Abstract
Non-human primate models have been useful in clarifying estradiol’s role in cognitive processing. These animal studies indicate estradiol impacts cognitive processes supported by regions within dorsolateral prefrontal cortex (DLPFC). Although human functional neuroimaging studies have begun to find similar relationships between estradiol in women for some forms of ‘cold’ cognitive control, to date no studies have examined the relationship between estradiol and DLPFC function in the context of active attempts to regulate one’s emotions. Here, we asked whether peripheral 17-beta estradiol levels in adolescent girls in different pubertal developmental stages (age = 14.9 years ± 1.74) were related to engagement of DLPFC regions during the use of a cognitive strategy for regulating emotion known as reappraisal using functional Magnetic Resonance Imaging. Findings indicated that higher estradiol levels predicted greater DLPFC activity during the down-regulation of negative emotion using reappraisal. This is the first report of an association between estradiol level and DLPFC activity during cognitive reappraisal of negative emotion. The study suggests a possibility that estradiol might positively contribute to regulatory function of a cortical system important for emotional experiences.
1. Introduction
Mounting evidence accumulated over the last four decades supports the influence of steroidal sex hormones on cortical and subcortical regions implicated in emotional and cognitive processing (Gurvich et al., 2018; Toffoletto et al., 2014). However, the exact neurobiological mechanisms behind ovarian hormones modulation of underlying neural circuitries of emotion and cognition still remain unclear. The most frequently studied of these hormones are 17β – estradiol (E2) and progesterone (P4), the end products of different synthesis pathways within the hypothalamic-pituitary-gonadal (HPG) endocrine system. According to a meta-analytic review (Toffoletto et al., 2014), both E2 and P4 mediate neural coupling of cortical and subcortical regions including amygdala during emotion processing. In addition to its well-described role in promoting growth of secondary sex characteristics after puberty, animal studies primarily from rodent studies demonstrate that estrogens can facilitate higher order cognitive functioning via a range of likely interrelated functions that involve multiple signaling pathways (Marrocco and McEwen, 2016). Estrogen-regulated synapse formation is mediated via both genomic and rapid, non-genomic mechanisms in cognitively relevant brain regions, including the hippocampus and prefrontal brain regions (Gurvich et al., 2018). Specifically, E2 (the major biologically-active estrogen) has been shown to modulate human dorsolateral prefrontal cortex (DLPFC) activity in both males and females from behavioral and imaging paradigms examining non-emotional working memory (Keenan et al., 2001) with mixed evidence of beneficial effects of E2 on cognitive performance in aging (Engler-Chiurazzi et al., 2017). Estrogen may alter some aspects of cognitive function, through cellular mechanisms that result in rapid effects through binding to membrane receptors or long lasting and sustained effects on neural function through binding classical nuclear receptors in the DLPFC (Shanmugan and Epperson, 2014). Many experimental studies in rhesus monkeys and human neuroimaging studies support a prefrontal locus for positive effect of E2 on cognitive performance (Hampson, 2018). Like E2, P4 exerts trophic effects on brain development throughout adolescence and adulthood. Both E2 and P4 are thought to act together to enhance neuronal function through mechanisms such as enhancing synaptic transmission (Gurvich et al., 2018).
Experiments in rhesus monkeys have identified a specific DLPFC region (Brodmann’s Area 46) as an important structure involved in sex hormone effects on brain and cognition (Bailey et al., 2011). For example, when ovariectomized older monkeys received E2 supplementation, they performed working memory tasks mediated by DLPFC as well as young adults with or without E2 (Bailey et al., 2011). Analysis of layer III pyramidal cells in DLPFC area 46 in the same monkeys showed that cyclical E2 increased the density of small, thin spines, possibly enabling a form of synaptic plasticity that may support working memory (Bailey et al., 2011). This beneficial role of E2 on DLPFC function appears to be more pronounced during puberty when there are substantial modifications in the cerebral cortex, including changes in the rate of synaptic formation and neuronal loss (Sanz et al., 2008). Rat studies show that estradiol modulates these neuronal differentiation and survival processes (Sanz et al., 2008). E2 is known to affect remodeling of cortico-subcortical neural circuits during puberty via both organization (permanent) and activational (temporary, context-specific) effects (Vigil et al., 2016). The strong evidence for a beneficial role of E2 on brain function from rodent and primate studies has supported a model in which E2 levels act on DLPFC structure or function in ways that are believed to generally augment various non-emotional forms of cognitive control (Bailey et al., 2011; Hara et al., 2014).
