. Int. J. Psychiatry Clin. Pract 18, 21–24. doi: 10.3109/13651501.2013.845222 [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Rabkin JG, Mcelhiney D,P, Rabkin MC, Mcgrath R, Ferrando PJ, Subsyndromal SJ, O., 2006. Placebo-Controlled Trial of Dehydroepiandrosterone (DHEA) for Treatment of Nonmajor Depression in Patients With HIV/AIDS. Am. J. Psychiatry 163, 59–66. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB, 1997. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet. Gynecol 90, 709–714. [DOI] [PubMed] [Google Scholar]
- Revelle WR, 2017. psych: Procedures for personality and psychological research. [Google Scholar]
- Rosseel Y, 2012. Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw 48, 1–36. [Google Scholar]
- Santoro N, Torrens J, Crawford S, Allsworth JE, Finkelstein JS, Gold EB, Korenman S, Lasley WL, Luborsky JL, McConnell D, Sowers MF, Weiss G, 2005. Correlates of circulating androgens in mid-life women: The Study of Women’s Health Across the Nation. J. Clin. Endocrinol. Metab 90, 4836–4845. doi: 10.1210/jc.2004-2063 [DOI] [PubMed] [Google Scholar]
- Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR, Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR, 2016. Reproductive Steroid Regulation of Mood and Behavior. Compr. Physiol 6, 1135–1160. doi: 10.1002/cphy.c150014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, Clair LSS, Murphy JH, Haq N, Rubinow DR, 2005. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch. Gen. Psychiatry 62, 154–162. [DOI] [PubMed] [Google Scholar]
- Schüle C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R, 2011. Neuroactive steroids in affective disorders: Target for novel antidepressant or anxiolytic drugs? Neuroscience 191, 55–77. doi: 10.1016/j.neuroscience.2011.03.025 [DOI] [PubMed] [Google Scholar]
- Schüle C, Nothdurfter C, Rupprecht R, 2014. The role of allopregnanolone in depression and anxiety. Prog. Neurobiol 113, 79–87. [DOI] [PubMed] [Google Scholar]
- Scott LV, Salahuddin F, Cooney J, Svec F, Dinan TG, 1999. Differences in adrenal steroid profile in chronic fatigue syndrome, in depression and in health. J. Affect. Disord 54, 129–137. doi: 10.1016/S0165-0327(98)00169-4 [DOI] [PubMed] [Google Scholar]
- Shidhaye R, Gangale S, Patel V, 2016. Prevalence and treatment coverage for depression: a population-based survey in Vidarbha, India. Soc. Psychiatry Psychiatr. Epidemiol 51, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Brain , Behavior , and Immunity Analysis of cortisol in hair – State of the art and future directions. BRAIN, Behav. Immun doi: 10.1016/j.bbi.2012.02.002 [DOI] [PubMed] [Google Scholar]
- T’sjoen GG, De Vos S, Goemaere S, Van Pottelbergh I, Dierick M, Van Heeringen C, Kaufman J, 2005. Sex steroid level, androgen receptor polymorphism, and depressive symptoms in healthy elderly men. J. Am. Geriatr. Soc 53, 636–642. [DOI] [PubMed] [Google Scholar]
- Trivedi J, Gupta P, 2010. An overview of Indian research in anxiety disorders. Indian J. Psychiatry 52, 210. doi: 10.4103/0019-5545.69234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson a, Uzunov DP, Costa E, Guidotti a, 1998. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. U. S. A 95, 3239–3244. doi: 10.1073/pnas.95.6.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N, De Rui M, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E, Sergi G, 2015. Serum dehydroepiandrosterone sulfate and incident depression in the elderly: The Pro.V.A. study. Am. J. Geriatr. Psychiatry 23, 863–871. doi: 10.1016/j.jagp.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Vos T, Abajobir AA, Abbafati C, Abbas KM, Abate KH, Abd-Allah F, …, Murray CJL, 2017. Global, regional, and national incidence , prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990 – 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–59. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Breidenstein J, Miller R, 2019a. Association of testosterone treatment with alleviation of depressive symptoms in men: A systematic review and meta-analysis. JAMA Psychiatry 76, 31–40. doi: 10.1001/jamapsychiatry.2018.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Phillip M, Lozza N, Ehlert U, 2016. The rate of change in declining steroid hormones : a new parameter of healthy aging in men ? Oncotarget 7, 1–28. doi: 10.18632/oncotarget.11752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Rice T, Kufert Y, Ehlert U, 2017. Neuroendocrinology of a Male-Specific Pattern for Depression Linked to Alcohol Use Disorder and Suicidal Behavior. Front. Psychiatry 7, 1–9. doi: 10.3389/fpsyt.2016.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Wasielewska JM, Leiter O, 2019b. The antidepressant effect of testosterone: An effect of neuroplasticity? Neurol. Psychiatry Brain Res 32, 104–110. doi: 10.1016/j.npbr.2019.05.004 [DOI] [Google Scholar]
- Weber B, Lewicka S, Deuschle M, Colla M, Heuser I, 2000. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology 25, 765–771. doi: 10.1016/S0306-4530(00)00023-8 [DOI] [PubMed] [Google Scholar]
- Weissman MM, Klerman GL, 1977. Sex differences and the epidemiology of depression. Arch. Gen. Psychiatry 34, 98–111. [DOI] [PubMed] [Google Scholar]
- Wennig R, 2000. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int 107, 5–12. [DOI] [PubMed] [Google Scholar]
- Westley CJ, Amdur RL, Irwig MS, 2015. High Rates of Depression and Depressive Symptoms among Men Referred for Borderline Testosterone Levels. J. Sex. Med 12, 1753–1760. doi: 10.1111/jsm.12937 [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO), 2017. Depression and Other Common Mental Disorders Global Health Estimates. doi:(WHO reference number: WHO/MSD/MER/2017.2) [Google Scholar]
- Wu FCW, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean MEJ, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT, 2010. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med 363, 123–135. doi: 10.1056/NEJMoa0911101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.