Abstract
Background:
High-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) at the time of myocardial infarction (MI) are strong predictors of prognosis. However, whether their pre-morbid (before MI occurrence) levels are associated with prognosis after incident MI is unknown.
Methods:
In 1,054 participants from the Atherosclerosis Risk in Communities Study with incident MI, we evaluated premorbid levels of hs-cTnT and NT-proBNP measured on average 5.8 [IQI 3.0-11.5] years prior to incident MI, and their associations with subsequent composite and individual outcomes of all-cause mortality, cardiovascular mortality, recurrent MI, heart failure, and stroke.
Results:
During a median follow-up of 3.0 years after MI, 801 participants developed the composite outcome. Both hs-cTnT and NT-proBNP were independently associated with the composite outcome after incident MI. Among individual outcomes, all-cause mortality, cardiovascular mortality, and heart failure showed significant associations with both cardiac markers. Overall, NT-proBNP demonstrated a more evident relationship than hs-cTnT. Indeed, the addition of premorbid NT-proBNP alone, but not hs-cTnT alone, to conventional predictors at incident MI significantly improved risk prediction of the composite outcome after incident MI (Δc-statistic 0.013 [95% CI 0.005-0.022] from 0.691 with conventional predictors).
Conclusions:
Premorbid levels of hs-cTnT and NT-proBNP assessed on average six years prior to incident MI were associated with adverse outcomes after incident MI. These results further highlight the importance of cardiac health at an earlier stage of life.
Keywords: high-sensitivity cardiac troponin T, natriuretic peptide, prognosis, myocardial infarction
INTRODUCTION
Cardiac troponin T (cTnT) and natriuretic peptides are useful to diagnose myocardial infarction (MI) and heart failure, respectively, since cTnT is released as a result of cardiomyocyte necrosis1 and natriuretic peptides are released from cardiac myocyte in response to myocardial stretch and volume overload.2 Furthermore, these cardiac markers predict the risk of adverse outcomes among patients with MI3-23 and are indicated to guide treatment selection particularly at the acute stage.24-26 This is reasonable since cTnT and natriuretic peptides at the acute stage would reflect severity of MI and cardiac overload. In addition, these cardiac biomarkers have been shown to also predict adverse outcomes among patients with stable coronary heart disease.27-33
Recently, these cardiac markers have been found to be elevated in some individuals without prevalent cardiovascular disease (CVD), particularly after a high-sensitive assay was developed for cTnT,34, 35 and to be associated with incident CVD.34-39 Although these cardiac markers are used for the risk stratification at the acute phase of MI,24-26 whether premorbid (i.e., prior to MI) levels of these cardiac markers are related to prognosis after the development of MI is unknown. It is possible that individuals with lower premorbid levels of cardiac markers may handle the occurrence of MI better than those with higher levels due to greater cardiac reserve. If we confirm this hypothesis, that would further support the importance of premorbid cardiac conditions and maintaining cardiac health at an earlier stage of life although some individuals may unfortunately develop MI despite optimal cardiac health.
Therefore, we examined the associations of premorbid levels of high-sensitivity cTnT (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) with the risk of adverse outcomes after incident MI at later life in a prospective community-based cohort.
METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective cohort of 15,792 individuals aged 45 to 64 years at visit 1 (1987-1989) from four US communities (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). There have been five follow-up examinations in 1990 to 1992 (visit 2), 1993 to 1995 (visit 3), 1996 to 1998 (visit 4), 2011 to 2013 (visit 5), and 2016 to 2017 (visit 6). hs-cTnT and NT-proBNP were measured at visit 2, visit 4 and visit 5.
Although our primary interest was the associations of premorbid levels of hs-cTnT and NT-proBNP with subsequent risk of adverse outcomes after MI, to fully acknowledge their associations with continuum pathophysiology of MI, we also quantified the associations of hs-cTnT and NT-proBNP with incident MI in the entire study population at visit 2 as well. For that analysis, of 14,348 ARIC study participants at visit 2, we excluded participants who had a history of coronary heart disease, stroke or heart failure at baseline (n=1,543), who were neither whites nor blacks (n=41), the small number of black participants from the Minnesota and Washington County centers (n=42), who did not have information on hs-cTnT and NT-proBNP (n=833), leaving a sample of 11,889 participants.
For the primary analysis, of 11,889 ARIC participants, we identified 1,204 incident MI cases occurring after visit 2 through the end of 2015, adjudicated by the ARIC physician panel. Then, we excluded those who were missing the latest premorbid data prior to MI for hs-cTnT or NT-proBNP (n=8), who had coronary heart disease, stroke, or heart failure prior to the measurement of these two cardiac markers in ARIC (n=91), and who had missing information on covariates at the time of MI (n=51), resulting in 1,054 participants for this study. We used the latest premorbid data on hs-cTnT and NT-proBNP from either of visit 2, 4 or 5 for each MI case (Web Figure 1). An ethics committee at each site approved the study protocol, and study participants provided informed consent at each study visit.
Measurement of cardiac biomarkers
hs-cTnT was measured using a high-sensitive sandwich immunoassay method (Roche Elecsys T; Roche Diagnostic). Stored serum samples obtained at visit 2 were assayed for hs-cTnT using a Roche Elecsys 2010 Analyzer (Roche Diagnostics). Stored plasma samples obtained at visit 4 and visit 5 were assayed for hs-cTnT using a Cobas e411 analyzer (Roche Diagnostics, Indianapolis, Indiana). For NT-proBNP, stored serum samples obtained at visit 2 were measured using a sandwich immunoassay method (Roche Diagnostics) implemented on a Roche Elecys 2010 Analyzer. Stored plasma samples collected at visit 4 and visit 5 were analyzed using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics). The as is measurable limit of hs-TnT and NT-proBNP were 3 ng/L and 5 pg/mL, respectively. We assigned half the lower limit of each marker for participants with unmeasurable levels.
Covariates
We considered the nine predictors in the TIMI Risk Score for Secondary Prevention (TRS2°P),40 a risk stratification tool for recent MI patients, as covariates. Specifically, TRS2°P includes the following factors at incident MI: age, smoking status (current vs. noncurrent), kidney dysfunction, and a history of heart failure, hypertension, diabetes, stroke, coronary artery bypass graft (CABG), or peripheral artery disease (PAD). Utilizing all data sources available in ARIC (data at visits, data from annual telephone calls during follow-up, and abstracted data from medical records at the time of the MI admission), we determined information on each predictor of TRS2°P. For each covariate in each participant, a data point closest to incident MI was used. Age was determined at the date of incident MI and modeled continuously. Smoking status was determined by data obtained within a year prior to incident MI. Information on smoking status, medical history (e.g., hypertension and diabetes), and medication use (e.g., antihypertensive and antidiabetic medication uses) were collected at all study visits using standard questionnaires and were updated through the annual telephone contacts and abstracted data from medical records at MI admission, when appropriate. Blood pressure was measured three times by certified technicians using a sphygmomanometer, and the average of the last two measurements was recorded at study visits except for visit 4, at which blood pressure was measured twice, and the average recorded. Blood samples were also collected at all study visits. Serum glucose levels were measured by the modified hexokinase/glucose-6-phosphate dehydrogenase method, and serum creatinine concentration was measured using a modified kinetic Jaffe method. We defined hypertension as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications. We defined diabetes as fasting glucose level ≥126 mg/dL, non-fasting glucose level ≥200 mg/dL, self-reported physician diagnosis of diabetes, or antidiabetic medication use. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation using serum creatinine measured within a year prior to incident MI.41 Kidney dysfunction was defined as eGFR <60 ml/min/1.73m2 or chronic kidney disease-related ICD-9 codes in hospitalization. Prior heart failure, CABG, and PAD were defined based on ICD-9 discharge and procedure codes in hospitalization. Prior stroke was defined as adjudicated definite or probable stroke cases. In addition to the TRS2°P predictors, sex and race-ARIC visit centers were also included as covariates.