While E2 or P4 modulation of cognition and brain function is less studied in humans than animals, there is an emerging literature that supports similar effects of E2 and P4 on human DLPFC-mediated executive cognitive abilities. For instance, E2 has been shown to improve cognitive task performance during naturally-fluctuating high-estrogen periods of the ovarian cycle (Maki et al., 2002) and during the estrogen phase of birth control (Mordecai et al., 2008) in premenopausal women. Estrogen deprivation due to pharmacological blockade or to surgical or natural menopause can worsen executive function (Shanmugan and Epperson, 2014). This effect is reversed following add-back estrogen treatment (Sherwin and Tulandi, 1996). Similar to the E2 effect, higher P4 levels were associated with greater resting state functional connectivity of DLPFC with the hippocampus (Arelin et al., 2015) or a reduction in the affective component of pain experiences (Vincent et al., 2018). But evidence for P4 effect is less consistent compared to the E2 effect (Graham et al., 2017). A unifying factor for these findings is that these studies typically the engagement of the DLPFC (Epperson et al., 2012). Moreover, four human neuroimaging studies have found that higher E2 is positively linked to DLPFC activity during sustained attention (Stevens et al., 2005), working memory (Dumas et al., 2010), memory retrieval (Shaywitz et al., 1999) or inhibition of positive words (Amin et al., 2006). The DLPFC is an important region in a cortical system engaged during reappraisal of emotion regulation (Buhle et al., 2014). It has been well-established that DLPFC actively maintains task-relevant goal information in mind and biases information processing in other cortical regions in a top-down manner (Miller and Cohen, 2001). These are operations that are equally relevant when one must reinterpret the meaning or personal relevance of emotion-eliciting stimuli. Importantly, there is empirical evidence that greater DLPFC activity is linked to successful reappraisal, as evidenced by lower self-rating of negative reactivity (Bastiaansen et al., 2018; Silvers et al., 2015; van der Meulen et al., 2017).
This study examined whether E2 and P4 in adolescent girls will be associated with DLPFC activity during reappraisal of emotion. We conducted an a priori hypothesis-driven region-of-interest analysis of DLPFC brain regions localized by a recently published cortical parcellation atlas (Glasser et al., 2016) to closely correspond to the DLPFC brain regions identified in animal research. We focused on regulation of negative emotions because dysregulation of negative emotions is closely associated with mood and anxiety disorders (Pico-Perez et al., 2017). Our primary hypothesis was that adolescent girls with higher E2 levels would show greater DLPFC activation levels when using reappraisal to either “Increase” or “Decrease” the intensity of negative emotional reactions. A secondary goal was to examine any influence of P4 on DLPFC function. A recent human neuroimaging review (Toffoletto et al., 2014) suggested that fluctuating both E2 and P4 across menstrual cycle could modulate cortical and subcortical regions supporting either cognitive control or emotion perception. There are fewer P4 studies than of E2, and the available evidence for its neuromodulatory role during cognitive processing is more mixed. However, research has shown it is possible for P4 to alter brain function – either directly or by amplifying E2’s neuroprotective effects (Singh and Su, 2013). P4 is also well documented to have neuroprotective effects against numerous insults in a variety of cell models, animal models, and in humans by improving neuronal survival and modulating inflammation and apoptosis (De Nicola et al., 2013). Therefore, we also predicted that progesterone levels might be positively associated with DLPFC activation. Finally, we explored whether there might be an interaction effect of E2 and P4 on DLPFC activation.
2. Material and Methods
2.1. Participants
The thirty-two adolescent girls in this study were recruited as a supplementary study from an ongoing NIMH-funded neuroimaging study (R01MH102854) performed at the Olin Neuropsychiatry Research Center (ONRC). Participant assent and parental permission were obtained. The Hartford Hospital Institutional Review Board approved all study procedures. Three girls with contraceptive use and one girl who declined to complete the fMRI session were excluded from the final sample of 28 peri-pubertal girls. No participants were left-handed. Participants’ lack of psychiatric diagnoses was confirmed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL-5 )(Kaufman et al., 1997) for Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM-5) (Association, 2013) conducted by experienced staff using standard administration guidelines. Diagnostic decisions were made in weekly consensus meetings supervised by a licensed clinical psychologist with over 16 years of K-SADS experience (MCS). The Peterson Development Scale (Petersen et al., 1988) showed that about 85% of the sample was advanced (n =15) or post-puberty (n = 9) while only 14% of the sample (n =4) was mid-puberty. Also, all participants completed self-report of Olin Menstrual Cycle Questionnaire, which asked information about contraceptive use and age at menarche. The mean age at menarche was 12.11 years (± 1.68), ranging from 9 to 15 years.
