Abstract
Objectives:
Excessive and insufficient sleep have been associated with cognitive dysfunction in older adults in U.S. and non-U.S. studies. However, the U.S. studies were not in nationally representative samples. We investigated the association between sleep duration and cognitive performance in a nationally representative sample of U.S. older adults.
Participants:
We studied 1,496 survey participants aged ≥60 years from the National Health and Nutrition Examination Survey (NHANES) 2013-14 dataset.
Measurements:
Our primary predictor was weekday (or workday) nighttime sleep duration, categorized as 2-4, 5, 6, 7 (reference), 8, 9, and ≥10 hours. We studied five cognitive outcomes: Consortium to Establish a Registry for Alzheimer’s Disease Word Learning (CERAD-WL) Immediate Recall, CERAD-WL Delayed Recall, Animal Fluency Test (AFT), Digital Symbol Substitution Test (DSST), and subjective cognitive problems (SCP).
Results:
After adjusting for age, sex, race, education, depressive symptoms, and sedative-hypnotic use, sleep duration of ≥10 hours was significantly associated with lower scores on CERAD-WL Immediate Recall, CERAD-WL Delayed Recall, AFT, and DSST, and greater odds of SCP; sleep duration of ≥8 hours was associated with lower CERAD-WL delayed recall scores: 8 hours, 9 hours, and ≥10 hours. After adjustment, there were no significant associations of shorter sleep duration with cognition.
Conclusions:
In U.S. adults aged ≥60 years, long nighttime weekday or workday sleep duration is associated with poorer verbal memory, semantic fluency, working memory, and processing speed in addition to greater odds of self-reported cognitive problems. Long sleep duration may be a marker of fragmented sleep or neurodegeneration in U.S. older adults.
Objective
A growing body of literature has highlighted the critical role sleep plays in the cognitive and general health in older adults.1,2 While there are numerous metrics of sleep quality and quantity, sleep duration is of particular interest given its conceptual accessibility to community-dwelling older adults and the relative ease with which it can be queried in large epidemiologic samples. The recommended sleep duration for adults aged 65 years and older, per National Sleep Foundation guidelines, is 7 to 8 hours per night.3 Although some studies suggest that community-dwelling older adults’ median sleep duration is about 7 hours,4 many older adults habitually sleep for more or fewer hours than recommended.5,6 This is important, because short and long sleep have been found to be associated with multiple health conditions, including diabetes, coronary heart disease-related events, and all-cause mortality.7–10
In the multinational, non-U.S., nationally representative Study on Global Aging and Adult Health (SAGE) of older adults (≥ 50 years old), Gildner et. al. observed both short and long sleep durations to be associated with poorer cognitive performance.11 More specifically, the extremes of sleep duration were associated with poorer scores on tests of verbal fluency, digit span (forwards and backwards), and verbal recall (immediate and delayed), compared to those with intermediate sleep durations of 6-9 hours.11
U.S and non-U.S. studies that were not nationally representative have produced similar results. For example, in a prospective study of older adults in Guangzhou, China, Xu et. al. found both short and long sleep to be associated with lower scores on both global cognitive function and verbal memory.12 In a cross-sectional telephone survey of older adults in Paris, France, Ohayon et. al. found that sleep duration of 6 hours or less was associated with worse cognitive impairment.4 In the Northern Manhattan Study (NOMAS), Ramos et. al. noted an association between long sleep of 9 hours or more and poorer global cognition scores.13
Although the aforementioned studies evaluated various segments of the older adult population either in the U.S. or abroad, there remains a gap in knowledge about the association between sleep duration and performance across cognitive domains in a nationally representative sample of community-dwelling older adults in the U.S. Such knowledge could help inform U.S. guidelines about sleep duration for cognitive health. In this study, we evaluated the association of self-reported weekday or workday sleep duration with four measures of cognitive performance, covering a range of cognitive domains, and subjective cognitive problems in a nationally representative sample of community-dwelling U.S. older adults.
Methods
Participants
The National Health and Nutrition Examination Survey (NHANES) is a bi-yearly national survey of the U.S. children and adults. The survey consists of an in-home interview and an examination at a Mobile Examination Center (MEC). The 2013-14 NHANES sample had 10,175 children and adult participants. Of these, 1,841 were aged 60 years or older and had undergone the general interview. Among participants aged 60 and older, 56 only completed the in-home interview without completing the MEC exam and 1,785 participated in both the in-home interview and the examination at the Mobile Examination Center (MEC). Of these 1,785 participants, 19 were ineligible for the cognitive testing because they required a proxy for it; 16 were ineligible because they did not understand the supported languages of the cognitive testing; and 64 had no testing done. Of the 1,686 who remained, 1,638 completed all three cognitive tests. We excluded 5 of these 1,638 participants without complete data on sleep duration and an additional 137 without complete data on subjective cognitive problems or the covariates of interest. Thus, our final analytic sample consisted of 1,496 participants (Figure 1).
Figure 1.