Because participants with lower levels of cardiac markers prior to MI may have less severe incident Mis, we also adjusted for MI severity as a sensitivity analysis. We determined MI severity based on a modified score of the Predicting Risk of Death in Cardiac Disease Toll (PREDICT) that uses several clinical variables at incident MI (cardiogenic shock, history of MI, stroke, or angina, age, severity of electrocardiographic changes, congestive heart failure, and Charlson Comorbidity Index) for a maximum score of 21 points, with a higher score indicating higher severity.42, 43
Follow-up and outcomes
The outcomes of interest were composite and individual outcomes of all-cause mortality, cardiovascular mortality, recurrent MI, heart failure, and stroke after incident MI. Cardiovascular events and death in the ARIC Study were ascertained by contacting participants annually (semiannually since 2012), identifying hospitalizations and deaths during the previous year, and surveying discharge lists from local hospitals and death certificates from state vital statistics offices for potential cardiovascular events. Cardiovascular mortality was defined as death from coronary heart disease, heart failure, or stroke. Recurrent MI and incident stroke were defined as adjudicated definite and probable cases after incident MI. Incident heart failure after MI was defined as either a hospitalization or death with heart failure diagnosis based on ICD-9 code 428 or ICD-10 code I50 in any position. Participants were followed until administrative censoring on December 31, 2016, date of outcomes of interest including death, or loss to follow-up whichever came first.
Statistical analysis
Premorbid hs-cTnT was categorized into five categories (<3, 3-5, 6-8, 9-13, and ≥14 ng/L) according to thresholds in the previous literature.34, 44 hs-cTnT ≥14 ng/L is considered clinically elevated.28 To fairly compare with hs-cTnT, premorbid NT-proBNP was categorized into five categories according to the same percentiles of hs-cTnT categories (<51.8, 51.8-86.8, 86.9-140.3, 140.4-249.2, and ≥249.3 pg/mL corresponding to 52nd, 74th, 88th and 96th percentiles in the entire study population; <35.3, 35.3-64.8, 64.9-119.6, 119.7-222.5, and ≥222.6 pg/mL corresponding to the 27th, 47th, 69th, and 85th percentiles in patients with MI)44 Baseline characteristics were summarized according to the five categories of hs-cTnT and NT-proBNP as mean (standard deviation) or median [interquartile interval, IQI] for continuous variables and proportion for categorical variables.
We first examined the associations of hs-cTnT and NT-proBNP with incident MI in the entire study population (n=11,889). Cumulative incidence of MI was estimated across categories of hs-cTnT and NT-proBNP using the Kaplan-Meier method.
Then, among those who developed MI during follow-up (n=1,054), we estimated cumulative incidence of composite outcome after MI across categories of hs-cTnT and NT-proBNP using the Kaplan-Meier method. We examined two discrete risk periods: 1) within 30 days and 2) >30 days after MI. Subsequently, we quantified the association of categories of premorbid hs-cTnT and NT-proBNP with composite and individual adverse outcomes after incident MI using Cox proportional hazards models. We evaluated the impact of potential confounders: sex (female vs. male), race (black vs. white), calendar year of incident MI (≥2005 vs. <2005 [median]), elapsed time between cardiac markers measurement and incident MI, and individual predictors in TRS2°P, as noted above,40 and each of the cardiac markers, as appropriate (i.e., adjusting for NT-proBNP for the analysis of hs-cTnT and vice versa). We also evaluated the continuous association of hs-cTnT and NT-proBNP with composite outcome using the restricted cubic splines. We selected 1.5 ng/L for hs-cTnT (unmeasurable level) and 18 pg/mL for NT-proBNP (a median value of the lowest category <35 pg/mL) as the reference. In addition, we assessed cross-categories of hs-cTnT and NT-proBNP. Because of small cell sizes, we created nine cross-categories (<6, 6-13, and ≥14 ng/L for hs-cTnT and <66.6, 66.6-222.5, and ≥222.6 pg/mL for NT-proBNP).
We conducted several sensitivity analyses to evaluate the robustness of our findings. First, we repeated our analyses in several subgroups to assess potential interactions by age at MI (≥75 vs. <75 years), sex (female vs. male), and race (black vs. white). Due to sparse data in some categories of cardiac markers within subgroups, we estimated hazard ratios (HRs) for a 2-fold increment of each cardiac marker in the subgroup analyses. Interactions were tested by a likelihood ratio test comparing models with and without product terms of interest. Second, we evaluated whether the adjustment for MI severity (represented by PREDICT score) altered results. Third, since those with higher levels of hs-cTnT and NT-proBNP were likely to develop MI earlier during follow-up than those with lower levels, leading to longer follow-up after MI to capture adverse outcomes, we restricted our analysis to 1, 3, or 5 years of follow-up after incident MI. Fourth, we evaluated sex-specific quintiles of hs-cTnT and NT-proBNP since the distribution of both cardiac markers may differ between men and women.45, 46 Finally, we also quantified the associations of quintiles of premorbid NT-proBNP among MI patients with adverse outcomes.
To assess the incremental value of hs-cTnT and NT-proBNP levels for risk prediction of composite and individual outcomes of all-cause mortality, cardiovascular mortality, recurrent MI, heart failure and stroke after incident MI, the c-statistic was computed from two models incorporating demographic variables and predictors in TRS2°P at the time of MI (base model) with and without continuous hs-cTnT and NT-proBNP (both were log-transformed) based on 3-year predicted risk. All analyses were conducted with Stata, version 14.2 (StataCorp LP), and P<0.05 was considered statistically significant.
RESULTS
Cardiac markers and incident MI in the entire study population
In the entire study population, the mean age at baseline was 57 years, 57% were female, and 24% were blacks. Over a median follow-up of 23.4 years, there were 1,204 incident MI cases. A higher level of hs-cTnT was associated with higher risk of incident MI in a graded manner. In contrast, only the highest category of NT-proBNP had an elevated higher risk of incident MI compared with other lower categories (Web Figure 2).