2.2. Salivary Assessment of Ovarian Hormone Levels
Participants completed their fMRI session and saliva was collected (later assayed for E2 and P4) on the same day. A number of studies of children and adolescents typically adopt salivary analytes as salivary collection does not require venipuncture (Granger et al., 2012). Menstrual cycle stage was not controlled, as we wanted to capture naturally-occurring variation in circulating endogenous E2 levels across the menstrual cycle. Detailed saliva sample instructions were given during the consent meeting and families took collection materials home. On the day of fMRI assessment, participants were, asked to collect ~2mL of whole saliva by passive drool within 30 minutes of waking before brushing their teeth or eating between 6:00 and 9:00 am. On the day of assay, samples were thawed, centrifuged (to remove mucins) and assayed using commercially available immunoassays without modification to the manufacturer’s recommended protocol (Salimetrics LLC, Carlsbad, CA). All samples were performed in duplicate with the average value from the two tests used in data analysis. Final salivary hormone data consisted of 25 girls’ E2 and 28 girls’ P4. Out of 28 girls, 3 girls’ E2 levels were missing due to insufficient quantity of saliva. The lower limit of sensitivity for E2 was 0.1 pg/mL and that for P4 was 5pg/mL. All observed values for salivary E2 and P4 were above the lower limit of sensitivity; raw E2 values ranged from 0.47 to 3.87pg/mL and raw P4 values ranged from 24.06 to 569.63 pg/mL). The intra-assay coefficients of variation for E2 and P4 were 5.6% and 7.1%; and Inter-assay coefficients of variation were 9.25% and 8.55%, respectively. Salivary E2 and P4 values were positively skewed; therefore, log-transformed values for E2 and P4 were used in main analyses. Mean values of E2 and P4 were 0.17 pg/mL (± 0.15, range: 0.58) and 2.23 pg/mL (± 0.34, range: 1.37), respectively
2.3. fMRI Reappraisal Emotion Regulation Task
We used typical reappraisal emotion regulation task based on previous studies (Buhle et al., 2014). Prior to MRI, participants completed approximately 20 minutes of instructed practice in applying cognitive reappraisal techniques. This ensured they became accustomed to the International Affective Pictures System (IAPS) stimuli used, could perform quickly and accurately, but were not experts in the techniques so that a range of meaningful individual differences across the sample could be quantified. In each of four fMRI task runs, participants were presented with positive (n=15) and negative (n=15) images normatively rated for high arousal/valence, and neutral images (n=5) with low arousal/valence in pseudo-random, GLM efficiency-optimized order. Two of the runs had “Decrease” instructions. For these, participants were cued at trial onset to either naturally experience their emotions (i.e., “Look”) or Decrease their initial emotional reaction to the positive, negative, or neutral stimuli using cognitive reappraisal for 10 seconds. Then, they were asked to rate on a 5-point Likert-scale how negative (or positive) each image made them feel within 3 seconds, followed by an inter-trial interval of 6-9 seconds (Figure 1). The other two “Increase” runs were similar, except the cues prompted participants to use reappraisal to increase the intensity of their emotional reaction. This fMRI task was implemented using E-Prime (Psychology Software Tools, Inc.). Run order was counter-balanced across participants, but the two Increase or Decrease runs were always presented together for instruction set contiguity (see Supplemental Table 1 for IAPS numbers used in current paradigm).
Figure 1.
Example trial of the fMRI emotion regulation reappraisal task
2.4. Functional Neuroimaging Acquisition and Data Analysis Procedures
All participants were scanned at the ONRC using a Siemens 3T Skyra. Urine collected prior to MRI was tested for the presence of drugs metabolites and pregnancy. MR sequences were chosen for compatibility with Human Connectome Project (HCP) pre-processing pipelines (Marcus et al., 2011), which provide highly accurate, structural image-guided brain atlas normalization for fMRI. The detailed parameters for MRI data acquisition are described in Supplemental Materials.
The HCP functional preprocessing pipelines (Glasser et al., 2013) included structural (Pre-FreeSurfer, FreeSurfer, Post-FreeSurfer) and functional (Volume, Surface, MSMAII, DedriftAndResample) scripts. Briefly, T1/T2 images were ACPC-oriented, brain extracted, B0 inhomogeneity-corrected, mutually co-registered, distortion fieldmap-corrected, and finally MNI152 atlas-registered using FSL FLIRT+nonlinear FNIRT algorithms (Jenkinson et al., 2012). We did FreeSurfer-based (Dale et al., 1999) registration, skull-stripping, and pial extraction on 1mm-downsampled T1/T2 data to create structural volume/cortical ribbon files. EPI data were registered to FreeSurfer cortical ribbon output, resampled to atlas space, intensity normalized, smoothed at 2mm FWHM, and high-pass temporal filtered (2000s) before being written as a timeseries for analysis. Additional timeseries denoising with aggressive full variance cleanup was performed using ICA-FIX (Salimi-Khorshidi et al., 2014). Data from the dense timeseries were averaged within parcels identified in a recently-developed, multi-modal cortical atlas parcellation from the HCP group (Glasser et al., 2016) prior to fMRI activation modeling.
All included participants met the rigorous fMRI data quality control criteria for both behavioral responses and head motion. Each participant had at least 3 out of 5 behavioral responses for each trial type. Framewise displacement (FD) values were estimated to quantify scan-to-scan motion using all six translation and rotation parameters. For all four fMRI runs, all fMRI timeseries had mean FD below 0.45 (Power, 2012).
Brain activation was estimated using multi-stage General Linear Modeling (GLM) within FSL-based FEAT software. This approach estimates brain activation using FMRIB’s Improved Linear Model (FILM). FSL tools were used to estimate the effects of each task condition in each participant using a fixed-effects model (e.g., “Decrease” or “Increase” negative image). Since we were interested in brain activity when using reappraising strategy relative to natural look, two task contrasts were used for hypothesis testing (i.e., Decrease versus Look for negative images; Increase versus Look for negative images). These task contrasts were entered into a random effect analysis using FSL’s FEAT. The group-level statistics were tested using Permutation Analysis of Linear Models (PALM; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM) with 5,000 permutations and appropriate FDR-correction.
