Abstract
Background:
Studies testing the relationship between cortisol levels, depression, and antidepressant treatment response have yielded divergent results suggesting the possibility of moderators of a cortisol effect. Several studies indicate that age may moderate the relationship between cortisol and depression. In patients with Major Depressive Disorder (MDD), we studied the interactive effects of age and cortisol in association with MDD diagnostic status and mood and memory response to antidepressant treatment.
Methods:
Serum cortisol levels in 66 unmedicated patients with MDD and 75 matched healthy controls (HC) were measured at baseline and retrospectively analyzed. Logistic regression was used to determine an association of age, cortisol and their interaction with MDD diagnosis in the pooled sample of MDD and HC participants. Thirty-four of the MDD participants (age range: 19- 65 years; median: 36) underwent treatment with a selective serotonin reuptake inhibitor (SSRI) for 8 weeks. Clinician and self-ratings of depression symptoms, as well as tests of verbal and visual delayed recall were obtained at baseline and post treatment. Moderation analyses determined the effect of age on the relationship between baseline cortisol and treatment outcome.
Results:
Cortisol, moderated by age, was associated with MDD diagnosis (p<.05), treatment-associated reduction of depression symptoms (p<.001) and improvement of delayed recall (p<.001). Modeling the Cortisol X Age interaction suggested that for participants below the median age of our sample, lower cortisol levels were associated with a lower rate of MDD diagnosis and higher antidepressant effects. On the contrary, in those above the median sample age, lower cortisol was associated with a higher rate of MDD and less improvement in depression symptoms and memory performance.
Conclusions:
Our results add to the body of literature suggesting that age might be an important factor in moderating the relationship between peripheral cortisol levels, depression, cognition, and prognosis. These results indicate that previous disparities in the literature linking peripheral cortisol levels with depression characteristics and treatment response may critically relate, at least in part, to the age of the participants studied.
Keywords: major depressive disorder, cortisol, aging, antidepressants, memory, HPA axis
1. Introduction
Because of the immense and growing cost of Major Depressive Disorder (MDD) to individuals (Ferrari et al., 2013) and society (Greenberg et al., 2015), uncovering physiological biomarkers associated with depression that may predict treatment response is of high importance. Data accumulated over four decades of research indicate that the hypothalamic-pituitary-adrenal (HPA) axis plays a key role in the pathophysiology of MDD (Stetler and Miller, 2011; Wolkowitz et al., 2009) and might have utility for prediction of depressive symptom response to psychological therapies (Fischer et al., 2017). However, inconsistent results in the relationship between HPA axis markers such as cortisol and MDD suggest the presence of moderators such as age (Stetler and Miller, 2011).
Several groups have observed an association between MDD and perturbations of the HPA axis such as elevations in circulating cortisol (O’Brien et al., 2004; Stetler and Miller, 2011; Vreeburg et al., 2009) or nonsuppression of cortisol levels with the dexamethasone suppression test (Raison and Miller, 2003), particularly in more serious variants such as severe melancholic (Lamers et al., 2013) and MDD with psychotic features (Gomez et al., 2006; Nelson and Davis, 1997; Rothschild et al., 1987). However, many studies of older adults find no difference in overall cortisol levels in individuals with MDD relative to healthy controls (Conrad et al., 2008; Fabian et al., 2001; Ferrari et al., 2001). Lower cortisol was even detected in a significant proportion of elderly depressed patients (Bremmer et al., 2007; Oldehinkel et al., 2001). Other conditions characterized by high degrees of negative affect in elderly populations, including bereavement (Ong et al., 2011) and post-traumatic stress disorder (Yehuda et al., 1995), have also been associated with lower cortisol levels.
Age may also moderate the effects of glucocorticoids on memory function (Heffelfinger and Newcomer, 2001; Lupien et al., 2009). For example, in elderly rats, basal corticosterone levels correlated negatively with spatial memory performance, but this was not true of young rats (Yau et al., 1995). In young rats, amitriptyline improved spatial memory and increased corticosteroid hippocampal gene expression, but these effects were not seen in older rats, suggesting some antidepressant mechanisms related to glucocorticoids may be moderated by age (Yau et al., 1995). Two studies in humans (with MDD status unspecified) suggested that higher cortisol levels have detrimental effects on memory in older participants. A longitudinal study found that older adult individuals (mean age 76.5 ± 4.3 years) who exhibited a rise in cortisol levels over the course of five years exhibited impairments in delayed memory relative to those with decreasing and currently moderate cortisol levels (Lupien et al., 1998); another longitudinal study found similar evidence for a negative effect of increasing cortisol levels on memory in women but not in men (Seeman et al., 1997).
An important age-dependent concern for antidepressant treatment, including with selective serotonin reuptake inhibitors (SSRIs) is the emergence of suicidal ideation and behavior. As identified by the U.S. Food and Drug Administration, antidepressant treatment relative to placebo increases the risk of suicidal thinking and behavior for individuals under 25 years of age (Hampton, 2004). The risk of suicidal ideation and behavior, however, is lower with antidepressant treatment than placebo for adults 65 years of age or older, and the risk appears to change from higher to lower in midlife (McCain, 2009). Whether SSRIs might result in different outcomes based on a biological difference between individuals of different ages or other factors is unclear. Although suicidal behavior and depression treatment outcome are not isomorphic clinically or biologically, the clear link between higher depression severity and increased suicidal ideation (Beck et al., 1993) and the age dependent link between suicidality and antidepressant treatment indicates a need to study how age interacts with biology in determining antidepressant treatment response. One recent informative meta-analysis of suicidal behavior in a heterogeneous population of participants with different diagnoses and treatments found that younger individuals with higher cortisol and older individuals with lower cortisol were at highest risk (O’Connor et al., 2016). Motivated by this literature, we decided to investigate the relationships between cortisol, age and antidepressant treatment response within a sample of depressed participants receiving treatment with selective serotonin reuptake inhibitors (SSRIs). We undertook a retrospective analysis to explore the relationship between the state of the HPA axis as indicated by cortisol level moderated by age, and depression characteristics including MDD diagnosis and SSRI treatment outcome (mood and memory). An a priori hypothesis was generated that age would moderate the effects of cortisol on depression treatment outcome, in that younger individuals (relative to our sample) with higher cortisol and older individuals with lower cortisol would be at higher risk for MDD and improve less (symptomatically and in regards to memory) after a course of antidepressants.
2. Material and methods
2.1. Study participants
67 unmedicated MDD patients and 75 matched healthy controls (HC) were recruited at the University of California, San Francisco (UCSF), in a pilot study (N=38) from 2006-2008 (clinicaltrials.gov ) and a larger study (N=104) of cellular aging markers for antidepressant treatment response () taking place from 2011– 2014. All procedures were approved by the Committee on Human Research of UCSF. Participants were recruited by flyers, bulletin board notices, newspaper ads, Craigslist postings, and clinical referrals. Study participants gave written informed consent to participate and were compensated for participating. Those receiving antidepressant treatment were not charged.