Premorbid cardiac markers and adverse outcomes after incident MI
In 1,054 ARIC participants who developed incident MI during follow-up, mean age at MI was 71 years, 46% were female, and 26% were blacks. Most incident MI patients (~85%) did not have clinically elevated premorbid levels of hs-cTnT (≥14 ng/L) and NT-proBNP (≥222.6 pg/mL), with median elapsed time between cardiac biomarker evaluation and incident MI of 5.8 years (IQI 3.0-11.5 years [mean 5.5 years]). Individuals with higher premorbid hs-cTnT levels were more likely to be male, black, and have heart failure, hypertension, diabetes, PAD, or kidney dysfunction at the time of incident MI (Table 1). However, they were less likely to be current smokers. The proportion of individuals with MI who had a history of stroke showed an inverse U-shape pattern across hs-cTnT categories. In general, similar patterns were observed across NT-proBNP categories whereas there were no evident associations of NT-proBNP levels with proportion of blacks, diabetes, and current smoker. Also, those with higher NT-proBNP levels were more likely to be female and have PAD at the time of MI compared to lower NT-proBNP levels. Similar patterns were generally seen at the time of the premorbid biomarker values (Web Table 1).
Table 1.
Baseline characteristics by hs-cTnT and NT-proBNP categories, N=1,054
| hs-cTnT, ng/L | |||||
| <3 | 3-5 | 6-8 | 9-13 | ≥14 | |
| N=287 | N=211 | N=228 | N=175 | N=153 | |
| Age at MI, years | 67.3 (8.5) | 69.7 (8.2) | 72.6 (8.0) | 73.3 (8.4) | 72.0 (7.6) |
| Black | 21.6% | 22.3% | 27.2% | 27.4% | 34.6% |
| Female | 61.3% | 52.6% | 36.8% | 36.0% | 31.4% |
| Elapsed time from measurement to MI | 7.4(5.3) | 7.6(5.3) | 8.0(5.5) | 7.4(5.2) | 5.7(4.6) |
| Prior heart failure | 5.2% | 6.6% | 14.5% | 14.9% | 16.3% |
| Hypertension | 70.7% | 78.2% | 85.5% | 89.7% | 91.5% |
| Diabetes | 30.0% | 36.5% | 46.9% | 45.1% | 59.5% |
| Prior stroke | 3.8% | 2.8% | 5.7% | 3.4% | 3.9% |
| CABG | 1.7% | 2.4% | 5.7% | 3.4% | 3.3% |
| PAD | 7.3% | 6.2% | 8.8% | 10.3% | 12.4% |
| Kidney dysfunction | 8.0% | 10.0% | 15.4% | 18.3% | 28.8% |
| Current smoking | 33.2% | 17.4% | 12.2% | 11.9% | 8.5% |
| PREDICT score* | 6.4 (3.1) | 7.6 (3.7) | 7.8 (3.4) | 8.3 (3.6) | 8.5 (3.4) |
| hs-cTnT, ng/L | 1.5 [1.5-1.5] | 4.0 [4.0-5.0] | 7.0 [6.0-8.0] | 10.0 [9.0-12.0] | 19.0 [16.0-28.0] |
| NT-proBNP, pg/mL | 57.2 [28.7-102.9] | 58.9 [27.3-129.8] | 67.1 [31.4-140.1] | 76.1 [36.5-146.1] | 130.9 [54.9-283.9] |
| NT-proBNP, pg/mL | |||||
| <35.3 | 35.3-64.8 | 64.9-119.6 | 119.7-222.5 | ≥222.6 | |
| N=286 | N=212 | N=228 | N=174 | N=154 | |
| Age at MI, years | 67.8 (8.1) | 69.5 (8.3) | 71.6 (8.4) | 72.9 (8.3) | 73.1 (8.3) |
| Black | 33.2% | 23.6% | 24.1% | 20.1% | 24.0% |
| Female | 29.0% | 42.9% | 48.7% | 57.5% | 63.0% |
| Elapsed time from measurement to MI | 7.7(5.4) | 7.1(5.2) | 7.6(5.2) | 7.4(5.4) | 6.6(5.2) |
| Prior heart failure | 5.2% | 9.0% | 9.2% | 16.1% | 19.5% |
| Hypertension | 78.0% | 74.1% | 81.1% | 86.2% | 94.2% |
| Diabetes | 44.1% | 40.6% | 42.5% | 37.4% | 42.2% |
| Prior stroke | 4.2% | 3.8% | 4.0% | 3.5% | 4.6% |
| CABG | 2.5% | 4.3% | 3.1% | 4.0% | 2.6% |
| PAD | 5.6% | 6.6% | 7.9% | 10.9% | 15.6% |
| Kidney dysfunction | 11.9% | 10.9% | 11.4% | 19.0% | 25.3% |
| Current smoking | 19.2% | 21.7% | 18.9% | 19.0% | 12.3% |
| PREDICT score* | 6.7 (3.0) | 7.2 (3.8) | 7.4 (3.3) | 8.4 (3.6) | 9.0 (3.4) |
| hs-cTnT, ng/L | 5.0 [1.5-8.0] | 5.0 [1.5-9.0] | 6.0 [1.5-10.0] | 6.0 [3.0-9.0] | 9.0 [5.0-16.0] |
| NT-proBNP, pg/mL | 18.2 [10.5-26.4] | 48.0 [40.7-56.4] | 86.3 [75.5-102.3] | 156.0 [133.8-181.0] | 384.6 [275.4-643.9] |
Values for continuous variables are given as mean (standard deviation) or median [interquartile interval]; values for categorical variables are given as percentage
Abbreviations: CABG, coronary artery bypass graft; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro-brain natriuretic peptide; PAD: peripheral artery disease
Participants who have information on PREDICT score are 715.
Over a median follow-up of 3.0 (IQI, 0.04-9.4) years, 801 cases of MI developed the composite outcome (623 all-cause deaths including 242 due to cardiovascular disease, 214 cases for recurrent MI, 497 cases for heart failure, and 108 cases for stroke). When examining two discrete risk periods, those with higher levels of hs-cTnT and NT-proBNP were measured, on average, 6 years earlier had a higher risk of composite outcome within 30 days and >30 days after MI, compared to those with lower levels (Web Figure 3).
The associations generally consistent after adjusting for demographic variables, comorbidities at the time of MI and each other cardiac marker (Table 2). For hs-cTnT, the top two categories showed a significant hazard ratio (HR) for the composite outcome (1.53 [95% CI 1.20-1.94] for ≥14 ng/L and 1.27 [1.00-1.61] for 9-13 ng/L). In contrast, the top three categories of NT-proBNP demonstrated significant HRs in a graded fashion (1.93 [95% CI 1.52-2.45] for ≥222.6 pg/mL, 1.47 [1.17-1.86] for 119.7-222.5 pg/mL and 1.25 [1.01-1.55] for 64.9-119.6 pg/mL). A steeper risk gradient for NT-proBNP than hs-cTnT was confirmed when these cardiac markers were modeled using restricted cubic splines (Web Figure 4). When we accounted for MI severity, the associations remained significant (Web Table 2). We observed generally similar patterns after restricting follow-up time 1, 3, and 5 years after incident MI (Web Table 3). We also confirmed that quintiles of premorbid NT-proBNP were significantly associated with composite outcome (Web Table 4).