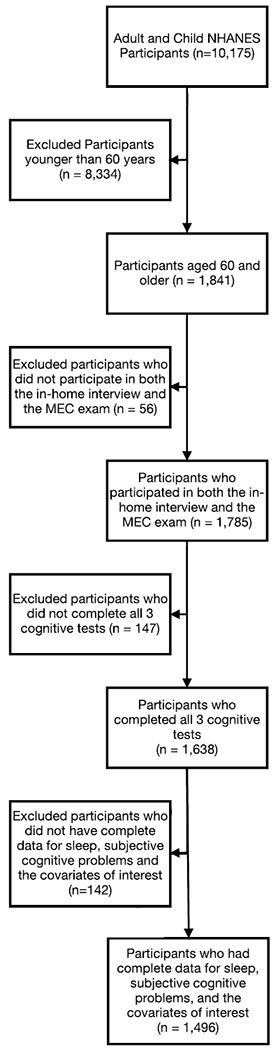
Participant Flow Diagram
Of these, there were 8 participants who required a proxy for the in-home interview (including the sleep measures), but not for the cognitive evaluation; these participants were included in the analysis. Since one of the eligibility requirements for the cognitive evaluation was the ability to complete the cognitive evaluation without a proxy, none of the NHANES participants had a proxy for the cognitive evaluation. A sensitivity analysis was performed excluding the 8 participants who required a proxy for the in-home interview. We did not find the results to be significantly different, so we retained them in our sample.
Measures
Demographics, sleep duration, and questionnaire data were obtained during in-home interviews. The age at the time of the survey was recorded in years for participants aged 80 and younger. For participants over the age of 80, their age was recorded as 80 in the dataset released by NHANES, to reduce the risk that the oldest participants could be identified. Cognitive data were acquired during MEC visits.
Sleep duration.
Survey participants (SP) or their proxies were asked “How much sleep {do you/does SP} usually get at night on weekdays or workdays?” Responses were given in units of hours. We categorized responses as ≤ 4 hours, 5 hours, 6 hours, 7 hours (reference), 8 hours, 9 hours, and ≥ 10 hours. We chose 7 hours as the reference, because it has been identified as the median sleep duration in older adults and has been used as a reference in prior studies of sleep duration and cognition in older adults.4,14
Cognitive tests.
Participants completed four cognitive tests: the Consortium to Establish a Registry for Alzheimer’s Disease Word Learning (CERAD-WL) (immediate and delayed recall),15 Animal Fluency Test (AFT),16 and Digital Symbol Substitution Test (DSST).17 Voice recordings were required for the CERAD-WL and Animal Fluency Tests (AFT). If consent was not obtained for voice recording, only the DSST was administered.
The CERAD-WL is a measure of verbal memory and consists of four trials. During the first three trials, participants were asked to read ten words aloud, and then to immediately recall them. The sum of correctly recalled words across the three trials served as the immediate recall score (possible range 0 to 30). Following completion of the AFT and DSST (approximately 8-10 minutes after starting the CERAD-WL), participants were asked to recall as many of the ten words as they could, and the total number of correctly recalled words served as their delayed recall score.
The AFT assesses semantic fluency. Participants were asked to name as many animals as they could within one minute. The total number of animals named served as their score.
The DSST, from the Wechsler Adult Intelligence Scale (WAIS III), is a test of sustained attention, processing speed, and working memory.17 Participants were given an examination sheet with a row of numbers and symbols at the top that served as a key. Below the key is an array of 122 numbers, and participants were asked to draw the appropriate symbol next to each number as quickly as they could within two minutes.
Subjective cognitive problems.
NHANES also collected information on subjective cognitive problems. The Medical Conditions Questionnaire asked participants or proxies about “…difficulties in thinking or remembering…like confusion or memory loss that are happening more often or getting worse” over the past 12 months, with response options of yes or no. They were also asked in the Physical Functioning Questionnaire, whether they are “limited in any way because of difficulty remembering [due to] periods of confusion?” We classified participants with responses of yes to either question as having subjective cognitive problems. Participants missing one of these items were excluded from analyses.
Other measures.
NHANES participants provided information on demographic characteristics. Depressive symptoms were measured using the Patient Health Questionnaire.18,19 Blood pressure and diabetes history were obtained during an in-home interview by asking “{Have you/Has SP} ever been told by a doctor or other health professional that {you/s/he} had hypertension, also called high blood pressure?” and “{Other than during pregnancy, {have you/has SP}/{Have you/Has SP}} ever been told by a doctor or health professional that {you have/{he/she/SP} has} diabetes or sugar diabetes?” Interviewers also collected information on prescription medications by first asking the participant whether they took prescription medications in the last 30 days. If they answered in the affirmative, the interviewer would ask the participant to show him or her the prescription bottles. If the bottles were not available, the interviewer relied on the participant’s verbal report. Participants were classified as being on sedative medications if at least one of their medications was categorized by NHANES as an anxiolytic, sedative, or hypnotic.