Depressed participants were diagnosed with a current major depressive episode, without psychotic features, with the Structured Clinical Interview for DSM IV-TR Axis I Disorders (SCID) (First et al., 2015) and this diagnosis was confirmed by clinical interview with a board-certified psychiatrist. They scored ≥17 on the 17-item Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960) indicating at least moderate severity. Exclusion criteria for MDD patients were psychotic symptoms during their current major depressive episode, history of psychosis outside of a mood disorder episode, bipolar disorder, post-traumatic stress disorder, any eating disorder or severe trauma within one month of entering the study, and substance abuse or dependence (including alcohol) within six months of entering the study. Co-morbid anxiety disorders, with the exception of PTSD, were not exclusionary if MDD was considered the primary diagnosis. None of the study participants had acute illnesses or infections, chronic inflammatory disorders, neurological disorders, or any other major medical conditions considered to be potentially confounding (e.g., cancer, HIV, diabetes, history of cardiovascular disease or stroke, etc.), as assessed by history, physical examination and routine blood screening.
All study participants were required to be free of any psychotropic medications, hormone supplements, ACE-inhibitors, steroid-containing birth control or other potentially interfering medications, and had not had any vaccinations, for at least six weeks prior to enrollment in the study. None were taking vitamin supplements above the U.S. recommended daily allowances (e.g., 90 mg/day for Vitamin C). Short-acting sedative-hypnotics were allowed as needed up to a maximum of three times per week, but none within one week prior to participation. Prior to each study visit, all participants tested negative for drugs of abuse with a urine toxicology screen (marijuana, cocaine, amphetamines, phencyclidine, opiates, methamphetamine, tricyclic antidepressants, and barbiturates), and women of child-bearing potential tested negative for pregnancy with a urine screen. Individuals taking part in the imaging study were excluded for any MRI contraindications.
2.2. Blood draw preparations
Participants were admitted as outpatients to the UCSF Clinical and Translational Science Institute between 8 a.m. and 11 a.m., having fasted (except water) since 10 p.m. the night before. Participants were instructed to sit quietly and relax for 25 - 45 minutes before blood samples were obtained. Blood was collected into serum separator tubes, and serum was saved and frozen at - 80 degrees C until assay to ensure stability (Kley et al., 1985) in a freezer equipped with an alarm system to notify of temperature conditions and ensure there were no temperature excursions. Cortisol assays for retrospective analysis were conducted in batches based on year of recruitment (2006 - 2008 and 2011 - 2014) as previously described (Hough et al., 2017). The first batch contributed 38 samples (18 MDD and 20 HC) and was assayed by Human ELISA (Immulite 2000, Siemens Healthcare Global, Erlangen, Germany, intra-assay CV 5.6%, inter-assay CV 7.7%). The second batch consisted of 104 samples (49 MDD and 55 HC) and was assayed with radioimmunoassay (Siemens Medical Solutions Diagnostics, Los Angeles, CA, intra-assay CV 3.4%, inter-assay CV 5.5%). Relative to the normal parameters of the cortisol assays (5ug/dL – 25ug/dL), in the HC sample, 13% of participants were below and 2% were above, whereas in MDD, 8% of participants were below and 0% of participants were above. Post blood draw, one MDD participant was found to be taking an ACE inhibitor and was excluded, resulting in 66 usable MDD participants and 75 HC.
2.3. Depression Symptom Measures
The severity of depressive symptoms was rated in depressed patients by self-report using the Quick Inventory of Depression Symptoms – Self Report (QIDS) (Rush et al., 2003), and by clinical observers using the HAMD, at baseline and (for treated patients) after 8 weeks of antidepressant medication. The QIDS was used as primary outcome because of its unidimensionality and high internal consistency (Reilly et al., 2015), and because it was normally distributed in our population whereas HAMD exhibited a leftward skew with most participants scoring near the cutoff point for study entry. Change in depression symptoms was calculated as post minus pre.
2.4. Cognitive Measures
Cognitive tests were administered to depressed patients in the treatment sample by research assistants trained by a licensed neuropsychologist with expertise in the delivery of research-grade assessment. The primary outcome studied was verbal and visual delayed recall (DR) due to previous work identifying a relationship between cortisol levels and delayed memory performance in aging (Lupien et al., 1998). The Hopkins Verbal Learning Test - Revised (HVLT) was used to assess verbal DR (Benedict et al., 1998) and the Brief Visuospatial Memory Test – Revised (BVMT) for visual DR (Benedict et al., 1996). To assess attention and working memory as a composite (attention-WM), WAIS-III forward and backward digit span tests were administered and the total score from both digit span trials was summed to provide an overall measure of performance (Wechsler, 1997). Raw scores for all measures were used. Not all participants completed cognitive measures: the HVLT was obtained on 27 MDD participants and the BVMT on 18 participants at follow up (Table 1). All BVMT participants were drawn from the 2011-2014 pool comprising batch 2.
Table 1.
Demographic and clinical variables
| MDD baseline (N = 66) |
HC (N = 75) | MDD treatment (N = 34) |
MDD treatment HVLT (N = 27) |
MDD treatment BVMT (N = 18) |
|
|---|---|---|---|---|---|
| Female sex (%) | 38 (58%) | 46 (61%) | 22 (66%) | 17 (63%) | 11 (61%) |
| Age (years) [range] | 38.8 ± 13.8 [19–68] | 37.3 ± 13.3 [20–69] | 37.4 ± 12.1 [19–65] | 36.0 ± 12.3 [20–65] | 36.0 ± 13.9 [19–62] |
| Race (%) | |||||
| Caucasian | 46 (70%) | 50 (67%) | 28 (82%) | 22 (82%) | 14 (78%) |
| African American / Black | 5 (8%) | 6 (8%) | 2 (6%) | 2 (7%) | 1 (6%) |
| Asian | 9 (14%) | 15 (20%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Multiracial American Indian | 1 (1%) | 2 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other | 5 (7%) | 2 (2%) | 3 (9%) | 3 (11%) | 3 (17%) |
| Ethnicity | |||||
| Hispanic (%) | 11 (17%) | 9 (12%) | 7 (20%) | 7 (26%) | 5 (28%) |
| Education (years) | 16.1 ± 2.2 | 16.7 ± 1.8 | 16.4 ± 2.2 | 16.3 ± 2.0 | 15.9 ± 2.3 |
| Smoking tobacco (%) | 17 (25%) | 6 (8%)* | 10 (30%) | 6 (22%) | 6 (33%) |
| Body Mass Index | 25.7 ± 4.5 | 24.5 ± 4.6 | 26.9 ± 4.9 | 26.6 ± 5.1 | 28.1 ± 4.6 |
| Age of MDD onset | 16.4 ± 8.7 | NA | 15.6 ± 9.2 | 16.3 ± 10.2 | 12.3 ± 4.7* |
| QIDS baseline | 13.1 ± 3.8 | 1.9 ± 1.3** | 13.3 ± 3.3 | 13.5 ± 3.4 | 13.4 ± 2.8 |
| HAMD baseline | 20.1 ± 3.3 | NA | 19.6 ± 3.0 | 19.9 ± 3.2 | 20.0 ± 3.4 |
| AM serum cortisol baseline (μg/dL) | 9.6 ± 4.6 | 9.7 ± 6.0 | 9.9 ± 4.7 | 10.6 ± 4.6 | 8.5 ± 4.1 |
BVMT=Binder Visual Memory Test; HAMD=Hamilton Depression Rating Scale 17 item; HC=healthy control; HVLT=Hopkins Verbal Learning Test; MDD=Major Depressive Disorder; NA=not available; QIDS=Quick Inventory of Depression Symptoms – Self Report; ug/dL=micrograms per deciliter. MDD baseline variables were compared to all other samples, with significant differences indicated as *p<.05 and **p<.01. No differences were found between variables of the three MDD treatment samples compared with each other.