Table 2.
Adjusted hazard ratios (95%CI)* of secondary adverse outcomes after MI for categories of hs-cTnT and NT-proBNP, N=1,054
| hs-cTnT, ng/L | <3 (N=287) | 3-5 (N=211) | 6-8 (N=228) | 9-13 (N=175) | ≥14 (N=153) | P trend |
| Composite outcome | ||||||
| Cases | 207 | 160 | 167 | 135 | 132 | |
| HR (95%CI) | Ref. | 1.14 (0.93-1.41) | 1.05 (0.84-1.31) | 1.27 (1.00-1.61) | 1.59 (1.23-2.05) | <0.01 |
| All-cause mortality | ||||||
| Cases | 145 | 122 | 128 | 114 | 114 | |
| HR (95%CI) | Ref. | 1.17 (0.91-1.49) | 1.13 (0.87-1.46) | 1.51 (1.15-1.98) | 1.95 (1.46-2.59) | <0.01 |
| Cardiovascular mortality | ||||||
| Cases | 50 | 53 | 46 | 46 | 47 | |
| HR (95%CI) | Ref. | 1.49 (0.99-2.22) | 1.14 (0.74-1.76) | 1.78 (1.14-2.77) | 2.13 (1.34-3.38) | <0.01 |
| Recurrent MI | ||||||
| Cases | 63 | 43 | 36 | 34 | 38 | |
| HR (95%CI) | Ref. | 0.99 (0.67-1.48) | 0.77 (0.49-1.20) | 1.20 (0.75-1.90) | 1.48 (0.92-2.39) | 0.13 |
| Heart failure | ||||||
| Cases | 106 | 105 | 107 | 89 | 90 | |
| HR (95%CI) | Ref. | 1.49 (1.13-1.96) | 1.29 (0.96-1.73) | 1.57 (1.15-2.14) | 1.79 (1.29-2.48) | <0.01 |
| Stroke | ||||||
| Cases | 31 | 17 | 27 | 16 | 17 | |
| HR (95%CI) | Ref. | 0.72 (0.39-1.31) | 1.14 (0.65-2.01) | 0.99 (0.51-1.91) | 1.33 (0.67-2.62) | 0.36 |
| NT-proBNP, pg/mL | <35.3 (N=286) | 35.3-64.8 (N=212) | 64.9-119.6 (N=228) | 119.7-222.5 (N=174) | ≥222.6 (N=154) | |
| Composite outcome | ||||||
| Cases | 198 | 152 | 174 | 136 | 141 | |
| HR (95%CI) | Ref. | 1.14 (0.92-1.42) | 1.25 (1.01-1.55) | 1.47 (1.17-1.86) | 1.93 (1.52-2.45) | <0.01 |
| All-cause mortality | ||||||
| Cases | 143 | 117 | 139 | 108 | 116 | |
| HR (95%CI) | Ref. | 1.13 (0.88-1.45) | 1.31 (1.02-1.66) | 1.54 (1.17-2.01) | 2.04 (1.55-2.68) | <0.01 |
| Cardiovascular mortality | ||||||
| Cases | 54 | 40 | 57 | 38 | 53 | |
| HR (95%CI) | Ref. | 1.02 (0.67-1.54) | 1.44 (0.98-2.12) | 1.51 (0.96-2.35) | 2.66 (1.74-4.05) | <0.01 |
| Recurrent MI | ||||||
| Cases | 66 | 42 | 46 | 30 | 30 | |
| HR (95%CI) | Ref. | 0.93 (0.62-1.38) | 1.07 (0.72-1.58) | 1.07 (0.67-1.70) | 1.19 (0.74-1.93) | 0.43 |
| Heart failure | ||||||
| Cases | 108 | 87 | 108 | 93 | 101 | |
| HR (95%CI) | Ref. | 1.15 (0.86-1.53) | 1.35 (1.02-1.77) | 1.69 (1.26-2.27) | 2.15 (1.59-2.91) | <0.01 |
| Stroke | ||||||
| Cases | 25 | 17 | 27 | 25 | 14 | |
| HR (95%CI) | Ref. | 0.93 (0.49-1.74) | 1.44 (0.82-2.53) | 2.10 (1.15-3.85) | 1.38 (0.68-2.79) | 0.05 |
HR: hazard ratio; CI: confidence interval
Adjusted for age at MI, female, race*field center, prior heart failure, hypertension, diabetes, prior stroke, coronary artery bypass graft, peripheral artery disease, chronic kidney disease, current smoking, calendar year of MI (≥2005 vs. <2005), elapsed time between premorbid biomarker measurements and incident MI, and each of the cardiac markers, as appropriate (NT-proBNP was incorporated in the analyses for hs-cTnT and vice versa)
For individual outcomes, we found that both hs-cTnT and NT-proBNP were significantly associated with all-cause mortality, cardiovascular mortality, and heart failure after incident MI (Table 2). For these outcomes, the highest category of both cardiac markers showed significant associations. Overall, the results for NT-proBNP appeared more robust and evident compared to those for hs-cTnT (e.g., HR in the highest category of NT-proBNP and hs-cTnT was 2.66 (1.74-4.05) and 2.13 (1.34-3.38) for cardiovascular mortality and 2.15 (1.59-2.91) and 1.79 (1.29-2.48) for heart failure, respectively). Although the second highest category of NT-proBNP showed a significant HR for stroke, neither of hs-cTnT or NT-proBNP demonstrated a significant relationship with recurrent MI. Again, we observed similar associations after the additional adjustment for MI severity (Web Table 2). The restriction of follow-up time demonstrated similar patterns (Web Table 3). We also confirmed that quintiles of NT-proBNP showed similar patterns (Web Table 4).
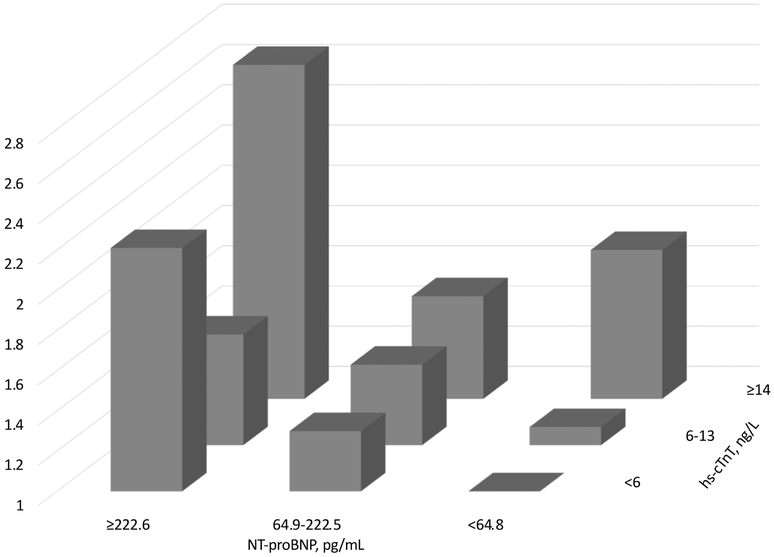
For cross-categories of hs-cTnT and NT-proBNP, we confirmed that higher levels of hs-cTnT and NT-proBNP were independently associated with higher risk for the composite outcome after incident MI, without significant interaction (P for interaction=0.39) (Figure 1 and Web Table 5). With hs-cTnT <6 ng/L and NT-proBNP <64.8 pg/mL as a reference, the middle category of NT-proBNP 64.8-222.5 pg/mL conferred significantly elevated risk even when hs-cTnT was <6 ng/L. However, this was not the case for the middle category of hs-cTnT with NT-proBNP <64.8 pg/mL.