Statistical Analyses
We computed descriptive statistics and compared participants across sleep duration categories using linear regression for continuous variables, logistic regression for binary variables, and Chi-squared tests for categorical variables. Covariates associated with self-reported sleep duration and at least 1 of the 4 measures of cognitive function or subjective cognitive problems at the p <0.10 level were included in the multiple regression models; sex and education (some college or higher) were also included for face validity. We performed unadjusted and adjusted multiple regression analyses with sleep duration as the predictor and cognitive test score or subjective cognitive problems as the outcome. We conducted tests for linear trend across sleep duration categories. All analyses were performed with Stata/IC 15.1 (Statacorp, College Station, TX) with sample weights applied to make results nationally representative. Because NHANES uses a complex multistage, probability sampling design, we also accounted for primary sampling units and strata, using Taylor series approximations.
Results
Participants had a mean age of 69.3 ± 0.3 years (mean ± standard error). The range of the participants’ ages had a lower limit of 60 years. However, the upper limit is unknown because participants whose age was greater than 80 years had their age recorded as 80 years in the dataset released by NHANES to reduce the risk of identifying participants. Per NHANES documentation, the weighted mean age for those aged above 80 was 84 years. In this sample, there were 245 participants who were recorded as 80 years of age. Overall, 46.1% were male, 79.3% identified themselves as White, and 63.4% reported at least some college education (Table 1). With respect to sleep duration, 61 (4.1%) reported 2-4 hours of sleep, 110 (7.4%) reported 5 hours of sleep, 318 (21.3%) reported 6 hours of sleep, 406 (27.1%) reported 7 hours of sleep, 430 (28.7%) reported 8 hours of sleep, 104 (7%) reported 9 hours of sleep, 67 (4.5%) reported 10 or more hours of sleep. Sleep duration was significantly associated with age, race, hypertension, depressive symptoms, and sedative use. Means ± standard errors of participants’ performance on the cognitive tests were also calculated by sleep-duration category (Table 2).
Table 1.
Participant Characteristics (mean ± standard error or n (%)) by Sleep Duration
| Sleep Duration (Hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 - 4 | 5 | 6 | 7 | 8 | 9 | ≥ 10 | F stat | df | p-value† | |
| n | 61 (4.1) | 110 (7.4) | 318 (21.3) | 406 (27.1) | 430 (28.7) | 104 (7.0) | 67 (4.5) | --- | ||
| Age | 66.5± 0.9 | 69.5 ± 1.0 | 68.9 ± 0.5 | 68.4 ± 0.4 | 70.0 ± 0.3 | 71.0 ± 0.8 | 70.2 ± 1.4 | 5.08 | 6, 10 | 0.0122 |
| Male | 21 (34.1) | 50 (37.3) | 147 (42.9) | 210 (52.6) | 208 (44.7) | 54 (50.2) | 27 (34.2) | 1.88 | 6, 10 | 0.1799 |
| White | 16 (55.7) | 35 (62.8) | 131 (69.9) | 229 (84.9) | 241 (82.2) | 68 (86.2) | 42 (84.1) | 7.97 | 6, 10 | 0.0024 |
| ≥ College | 20 (47.8) | 47 (55.4) | 165 (61.1) | 235 (69.0) | 233 (61.4) | 64 (69.8) | 26 (56.7) | 1.88 | 6, 10 | 0.1796 |
| Obese | 27 (47.4) | 47 (51.7) | 118 (41.4) | 148 (36.8) | 146 (33.5) | 42 (41.8) | 25 (37.5) | 2.07 | 6, 10 | 0.1477 |
| DM | 12 (13.3) | 29 (22.8) | 79 (25.7) | 86 (17.3) | 93 (17.6) | 29 (20.0) | 16 (22.3) | 1.28 | 6, 10 | 0.3492 |
| HTN | 37 (41.4) | 77 (72.9) | 210 (65.1) | 234 (53.1) | 281 (63.9) | 71 (65.2) | 44 (70.8) | 2.34 | 6, 10 | 0.1131 |
| Dep. Sx. | 8.1 ± 1.1 | 4.6 ± 0.5 | 4.0 ± 0.2 | 2.2 ± 0.2 | 2.9 ± 0.3 | 3.8 ± 0.6 | 3.0 ± 0.5 | 6.02 | 6, 10 | 0.0067 |
| Sedative Use | 5 (6.4) | 12 (17.3) | 25 (8.1) | 25 (6.3) | 40 (11.6) | 16 (22.2) | 8 (12.3) | 6.37 | 6, 10 | 0.0055 |
Key: ≥ College = have at least some college education, Dep. Sx. = Depressive Symptoms, DM = Diabetes Mellitus, HTN = Hypertension
Survey-weighted linear and logistic regression; P-value is of the F-statistic, df = degrees of freedom, F stat = F statistic
Table 2.