2.5. Antidepressant treatment
34 of the MDD participants were provided with open-label SSRI antidepressant treatment for 8 weeks. The end-of-study blood draw, clinical assessment and depression ratings were performed at the end-of-study visit, while the subjects were still on their standing doses of medication. The decision to treat 34 of the larger group of MDD subjects was not based on randomization nor on any systematic selection criteria: the larger study added a treatment phase about midway through the study, and all of the treated subjects who had cortisol data and depression outcome measures were included in the present report. Doses of medications, including maximum tolerated doses, were based on clinical practice of a Board-Certified psychiatrist with over 35 years experience in prescribing antidepressants to depressed patients according to standard clinical guidelines (American Psychiatric Association, 2010); therefore the dosing schedules were reflective of real-world clinical practice. Participants were begun at a low dose which was titrated to therapeutic range during the eight-week period as clinically indicated. All subjects were started on sertraline unless this was deemed clinically inappropriate such as for prior failed trial or previous history of side effects. Of the 34 subjects, 28 received sertraline (dose range 25 to 200mg, final dose 110±38mg), 2 escitalopram (dose range 10 to 20mg, final dose 15±7mg), 2 citalopram (dose range 20 to 40mg, final dose 35±7mg), and 2 fluoxetine (dose range 20 to 40mg, final dose 35±7mg). No subjects required SSRI switch. In general, maximum tolerated doses were held constant for at least the last 4 weeks of the 8-week trial. Compliance with medication was assessed by telephone check-ins at Week 1, and in-person visits at week 4 and 8 with pill counts. Plus, antidepressant levels were measured in blood, and in every case (with the exception of one subject, a treatment responder, who tolerated only 25 mg/day of sertraline), the blood levels were within the expected ranges, consistent with very good medication compliance. Short-acting benzodiazepines (t-1/2 ≤ 12 hours) were allowed for sleep in the MDD group on an “as needed” basis up to three times per week, but none within one week of any testing or blood drawing session.
2.6. Statistical analysis
Statistical analysis was conducted with R version 3.2.3 (R Core Team, 2016). Baseline demographic variables included age, sex, cortisol, body mass index (BMI), race, ethnicity (Hispanic), current smoking (tobacco), Cortisol levels were log transformed and then converted to z scores by batch to facilitate comparison. The baseline variables were examined for outliers and one participant was identified who scored more than 3 standard deviations below the mean on multiple cognitive tests at baseline (HVLT, BVMT, Digit Span), and this participant was removed from analysis of cognitive results. Model terms for all analyses were presented as Beta ± one standard error (SE).
2.6.1. Preliminary Analyses
Preliminary analyses were conducted to determine the comparability of the larger samples with subsamples used for specific outcomes analyses, using Chi-square tests for categorical variables and t-tests for continuous measures. Analyses were also conducted to determine which covariates (aside from sex, which was always included due to its well-known association with MDD) should be included in the models of Cortisol X Age with each outcome; and whether cortisol, age or sex were individually associated with outcomes. Specifically, logistic regression was used to determine associations between smoking, sample batch, cortisol, age, sex, and BMI, with MDD diagnosis. Linear regression was used to determine associations between changes in longitudinal outcome measures (QIDS, HAMD, BVMT and HVLT delayed recall) and smoking, sample batch, cortisol, age, sex, BMI, and baseline scores on the outcome measures. Longitudinal changes in symptoms from before to after treatment were characterized with t-tests and Cohen’s d effect size.
2.6.2. Primary Analyses
A priori, three outcomes were tested as primary: MDD diagnosis, self-reported depression symptom change, and delayed recall change. Logistic regression was employed to model the relationship between Cortisol X Age with baseline MDD diagnosis in the pooled sample of MDD and HC participants, using sex and smoking as covariates (smoking because it differed at baseline between MDD and HC.) A linear model was utilized to determine the association of QIDS change with Cortisol X Age, using sex and baseline QIDS as covariates. A linear model was also employed to determine the association of verbal (HVLT) and visual (BVMT) DR change with Cortisol X Age, adjusting for sex and baseline score as covariates, and the interaction term was considered significant if p<.025 to correct for two comparisons.
2.6.3. Secondary Analyses
Cortisol X Age was studied in association with change in HAMD and attention-WM, using sex and baseline attention-WM as covariates. To determine whether the DR change results could be accounted for by change in attention-WM, change in attention-WM was added as a covariate to the primary analysis models of change in DR.
2.6.4. Visualization of longitudinal outcome interaction models
To visually model how the relationship between cortisol and clinical outcome was moderated by age, the results were plotted using a sliding window in a manner analogous to signal processing algorithms that model variables of interest based on when they occur (Kaiser et al., 2018; Sugase et al., 1999). Participants were first ordered from 1 to 34 on the basis of age. Sliding windows of 9 consecutive participants (e.g., participants 1 through 9, 2 through 10, 3 through 11, etc.) were obtained to cluster participants of similar age together. For each window of similarly aged participants, a linear regression model was performed using the relevant outcome as the dependent variable and cortisol as the independent variable. To facilitate visualization of the results, the beta values (for the slope of cortisol associated with the outcome variable in each sliding window) were plotted on the y axis versus the median of age of each sliding window on the x axis.
3. Results
3.1. Preliminary analyses
3.1.1. Clinical and treatment samples
The clinical MDD sample and matched HC were comparable on baseline demographic measures (Table 1). On average, participants were female, college educated, and Caucasian. Depression severity of MDD participants was on average moderate as assessed by both the HAMD and the QIDS, whereas the low score of HC on the QIDS was consistent with the absence of depression (these participants were not scored with the HAMD.) The MDD treatment subsamples did not differ on baseline variables from the overall MDD sample or from each other except for a younger age of onset in the subset to whom the BVMT was administered relative to the overall MDD sample (p<.05, Table 1).
3.1.2. Association of individual baseline variables with MDD diagnosis and treatment outcomes
To determine how baseline variables associated with MDD status, we pooled the data from MDD participants (termed “MDD base”) and HC samples (Table 1) and coded for presence of MDD in a binary fashion. With logistic regression, only smoking was associated with MDD diagnosis, but not other baseline variables (age, sex, BMI, cortisol, batch, Table 2). This also illustrated that our MDD sample was well controlled on pre-specified age and sex variables.