Figure 1.
Adjusted hazard ratios of composite outcome by combined hs-cTnT and NT-proBNP (P-for interaction=0.30). Adjusted for age at MI, female, race*field center, prior heart failure, hypertension, diabetes, prior stroke, coronary artery bypass graft, peripheral artery disease, chronic kidney disease, current smoking, and calendar year of MI (≥2005 vs. <2005), and elapsed time between premorbid biomarker measurements and incident MI.
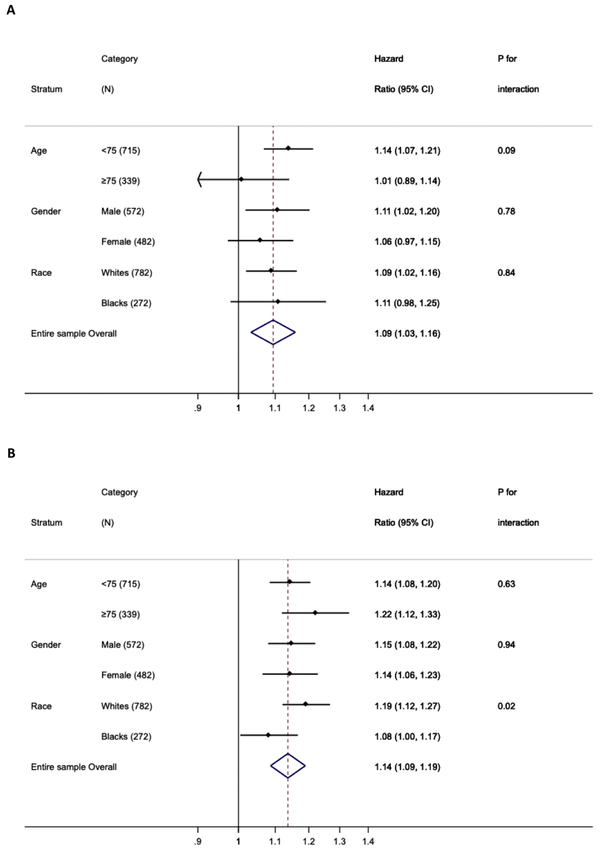
Both cardiac markers were significantly associated with the composite outcome in all subgroup tested (Figure 2). Although the association for hs-cTnT was not statistically significant in a few subgroups, NT-proBNP was significantly associated with the composite outcome in every subgroup. For individual outcomes, we observed consistent results across subgroups with an exception of a stronger association between NT-proBNP and heart failure in men and whites than their counterparts (Web Table 6). When sex-specific quintiles of cardiac markers were evaluated, we observed generally similar patterns in men and women (Web Table 7 and 8).
Figure 2.
Adjusted hazard ratios (95% CI)* of composite outcome for 2-fold increment of hs-cTnT (A) and NT-proBNP (B) in subgroups. Adjusted for age at MI, female, race*field center, prior heart failure, hypertension, diabetes, prior stroke, coronary artery bypass graft, peripheral artery disease, chronic kidney disease, current smoking, and calendar year of MI (≥2005 vs. <2005), elapsed time between premorbid biomarker measurements and incident MI and each of the cardiac markers, as appropriate (NT-proBNP was incorporated in the analyses for hs-cTnT and vice versa)
C-statistic for the composite outcome based on conventional risk factors at the time of the MI was 0.691 (95% CI 0.669-0.713) (Table 3). The addition of premorbid NT-proBNP improved risk prediction of the composite outcome (c-statistic differences of 0.013 [95% CI 0.005-0.021]), but the addition of hs-cTnT did not. For individual outcomes, c-statistics of all-cause mortality, cardiovascular mortality and heart failure were significantly improved when we added NT-proBNP (c-statistic differences of 0.010 [0.007-0.028] from 0.728 with conventional risk factors, 0.017 [0.001-0.033] from 0.729, 0.017 [0.007-0.028] from 0.723, respectively). The simultaneous addition of hs-cTnT and NT-proBNP significantly improved c-statistic only for the composite outcome, all-cause mortality and heart failure (0.013 [95%CI 0.005-0.022], 0.011 [0.002-0.020], and 0.017 [0.006-0.028] respectively), but the improvement was not superior to the sole addition of NT-proBNP.
Table 3.
C-statistics (95% CI) with the addition of continuous hs-cTnT or NT-proBNP to basic model
| Base model | + hs-cTnT | + NT-proBNP | + hs-cTnT and NT-proBNP |
|
|---|---|---|---|---|
| Composite outcome | ||||
| C statistics (95% CI) | 0.691 (0.669, 0.713) | 0.694 (0.672, 0.716) | 0.704 (0.683, 0.726) | 0.704 (0.683, 0.726) |
| Difference (95%CI) | - | 0.003 (−0.002, 0.008) | 0.013 (0.005, 0.021) | 0.013 (0.005, 0.022) |
| All-cause mortality | ||||
| C statistics (95% CI) | 0.728 (0.699, 0.757) | 0.731 (0.702, 0.761) | 0.738 (0.708, 0.767) | 0.739 (0.709, 0.768) |
| Difference (95%CI) | - | 0.003 (−0.004, 0.010) | 0.010 (0.001, 0.018) | 0.011 (0.002, 0.020) |
| Cardiovascular mortality | ||||
| C statistics (95% CI) | 0.729 (0.685, 0.772) | 0.729 (0.685, 0.773) | 0.746 (0.702, 0.789) | 0.744 (0.702, 0.789) |
| Difference (95%CI) | - | 0.000 (−0.011, 0.011) | 0.017 (0.001, 0.033) | 0.015 (−0.001, 0.032) |
| Recurrent MI | ||||
| C statistics (95% CI) | 0.671 (0.621, 0.721) | 0.675 (0.624, 0.725) | 0.672 (0.622, 0.722) | 0.676 (0.625, 0.726) |
| Difference (95%CI) | - | 0.004 (−0.007, 0.014) | 0.001 (−0.005, 0.006) | 0.004 (−0.007, 0.015) |
| Heart failure | ||||
| C statistics (95% CI) | 0.706 (0.680, 0.733) | 0.709 (0.683, 0.735) | 0.723 (0.680, 0.749) | 0.723 (0.698, 0.749) |
| Difference (95%CI) | - | 0.003 (−0.004, 0.009) | 0.017 (0.007, 0.028) | 0.017 (0.006, 0.028) |
| Stroke | ||||
| C statistics (95% CI) | 0.707 (0.643, 0.770) | 0.706 (0.642, 0.769) | 0.721 (0.659, 0.784) | 0.722 (0.659, 0.785) |
| Difference (95%CI) | - | −0.001 (−0.004, 0.002) | 0.015 (−0.004, 0.033) | 0.015 (−0.003, 0.034) |
Base model included age at MI, female, race*field center, prior heart failure, hypertension, diabetes, prior stroke, coronary artery bypass graft, peripheral artery disease, chronic kidney disease, current smoking, and calendar year of MI (≥2005 vs. <2005), and elapsed time between premorbid biomarker measurements and incident MI.