Participant Performance on Cognitive Tests (mean ± standard error or n (%)) by Sleep Duration
| Sleep Duration (Hours) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 - 4 | 5 | 6 | 7 | 8 | 9 | ≥10 | F stat | df | p-value† | |
| n | 61 (4.1) | 110 (7.4) | 318 (21.3) | 406 (27.1) | 430 (28.7) | 104 (7.0) | 67 (4.5) | --- | ||
| CERAD Im. | 20.2 ± 0.7 | 19.9 ± 0.7 | 20.4 ± 0.3 | 21.3 ± 0.4 | 20.2 ± 0.2 | 19.8 ± 0.6 | 18.7 ± 0.8 | 4.96 | 6, 10 | 0.0132 |
| CERAD Del. | 6.5 ± 0.3 | 6.6 ± 0.4 | 6.7 ± 0.1 | 7.0 ± 0.2 | 6.4 ± 0.1 | 6.0 ± 0.3 | 5.5 ± 0.5 | 3.93 | 6, 10 | 0.0279 |
| AFT | 17.1 ± 0.6 | 17.8 ± 1.0 | 17.5 ± 0.4 | 18.9 ± 0.5 | 18.1 ± 0.2 | 17.7 ± 0.4 | 15.1 ± 0.8 | 6.66 | 6, 10 | 0.0047 |
| DSST | 49.1 ± 2.9 | 49.1 ± 2.4 | 51.1 ± 1.1 | 54.7 ± 1.3 | 50.9 ± 1.1 | 52.4 ± 3.0 | 44.6 ± 2.0 | 5.63 | 6, 10 | 0.0086 |
Key: CER. Im. = CERAD-WL Immediate recall, CER. Del. = CERAD-WL Delayed Recall, AFT = Animal Fluency Test, DSST = Digit Symbol Substitution Test
Survey-weighted linear regression; P-value is of the F-statistic, df = degrees of freedom, F stat = F statistic
CERAD-WL Immediate Recall
Compared to participants reporting 7 hours of sleep, performance on CERAD-WL immediate recall was poorer among those reporting sleep durations of 5 hours (B = −1.36, 95% confidence interval (CI) −2.59, −0.13), 8 hours (B = −1.04, 95% CI −1.82, −0.27), and 10 or more (B = −2.57, 95% CI −3.79, −1.36) (Table 3). After adjusting for age, sex, race, education, depressive symptoms, and sedative medications, only sleep duration of 10 or more hours remained significantly associated with lower CERAD-WL immediate recall score (B = −2.11, 95% CI −3.43, −0.80). There was a linear trend, such that, among participants sleeping ≥ 7 hours those with longer sleep had poorer CERAD-WL immediate recall scores, even after adjustment.
Table 3.
CERAD-WL Immediate and Delayed Recall Performance by Sleep Duration (B, 95% CI)
| Linear trend† (≤7h) | Sleep Duration, Hours per Day | Linear trend† (≥7 h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F stat | p-value | 2-4 | 5 | 6 | 7 | 8 | 9 | ≥10 | F stat | p-value | |||
| df | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | df | ||||||
| CERAD Word Learning Immediate Recall | |||||||||||||
| Unadjusted | 2.08 | 1, 15 | 0.1696 | −1.03 (−2.60, 0.54) | −1.36 (−2.59, −0.13)* | −0.84 (−1.71, 0.03) | Ref. | −1.04 (−1.82, −0.27)* | −1.51 (−3.21, 0.19) | −2.57 (−3.79, −1.36)* | 22.90 | 1, 15 | 0.0002 |
| Adjusted‡ | 0.32 | 1, 15 | 0.5784 | −0.50 (−1.78, 0.78) | −0.68 (−1.70, 0.35) | −0.43 (−1.25, 0.39) | Ref. | −0.65 (−1.33, 0.03) | −0.88 (−2.28, 0.53) | −2.26 (−3.85, −0.68)* | 8.84 | 1, 15 | 0.0095 |
| CERAD Word Learning Delayed Recall | |||||||||||||
| Unadjusted | 4.48 | 1, 15 | 0.0514 | −0.58 (−1.22, 0.06) | −0.41 (−1.05, 0.22) | −0.29 (−0.71, 0.13) | Ref. | −0.66 (−1.10, −0.22)* | −1.01 (−1.84, −0.19)* | −1.58 (−2.55, −0.62)* | 14.66 | 1, 15 | 0.0016 |
| Adjusted‡ | 0.13 | 1, 15 | 0.7285 | −0.34 (−1.06, 0.38) | −0.09 (−0.51, 0.34) | −0.10 (−0.55, 0.35) | Ref. | −0.46 (−0.87, −0.05)* | −0.69 (−1.34, −0.04)* | −1.41 (−2.56, −0.27)* | 7.04 | 1, 15 | 0.0181 |
P < 0.05
Adjusted for age, sex, race, education, depressive symptoms, and sedative medication use
Contrast of marginal linear predictions, df = degrees of freedom, F stat = F statistic
CERAD-WL Delayed Recall
In unadjusted models, CERAD-WL delayed recall scores were also poorer among those with longer sleep compared to those sleeping 7 hours, specifically those reporting 8 hours (B = −0.66, 95% CI −1.10, −0.22), 9 hours (B = −1.01, 95% CI −1.84, −0.19), and 10 or more hours (B =−1.58, 95% CI −2.55, −0.62). In adjusted multiple regression analysis, compared to those reporting 7 hours sleep, scores were lower among participants reporting sleep durations of 8 hours (B = −0.45, 95% CI −0.84, −0.06), 9 hours (B = −0.69, 95% CI −1.33, −0.05) and ≥ 10 hours (B = −1.36, 95% CI −2.42, −0.30). Among participants reporting ≥ 7 hours of sleep, those reporting longer sleep had worse CERAD-WL delayed recall scores, even after adjustment (Table 3).