Table 2.
Associations of baseline variables with MDD diagnosis in pooled MDD/HC sample
| Individual Variable Models | |||
|---|---|---|---|
| Variable (N) | β | SE | P |
| Age (144) | 0.008 | 0.01 | 0.52 |
| Cortisol (141) | 0.08 | 0.17 | 0.66 |
| Batch (144) | -0.008 | 0.38 | 0.98 |
| Sex (144) | 0.06 | 0.34 | 0.85 |
| BMI (144) | 0.06 | 0.04 | 0.11 |
| Tobacco Use (144) | 1.19 | 0.49 | 0.01 |
| Interaction Model (N=141) | |||
| Age | 0.01 | 0.013 | 0.45 |
| Cortisol | 1.34 | 0.63 | 0.03 |
| Cortisol X Age | -0.04 | 0.02 | 0.04 |
| Model: χ2(3) =4.8, p = 0.19 | |||
| Interaction Model Adjusting for Smoking and Sex (N=141) | |||
| Age | 0.02 | 0.01 | 0.26 |
| Cortisol | 1.45 | 0.64 | 0.02 |
| Sex | 0.05 | 0.37 | 0.88 |
| Smoking (tobacco) | 1.50 | 0.52 | 0.004 |
| Cortisol X Age | -0.04 | 0.02 | 0.03 |
| Model: χ2(3) =12.6, pi = 0.005 | |||
BMI=Body Mass Index; HC=Healthy Controls; MDD=Major Depressive Disorder
Correlations between baseline outcome scores and change in outcome scores were consistently significant, with higher depression symptom scores and lower DR scores associated with greater improvement (p<.001 for QIDS, p=.01 for HAMD, p<.0001 for both BVMT and HVLT DR, Table 4). Age was negatively correlated with improvement in QIDS (p<.05) and positively correlated with improvement in BVMT DR (p<.05) (Table 4). Other tested variables were not individually associated with treatment outcomes (Table 4).
Table 4.
Correlates of change in depression and cognition
| Change in QIDS (N=34) | Change in HAMD (N=34) | |||||||
|---|---|---|---|---|---|---|---|---|
| Individual Variable Models | Individual Variable Models | |||||||
| Variable (df) | β | SE | p | Variable (df) | β | SE | p | |
| Age | 0.13 | 0.05 | 0.02 | Age | 0.11 | 0.08 | 0.19 | |
| Cortisol | -0.24 | 0.72 | 0.74 | Cortisol | 0.9 | 1.03 | 0.39 | |
| QIDS baseline | -0.72 | 0.16 | 0.001 | HAMD baseline | -0.78 | 0.3 | 0.01 | |
| Batch | -1.46 | 1.34 | 0.29 | Batch | 0.75 | 1.97 | 0.7 | |
| Sex | 0.48 | 1.41 | 0.73 | Sex | 0.64 | 2.05 | 0.76 | |
| BMI | 0.14 | 0.14 | 0.3 | BMI | 0.3 | 0.19 | 0.14 | |
| Tobacco Use | -0.08 | 1.49 | 0.96 | Tobacco Use | -1.97 | 2.13 | 0.36 | |
| Interaction Model | Interaction Model | |||||||
| Age | 0.19 | 0.05 | 0.001 | Age | 0.2 | 0.08 | 0.02 | |
| Cortisol | 7.44 | 1.98 | 0.001 | Cortisol | 8.36 | 3.37 | 0.02 | |
| Cortisol X Age | -0.18 | 0.05 | 0.001 | Cortisol X Age | -0.17 | 0.08 | 0.04 | |
| Model: F(3,30)=7.85, adj R2=.38, p<.001 | Model: F(3,30)=2.98, adj R2=.15, p<.05 | |||||||
| Interaction Model Adjusting for Baseline Symptoms and Sex | Interaction Model Adjusting for Baseline Symptoms and Sex | |||||||
| Age | 0.12 | 0.06 | 0.04 | Age | 0.14 | 0.09 | 0.12 | |
| Cortisol | 5.55 | 2.07 | 0.01 | Cortisol | 6.94 | 3.43 | 0.05 | |
| Sex | 0.17 | 1.06 | 0.88 | Sex | -0.51 | 1.87 | 0.79 | |
| QIDS baseline | -0.45 | 0.19 | 0.03 | HAMD baseline | -0.59 | 0.33 | 0.09 | |
| Cortisol X Age | -0.13 | 0.05 | 0.02 | Cortisol X Age | -0.14 | 0.08 | 0.12 | |
| Model: F(5,28)=6.04, adj R2=.44, p<.001 | Model: F(5,28)=2.64, adj R2=.20, p<.05 | |||||||
| Change in HVLT DR (N=26) | Change in BVMT DR (N=18) | |||||||
| Individual Variable Models | Individual Variable Models | |||||||
| Variable (df) | β | SE | p | Variable (df) | β | SE | p | |
| Age | -0.008 | 0.03 | 0.21 | Age | 0.05 | 0.02 | 0.02 | |
| Cortisol | 0.74 | 0.37 | 0.06 | Cortisol (z score) | -0.009 | 0.26 | 0.97 | |
| HVLT DR baseline | -0.60 | .09 | 0.0001 | BVMT DR baseline | -0.77 | 0.10 | 0.0001 | |
| Sex | -0.06 | 0.71 | 0.94 | Sex | 0.01 | 0.64 | 0.98 | |
| BMI | -0.03 | 0.06 | 0.67 | BMI | -0.10 | 0.06 | 0.16 | |
| Tobacco Use | 1.19 | 0.79 | 0.15 | Tobacco Use | 0.08 | 0.66 | 0.90 | |
| Interaction Model | Interaction Model | |||||||
| Age | -0.02 | 0.02 | 0.49 | Age | 0.04 | 0.02 | 0.10 | |
| Cortisol | -2.35 | 1.21 | 0.06 | Cortisol | -0.76 | 0.90 | 0.41 | |
| Cortisol X Age | .08 | .03 | .01 | Cortisol X Age | 0.02 | 0.02 | 0.31 | |
| Model: F(3,22)=4.01, adj R2=.27, p<.05 | Model: F(3,14)=2.74, adj R2=.23, p=.08 | |||||||
| Interaction Model Adjusting for Baseline Symptoms and Sex | Interaction Model Adjusting for Baseline Symptoms and Sex | |||||||
| Age | -.04 | 0.02 | 0.01 | Age | -0.03 | 0.02 | 0.09 | |
| Cortisol | -1.54 | 0.74 | 0.05 | Cortisol | -1.30 | 0.46 | 0.02 | |
| Sex | -0.03 | 0.40 | 0.95 | Sex | -0.36 | 0.32 | 0.29 | |
| HVLT DR baseline | -0.57 | 0.09 | 0.00001 | BVMT DR baseline | -0.87 | 0.13 | 0.0001 | |
| Cortisol X Age | 0.05 | 0.02 | 0.02 | Cortisol X Age | 0.03 | 0.01 | 0.01 | |
| Model: F(5,20)=14.83, adj R2=.73, p<.00001 | Model: F(5,12)= 17.11, adj R2=.83, p<.0001 | |||||||
Association of change in outcome measure with individual variables at baseline, Cortisol X Age interaction model, and Cortisol X Age adjusting for baseline symptoms and sex. BMI = body mass index; BVMT = Binder Visual Memory Test; DR = delayed recall; HAMD = Hamilton Depression Rating Scale 17-item; HVLT = Hopkins Verbal Learning Test; QIDS = Quick Inventory of Depression Symptoms - Self Report
3.1.3. Effect of treatment
With treatment, self- and clinician-rated depression symptoms declined significantly as expected for a single group, open label treatment (Table 3). HVLT total learning score and DR improved, but there were no changes in other verbal memory measures, visual learning or attention-WM (Table 3).