DISCUSSION
In this community-based cohort study, we found that higher premorbid levels of hs-cTnT and NT-proBNP, assessed on average six years prior to incident MI, were associated with adverse outcomes after incident MI. In terms of individual outcomes, all-cause mortality, cardiovascular mortality, and heart failure demonstrated particularly close relationships to both cardiac markers even at their mildly elevated levels. Robust results were observed even after accounting for severity of MI or restricting the follow-up to 1 to 5 years after incident MI. These associations were largely consistent across different demographic subgroups although a stronger association between NT-proBNP and heart failure was observed in men and in whites than their counterparts. Overall, the associations appeared to be more evident for NT-proBNP than hs-cTnT. Indeed, the addition of NT-proBNP alone, but not hs-cTnT alone, to conventional predictors significantly improved risk prediction of adverse outcomes after incident MI. When we added hs-cTnT and NT-proBNP simultaneously, the improvement was not superior to the sole addition of NT-proBNP.
A number of previous studies have described that these cardiac markers obtained during the first few days after the onset of MI3-8, 10-23 or in stable patients with previous MI9, 27-33 were associated with a worse prognosis after incident MI. However, to the best of our knowledge, our study is the first to evaluate premorbid levels of hs-cTnT and NT-proBNP, measured on average approximately 6 years prior to incident MI, and their association with adverse outcomes after incident MI. 90% of MI patients in the highest categories for both premorbid cardiac markers had adverse outcomes after incident MI. Of note, we observed worse prognosis even in the group of MI patients who had mildly evaluated premorbid levels of both cardiac markers.
There are several plausible mechanisms to explain these associations we observed for premorbid levels of cardiac markers with post-MI adverse outcomes. Premorbid levels of hs-cTnT and NT-proBNP in patients with MI may reflect comorbidities such as hypertension, diabetes, or kidney dysfunction,47, 48 and thereby increasing risk of secondary outcomes. Also, it is possible that those with higher premorbid levels of cardiac markers may have more severe MI. Nonetheless, these associations remained significant after accounting for conventional risk factors as well as MI severity in our study. Both cardiac markers may reflect clinically unrecognized cardiac conditions (e.g., ischemia and altered left ventricular structure and function),28, 29, 35, 47 and thus persons with higher premorbid hs-cTnT and NT-proBNP may have less cardiac reserve to overcome an episode of MI. Also, it is possible that these cardiac markers reflect systemic organ damage, leading to elevated risk of adverse outcomes in general. In fact, these cardiac markers have been associated with some non-cardiovascular conditions like lung function and infection.49, 50
In terms of individual cardiovascular outcomes after MI, premorbid cardiac markers were particularly associated with all-cause mortality, cardiovascular mortality, and heart failure. The observation for NT-proBNP seems intuitive since NT-proBNP is a marker of heart failure, and heart failure is a lethal condition.36-38 In contrast, the finding for hs-cTnT may require some discussion. Although hs-cTnT is clinically considered as a diagnostic marker of MI, mechanisms behind its elevation in persons without MI are not fully understood. Interestingly, the closer relationship of hs-cTnT to incident heart failure than incident MI has been seen in the general population without a history of CVD,34-38 and higher levels of hs-cTnT are associated with structural changes in the heart especially increased left ventricular mass which is a risk for subsequent heart failure.39 Although future studies are needed to understand why hs-cTnT and NT-proBNP may be elevated in some individuals without CVD, our findings show that premorbid levels of hs-cTnT and NT-proBNP as important markers for heart failure and mortality even after the development of MI.
The sex- and race-differences in the association between NT-proBNP and heart failure seem to deserve some discussion. Regarding the sex-difference, although the interaction was statistically significant, it is of note that higher levels of NT-proBNP were significantly associated with the risk of heart failure in both men and women. The race-difference may reflect the complex interplay among race, natriuretic peptide, and obesity. For example, an inverse relationship between obesity and natriuretic peptides is well-known, and blacks are more likely to be obese than whites in general.51 Indeed, blacks are shown to have lower natriuretic peptide levels than whites.52, 53 Also, some studies have shown that natriuretic peptides may be less prognostic in obese individuals than those with normal weight.54 Nonetheless, we should keep in mind that our subgroup analysis was performed without a prespecified hypothesis and thus was hypothesis generating.
Overall, NT-proBNP showed more evident results than hs-cTnT in our study. We found that assessment of NT-proBNP in addition to conventional risk factors yielded modest incremental improvement in risk discrimination for adverse outcomes after MI, but not hs-cTnT alone. There may be a few reasons behind this observation. First of all, this may reflect the importance of heart failure as an outcome in individuals with incident MI55 since NT-proBNP is a potent predictor of heart failure.36-38 Indeed, NT-proBNP level reflects response to wall stress from volume or pressure overload.2 Also, NT-proBNP may reflect cardiac conditions better than hs-cTnT particularly at premorbid stage. For example, there were ~30% of participants with hs-cTnT levels below the level of limit of detection (<3 ng/L) whereas 96.2% of our study population had measurable levels of NT-proBNP.
The improvements in c-statistics by adding NT-proBNP to prediction models were approximately 0.01-0.02, which might seem small. However, we should note that c-statistic is considered as a conservative statistic and this amount of improvement is shown for many novel predictors. For example, even cardiac markers at the time of MI demonstrated similar degree of improvement in c-statistic for secondary outcomes after MI in previous studies.56-58
There are several clinical and pathophysiological implications from our study. Although both cardiac markers are currently used as a prognostic marker in patients with MI, premorbid data of these cardiac markers are not readily available at this moment. However, this situation may be different in the future according to wide spread of electronic medical record as well as some experts proposing to evaluate these cardiac markers for cardiovascular risk prediction for primary prevention. Premorbid cardiac conditions may contribute to prognosis not only in primary prevention setting but also secondary prevention setting. Thus, our finding further supports the continuum of cardiovascular cascade (the concept of sequence of pathophysiologic cardiovascular event).59, 60 Our study suggests the importance of earlier prevention and management of cardiovascular risk in this continuum. For example, individuals with high premorbid levels of cardiac markers may benefit from more aggressive preventive therapies or preventive therapies with particular effects on heart failure risk such as beta blockers, thiazide diuretics, or renin-angiotensin system inhibitors when indicated (e.g., hypertension).61-63
Our study has several limitations. First, elapsed time from premorbid data to incident MI and follow-up time after incident MI varied across participants. Second, the elapsed time was on average approximately six years. Thus, the results may be different if premorbid levels could be measured more closely to MI occurrence. Third, the use of hospitalization and discharge codes for the diagnosis of incident heart failure may have resulted in some misclassification. However, the use of the heart failure cases in cohort studies has been associated with relatively high diagnostic specificity.64 Fourth, levels of troponins and natriuretic peptides at MI occurrence were not systematically assessed in ARIC, and thus we could not fully explore whether premorbid levels of these cardiac markers provide additional information beyond their levels at MI occurrence. Finally, there is a possibility that residual confounding could bias our results.