Animal Fluency Test
In the unadjusted model, compared to 7 hours of sleep, both shorter and longer sleep durations were associated with poorer AFT performance: 2-4 hours (B = −1.86, 95% CI −3.19, −0.52), 6 hours (B = −1.39, 95% CI −2.47, −0.30), and 10 or more hours (B = −3.78, 95% CI −6.16, −1.40) (Table 4). In the adjusted model, however, only sleep of 10 hours or more was associated with poorer AFT performance (B = −3.20 95% CI −5.77, −0.62). There was again a linear trend, such that, among participants sleeping ≥ 7 hours, those with longer sleep duration had poorer performance on the AFT, even after adjustment (Table 4).
Table 4.
Animal Fluency Test and Digit Symbol Substitution Test Performance by Sleep Duration (B, 95% CI)
| Linear trend† (≤7 h) | Sleep Duration, Hours per Day | Linear trend† (≥7 h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F stat | df | p-value | 2-4 | 5 | 6 | 7 | 8 | 9 | ≥10 | F stat | df | p-value | |
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | ||||||||
| Animal Fluency Test | |||||||||||||
| Unadjusted | 3.83 | 1, 15 | 0.0691 | −1.86 (−3.19, −0.52)* | −1.09 (−3.50, 1.32) | −1.39 (−2.47, −0.30)* | Ref. | −0.84 (−2.05, 0.37) | −1.18 (−2.38, 0.02) | −3.78 (−6.16, −1.40)* | 14.38 | 1, 15 | 0.0018 |
| Adjusted‡ | 0.10 | 1, 15 | 0.7613 | −0.22 (−1.61, 1.16) | 0.45 (−1.41, 2.31) | −0.42 (−1.45, 0.60) | Ref. | −0.10 (−1.30, 1.10) | −0.46 (−1.66, 0.74) | −2.90 (−5.68, −0.12)* | 5.54 | 1, 15 | 0.0327 |
| Digit Symbol Substitution Test | |||||||||||||
| Unadjusted | 3.73 | 1, 15 | 0.0726 | −5.58 (−11.67, 0.52) | −5.59 (−10.41, −0.77)* | −3.60 (−7.07, −0.13)* | Ref. | −3.82 (−7.24, −0.39)* | −2.33 (−9.58, 4.93) | −10.05 (−14.54, −5.56)* | 15.47 | 1, 15 | 0.0013 |
| Adjusted‡ | < 0.01 | 1, 15 | 0.9977 | 0.06 (−4.88, 4.99) | −0.10 (−3.90, 3.70) | −0.25 (−3.26, 2.76) | Ref. | −1.17 (−3.87, 1.52) | 1.12 (−4.08, 6.32) | −7.47 (−12.04, −2.91)* | 8.84 | 1, 15 | 0.0095 |
P < 0.05
Adjusted for age, sex, race, education, depressive symptoms, and sedative medication use
Contrast of marginal linear predictions, df = degrees of freedom, F stat = F statistic, CI = Confidence interval
Digit Symbol Substitution Test
In unadjusted analyses, compared to participants sleeping 7 hours, lower scores were observed among those reporting sleep durations of 5 hours (B = −5.59 95% CI −10.41, −0.77), 6 hours (B = −3.60 95% CI −7.07, −0.13), 8 hours (B = −3.82 95% CI −7.24, −0.39), and 10 or more hours (B = −10.05 95% CI −14.54, −5.56). After adjustment for covariates, only sleep durations of 10 hours or greater were associated with poorer DSST scores (B = −7.56 95% CI −11.52, −3.60). Among participants reporting ≥ 7 hours of sleep a night, those with longer sleep durations had poorer DSST scores, even after adjustment (Table 4).
Subjective Cognitive Problems
In unadjusted analyses, relative to those who slept 7 hours, self-reported cognitive problems were more prevalent among those who slept 2-4 hours (OR = 5.06, 95% CI 1.80, 14.26), 6 hours (OR = 1.83, 95% CI 1.02, 3.30), 8 hours (OR = 1.91, 95% CI 1.21,3.01), 9 hours (OR = 2.31, 95% CI 1.33, 4.00), and 10 or more hours (OR = 4.19, 95% CI 1.76, 9.93). After adjusting for age, sex, race, education, depressive symptoms, and sedative medications, compared to those sleeping 7 hours, sleep durations of 10 or more hours (OR = 3.61, 95% CI 1.42, 9.22) were associated with higher odds of subjective cognitive problems (Table 5). There was a linear trend among participants sleeping ≥ 7 hours, such that those with longer sleep had greater odds of reporting subjective cognitive problems, even after adjustment (Table 5).
Table 5.
