Abstract
Background
Hodgkin lymphoma (HL) is a type of lymphoma that arises from the B lymphocytes. The four main subtypes of HL are the nodular sclerosing, mixed cellularity, lymphocyte rich and the lymphocyte depleted. Nodular sclerosis subtype accounts for majority of all classical HL, whereas lymphocytic depletion type accounts for less than 1%. The main objective of reporting this case is to share with the medical fraternity a rare presentation of abdominal lymphocyte-depleted classical Hodgkin lymphoma.
A 47-year-old gentleman of Malay ethnicity with no known pre-morbidities, presented to the haematology unit with a 2-month history of night fever, loss of weight, malaise, anorexia and abdominal swelling. Abdominal examination revealed a periumbilical and lower epigastric swelling measuring 6x6 cms. The swelling was non-tender, firm in consistency and smooth on palpation. The Contrast Enhanced Computed Tomography (CECT) imaging revealed an enlarged mesenteric mass measuring 5.8x6.9x5.7 cm and multiple enlarged aorta-caval lymph nodes. The mesenteric tumour histology and immunohistochemistry were consistent with lymphocyte depleted HL. He completed six cycles of intravenous ABVD polychemotherapy consisting of doxorubicin (Adriamycin) 25mg/m2, Bleomycin 10mg/m2, Vinblastine 6mg/m2 and Dacarbazine 375mg/m2. The Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG PET /CT) imaging post 2 cycles and 6 cycles of ABVD polychemotherapy showed complete metabolic response to chemotherapy.
Conclusion
Lymphocyte-depleted classical Hodgkin lymphoma (LDcHL) is a rare entity and is mostly diagnosed at a later stage rendering it a disease with poor prognostic outcomes. Early detection and prompt institution of therapy is crucial in the management of this disease.
Keywords: lymphocyte depleted, mesenteric tumour, Hodgkin lymphoma, polychemotherapy
Background
Hodgkin lymphoma (HL) is a type of lymphoma that arises from the B lymphocytes. There are two types of Hodgkin lymphoma; classical Hodgkin lymphoma and nodular lymphocyte predominant Hodgkin lymphoma. Classical Hodgkin lymphoma (excluding nodular lymphocyte predominant Hodgkin lymphoma) can be classified into four main pathologic subtypes based upon Reed–Sternberg cell morphology and the composition of the reactive cell infiltrate seen in the lymph node biopsy specimen.1 The four main subtypes are the nodular sclerosing, mixed cellularity, lymphocyte rich and the lymphocyte depleted. Internationally, nodular sclerosis subtype accounts for majority of all classical HL, whereas lymphocytic depletion type accounts for less than 1%.2 There is a male predominance for Hodgkin lymphoma. Cervical lymph nodes are frequently involved in approximately 75% of the cases. Lymphocyte-Depleted classical Hodgkin lymphoma (LDcHL) is more common in developing countries and is often Human Immunodeficiency Virus (HIV) associated.3 LDcHL has a predilection for abdominal organs, retroperitoneal lymph nodes and bone marrow.3 The main objective of reporting this case is to share with the medical fraternity of a rare presentation of abdominal lymphocyte-depleted classical Hodgkin lymphoma.
Case Presentation
A 47-year-old gentleman of Malay ethnicity with no known pre-morbidities, presented to the hematology unit with a two-month history of night fevers, loss of weight, anorexia and abdominal swelling. He complained of a gradual increase of the abdominal swelling. He had a normal bowel habit. He is a non-smoker, a teetotaller and has no history of other substance abuse. He has no significant family history. He works as a teacher in a secondary school. Besides that, this gentleman has no history of consuming traditional or herbal medications.
On examination, he was alert and had a thin body habitus. Abdominal examination revealed a periumbilical and lower epigastric swelling measuring 6×6 cm. The swelling was nontender, firm in consistency and smooth on palpation. The liver and spleen were both not palpable. The kidneys were not ballotable. The cardiovascular, respiratory and neurological examinations were all unremarkable. There were no other palpable lymph nodes in the neck, axillae and groin.