In conclusion, premorbid levels of hs-cTnT and NT-proBNP measured on average approximately six years prior to incident MI were independently associated with adverse outcomes after incident MI, with more evident relations for NT-proBNP. Our findings suggest the importance of pre-MI cardiac condition among MI patients.
Supplementary Material
Acknowledgement:
The authors thank the staff and participants of the ARIC study for their important contributions. Reagents for the NT-proBNP and high sensitivity cardiac troponin T assays were donated by Roche Diagnostics.
Sources of Funding: The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021). This research was supported in part by NIH/NIDDK grant R01DK089174 to Dr. Selvin and NIH/NHLBI R01HL134320 grant to Drs. Ballantyne and Selvin. Dr. Selvin was also supported by NIH/NIDDK grant K24DK106414.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gupta S, de Lemos JA. Use and misuse of cardiac troponins in clinical practice. Prog Cardiovasc Dis 2007;50(2):151–65. [DOI] [PubMed] [Google Scholar]
- 2.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994;90(1):195–203. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA, de Lemos JA, Sabatine MS, Murphy SA, Demopoulos LA, DiBattiste PM, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol 2003;41(8):1264–72. [DOI] [PubMed] [Google Scholar]
- 4.Tapanainen JM, Lindgren KS, Makikallio TH, Vuolteenaho O, Leppaluoto J, Huikuri HV. Natriuretic peptides as predictors of non-sudden and sudden cardiac death after acute myocardial infarction in the beta-blocking era. J Am Coll Cardiol 2004;43(5):757–63. [DOI] [PubMed] [Google Scholar]
- 5.Richards AM, Nicholls MG, Yandle TG, Ikram H, Espiner EA, Turner JG, et al. Neuroendocrine prediction of left ventricular function and heart failure after acute myocardial infarction. The Christchurch Cardioendocrine Research Group. Heart 1999;81(2):114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards AM, Nicholls MG, Yandle TG, Frampton C, Espiner EA, Turner JG, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation 1998;97(19):1921–9. [DOI] [PubMed] [Google Scholar]
- 7.Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, et al. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 1996;93(11):1963–9. [DOI] [PubMed] [Google Scholar]
- 8.Arakawa N, Nakamura M, Aoki H, Hiramori K. Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol 1996;27(7):1656–61. [DOI] [PubMed] [Google Scholar]
- 9.Gerber Y, Jaffe AS, Weston SA, Jiang R, Roger VL. Prognostic value of cardiac troponin T after myocardial infarction: a contemporary community experience. Mayo Clin Proc 2012;87(3):247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omland T, Persson A, Ng L, O'Brien R, Karlsson T, Herlitz J, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation 2002;106(23):2913–8. [DOI] [PubMed] [Google Scholar]
- 11.Mega JL, Morrow DA, De Lemos JA, Sabatine MS, Murphy SA, Rifai N, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE-TIMI-23 substudy. J Am Coll Cardiol 2004;44(2):335–9. [DOI] [PubMed] [Google Scholar]
- 12.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 2001;345(14):1014–21. [DOI] [PubMed] [Google Scholar]
- 13.Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, et al. B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 2003;107(22):2786–92. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl B, Diderholm E, Lagerqvist B, Venge P, Wallentin L, Investigators FI. Mechanisms behind the prognostic value of troponin T in unstable coronary artery disease: a FRISC II substudy. J Am Coll Cardiol 2001;38(4):979–86. [DOI] [PubMed] [Google Scholar]
- 15.James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)-IV substudy. Circulation 2003;108(3):275–81. [DOI] [PubMed] [Google Scholar]
- 16.Bohula May EA, Bonaca MP, Jarolim P, Antman EM, Braunwald E, Giugliano RP, et al. Prognostic performance of a high-sensitivity cardiac troponin I assay in patients with non-ST-elevation acute coronary syndrome. Clin Chem 2014;60(1):158–64. [DOI] [PubMed] [Google Scholar]
- 17.Grinstein J, Bonaca MP, Jarolim P, Conrad MJ, Bohula-May E, Deenadayalu N, et al. Prognostic implications of low level cardiac troponin elevation using high-sensitivity cardiac troponin T. Clin Cardiol 2015;38(4):230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohman EM, Armstrong PW, White HD, Granger CB, Wilcox RG, Weaver WD, et al. Risk stratification with a point-of-care cardiac troponin T test in acute myocardial infarction. GUSTOIII Investigators. Global Use of Strategies To Open Occluded Coronary Arteries. Am J Cardiol 1999;84(11):1281–6. [DOI] [PubMed] [Google Scholar]
- 19.Ottani F, Galvani M, Nicolini FA, Ferrini D, Pozzati A, Di Pasquale G, et al. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 2000;140(6):917–27. [DOI] [PubMed] [Google Scholar]
- 20.Aviles RJ, Askari AT, Lindahl B, Wallentin L, Jia G, Ohman EM, et al. Troponin T levels in patients with acute coronary syndromes, with or without renal dysfunction. N Engl J Med 2002;346(26):2047–52. [DOI] [PubMed] [Google Scholar]
- 21.Ndrepepa G, Braun S, Schulz S, Byrne RA, Pache J, Mehilli J, et al. Comparison of prognostic value of high-sensitivity and conventional troponin T in patients with non-ST-segment elevation acute coronary syndromes. Clin Chim Acta 2011;412(15-16):1350–6. [DOI] [PubMed] [Google Scholar]
- 22.Jernberg T, Stridsberg M, Venge P, Lindahl B. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol 2002;40(3):437–45. [DOI] [PubMed] [Google Scholar]
- 23.Lindahl B, Venge P, James S. The new high-sensitivity cardiac troponin T assay improves risk assessment in acute coronary syndromes. Am Heart J 2010;160(2):224–9. [DOI] [PubMed] [Google Scholar]
- 24.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284(7):835–42. [DOI] [PubMed] [Google Scholar]
- 25.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163(19):2345–53. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Park HS, Chae SC, Cho Y, Yang DH, Jeong MH, et al. Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Am J Cardiol 2009;104(2):182–9. [DOI] [PubMed] [Google Scholar]
- 27.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med 2005;352(7):666–75. [DOI] [PubMed] [Google Scholar]
- 28.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361(26):2538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. B-type natriuretic peptide and ischemia in patients with stable coronary disease: data from the Heart and Soul study. Circulation 2003;108(24):2987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bibbins-Domingo K, Gupta R, Na B, Wu AH, Schiller NB, Whooley MA. N-terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP), cardiovascular events, and mortality in patients with stable coronary heart disease. JAMA 2007;297(2):169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyngbaek S, Winkel P, Gotze JP, Kastrup J, Gluud C, Kolmos HJ, et al. Risk stratification in stable coronary artery disease is possible at cardiac troponin levels below conventional detection and is improved by use of N-terminal pro-B-type natriuretic peptide. Eur J Prev Cardiol 2014;21(10):1275–1284. [DOI] [PubMed] [Google Scholar]