Association of Sleep Duration with Subjective Cognitive Problems (OR, 95% CI)
| Linear trend† (≤7 h) | Sleep Duration, Hours per Day | Linear trend† (≥7 h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F stat | p-value | 2-4 | 5 | 6 | 7 | 8 | 9 | >=10 | F stat | p-value | |||
| df | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | df | ||||||
| Unadjusted | 9.64 | 1, 15 | 0.0073 | 5.06 (1.80, 14.26)* | 1.66 (0.66, 4.15) | 1.83 (1.02, 3.30)* | Ref. | 1.91 (1.21, 3.01)* | 2.31 (1.33, 4.00)* | 4.19 (1.76, 9.93)* | 12.83 | 1, 15 | 0.0027 |
| Adjusted‡ | 1.39 | 1, 15 | 0.2563 | 1.95 (0.62, 6.12) | 0.81 (0.32, 2.08) | 1.21 (0.60, 2.45) | Ref. | 1.51 (0.99, 2.30) | 1.33 (0.68, 2.60) | 3.61 (1.42, 9.22)* | 8.73 | 1, 15 | 0.0098 |
P < 0.05
OR = odds ratio, CI = confidence interval
Adjusted for age, sex, race, education, depressive symptoms, and sedative medication use
Contrast of marginal linear predictions, df = degrees of freedom, F stat = F statistic
Conclusions
We investigated the association of self-reported sleep duration with performance on objective cognitive tests and subjective cognitive problems in a nationally representative sample of community-dwelling U.S. adults aged 60 and older. After accounting for several potential confounders, we found that longer, but not shorter sleep duration, was associated with poorer cognitive performance and subjective cognitive problems. Notably, participants reporting 10 hours or more sleep had lower scores in all four cognitive tests, which measured immediate and delayed verbal memory, semantic fluency, and sustained attention, processing speed, and working memory; they also had greater odds of subjective cognitive problems. Additionally, a linear trend was found across normal-to-longer sleep duration (≥ 7 hours), but not normal-to-shorter sleep duration (≤ 7 hours), such that longer sleep was associated with poorer performance on all four cognitive tests and greater odds of subjective cognitive problems, even after adjustment for covariates.
Our findings are consistent with those from several prior studies. For instance, similar to our results, Ramos et. al. found that among older adults in the NOMAS, those reporting ≥9 hours of sleep had lower MMSE scores after adjustment for demographics, medications, vascular risk factors, and sleep-disordered breathing risk factors.13 Also, consistent with our results, they found no significant association between shorter sleep duration and cognitive performance.13 Likewise, in a study of non-institutionalized older adults in Spain, Faubel et. al. found that self-reported long sleep (≥11 hours), but not short sleep (<7 hours) over a 24-hour period, was associated with lower performance on a Spanish version of the MMSE.14 Although the magnitudes of the differences in cognitive performance we observed among the sleep duration groups compared to the reference group were small, they are comparable to those found in other studies of self-reported sleep duration and cognition, including those by Gildner et al., Xu et al., and Ramos et al.11–13 Future longitudinal studies in nationally representative samples of U.S. older adults may elucidate whether these differences remain subtle or increase over time.
Where objectively measured sleep is concerned, actigraphy-based studies have found associations between long sleep duration and poorer cognitive performance, but not short sleep duration. In the Study of Osteoporotic Fractures (SOF), among older women who completed wrist actigraphy, those in the longest tertile of total sleep time had poorer scores on the Modified Mini-Mental Status Exam, and measures of both semantic and phonemic fluency than those in the middle tertile; the performance of those in the shortest and middle tertiles did not differ.20
The association between longer sleep and poorer cognitive scores suggests one of two possibilities: either long sleep is a marker of poor health21 or long sleep is itself detrimental for cognitive performance. Given the lack of biological plausibility, the latter is unlikely. The more plausible explanation is that longer sleep is a marker of sleep fragmentation,22 which in turn has been linked to poorer cognitive performance.23 Fragmented sleep may be a marker of either underlying neuropathology or sleep apnea.24 In a prospective study, long sleep duration was demonstrated to be a risk factor for incident dementia and may have been an early proxy marker for underlying neurodegenerative disease.6 Also, obstructive sleep apnea, which can lead to sleep fragmentation,22 has likewise been shown to be a risk factor for dementia.2,25 The differential diagnosis of long sleep includes but is not limited to obstructive sleep apnea and potentially early, preclinical stages of neurodegenerative disorders, such as Alzheimer’s disease.2,6,22–25
It is of interest that the results of this study differed from studies that found a statistically significant association between short sleep and poorer cognition. In a study of older adults in the Chinese province of Guangzhou by Xu. et. al., both self-reported short and long sleep durations were found to be associated with lower scores on global cognitive function and delayed verbal recall.12 Sleep durations ≤ 6 hours and ≥ 9 hours were associated with lower scores on MMSE; sleep durations of ≤ 5 hours and ≥ 10 hours were associated with lower scores on delayed verbal recall.12 Additionally, in a 2014 nationally representative non-U.S. study of sleep duration and sleep quality among older adult participants of the SAGE study in India, China, Russian Federation, Ghana, Mexico, and South Africa, Gildner et. al. found that both those with self-reported short sleep duration and those with self-reported long sleep duration had poorer performance on tests of verbal fluency, backwards and forwards digit span, and immediate and delayed verbal recall.11 The study of older adults in Paris by Ohayon et. al. had revealed that self-reported sleep durations of 4 hours or less were associated with cognitive impairment, as gauged by the Cognitive Difficulties scale.4 There are several potential reasons we did not find a statistically significant association between short sleep and cognitive performance. One possibility is that our largely null results for shorter sleep duration may be due to insufficient statistical power. The Guangzhou study had 13,888 participants, and the SAGE study had 35,552, whereas our study sample was comprised of 1,496 respondents. Additionally, these studies may have had different findings because they employed different cognitive batteries. Also, we did not exclude the extremes of self-reported sleep duration as Xu et. al. had. We included more extreme sleep durations in our analysis to deliver a more comprehensive representation of the older population and to preserve statistical power. Lastly, age differences may account for some of the discrepant findings. For example, the studies by Xu et. al. and the Gildner et. al. included adults age ≥ 50 years, but we only studied adults aged ≥ 60 years.
