Abstract
Dietary antioxidant supplements such as L-glutathione have gained considerable attention in dermatology and cosmeceutical fields. L-glutathione possesses antiaging, antimelanogenic, antioxidant, and anticancer properties. This study aimed to investigate the inhibitory effects of L-glutathione on melanogenesis activity and oxidative stress in ultraviolet B (UVB)-irradiated BALB/c mice. Eighteen female BALB/c mice were randomly divided into 3 groups: a control group (n=6), a group without UVB irradiation and L-glutathione administration; a UVB irradiated group (n=6), a group irradiated with a UVB dose of 250 mJ/cm2 for 3 min; and a treatment group (n=6), a group irradiated with UVB and treated with 100 mg/kg of L-glutathione by oral gavage. Treatment was given for 14 days, and UVB irradiation was given on days 9, 11, and 13. Oral L-glutathione significantly (P<0.05) reduced lipid peroxidation and elevated superoxide dismutase activity the and glutathione level. L-glutathione also inhibited melanin content and tyrosinase activity significantly (P<0.05) as compared with the UVB-irradiated group. Histopathological examination also showed that L-glutathione reduced the deposition of melanin pigment in the basal layer of the epidermis as compared with that in UVB-irradiated mice. All in all, the present study demonstrated that L-glutathione has the potential to be developed as a photoprotection agent against UVB-induced oxidative stress and melanogenesis.
Keywords: L-glutathione, melanogenesis, oxidative stress, photoprotection, ultraviolet B (UVB)
Introduction
The skin is the largest organ of the body and comprises two primary layers, which include epidermis and dermis, which together are made up of epithelial, mesenchymal, glandular, and neurovascular components [8]. Skin functions as the primary first-line defense against external antigens and environmental stressors such as ultraviolet (UV) radiation [13]. UV radiation includes UVA (315–400 nm), UVB (280–315 nm), and UVC (200–280 nm). Among all of the UV spectrum, UVB is the most genotoxic and damaging to the skin [22]. Excessive exposure to UVB can lead to hyperpigmentation, erythema, cutaneous edema, and even skin cancer [16].
Melanin is the skin-protective barrier that acts by absorbing UV radiation [25]. It is formed from melanogenesis conducted in melanocytes located in the basal layer of the epidermis [7]. Prolonged exposure to UV radiation may cause the accumulation of abnormal melanin induces pigmentation disorders, such as melasma, freckles and ephelide. One of the noninvasive strategies to treat these disorders is to control melanogenesis by inhibiting tyrosinase gene expression. Tyrosinase is the key enzyme that initiates the melanogenesis biosynthesis pathway [14].
Additionally, acceleration of melanogenesis activity due UV radiation produces hydrogen peroxide (H2O2) and other prooxidants such as reactive oxygen species (ROS) that would expose the melanocyte to high levels of oxidative stress. ROS scavengers have the ability to downregulate the UV-induced melanogenesis [17, 32].
Therefore, the use of antioxidant supplementation has become a new trend in dermatology and skin care products in recent years[1, 18]. L-glutathione, also known as reduced glutathione, low-molecular-weight, water-soluble tripeptide antioxidant. L-glutathione plays a vital role in detoxification of metabolic products such as lipid peroxides and xenobiotic compounds like pollutants, heavy metals, and drugs [1, 9]. Several studies have also proven that L-glutathione is able to slows down carcinogenesis, attenuate immune response, and remove toxic xenobiotics [11, 15, 24]. Besides, its role as anti-melanogenic properties have led to its promotion as a skin lightening agent [26]. In the present study, we aimed to determine the inhibitory effect of L-glutathione on melanogenesis activity and oxidative stress in UVB irradiated BALB/c mice.
Materials and Methods
Preparation of L-glutathione
L-glutathione was a gift from Dr. Nabisarr Mustan, Cambridge Herbal Sdn Bhd (Sungai Buloh, Malaysia). Mice were treated with L-glutathione at a dose of 100 mg/kg [31]. The dose was freshly prepared daily by dissolving L-glutathione in corn oil and then vortexing it until the powder dissolved completely. All mice were fasted for 4 h before being treated with 0.2 ml of dissolved glutathione by oral gavage [19].
Animals and experimental design
Eighteen female BALB/c mice were supplied by the Faculty of Veterinary Medicine, University Putra Malaysia. The animals were kept in the animal house of the Department of Biomedical Science, Centre of Health and Applied Sciences, Universiti Kebangsaan Malaysia, under 12-h light/dark cycles at controlled room temperatures. All mice were given free access to a standard pellet diet and drinking water.
The animals were randomly divided into three groups: a vehicle control group (n=6), which was not exposed to UVB irradiation and not treated with L-glutathione; an exposure group (n=6), which was exposed to UVB irradiation only; and a treatment group (n=6), which was exposed to UVB irradiation and also treated with 100 mg/kg L-glutathione. The L-glutathione was administered throughout the treatment period. The treatment was administered for 14 successive days. Briefly, the dorsal parts of the mice were shaved using an electric shaver (Phillips, Malaysia). Mice from both groups (exposure and treatment groups) were then exposed to UVB irradiation for 3 min at a dose of 250 mJ/cm2 on the 9th, 11th, and 13th days of treatment [21]. The source of irradiation was a 15 watt lamp emitting UV light with a wavelength of 312 nm (UVP, Upland, CA, USA).