Table 3.
Depression and cognition changes after 8 weeks of treatment
| pre | post | t( df) | P | |
|---|---|---|---|---|
| QIDS | 13.3 ± 3.3 | 7.21 ± 3.21 | 9.21 (33) | 10−9 |
| HAM-D | 19.62 ± 3.04 | 9.71 ± 5.16 | 10.24 (33) | 10−10 |
| HVLT Total | 27.42 ± 4.90 | 28.93 ± 3.71 | 2.44 (25) | 0.02 |
| HVLT Delayed Recall | 10.09 ± 2.05 | 10.81 ± 1.30 | 3.24 (25) | 0.003 |
| HVLT Recognition | 10.52 ± 3.33 | 11.19 ± 1.30 | 1.27 (24) | 0.22 |
| HVLT Retention | 96.94 ± 13.56 | 98.91 ± 10.37 | 1.21 (25) | 0.24 |
| BVMT Total | 26.79 ± 7.69 | 29.67 ± 3.45 | 1.27 (17) | 0.22 |
| BVMT Delayed Recall | 10.05 ± 2.82 | 10.89 ± .68 | .92 (17) | 0.37 |
| BVMT Retention | 82.34 ± 35.32 | 95.73 ± 5.25 | 1.23 (17) | 0.23 |
| Digit Span | 19.26 ± 4.72 | 19.67 ± 3.89 | .67 (32) | 0.51 |
BVMT=Binder Visual Memory Task; df=degrees of freedom; HAMD=Hamilton Depression Rating Scale 17 item; HC=healthy control; HVLT=Hopkins Verbal Learning Task; MDD=Major Depressive Disorder; t=paired t-test statistic; QIDS=Quick Inventory of Depression Symptoms – Self Report
3.2. Primary Outcomes
3.2.1. Association of Cortisol X Age with MDD diagnosis
Age was a moderator of the association of cortisol with MDD diagnosis (Table 2), and this was not influenced by adjustment for smoking or sex. To characterize the pattern of moderation, we performed median splits on cortisol (z score = 0) and age (36 years) and determined the proportion of participants with MDD in each group. This yielded the following percentages of participants with MDD: low age and low cortisol 39% (n=12/31), low age and high cortisol 48% (n=19/41), high age and low cortisol 56% (n=25/45), high age and high cortisol 42% (n=11/26).This suggested that the significance of the Cortisol X Age interaction was due to a modestly increased risk of MDD in those younger participants with higher cortisol levels and older participants with lower cortisol levels. Post hoc Chi-square test, however, was not significant for differences among these subgroups (X(3) = 2.4, p=.5), possibly due to limited power when subdividing the sample into four discrete classifications.
3.2.2. Age moderates association of cortisol with self-reported depression symptom outcome
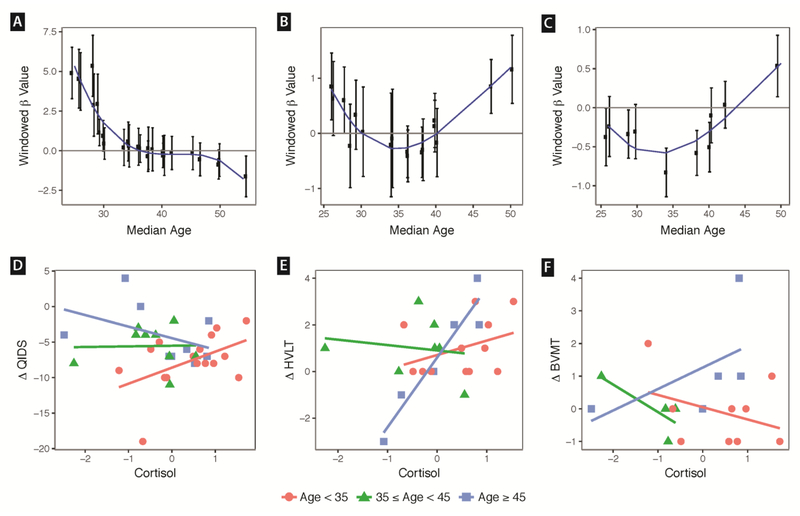
For change in QIDS, the linear model of Cortisol X Age with sex and baseline symptom covariates was significant (p<.001), and the overall model accounted for 43% of the variance in change in QIDS (Table 4). Significant terms included cortisol, QIDS baseline score, and Cortisol X Age (p<.05 for all). Removing covariates did not alter the significance of the Cortisol X Age term (Table 4). The pattern of interaction suggested greater improvement in participants of younger age with lower cortisol and older age with higher cortisol (Fig 1 A, D).
Figure 1.
Panels A – C. Sliding window modeling of how cortisol was associated with outcome in each age group. Participants were ordered on age and grouped into overlapping windows of 9 participants. Successive windows iterated by one participant. Each window corresponds to one data point. Y axis shows Beta coefficient ± one standard error of outcome change regressed on cortisol within each sliding window. X-axis indicates median age for the window of 9 participants in which the cortisol-outcome regression was performed. A third order polynomial was used to model the curve. For panel A, negative Beta value indicates higher cortisol was associated with improvement in depression symptoms. For panels B and C, positive Beta indicates that higher cortisol was associated with improvement in verbal and visual delayed recall, respectively. Panels D-F. Data points for cortisol (z scores) versus change in clinical outcome (pre - post). A negative delta for QIDS indicates clinical depression improvement, whereas a positive delta for HVLT and BVMT indicates memory improvement. Red circles show participants less than 35 years of age. Green triangles show participants between 35 and 44. Blue squares show participants 45 and older. The trend line was modeled within each age range. QIDS=Quick Inventory of Depression Symptoms – Self Report; BVMT=Binder Verbal Memory Test Delayed Recall; HVLT=Hopkins Verbal Learning Test Delayed Recall.
3.2.3. Age moderates association of cortisol with improvement in delayed recall
In the HVLT verbal DR interaction model, all terms (Age, Cortisol, baseline symptoms, and Cortisol X Age) were significant except for sex, and the overall model accounted for 73% of the variance in outcome (p<.00001). Neither adjusting for change in attention-WM performance nor for sample batch reduced the significance of the model (Supplementary Results). The pattern of interaction suggested greater improvement in participants of older age with higher cortisol (Fig 1 B,E).