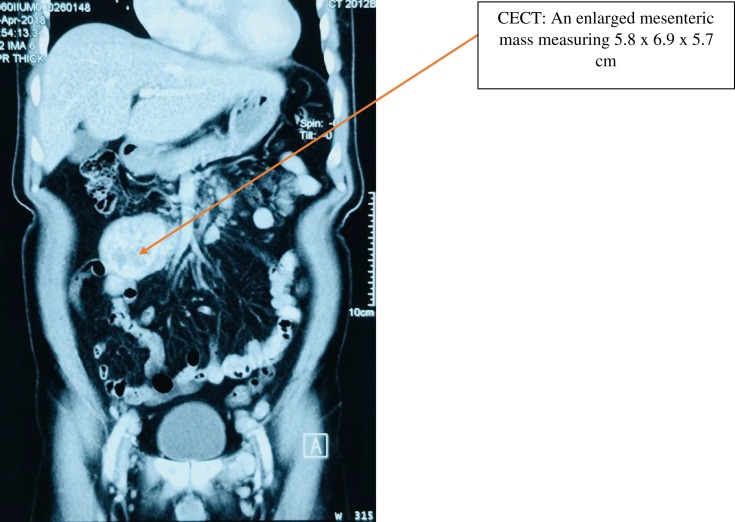
The laboratory parameters are tabulated in Table 1. The Whole-body Computed Tomography (Figure 1) revealed an enlarged mesenteric mass measuring 5.8×6.9×5.7 cm. Multiple enlarged aorta-caval lymph nodes, the largest measuring 2.1 cm×2.4 cm were seen displacing the inferior vena cava. No other lymphadenopathies were seen elsewhere. His retroviral and hepatitis viral screening were all non-reactive.
Table 1.
Tabulation Of Laboratory Parameters
| Laboratory Parameters | Value (Unit With Normal Range) |
|---|---|
| Hemoglobin | 10.6 (13.5–16.5 g/dL) |
| Total White Cell Count | 7.2 (4–10×109/L) |
| Absolute Lymphocyte Count | 1.5 (1–3×109/L) |
| Platelet | 220 (150–400×109/L) |
| Creatinine | 80 (74–100 umol/L) |
| Albumin | 30 (>40 g/L) |
| Lactate Dehydrogenase | 120 (97–180 U/L) |
| Erythrocyte Sedimentation Rate (ESR) | 80 (0–20 mm/hr) |
| Anti- (HIV)-1,2 | Not detected |
| Epstein Barr Virus IgM | Not detected |
| Epstein Barr PCR | Not detected |
Figure 1.
Whole body CT shows an enlarged mesenteric mass measuring 5.8×6.9×5.7 cm.
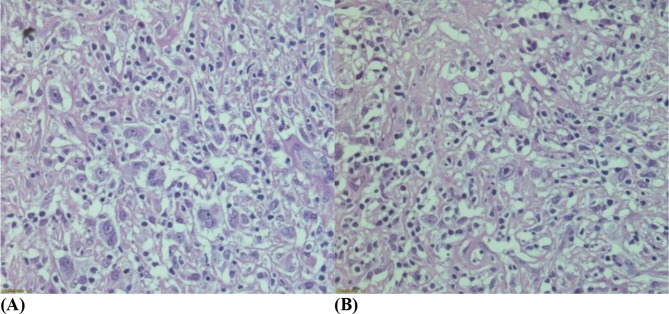
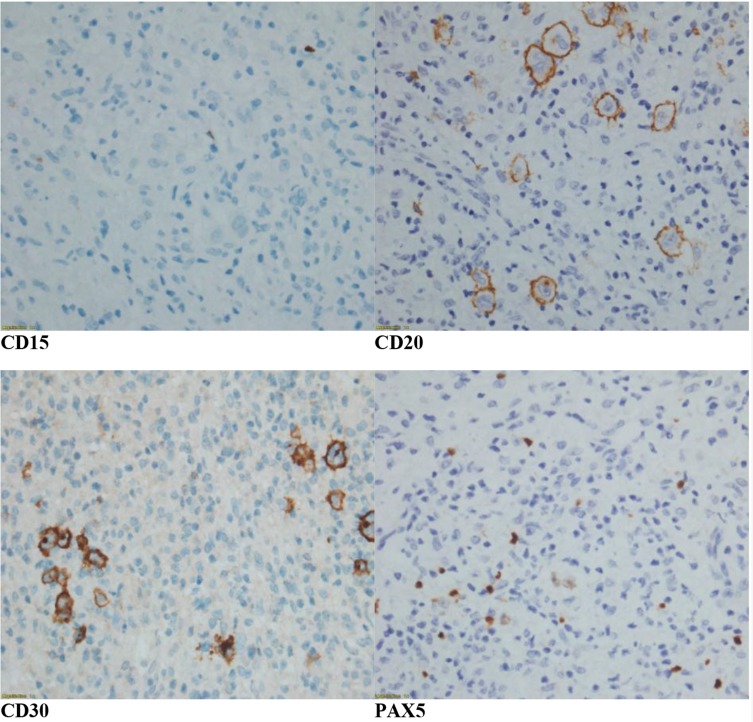
Subsequently, he underwent a diagnostic laparotomy and excision of the mesenteric tumour. The mesenteric tissue histology (Figure 2A and B) showed pleomorphic Hodgkin and Reed-Sternberg cells (HRS) with diffuse polymorphic cellular population in a background of widespread fibrosis. CD68+ve histiocytes were admixed with a few small lymphocytes. Immunohistochemistry studies (Figure 3) revealed neoplastic cells expressing positivity for CD30, weak positivity for CD20, dim positivity for PAX5 and negativity for CD15. Ki 67 proliferation index was approximately 50%. The mesenteric tumour histology and immunohistochemistry were consistent with lymphocyte-depleted Hodgkin lymphoma. Bone marrow trephine biopsy did not show any disease infiltration.