We focused on four bilateral DLPFC parcels (BA46, BA9/46) from the cortical atlas (Glasser et al., 2016) that most closely corresponded to the cytoarchtectonically-identified lateral prefrontal area 46 in rhesus monkey studies (Petrides and Pandya, 1999). To evaluate the association between brain activation and hormones, two regression analyses were conducted with E2 or P4 level as a predictor and DLPFC activity as a dependent variable. Our E2 measurements were correlated with age (r = 0.42, p =.03) and P4 (r = 0.36, p =.05), but there were no associations with pubertal status for E2 (r = 0.03, p =.89) or P4 (r = 0.06, p =.79 for P4). As such, we included age only as a covariate in each regression model to dissociate hormone effects from generalized developmental maturation. All predictors were mean-centered. An FDR-corrected (Benjamini and Hochberg, 1995) q-value of less than 0.025 (= 0.05 / 2 contrasts of interest) was required for statistical significance, as each contrast of interest for E2, P4, or their interaction represented a separate “family” of parcel tests that can be evaluated independently of each other without compromising proper Type I error control. Significant effects showing the association between hormone value and DLPFC activity controlling for age were presented with Cohen’s d effect size. Two additional analyses were conducted for completeness (see Supplemental Materials). First, participant’s average self-ratings of emotional intensity for each task condition were calculated so they could be included in post hoc correlations with estimated brain function. Second, we conducted exploratory whole brain analysis using 391 parcels (Glasser et al., 2016) at uncorrected p <0.01 to gain a preliminary understanding of whether or not E2 or P4 levels might affect brain activity in other regions. The whole brain analyses revealed that there were many prefrontal regions including DLPFC and anterior cingulate cortex showing associations with E2 level during down-regulation of negative emotion at uncorrected p-value of .01. Similar associations between P4 and brain activity in anterior cingulate cortex and inferior frontal cortex were observed during down-regulation of negative emotion at uncorrected p-value of .01. However, these results were exploratory analyses and the effects were not statistically significant (see Supplemental Materials for details about whole brain analysis).
3. Results
3.1. Task validity check
The repeated measures ANOVA was conducted to determine whether participants’ emotional intensity ratings during the task varied as a function of reappraisal instruction. The repeated measures ANOVA revealed that there was a significant main effect of instruction [F(2,54) = 154.99, p < .0001, ηp2: 0.85]. According to post hoc paired t-tests, adolescent girls rated IAPS negative images more positively when instructed to “Decrease” compared to “Look” (t(27) = 5.84, p < .001) and more negatively when instructed to “Increase” compared to “Look” (t(27) = −2.16, p = .04).
3.2. The associations between E2 or P4 level and self-reported emotion regulation
We tested whether participants’ self-rating scores were associated with difference of average self-reported emotions in E2 or P4 log transformed values. Pearson correlations between E2 or P4 level and the difference of average self-reported emotions during “Increase” or “Decrease” versus “Look” on negative emotion trials were not significantly associated with E2 and P4 (all ps > .05).
3.3. E2 effects on DLPFC activation
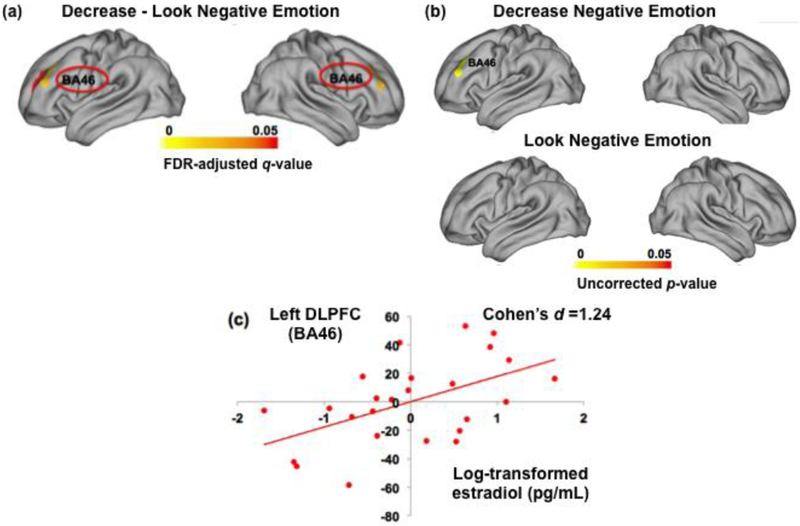
There was a significant main effect of E2 for DLPFC parcels only during Decrease versus Look negative emotion condition, but not during Increase versus Look of task condition (left DLPFC (BA46): t(24) = −0.43, p = .69, left DLPFC (BA 9,46): t(24) =0.04, p = .69, right DLPFC (BA46): t(24) = −0.52, p = .69, right DLPFC (BA9,46): t(24) = −0.16, p = .69) . As shown in Figure 2a, there were bilateral DLPFC parcels showing significant positive associations between estradiol level and left DLPFC (t(24) = 2.90, FDR-corrected q = .01) and, right DLPFC (t(24) = 2.54, q = .02) during Decrease versus Look negative emotion. To make sure that this association came from brain activity during Decrease but not during the Look control condition, we confirmed that the association between estradiol level and DLPFC activity during Look negative emotion was not significant at an uncorrected p-value of .05. Consistent with our expectations, significant DLPFC parcel was found only during Decrease negative emotion (t(24) = −1.30, uncorrected p-value = 0.02), but not during Look condition (Figure 2b). These relationships were visualized using a post hoc correlation showing the linear effect. As presented in Figure 2c, higher estradiol levels were positively correlated with greater left DLPFC activity (r = .52, p =.008, d =1.24) during down-regulation of negative emotions. The same pattern of positive association was found in right DLPFC (BA46) (r = .47, p =.018, d =1.09).