Because change in the verbal DR measure was associated with Cortisol X Age, we next tested whether Cortisol X Age was associated with change in delayed visual recall using the BVMT. In the interaction model adjusting for baseline symptoms and sex, cortisol, baseline performance, and Cortisol X Age were significant and the model accounted for 83% of the variance in outcome (Table 4, p<.0001). Adjusting for change in attention-WM did not impact the significance of these findings (Supplementary Results). The pattern of interaction suggested greater improvement in participants of older age with higher cortisol (Fig 1 C,F).
3.3. Secondary Outcomes
We tested whether age moderated the relationship between cortisol and clinician-rated depression symptoms. Although age moderated the effect of cortisol on HAMD in the model without covariates, when adding sex and baseline HAMD the interaction term was no longer significant (Table 4). While the overall model with covariates was statistically significant (p<.05) as was the cortisol term (p<05), the model accounted for a lower proportion of the variance (20%) than noted above for self-reported symptoms.
With respect to change in attention-WM, we found no effect of age, cortisol, or their interaction (p>.5 for all independent variables). However, adding baseline attention-WM score did result in significance of the overall model, with baseline attention-WM score as the only significant variable (Supplementary Results).
4. Discussion
In a sample of MDD individuals who were physically healthy and unmedicated at study entry, age moderated the association of cortisol with baseline MDD status and several important outcomes: depression symptomatic improvement with antidepressant treatment, and delayed recall with both verbal and visual stimuli. Confirming our hypotheses, for adults younger than the median age of our sample, modeling of the data indicated that higher levels of cortisol were associated with worse treatment response, while for participants older than the median, higher levels of cortisol were associated with better treatment response. For cognitive outcomes, there appeared to be little association with cortisol at lower ages but a strong association in the upper age range, most prominently in those over 45 years of age, in which relatively higher baseline levels of cortisol were associated with improvement in both verbal and visual delayed memory. This partially confirmed our hypothesis in older, but not younger adults in our sample. The association of Cortisol X Age with improvements in memory and mood could neither be explained by covariates such as sex or baseline symptoms, nor by a commensurate change in a composite score of attention and working memory. To our knowledge, this is the first study to find a consistent, age-dependent biomarker (cortisol level) that is associated with MDD diagnostic status, reduction in depression symptoms and memory improvement.
Though observed in a limited sample of 34 individuals, the Cortisol X Age association with depression treatment outcome is consistent with two recent large meta-analyses that assessed the impact of age in moderating cortisol on prediction of outcome in varied clinical populations. First, O’Connor et al (2016) demonstrated a similar relationship between age and cortisol in predicting suicidal behavior. This meta-analysis analyzed 27 studies including over 2000 individuals and found an interactive effect between age and basal cortisol in predicting suicide attempt across patients with different diagnoses: those patients under 40 with higher cortisol and those over 40 with lower cortisol were at greatest risk. Interestingly, the inflection point for antidepressant treatment outcome in this study was also at about 40 years of age. The reason for this shift in midlife is unclear. Although the curve is roughly similar to that of fertility, inclusion of sex in our model did not reduce the significance of our findings or clearly suggest a sex-specific effect.
The finding that age moderated the association of cortisol with MDD diagnostic status is also broadly consistent with a meta-analysis of 26 studies containing over 5000 individuals that found a similar Cortisol X Age interaction to predict post-traumatic stress disorder (PTSD) risk (Morris et al., 2016). In this meta-analysis, younger individuals with higher cortisol and older individuals with lower cortisol in the period following a traumatic event were at greater risk for later development of PTSD. However, the inflection point in their study was approximately 30 years of age, perhaps suggesting that the physiological process underlying this finding might be accelerated in those with significant trauma reactivity, relative to those without PTSD (as comprised our sample).
Consistent with several prior reports, higher cortisol levels in younger individuals was associated with higher MDD and worse treatment response. Stetler et al. (2011), for example, found that higher cortisol levels were evident with a moderate effect size in MDD using metaanalysis. Prior studies of cortisol as a predictor of MDD treatment with psychological therapies (Fischer et al., 2017) and antidepressant treatment (Kin et al., 1997) have suggested that higher levels are a poor prognostic sign. Our results in adults over the median age of our sample, which in contrast indicated that lower levels of circulating cortisol were associated with higher rates of depression diagnosis are consistent with some prior reports (Bremmer et al., 2007; Oldehinkel et al., 2001). Potential mechanisms related to these findings are unclear but we suggest two possibilities related to changes in the HPA Axis with aging and stress. An overall reduction in HPA activity could be associated with lower cortisol levels in adults past midlife who are particularly vulnerable to treatment resistant depression. Alternatively, increased neural glucocorticoid receptor sensitivity, perhaps due to chronic HPA axis activation from stress earlier in life, could result in lower peripheral cortisol levels via feedback inhibition. Both of these mechanisms have been postulated to underlie clinical symptoms often comorbid with depression, including somatic symptoms and pain syndromes, chronic fatigue, and stress related disorders, albeit irrespective of age (Fries et al., 2005; Heim et al., 2000; Raison and Miller, 2003).
The findings that, for younger adults in our sample, cortisol levels were not apparently associated with delayed recall improvement with antidepressants, while for adults past midlife increasing cortisol was associated with improved delayed recall, are challenging to explain apart from the aforementioned general mechanisms possibly associated with antidepressant response. That changes in attention and working memory did not account for the effect of Cortisol X Age on delayed recall suggests a specific memory-related mechanism, perhaps mediated by medial temporal lobe structures. Recent work has suggested that higher circulating cortisol is associated with reduction in amygdala volume in older but not younger adults (Ennis et al., 2019), which might be thought to most impact an emotional learning difference between age groups. However, volumetric differences with age were not seen in the hippocampus which is the critical structure implicated in delayed recall (Smith and Milner, 1981). Lupien and colleagues (1998) demonstrated negative associations between chronically elevated cortisol, reduction in hippocampal volume and delayed recall impairment in an aging population. SSRIs have been shown to increase BDNF derived neural progenitor cells in the hippocampus (Boldrini et al., 2009), perhaps counteracting the effects of elevated basal cortisol in older adults. Whether this would be thought to differ in younger adults, however, is unclear. Other authors have speculated that older adults might be more vulnerable than younger individuals to hippocampal microglial neuroinflammatory pathways that are primed by cortisol (Barrientos et al., 2012). Such microglial activation might be reversed by SSRIs (Farooq et al., 2018; Tynan et al., 2012). Thus, older adults with relatively higher levels of basal cortisol might be those more likely to have putative depression mechanisms, such as microglial activation, modulable by SSRIs. It should be noted that the effects seen might not extend to paroxetine, which was not provided to our subjects and which uniquely among the SSRIs (probably due to its anticholinergic effects) has been associated with worsened delayed recall (Furlan et al., 2001; Schmitt et al., 2001).