This study also had some limitations. First, the NHANES uses a cross-sectional design which precludes a longitudinal analysis that might provide insight into the directionality and time course of the link between sleep and cognitive performance. In addition, our sample contained only non-institutionalized participants, and results may not generalize to institutionalized older adults. Further, our data were based on self-reported weekday or workday nighttime sleep duration, and the NHANES did not assess sleep duration on weekends or days off, daytime sleep duration, or include objective sleep measures in the 2013-2014 sample. Our data also did not include sleep diaries or polysomnography. Previous studies have found that objective and subjective measures frequently disagree, and that in older people this may be due to cognitive problems.26 Therefore, the self-reported sleep durations collected in our sample may not have captured all participants’ true sleep durations. Further, our sample included 8 participants who required proxies for the in-home interview, which includes the sleep questionnaire. Although the validity of proxy-reported sleep duration in older adults in unknown, it is likely to be vulnerable to the aforementioned inaccuracies of self-reported sleep without proxy in addition to the inaccuracies that may come with proxy reporting. However, it is also possible that a proxy observer may provide a more accurate history if a survey participant has memory problems. We completed a sensitivity analysis excluding those with proxies and did not find a significant change in the results, so we included them in our sample. In addition, the ages of participants older than 80 years were recorded as 80 in the datasets released by NHANES to reduce the risk of identifying participants in this age group. Per NHANES documentation, the average age of participants aged over 80 was 84 years. In our study, 245 out of the 1,496 participants were recorded as having age 80 years. This measure against disclosure risk protects participants but likely results in an underestimation of the ages of participants and may have limited this study’s ability to adjust for age, an important risk factor for cognitive decline. Also, our study population was relatively young. Although we cannot be certain of the exact ages of those recorded as aged 80 years, 1,251 out of 1,496 participants were aged 60 to 79 years. Thus, findings may not generalize to adults who are significantly older than our study population. Further, the NHANES 2013-14 sleep questionnaire did not collect data regarding obstructive sleep apnea, which is an important potential confounder of the relationship between sleep duration and cognition. Additionally, the measures of subjective cognitive problems were based on questions about memory and confusion from the medical condition and physical function questionnaire; the psychometric properties of these items are unknown and require further study. Finally, the classifications of participants as taking or not taking sedative medications was limited by the method of data collection, which relied on self-report if participants’ medication bottles were not available. A more objective means of assessment would enhance the measurement of sedative use.
Our study also has important strengths. First, prior studies in this area include several U.S. studies that were not nationally representative and a nationally representative non-U.S. study. The present study, however, investigated sleep duration-cognition links in a nationally representative sample of non-institutionalized U.S. older adults. Although the National Sleep Foundation has published sleep duration recommendations by age that include older people,3 the Sleep Research Society and the American Academy of Sleep Medicine have not yet issued guidelines for sleep duration for individuals over age 60.27 Our findings could be used to inform these recommendations with regard to the outcome of cognitive health. Second, because NHANES collected data on performance on well-studied cognitive tests from different domains and a measure of subjective cognitive problems, this yielded a more nuanced picture of how various cognitive domains vary in their association with sleep duration than have studies using global cognitive tests such as the MMSE. Although subjective cognitive impairment (SCI) is still controversial, it may be a useful marker of underlying brain pathology and thus warrants investigation.28
In conclusion, compared to community-dwelling U.S. older adults reporting seven hours of nighttime sleep on weekdays or workdays, those reporting longer sleep duration have poorer objective and subjective cognitive performance. Future studies of both daytime and nighttime sleep in relation to cognitive performance and decline in representative samples are needed, particularly those involving objective measures (e.g., wrist actigraphy). Such studies would add to our understanding of the link between sleep duration and cognitive outcomes. This knowledge could be used to inform public health policy, clinical guideline development, and both clinical assessment and practice.
1). What is the primary question addressed by this study?—
The question addressed by the study must limited to only one sentence.
Although studies have found links between the extremes of sleep duration and cognitive performance, there is a lack of these studies in nationally representative samples of U.S. older adults.
2). What is the main finding of this study?—
The finding must be limited to two sentences.