Figure 2.
Relationships between estradiol levels and DLPFC activity during down-regulation of negative emotion
Note. DLPFC = Dorsolateral Prefrontal cortex. (b) This shows relationship between estradiol level and DLPFC activity when subjects were instructed to “Decrease” (top) “Look” (bottom) emotional reactions to negative images. (c) Estradiol values used in main analyses represent log-transformed ones.
3.4. P4 effects on DLPFC activation
There was no main effect of P4 during any task contrasts of interest (see Supplementary Table 2).
3.5. E2 and P4 Interaction and E2, P4 and Age Interaction Effects
Both of the right DLPFC parcels (BA46, BA9, 46) showed an interaction effect of E2 and P4 during Increase versus Look negative emotions at uncorrected p-value of 0.05 while controlling for age. However, these interaction effects did not survive with FDR-corrected q-value (all q-value >.40). There were no significant interaction effects of E2 and P4, nor were there any significant interactions of age, E2 and P4 for any task conditions with FDR-correction (see Supplementary Table 3).
4. Discussion
The purpose of this study was to determine whether endogenous E2 levels were associated with DLPFC activity in adolescent girls while they used cognitive reappraisal strategies to alter the intensity of negative emotional reactions. As predicted and consistent with primate studies, we found a strong association between E2 and neural activity in bilateral DLPFC (BA 46) (Cohen’s d 1.15~1.35) when participants down-regulated negative emotions elicited by viewing unpleasant pictures. This association with E2 appears to be specific to down-regulation of negative emotion, because the same association with E2 was not observed during up-regulation of negative emotions even at liberal statistical thresholds. However, we did not run an interaction analysis to test this conclusion statistically.
The current findings suggest that circulating E2 levels in adolescent girls influence a DLPFC region of a cortical system during emotion regulation in similar ways to how E2 may enhance DLPFC activity supporting cognitive control in non-emotional contexts. This highlights interesting parallels between the non-human primate literature on estrogen modulation of cognitive control and estrogen’s effects on human emotion regulation. As a key region of cognitive control, the DLPFC has extensive reciprocal projections with the sensory and motor association cortices that are necessary for guiding thoughts, attention and goal-directed actions. Also, it has direct connections with the posterior hippocampus and also has widespread subcortical projections down to caudate, thalamus and to the cerebellar cortex via pons (Datta and Arnsten, 2018). Neurons in DLPFC can maintain firing and represent information in the absence of sensory stimulation. These neurons are able to maintain firing across the delay period when information must be held in mind without sensory stimulation in monkey studies (Datta and Arnsten, 2018). According to several neural models of emotion regulation, DLPFC has been consistently implicated as critical site of explicit cognitive regulation of emotion (Buhle et al., 2014). Such models suggest that successful reappraisal engages selective attention and working memory arguably supported by DLPFC to direct attention to reappraisal-relevant stimulus features and hold in mind reappraisal goals as well as the content of one’s reappraisal (Buhle et al., 2014). Our study did not attempt to dissociate generalized cognitive control processes from those that might be more specific to emotion processing or to reappraisal. However, the current results provide a foundation to explore such specific effects in new studies requiring various cognitive processes during reappraisal.
Different from our expectation, we did not find the association between P4 and DLPFC activity during reappraisal of emotion regulation. But we cannot necessarily come to the conclusion of no role of P4 in emotion regulation. Endogenous E2 and P4 levels are known to co-fluctuate (Becker et al., 2005); indeed, E2 and P4 levels in current study were highly correlated (r = .49, p = .01). Thus, we could not conclusively dissociate the potential role of P4 in emotion regulation. It is possible that P4 may modulate other PFC regions, not specific to DLPFC during down-regulation of negative emotions. Indeed, our exploratory data analysis supports this possibility, as evidenced by P4 effect in anterior cingulate cortex and inferior frontal cortex during down-regulation of negative emotion at uncorrected level. Pharmacological manipulations where E2 is augmented in isolation from P4 are required to provide more compelling evidence for a discrete role for E2 in reappraisal of emotion regulation.