Figure 2.
(A, B) Hematoxylin & Eosin ×40: The mesenteric tissue histology shows pleomorphic Hodgkin and Reed-Sternberg cells (HRS) with diffuse polymorphic cellular population in a background of widespread fibrosis.
Figure 3.
Immunohistochemistry studies ( ×40 magnifications) show positivity for CD30, dim positivity for PAX 5, weak positivity for CD20 and negativity for CD15.
He was diagnosed with lymphocyte-depleted classical Hodgkin lymphoma Stage II (infradiaphragmatic non-bulky). International Prognostic Score (IPS): 3 (Age >45, Gender: Male, Albumin: 30g/L)
He was treated with six cycles of ABVD polychemotherapy on days 1 and 15 which consisted of intravenous doxorubicin (Adriamycin) 25mg/m2, intravenous Bleomycin 10mg/m2, intravenous Vinblastine 6mg/m2 and intravenous Dacarbazine 375mg/m2. Each cycle consisted of giving the patient these 4 drugs twice (on days 1 and 15). The cycle was repeated every 28 days. He did not suffer from any significant complications of the treatment.
The Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (FDG PET/CT) imaging post 2 cycles and 6 cycles of ABVD polychemotherapy showed complete metabolic response to chemotherapy. He has been in complete remission for the past 2 years and is on regular follow-up at the hematology clinic.
Discussion
This case represents a rare entity of lymphocyte-depleted classical Hodgkin lymphoma. The literature suggest that the overall incidence of LDcHL is decreasing over time. The reasons for this slow decline are not very well understood. LDcHL usually carries a poor prognosis and occurs in older males. Patients with LDcHL present more often with unfavorable characteristics such as advanced stage, B symptoms, large mediastinal mass, extranodal disease, high ESR, involvement of three or more lymph node areas, and higher IPS; in addition, organ involvement of bone marrow and liver was also more frequent.4 About half of the cases of Hodgkin lymphoma is associated with Epstein Barr Virus infection.5 However, in our patient, he did not have any evidence of HIV or Epstein Barr virus infection based on serology and peripheral blood for Polymerase Chain Reaction (PCR) analysis.
Histological diagnosis of LDcHL is often not straightforward, and its distinction from aggressive non-Hodgkin lymphomas such as anaplastic large cell lymphoma can be difficult and often poses a diagnostic dilemma. Prior to 1980, patients with aggressive non-Hodgkin Lymphoma were sometimes misdiagnosed as having HL and classified as lymphocyte depleted or mixed cellularity subtypes.6 Today, expert pathologists more reliably distinguish LDcHL from other entities including diffuse large B-cell lymphoma, anaplastic large-cell lymphoma, or gray zone lymphoma by means of morphology, immunophenotyping, and molecular genetic analyses.7
Histologically, LDcHL is consistent with predominance of Hodgkin and Reed-Sternberg cells (HRS) in relation to the cell density of the background. The background is often characterised by widespread fibrosis and significant depletion in reactive lymphocytes.7 The HRS cells are usually pleomorphic and they exhibit sarcomatous appearance. Immunohistochemistry studies reveal HRS cells which stain positive for CD15, CD30, PAX5 and MUM1/IRF4.7 They may show weak positivity for CD20. These features on histopathology and immunohistochemistry are consistent with the findings seen in our patient. EBV is found in almost all cases of HIV associated LDcHL suggesting that EBV plays an important role in the pathogenesis of the disease via the expression of Latent Membrane Protein-1 (LMP-1) which activates critical signalling pathways.