We found that longer self-reported sleep duration was linked to poorer cognitive performance and greater odds of subjective cognitive problems in a nationally representative sample of U.S. older adults.
3). What is the meaning of the finding?—
The meaning of the finding must be limited to one sentence.
Long sleep duration may be a marker of disturbed (e.g., fragmented) sleep, which may be due to sleep apnea or neurodegeneration, in U.S. older adults.
Acknowledgments
Sources of Support:
Dr. Dominique Low: NIHLBI T32HL110952
Dr. Adam Spira: NIA (R01AG050507, RF1AG050745, R01AG049872, U01AG052445, R01AG054771)
Dr. Mark Wu: NIA R01AG054771
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest declaration: Adam Spira received an honorarium from Springer Nature Switzerland AG for Guest Editing a Special Issue of Current Sleep Medicine Reports. Previous presentation: An early version of this paper was presented as a poster at and published as an abstract in the abstract supplement of the 2018 Annual Meeting of the American Academy of Neurology in Boston, MA which took place from April 21, 2018 to April 27, 2018.
References
- 1.Newman AB, Enright PL, Manolio TA, et al. : Sleep Disturbance, Psychosocial Correlates, and Cardiovascular Disease in 5201 Older Adults: The Cardiovascular Health Study. J Am Geriatr Soc 1997; 45: 1–7 [DOI] [PubMed] [Google Scholar]
- 2.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol 2014; 13:1017–1028 [DOI] [PubMed] [Google Scholar]
- 3.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015; 1:40–43 [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep 2005; 28:981–989 [PubMed] [Google Scholar]
- 5.Mesas AE, López-García E, León-Muñoz LM, et al. The association between habitual sleep duration and sleep quality in older adults according to health status. Age Ageing 2011; 40:318–323 [DOI] [PubMed] [Google Scholar]
- 6.Benito-León J, Bermejo-Pareja F, Vega S, et al. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 2009; 16:990–997 [DOI] [PubMed] [Google Scholar]
- 7.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003; 26:380–384 [DOI] [PubMed] [Google Scholar]
- 8.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med 2003; 163:205–209 [DOI] [PubMed] [Google Scholar]
- 9.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta analysis. J Sleep Res 2009; 18:148–158 [DOI] [PubMed] [Google Scholar]
- 10.Kripke DF, Garfinkel L, Wingard DL, et al. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 2002; 59:131–136 [DOI] [PubMed] [Google Scholar]
- 11.Gildner TE, Liebert MA, Kowal P, et al. Associations between sleep duration, sleep quality, and cognitive test performance among older adults from six middle income countries: results from the Study on Global Ageing and Adult Health (SAGE). J Clin Sleep Med 2014; 10:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu L, Jiang CQ, Lam TH, et al. Sleep Duration and Memory in the Elderly Chinese: Longitudinal Analysis of the Guangzhou Biobank Cohort Study. Sleep 2014; 37:1737–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the minimental score: the Northern Manhattan study. J Clin Sleep Med 2013; 9:669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faubel R, López-Garcia E, Guallar-Castillón P, et al. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res 2009; 18:427–435 [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989; 39:1159–1165 [DOI] [PubMed] [Google Scholar]
- 16.Strauss E, Sherman EM, Spreen O: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY, Oxford University Press, 2006 [Google Scholar]
- 17.Wechsler D WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale—Third Edition. San Antonio, TX, Psychological Corporation, 1997 [Google Scholar]
- 18.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a Brief Depression Severity Measure. J Gen Intern Med 2001; 16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL. The PHQ-9: A New Depression Diagnostic and Severity Measure. Psychiatr Ann 2002; 32:509–515. [Google Scholar]
- 20.Spira AP, Stone KL, Redline S, et al. Actigraphic Sleep Duration and Fragmentation in Older Women: Associations with Performance Across Cognitive Domains. Sleep 2017; 40:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benito-León J, Louis ED, Bermejo-Pareja F. Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES). J Psychiatr Res 2013; 47:1998–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev 2004; 8:159–174 [DOI] [PubMed] [Google Scholar]
- 23.Lim AS, Yu L, Costa MD, et al. Increased Fragmentation of Rest-Activity Patterns is associated with a Characteristic Pattern of Cognitive Impairment In Older Individuals. Sleep 2012; 35:633–640B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinnunen KM, Vikhanova A, Livingston G. The Management of Sleep Disorders in Dementia: An Update. Curr Opin Psychiatry 2017; 30:491. [DOI] [PubMed] [Google Scholar]
- 25.Buratti L, Luzzi S, Petrelli C, et al. Obstructive Sleep Apnea Syndrome: An Emerging Risk Factor for Dementia. CNS Neurol Disord Drug Targets 2016; 15:678–682 [DOI] [PubMed] [Google Scholar]
- 26.Van Den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res 17, 295–302 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Watson NF, Badr MS, Belenky G, et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep 38, 1161–1183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart R Subjective Cognitive Impairment. Curr Opin Psychiatry 2012; 25:445. [DOI] [PubMed] [Google Scholar]