One way for future emotion regulation research to examine this association between E2 and DLPFC function is to better learn the ways in which different sex hormone levels influence the cortical regulatory and subcortical emotion generating brain systems. The present results converge with prior E2 neuroimaging studies to suggest a context-invariant effect of E2 on DLPFC activity that applies equally to ‘cold’ and ‘hot’ forms of information processing. Work from prior primate studies of cognitive control suggests several potential cellular mechanisms that might underlie this E2 effect (Sellers et al., 2015). One proposed way that E2 might enhance cognitive function is by altering connectivity among neurons, which can be achieved by changing number, strength or type of functional synapses between two cells (Chklovskii et al., 2004). For instance, prior primate studies implicate cellular alterations to pyramidal neurons on Area 46. Specifically, cyclical E2 has been shown to increase the density of small spines in the monkeys during cognitive performance (Bailey et al., 2011). Such synaptic plasticity may induce measurable changes in the functional connectivity of the neocortical network supporting the higher-order cognitive functions engaged for reappraisal (Shimoura et al., 2015). Indeed, a review of resting-state and structural functional connectivity studies (Peper et al., 2011) found that E2 may enhance both cortico-cortical connections and subcortico-cortical functional connectivity (Peper et al., 2011). Moreover, recent resting-state functional connectivity studies suggest a possibility that the strength of resting-state functional connectivity of cortical-cortical and/or cortical-subcortical brain areas may differ across the menstrual cycle, although such evidence is not conclusive (Hjelmervik et al., 2014; Petersen et al., 2014). However, no studies have yet directly observed network connectivity by E2 during emotion regulation in a way that might be unique to females who have cyclically fluctuating hormone levels across different phases of menstrual cycle. This is an important issue, but was not purpose of current study and waits for testing in future studies. Higher levels of E2 in women might predict greater strength of functional connectivity between cortical regulatory and subcortical emotion-generating regions such as amygdala. Exploring these ideas in future studies ideally would involve experimental E2 level manipulation in women, either by tracking natural cyclic changes throughout the menstrual cycle or by E2 augmentation.
The examination of how sex hormones interact with brain function supporting emotion regulation has important implications for efforts to understand the role of estrogen in heightened vulnerability of mood disorders for women. Difficulty in emotion regulation is a proposed factor in the development and maintenance of Major Depressive Disorder (Visted et al., 2018). Sex differences in mood disorders emerge after puberty (Paus et al., 2008) when E2 and P4 dramatically increase. It is not yet known whether modulation of DLPFC activity by E2 levels contributes to mood dysregulation in some women. There is some evidence for a relationship. For instance, ovariectomy and ovarian suppression induced by gonadotropin-releasing hormone agonists eliminate symptoms of premenstrual syndrome such as mood disturbances and/or irritability (Wyatt et al., 2004). E2 has been used as a treatment with antidepressant effects for peri-menopausal women although antidepressant actions of E2 are inconclusive (Gordon et al., 2018). We can speculate that dramatic changes in E2 level in late follicular phase after very low level of E2 at menstruation may boost effects of E2 on DLPFC function supporting emotion regulation rather than stable level of E2 above a threshold. Indeed, strong enhancement of E2 in cognitive function has been observed in studies when rodents received estrogen treatment after ovariectomy, which reduces E2 level into near zero (Chakraborty and Gore, 2004). If this idea is correct, it might be that adolescent girls’ relatively low level of E2 compared to P4 level in luteal phases serves to increase vulnerability of girls’ mood dysregulation by altering the way in which DLPFC or its connected networks engage to mediate negative emotional reactions. However, such a speculative model requires careful testing by manipulating different menstrual cycle phases. This was beyond the scope of current study. Numerous unanswered questions remain regarding whether this link between E2 and DLPFC function truly underlies mood regulation in ways that are translationally useful. For example, the E2/DLPFC association found here should be bolstered by evidence for a causal relationship between E2 and DLPFC levels. There also is a need to determine if there is an optimal level of E2 for successful emotion regulation, to characterize the exact influence of E2 on DLPFC-mediated emotion regulation capacity throughout the menstrual cycle, and eventually to learn what sort of exogenous treatment might maximize the ability of DLPFC emotion regulation-related neural circuits to function.
It is of note that the association effect of E2 on DLPFC activity was differentiated from maturational changes associated with age. Some studies suggest that developmental increases in estradiol might underlie increases in dysphoric mood or emotion (Balzer et al., 2015). Here however, the relationship between E2 on DLPFC activity was distinct from age-related developmental changes in current study. This suggests that E2’s influences are over and above any risk for mood dysregulation that might occur simply as a function of development. The absence of statistical interactions with age in our regression models reinforces this conclusion, as it reflects an association effect that occurred equally at all ages. Puberty may be another factor that might influence estradiol changes in development of DLPFC function during adolescence. There are some arguments that pubertal maturation is an important variable to consider in terms of understanding sex differences in human brain development, as physical and hormonal changes during puberty are closely linked with changes in gray and white matter development (Herting and Sowell, 2017). The unequal ratio of participants in each pubertal developmental status in our sample might have masked possible estradiol maturational changes associated with pubertal development. Also, this study is limited by a relatively small sample size considering different range of pubertal stages in the participants. Future research should investigate how pubertal developmental stages would modulate estradiol maturational changes in DLPFC activity with equal ratio of participants in each pubertal developmental status. More information is needed to understand exactly how this association is relevant to adolescent vulnerability to mood dysregulation. The estradiol-DLPFC link found here also opens the door to future experimental consideration of other endocrine factors known to influence both organizational and activation effects of sex hormones on the cortical regulatory system engaged for different types of emotion regulation. As one example, many animal studies show that insulin-like growth factor-1 (IGF-I) is a potential modulator of mood homeostasis (Santi et al., 2018) signaling in the PFC that becomes more sensitive to estrogen regulation during puberty (Sanz et al., 2008).