The current study’s findings should be interpreted in light of its strengths and limitations. Strengths of our study included that our participants were medically healthy and unmedicated for a minimum of 6 weeks before baseline testing. A limitation of this study is that comorbid Axis II disorders, presence of melancholia and prior episodes of MDD with psychosis were not evaluated, although subjects with current psychotic symptoms or psychotic disorders were excluded and all subjects were recruited from outpatient settings. A second limitation of this work is reliance on a single basal morning cortisol level. Although variability of setting was mitigated by laboratory draw after a period of rest, the pulsatile nature of cortisol secretion could lead to variability of measurement across days. A third limitation is that our sample sizes for the retrospective clinical outcomes analyses were limited. In part to address this limitation, outcomes utilized continuous measures as opposed to splitting the sample into subgroups (for example, based on remission status). The use of continuous outcome measures also served to increase our power to find an effect. Fourth, while the open-label nature of our study more closely resembles common clinical practice, it makes mechanistic interpretations difficult. Specifically, effects seen after SSRI treatment could be due to the SSRI or to non-specific factors such as time or degree of comfort in the test setting. Finally, participants were all younger than 70 years and our main findings may not apply to those over 70 years of age.
5. Conclusions
These retrospective results are in keeping with other observations about a possible role of patient age moderating the interaction between cortisol level, MDD symptoms and antidepressant treatment outcome, with a threshold effect somewhere between early and middle adulthood. Further confirmatory research on the moderation of cortisol by age in association with MDD and treatment outcome should sample cortisol at multiple times across several days in a home environment and in the lab both before and after treatment, and it should involve larger samples across a broader range of ages. As a moderation effect of age on cortisol has been observed in association with limbic system architecture (Ennis et al., 2019), further research may elaborate whether there are common brain structural or functional changes in those with MDD who are younger with higher cortisol levels and older with lower cortisol levels. Further study of molecular mechanisms underlying these effects might help elucidate targets of antidepressant treatments such as glucocorticoid receptor blockers and other HPA axis modulators (Block et al., 2018; Menke, 2019; Wolkowitz and Reus, 1999). We suggest that further research of age-dependent changes of the HPA axis might inform an understanding of biological differences in response to antidepressant treatments across different stages of adulthood.
Supplementary Material
Highlights.
Cortisol, moderated by age, was associated with major depression diagnosis.
Cortisol, moderated by age, was associated with improvement in major depression symptoms
Cortisol, moderated by age, was associated with change in verbal and visual delayed recall
7. Financial support and acknowledgments
Data acquisition was funded by a grant from the National Institute of Mental Health (Grant Number R01 - MH083784). This project was also supported by National Institutes of Health/National Center for Research Resources (NIH/NCRR) and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF- CTSI Grant Number UL1 RR024131. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934. Author TTY was supported by the Brain and Behavior Research Foundation (formerly NARSAD), grant number R01MH085734-05 from the National Institute of Mental Health, and grant numbers R21AT009173 and 1R61AT009864-01A1 from the National Center for Complementary and Integrative Health. Author FAJ was supported by grant #R21AG051970 from the National Institute on Aging. Author CMH is supported by the Graduate Division of the University of California, Los Angeles, and the National Science Foundation Graduate Research Fellowship Program (NSF Grant Number DGE-1650604). Additional financial support was obtained from the Tinberg Family and the O’Shaughnessy Foundation.
The authors gratefully acknowledge statistical consultation by Kevin Delucchi, PhD, the nursing and other staff of the UCSF CTSI Clinical Research Center, and administrative assistance of Brenton Nier and Mina Cheema. None of the granting or funding agencies had a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript. The Co-Principal Investigators, OMW, ESE, and SHM, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association, 2010. Practice Guideline for the Treatment of Patients With Major Depressive Disorder, Third Edition. American Psychiatric Association, Arlington (VA). [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF, 2012. Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition. Horm. Behav. 62, 219–27. 10.1016/j.yhbeh.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Beck JS, Newman CF, 1993. Hopelessness, Depression, Suicidal Ideation, and Clinical Diagnosis of Depression. Suicide Life-Threatening Behav. 23, 139–145. 10.1111/J.1943-278X.1993.TB00378.X [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J, 1998. Hopkins Verbal Learning Test Revised: Normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 12,43–55. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B, 1996. Revision of the brief visuospatial memory test: Studies of normal performance, reliability, and validity. Psychol. Assess. 8, 145–153. [Google Scholar]
- Block TS, Kushner H, Kalin N, Nelson C, Belanoff J, Schatzberg A, 2018. Combined Analysis of Mifepristone for Psychotic Depression: Plasma Levels Associated With Clinical Response. Biol. Psychiatry 84, 46–54. 10.1016/j.biopsych.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V, 2009. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 34, 2376–2389. 10.1038/npp.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer MA, Deeg DJ, Beekman AT, Penninx BW, Lips P, Hoogendijk WJ, 2007. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry 62, 479–486. 10.1016/j.biopsych.2006.11.033 [DOI] [PubMed] [Google Scholar]
- Conrad A, Wilhelm FH, Roth WT, Spiegel D, Taylor CB, 2008. Circadian affective, cardiopulmonary, and cortisol variability in depressed and nondepressed individuals at risk for cardiovascular disease. J. Psychiatr. Res. 42,769–777. 10.1016/J.JPSYCHIRES.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis GE, Quintin E-M, Saelzler U, Kennedy KM, Hertzog C, Moffat SD, 2019. Cortisol relates to regional limbic system structure in older but not younger adults. Psychoneuroendocrinology 101, 111–120. 10.1016/j.psyneuen.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian TJ, Dew MA, Pollock BG, Reynolds CF, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trottini M, Kroboth PD, 2001. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol. Psychiatry 50, 767–74. [DOI] [PubMed] [Google Scholar]
- Farooq RK, Tanti A, Ainouche S, Roger S, Belzung C, Camus V, 2018. A P2X7 receptor antagonist reverses behavioural alterations, microglial activation and neuroendocrine dysregulation in an unpredictable chronic mild stress (UCMS) model of depression in mice. Psychoneuroendocrinology 97, 120–130. 10.1016/j.psyneuen.2018.07.016 [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, Whiteford HA, 2013. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med 43, 471–481. 10.1017/S0033291712001511 [DOI] [PubMed] [Google Scholar]