Treatment for LDcHL should include a combination of polychemotherapy and radiotherapy. One of the frequently raised questions is whether LDcHL should be treated more aggressively with dose-intensive chemotherapy. The literature suggest that six cycles of chemotherapy consisting of doxorubicin (Adriamycin), bleomycin, vinblastine and dacarbazine (ABVD) is the appropriate standard regimen and involved field radiotherapy (IFRT) with an intermediate dosage (25–40 Gy) targeting the Waldeyer’s ring and cervical lymph nodes should be the first line of treatment for patients with HL.8 Some authors conclude that chemotherapy alone with ABVD without radiotherapy can be an option in the treatment of non-bulky Hodgkin disease.9
A Comprehensive Analysis of The German Hodgkin Lymphoma Group published in September 2016 which included a total of 12,155 patients with biopsy-proven Hodgkin Lymphoma, of which, 10,019 patients underwent central expert pathology review. Eighty-four patients (<1%) were confirmed as LDcHL. Eighty-three of these 84 patients who were confirmed with LDcHL had early unfavourable or advanced disease with only 1 having early favourable disease. The overall results of this study implied that patients confirmed with LDcHL (early unfavourable and advanced stage with bulky disease) should receive a dose-intense chemotherapy such as Escalated BEACOPP regimen. Recommendation for using dose-intense treatment strategies is limited to patients with HL younger than 60 years of age. Older patients are at increased risk of acute toxicity due to Escalated BEACOPP chemotherapy.10 Previous reports have demonstrated that better tumour control achieved with BEACOPP was offset by higher toxicity and did not translate into better outcome.11
Conclusion
LDcHL is a rare entity and is often diagnosed at a later stage rendering it a disease with poor prognostic outcome. Early detection and prompt institution of therapy is crucial in the management of LDcHL as this disease frequently suffers a worse prognosis as compared to other subtypes of classical Hodgkin Lymphoma. Further research studies are necessary to evaluate the pathophysiology and genomic profile of this disease.
Consent For Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Banerjee D. Recent advances in the pathobiology of Hodgkin’s lymphoma: potential impact on diagnostic, predictive, and therapeutic strategies. Adv Hematol. 2011;439456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurtado-Cordovi JM, Verma V, Gotlieb V, Frieri M. Lymphocyte rich Hodgkin’s lymphoma presented with warm hemolytic anemia: a case report and literature review. Case Rep Hematol. 2011;2011:385–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klimm B, Franklin Jeremy, et al. Lymphocyte-depleted classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin study group. J Clin Oncol. 2011;29(29):3914–3920. doi: 10.1200/JCO.2011.36.4703 [DOI] [PubMed] [Google Scholar]
- 5.Stewart BW and Wild CP. World Cancer Report 2014. World Health Organization. 2014; Chapter 2.4.
- 6.Miller TP, Byrne GE, Jones SE. Mistaken clinical and pathologic diagnoses of Hodgkin’s disease: a Southwest oncology group study. Cancer Treat Rep. 1982;66:645–651. [PubMed] [Google Scholar]
- 7.Slack GW, Ferry JA, Hasserjian RP, et al. Lymphocyte depleted Hodgkin lymphoma: an evaluation with immunophenotyping and genetic analysis. Leuk Lymphoma. 2009;50:937–943. doi: 10.1080/10428190902930488 [DOI] [PubMed] [Google Scholar]
- 8.Singh R, Sheikh S, Pallagatti S, et al. Lymphocyte depleted Hodgkin’s lymphoma presented with haemolytic anemia: a case report and literature review. J Maxillofac Oral Surg. 2015;14(Suppl 1):317–322. doi: 10.1007/s12663-013-0528-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canello George P. Chemotherapy alone for early Hodgkin’s lymphoma: an emerging option. J Clin Oncol. 2005;23:4574–4576. doi: 10.1200/JCO.2005.01.911 [DOI] [PubMed] [Google Scholar]
- 10.Klimm B, Diehl V, Engert: A. Hodgkin’s lymphoma in the elderly: a different disease in patients over 60. Oncology (Williston Park). 2007;21:982–990. [PubMed] [Google Scholar]
- 11.Halbsguth TV, Nogová L, Mueller H, et al. Phase 2 study of BACOPP (Bleomycin, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone) in older patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood. 2010;116:2026–2032. doi: 10.1182/blood-2009-11-253211 [DOI] [PubMed] [Google Scholar]