In conclusion, the current study shows an association between E2 and the DLPFC activity during cognitive reappraisal of negative emotions for the first time. The primary study limitations were due to the small sample size. Although we chose to focus solely on DLPFC in this study to directly bridge animal and human literature, we also were cognizant that the available statistical power only permitted us to test brain regions most likely to be modulated by E2 only during limited number of task contrasts. Indeed, our exploratory analyses indicate that smaller E2 and P4 effects likely exist in other brain regions and for up-regulation of negative emotion – possible effects that await a replication study with greater statistical power even during regulation of positive emotion. Our modest sample also precluded an attempt to determine the effect of pubertal status on the relationship between E2 and DLPFC activity, as the majority of adolescent girls we recruited had advanced or post-pubertal status. Another limitation is that we did not measure allopregnanolone along with P4. The lack of P4 modulation in brain activity during emotion regulation does not necessarily indicate no effect of allopregnanolone during emotion regulation. In fact, other studies have found evidence that allopregnanolone plays a role in mood regulation (Schiller et al., 2014). One final noteworthy limitation is that this study is associational in nature only. Future studies should manipulate E2 and P4 levels either through experimental augmentation or by quasi-experimental efforts to measure hormone levels during the menstrual cycle to confirm hypothesized changes in DLPFC activation or emotion-relevant behavior or objective measurements of arousal (e.g., electrodermal responses, pupilometry, etc). While the current finding begins to lay a foundation for future study of sex hormones’ effect on human emotional processing, we should remain cautious about generalization of the current results until replication. Future studies should build on these findings to determine if E2 levels modulate activity in other brain regions in ways that might be relevant to emotion regulation.
Supplementary Material
Highlights.
Higher estradiol levels in adolescent girls predicted greater DLPFC activity during cognitive reappraisal of negative emotion.
No association between progesterone and DLPFC activity during cognitive reappraisal of negative emotion during adolescence.
The association effect of estradiol on DLPFC was differentiated from maturational changes associated with age.
Acknowledgements
We would like to thank research staff, Stephanie Novotny, Karen Kesten, and Julie Reid for their help recruitment participants and collecting data for this study.
Funding Source
This study was funded by National Institute of Mental Health grant RO1MH102854.
Abbreviations
- 8Ad
DorsoLateral Prefrontal Cortex
- A5
Auditory Association Cortex
- STSvp
Auditory Association Cortex
- a10P
Orbital and Polar Frontal Cortex
- POS2
Posterior Cingulate Cortex
- 47s
Orbital and Polar Frontal Cortex
- TE1m
Lateral Temporal Cortex
- 2
Somatosensory and Motor Cortex
- 8Av
DorsoLateral Prefrontal Cortex
- p47r
Inferior Frontal Cortex
- PGs
Inferior Parietal Cortex
- PFm
Inferior Parietal Cortex
- TE1p
Lateral Temporal Cortex
- d23ab
Posterior Cingulate Cortex
- 10v
Anterior Cingulate and Medial Prefrontal Cortex
- 24dd
Paracentral Lobular and Mid Cingulate Cortex
- 23d
Posterior Cingulate Cortex
- p32
Anterior Cingulate and Medial Prefrontal Cortex
- ProS
Posterior Cingulate Cortex
- 11l
Orbital and Polar Frontal Cortex
- LBelt
Early Auditory Cortex
- TE1m
Lateral Temporal Cortex
- TE2a
Lateral Temporal Cortex
- 10r
Anterior Cingulate and Medial Prefrontal Cortex
- IFJa
Inferior Frontal Cortex
- IFSp
Inferior Frontal Cortex
- PHA3
Medial Temporal Cortex
- PreS
Medial Temporal Cortex
- P24pr
Anterior Cingulate and Medial Prefrontal Cortex
- 31pd
Posterior Cingulate Cortex
- a24
Anterior Cingulate and Medial Prefrontal Cortex
- 8Ad
DorsoLateral Prefrontal Cortex
- PGi
Inferior Parietal Cortex
- TE1a
Lateral Temporal Cortex
- TGd
Lateral Temporal Cortex
- TGv
Lateral Temporal Cortex
- POS1
Posterior Cingulate Cortex
- TGd
Lateral Temporal Cortex
- TGv
Lateral Temporal Cortex
Footnotes
All authors have confirmed they have no financial interests or potential conflicts of interest except Dr. Douglas A. Granger (DAG). DAG is the founder and chief Scientific and Strategy Advisor at Salimetrics LLC and Salivabio LLC. The nature of these relationships to his research is managed by committees on conflict of interest at Johns Hopkins University School of Medicine and the University of California at Irvine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin Z, Epperson CN, Constable RT, Canli T, 2006. Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage 32, 457–464. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH, 2011. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience 191, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer BW, Duke SA, Hawke CI, Steinbeck KS, 2015. The effects of estradiol on mood and behavior in human female adolescents: a systematic review. European journal of pediatrics 174, 289–298. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discoverty rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 57, 289–300. [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN, 2014. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral cortex (New York, N.Y. : 1991) 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC, 2004. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Experimental biology and medicine (Maywood, N.J.) 229, 977–987. [DOI] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K, 2004. Cortical rewiring and information storage. Nature 431, 782–788. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Datta D, Arnsten AFT, 2018. Unique Molecular Regulation of Higher-Order Prefrontal Cortical Circuits: Insights into the Neurobiology of Schizophrenia. ACS chemical neuroscience 9, 2127–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nicola AF, Gonzalez Deniselle MC, Garay L, Meyer M, Gargiulo-Monachelli G, Guennoun R, Schumacher M, Carreras MC, Poderoso JJ, 2013. Progesterone protective effects in neurodegeneration and neuroinflammation. Journal of neuroendocrinology 25, 1095–1103. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA, 2010. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Hormones and behavior 58, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J, 2012. Interactive Effects of Estrogen and Serotonin On Brain Activation During Working Memory and Affective Processing in Menopausal Women. Psychoneuroendocrinology 37, 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R, 1998. Sex steroid control of mood, mental state and memory. Clinical and experimental pharmacology & physiology 25, 764–775. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, 2013. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, Girdler SS, 2018. Efficacy of Transdermal Estradiol and Micronized Progesterone in the Prevention of Depressive Symptoms in the Menopause Transition: A Randomized Clinical Trial. JAMA psychiatry 75, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D, 2012. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. Journal of adolescence 35, 1081–1095. [DOI] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH, 2014. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proceedings of the National Academy of Sciences of the United States of America 111, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER, 2017. Puberty and structural brain development in humans. Frontiers in neuroendocrinology 44, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmervik H, Hausmann M, Osnes B, Westerhausen R, Specht K, 2014. Resting states are resting traits--an FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PloS one 9, e103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM, 2012. FSL. NeuroImage 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ, 2001. Prefrontal cortex as the site of estrogen's effect on cognition. Psychoneuroendocrinology 26, 577–590. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS, 2002. Implicit memory varies across the menstrual cycle: estrogen effects in young women. Neuropsychologia 40, 518–529. [DOI] [PubMed] [Google Scholar]
- Marcus DS, Harwell J, Olsen T, Hodge M, Glasser MF, Prior F, Jenkinson M, Laumann T, Curtiss SW, Van Essen DC, 2011. Informatics and data mining tools and strategies for the human connectome project. Frontiers in neuroinformatics 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annual review of neuroscience 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Mordecai KL, Rubin LH, Maki PM, 2008. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Hormones and behavior 54, 286–293. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN, 2008. Why do many psychiatric disorders emerge during adolescence? Nature reviews. Neuroscience 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J, 2011. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology 36, 1101–1113. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, 1988. A self-report measure of pubertal status: Reliability, Validity, and Initial Norms. Journal of youth and adolescence 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L, 2014. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage 90, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN, 1999. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. The European journal of neuroscience 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Power JD, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM, 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage 90, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi A, Bot M, Aleman A, Penninx B, Aleman IT, 2018. Circulating insulin-like growth factor I modulates mood and is a biomarker of vulnerability to stress: from mouse to man. Translational psychiatry 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Carrero P, Pernia O, Garcia-Segura LM, 2008. Pubertal maturation modifies the regulation of insulin-like growth factor-I receptor signaling by estradiol in the rat prefrontal cortex. Developmental neurobiology 68, 1018–1028. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Schmidt PJ, Rubinow DR, 2014. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology 231, 3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Raval P, Srivastava DP, 2015. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Frontiers in neuroendocrinology 36, 72–89. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN, 2014. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in the menopause transition. Human brain mapping 35, 847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC, 1999. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. Jama 281, 1197–1202. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T, 1996. "Add-back" estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. The Journal of clinical endocrinology and metabolism 81, 2545–2549. [DOI] [PubMed] [Google Scholar]
- Shimoura RO, Pena RFO, Roque AC, 2015. Effect of synaptic plasticity on functional connectivity and global activity of a neocortical network model. BMC Neuroscience 16, P210. [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama 289, 2651–2662. [DOI] [PubMed] [Google Scholar]
- Singh M, Su C, 2013. Progesterone and neuroprotection. Hormones and behavior 63, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Clark VP, Prestwood KM, 2005. Low-dose estradiol alters brain activity. Psychiatry research 139, 199–217. [DOI] [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundstrom-Poromaa I, Comasco E, 2014. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology 50, 28–52. [DOI] [PubMed] [Google Scholar]
- Vigil P, del Rbío JP, Carrera B, ArÁnguiz FC, Rioseco H, Cortés ME, 2016. Influence of sex steroid hormones on the adolescent brain and behavior: An update. The Linacre quarterly 83, 308–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visted E, Vollestad J, Nielsen MB, Schanche E, 2018. Emotion Regulation in Current and Remitted Depression: A Systematic Review and Meta-Analysis. Frontiers in psychology 9, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt KM, Dimmock PW, Ismail KM, Jones PW, O'Brien PM, 2004. The effectiveness of GnRHa with and without 'add-back' therapy in treating premenstrual syndrome: a meta analysis. BJOG : an international journal of obstetrics and gynaecology 111, 585–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.