- 32.Koenig W, Breitling LP, Hahmann H, Wusten B, Brenner H, Rothenbacher D. Cardiac troponin T measured by a high-sensitivity assay predicts recurrent cardiovascular events in stable coronary heart disease patients with 8-year follow-up. Clin Chem 2012;58(8):1215–24. [DOI] [PubMed] [Google Scholar]
- 33.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61(12):1240–9. [DOI] [PubMed] [Google Scholar]
- 34.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation 2011;123(13):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304(22):2503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350(7):655–63. [DOI] [PubMed] [Google Scholar]
- 37.Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail 2012;5(6):727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marz W, Tiran B, Seelhorst U, Wellnitz B, Bauersachs J, Winkelmann BR, et al. N-terminal pro-B-type natriuretic peptide predicts total and cardiovascular mortality in individuals with or without stable coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health Study. Clin Chem 2007;53(6):1075–83. [DOI] [PubMed] [Google Scholar]
- 39.Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JA, et al. High Sensitive Cardiac Troponin T as an Early Biochemical Signature for Clinical and Subclinical Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Circulation 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohula EA, Bonaca MP, Braunwald E, Aylward PE, Corbalan R, De Ferrari GM, et al. Atherothrombotic Risk Stratification and the Efficacy and Safety of Vorapaxar in Patients With Stable Ischemic Heart Disease and Previous Myocardial Infarction. Circulation 2016;134(4):304–13. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs DR Jr., Kroenke C, Crow R, Deshpande M, Gu DF, Gatewood L, et al. PREDICT: A simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation 1999;100(6):599–607. [DOI] [PubMed] [Google Scholar]
- 43.Watkins S, Thiemann D, Coresh J, Powe N, Folsom AR, Rosamond W. Fourteen-year (1987 to 2000) trends in the attack rates of, therapy for, and mortality from non-ST-elevation acute coronary syndromes in four United States communities. Am J Cardiol 2005;96(10):1349–55. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y, Matsushita K, Sang Y, Grams ME, Skali H, Shah AM, et al. Association of high-sensitivity cardiac troponin T and natriuretic peptide with incident ESRD: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2015;65(4):550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014;63(14):1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40(5):976–82. [DOI] [PubMed] [Google Scholar]
- 47.Wallace TW, Abdullah SM, Drazner MH, Das SR, Khera A, McGuire DK, et al. Prevalence and determinants of troponin T elevation in the general population. Circulation 2006;113(16):1958–65. [DOI] [PubMed] [Google Scholar]
- 48.Rubin J, Matsushita K, Lazo M, Ballantyne CM, Nambi V, Hoogeveen R, et al. Determinants of minimal elevation in high-sensitivity cardiac troponin T in the general population. Clin Biochem 2016;49(9):657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM, Investigators AS. Troponin T, B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol 2013;23(2):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawagoe C, Sato Y, Toida T, Nakagawa H, Yamashita Y, Fukuda A, et al. N-terminal-pro-B-type-natriuretic peptide associated with 2-year mortality from both cardiovascular and non-cardiovascular origins in prevalent chronic hemodialysis patients. Ren Fail 2018;40(1):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129(3):399–410. [DOI] [PubMed] [Google Scholar]
- 52.Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial Differences in Natriuretic Peptide Levels: The Dallas Heart Study. JACC Heart Fail 2015;3(7):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta DK, Claggett B, Wells Q, Cheng S, Li M, Maruthur N, et al. Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. J Am Heart Assoc 2015;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorgis L, Cottin Y, Danchin N, Mock L, Sicard P, Buffet P, et al. Impact of obesity on the prognostic value of the N-terminal pro-B-type natriuretic peptide (NT-proBNP) in patients with acute myocardial infarction. Heart 2011;97(7):551–6. [DOI] [PubMed] [Google Scholar]
- 55.Gerber Y, Weston SA, Enriquez-Sarano M, Berardi C, Chamberlain AM, Manemann SM, et al. Mortality Associated With Heart Failure After Myocardial Infarction: A Contemporary Community Perspective. Circ Heart Fail 2016;9(1):e002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahluwalia N, Blacher J, Szabo de Edelenyi F, Faure P, Julia C, Hercberg S, et al. Prognostic value of multiple emerging biomarkers in cardiovascular risk prediction in patients with stable cardiovascular disease. Atherosclerosis 2013;228(2):478–84. [DOI] [PubMed] [Google Scholar]
- 57.Hochholzer W, Valina CM, Stratz C, Amann M, Schlittenhardt D, Buttner HJ, et al. High-sensitivity cardiac troponin for risk prediction in patients with and without coronary heart disease. Int J Cardiol 2014;176(2):444–9. [DOI] [PubMed] [Google Scholar]
- 58.Sato A, Hiroe M, Akiyama D, Hikita H, Nozato T, Hoshi T, et al. Prognostic value of serum tenascin-C levels on long-term outcome after acute myocardial infarction. J Card Fail 2012;18(6):480–6. [DOI] [PubMed] [Google Scholar]
- 59.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol 1987;59(7):23C–30C. [DOI] [PubMed] [Google Scholar]
- 60.Majmudar MD, Nahrendorf M. Cardiovascular molecular imaging: the road ahead. J Nucl Med 2012;53(5):673–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricci F, Di Castelnuovo A, Savarese G, Perrone Filardi P, De Caterina R. ACE-inhibitors versus angiotensin receptor blockers for prevention of events in cardiovascular patients without heart failure - A network meta-analysis. Int J Cardiol 2016;217:128–34. [DOI] [PubMed] [Google Scholar]
- 62.Gu J, Fan YQ, Bian L, Zhang HL, Xu ZJ, Zhang Y, et al. Long-term prescription of beta-blocker delays the progression of heart failure with preserved ejection fraction in patients with hypertension: A retrospective observational cohort study. Eur J Prev Cardiol 2016;23(13):1421–8. [DOI] [PubMed] [Google Scholar]
- 63.Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin-angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014;2(6):663–70. [DOI] [PubMed] [Google Scholar]
- 64.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail 2012;5(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.