- Ferrari E, Casarotti D, Muzzoni B, Albertelli N, Cravello L, Fioravanti M, Solerte SB, Magri F, 2001. Age-related changes of the adrenal secretory pattern: possible role in pathological brain aging. Brain Res. Brain Res. Rev. 37, 294–300. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. : Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Fischer S, Strawbridge R, Vives AH, Cleare AJ, 2017. Cortisol as a predictor of psychological therapy response in depressive disorders: Systematic review and metaanalysis. Br. J. Psychiatry 210, 105–109. 10.1192/bjp.bp.115.180653 [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH, 2005. A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016. 10.1016/j.psyneuen.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Furlan PM, Kalian MJ, Ten Have T, Pollock BG, Katz I, Lucki I, 2001. Cognitive and psychomotor effects of paroxetine and sertraline on healthy elderly volunteers. Am. J. Geriatr. Psychiatry 9, 429–38. [PubMed] [Google Scholar]
- Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, Solvason B, Schatzberg AF, 2006. The Neuropsychological Profile of Psychotic Major Depression and its Relation to Cortisol. Biol. Psychiatry 60,472–478. 10.1016/J.BIOPSYCH.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC, 2015. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76, 155–162. 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T, 2004. Suicide Caution Stamped on Antidepressants. JAMA 291, 2060 10.1001/jama.291.17.2060 [DOI] [PubMed] [Google Scholar]
- Heffelfinger AK, Newcomer JW, 2001. Glucocorticoid effects on memory function over the human life span. Dev. Psychopathol 13,491–513. 10.1017/S0954579401003054 [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH, 2000. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 25, 1–35. [DOI] [PubMed] [Google Scholar]
- Hough CM, Lindqvist D, Epel ES, Denis MS, Reus VI, Bersani FS, Rosser R, Mahan,, Burke HM, Wolkowitz OM, Mellon SH., 2017. Higher serum DHEA concentrations before and after SSRI treatment are associated with remission of major depression. Psychoneuroendocrinology 77, 122–130. 10.1016/j.psyneuen.2016.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Clegg R, Goer F, Pechtel P, Beltzer M, Vitaliano G, Olson DP, Teicher MH, Pizzagalli DA, 2018. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol. Med. 48, 1157–1166. 10.1017/S0033291717002628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin NM, Nair NP, Amin M, Schwartz G, Ahmed SK, Holm P, Katona C, Kragh-Sorensen P, Klitgaard N, Song WY, West TE, Stage K, 1997. The dexamethasone suppression test and treatment outcome in elderly depressed patients participating in a placebo-controlled multicenter trial involving moclobemide and nortriptyline. Biol. Psychiatry 42, 925–31. [DOI] [PubMed] [Google Scholar]
- Kley HK, Schlaghecke R, Kruskemper HL, 1985. [Stability of steroids in plasma over a 10- year period], J. Clin. Chem. Clin. Biochem. 23, 875–8. [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW, 2013. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 18, 692–699. 10.1038/mp.2012.144 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ, 1998. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1,69–73. 10.1038/271 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10, 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- McCain JA, 2009. Antidepressants and suicide in adolescents and adults: a public health experiment with unintended consequences? P T 34, 355–78. [PMC free article] [PubMed] [Google Scholar]
- Menke A, 2019. Is the HPA Axis as Target for Depression Outdated, or Is There a New Hope? Front. Psychiatry 10, 101 10.3389/fpsyt.2019.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Hellman N, Abelson JL, Rao U, 2016. Cortisol, heart rate, and blood pressure as early markers of PTSD risk: A systematic review and meta-analysis. Clin Psychol Rev 49, 79–91. 10.1016/j.cpr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JC, Davis JM, 1997. DST Studies in Psychotic Depression: A Meta-Analysis. Am. J. Psychiatry 154, 1497–1503. 10.1176/ajp.154.11.1497 [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N, 2004. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am. J. Psychiatry 161, 2081–2090. 10.1176/appi.ajp.161.11.2081 [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Ferguson E, Green JA, O’Carroll RE, O’Connor RC, 2016. Cortisol levels and suicidal behavior: A meta-analysis. Psychoneuroendocrinology 63, 370–379. 10.1016/j.psyneuen.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, van den Berg MD, Flentge F, Bouhuys AL, ter Horst GJ, Ormel J, 2001. Urinary free cortisol excretion in elderly persons with minor and major depression. Psychiatry Res 104, 39–47. [DOI] [PubMed] [Google Scholar]
- Ong AD, Fuller-Rowell TE, Bonanno GA, Almeida DM, 2011. Spousal loss predicts alterations in diurnal cortisol activity through prospective changes in positive emotion. Health Psychol. 30, 220–7. 10.1037/a0022262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. R Core Team R, R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Raison CL, Miller AH, 2003. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160, 1554–1565. 10.1176/appi.ajp.160.9.1554 [DOI] [PubMed] [Google Scholar]
- Reilly TJ, MacGillivray SA, Reid IC, Cameron IM, 2015. Psychometric properties of the 16-item Quick Inventory of Depressive Symptomatology: a systematic review and metaanalysis. J. Psychiatr. Res. 60, 132–40. 10.1016/j.jpsychires.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Rothschild AJ, Schatzberg AF, Langlais PJ, Lerbinger JE, Miller MM, Cole JO, 1987. Psychotic and nonpsychotic depressions: I. comparisons of plasma catecholamines and cortisol measures. Psychiatry Res. 20,143–153. 10.1016/0165-1781(87)90006-0 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 54, 573–583. 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Schmitt JAJ, Kruizinga MJ, Riedel WJ, 2001. Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. J. Psychopharmacol. 15, 173–179. 10.1177/026988110101500304 [DOI] [PubMed] [Google Scholar]
- Seeman TE, Mcewen BS, Singer BH, Albert MS, Rowe JW, 1997. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J. Clin. Endocrinol. Metab. 82, 2458–2465. 10.1210/jc.82.8.2458 [DOI] [PubMed] [Google Scholar]
- Smith M Lou, Milner B, 1981. The role of the right hippocampus in the recall of spatial location. Neuropsychologia 19, 781–793. 10.1016/0028-3932(81)90090-7 [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE, 2011. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73, 114–126. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- Sugase Y, Yamane S, Ueno S, Kawano K, 1999. Global and fine information coded by single neurons in the temporal visual cortex. Nature 400, 869–873. 10.1038/23703 [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR, 2012. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain. Behav. Immun. 26,469–479. 10.1016/j.bbi.2011.12.011 [DOI] [PubMed] [Google Scholar]
- Vreeburg SA., W.J.G.. b, Van Pelt J, DeRijk RH, Verhagen JCM, Van Dyck R, Smit JH, Zitman FG, Penninx BWJH, 2009. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 66, 617–626. 10.1001/archgenpsychiatry.2009.50 [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1997. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale, Third, ed. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wolkowitz OM, Burke H, Epel ES, Reus VI, 2009. Glucocorticoids. Mood, memory, and mechanisms. Ann N Y Acad Sci 1179,19–40. 10.1111/j.1749-6632.2009.04980.x [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, 1999. Treatment of depression with antiglucocorticoid drugs. Psychosom. Med. 61, 698–711. [DOI] [PubMed] [Google Scholar]
- Yau JLW, Olsson T, Morris RGM, Meaney MJ, Seckl JR, 1995. Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience 66, 571–581. 10.1016/0306-4522(94)00612-9 [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL, 1995. Low urinary cortisol excretion in holocaust survivors with posttraumatic stress disorder. Am. J. Psychiatry 152, 982–986. 10.1176/ajp.152.7.982 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.