The usage of the mice was approved by the Universiti Kebangsaan Malaysia Animal Ethics Committee (UKMAEC; UKMAEC No: FSK/2017/AHMAD ROHI/22-NOV./887-NOV.-2017-JULY-2018), and guidelines were strictly followed.
Preparation of skin tissue homogenate
The skin tissue was washed with normal saline and then minced using a pestle and mortar. After being weighed, the skin was then homogenized in phosphate buffer solution at pH 7.8. After centrifugation, the supernatant was removed and stored at −40°C. The whole procedure was conducted under cold conditions. This homogenate was used for evaluation of oxidative stress and melanogenesis activity evaluation.
Determination of glutathione (GSH) level
GSH level was measured in the skin homogenate based on the Ellman method [10]. with some modifications. Skin homogenate was treated with metaphosphoric acid solution and centrifuged at 3,000 xg for 10 min at 4°C. The obtained supernatant was mixed with reaction buffer at pH 8.0 and DTNB for 15 min. Then it was measured at 412 nm using a microplate reader. The GSH level is expressed as µmol/mg protein.
Determination of superoxide dismutase (SOD) activity
SOD activity was measured in the skin homogenate based on the Beyer and Fridovich method [4]. Briefly, an aliquot of (20 µl) of the homogenate was mixed with a substrate, which contained PBS (EDTA), L-methionine, NBT.2HCl,Triton-X, and riboflavin. The mixture was incubated in an aluminium box under 20 watt lamp for 7 min. The SOD activity was measured spectrophotometrically at 560 nm. SOD activity was calculated and expressed in U/min/mg protein.
Determination lipid peroxidation activity
Lipid peroxidation activity was measured in the form of malondialdehyde using thiobarbituric acid based on Stocks and Dormandy method with some modifications [27]. Briefly, a 0.5 ml aliquot of skin homogenate was added to a TCA/HCl solution, vortexed, and incubated at room temperature for 15 min. The mixture was added to TBA/NaOH, vortexed and heated in a boiling water bath (100°C) for 30 min. The malondialdehyde (MDA) in the sample reacted with the thiobarbituric acid to form a pink chromogen containing thiobarbituric acid reactive substances (TBARS). The level of TBARS in the supernatant was measured using a spectrophotometer at 532 nm. The MDA concentration was expressed as nmol/g protein of tissue protein.
Determination of dopa oxidase activity
The dopa oxidase assay used was the new 3-methyl-2-benzothiazolinonehydrazone hydrochloride (MBTH) assay based on the Winder and Harris method with a minor modification [30]. The rate production of the pink pigment was used as a quantitative measure of enzyme activity. The reaction mixture (1 ml) contained 490 µl assay buffer A (100 mM sodium phosphate pH 7.1, 4% by volume N, N’-dimethylformamide), 200 µl 5 mM L-DOPA, and 290 µL 20.7 mM MBTH. The reaction mixture, without enzyme, was prepared up in a 1.6 ml plastic cuvette and incubated at 37°C for 10 min. The temperature of the reaction mixture was measured using a thermometer, 20 µl of homogenate was added into the cuvette, and the cuvette contents were mixed by inversion. The initial rate of absorbance increase at 505 nm was monitored spectrophotometrically, routinely over a period of 5 min. The temperature of the assay mixture was rechecked at the end of the assay.
Determination of melanin content level
Melanin content was measured using the Talwar method [28]. Melanin in the resulting homogenate (0.35 ml) was solubilized by treatment with 2 N NaOH at 60°C for 4 days. An equal volume of distilled water was then added, followed by the addition of 0.7 ml of chloroform:phenol (1:1). The mixture was vortexed and centrifuged at 5,000 × g for 10 min to separate the phases. Melanin content in the water layer was pipetted into a 96-well microplate and measured by a microplate reader at 400 nm. Melanin content is expressed as micrograms of melanin per milligram wet weight of skin.
Histological evaluation
At the end of the experiment, the animals were anasthesized with by ketamine and xylazine and euthanized by cervical dislocation. Tissue samples were taken from the dorsal part of skin. The tissue specimens were fixed using 10% neutral buffered formalin for 24 h and embedded in paraffin blocks. Sections were cut at thickness of 5 µm for histopathological observation. Fontana Masson staining was performed to assess the melanin pigmentation on the mouse skin. In the histological examination, melanin was quantitated based on using Billings method [5].
Statistical analysis
The data analysis was performed using the IBM SPSS Statistics version 23.0 and values were expressed as the mean ±SEM. One-way ANOVA was used to compare the GSH, SOD, MDA, dopa oxidase, and melanin content level between groups. Differences among means were examined using Tukey’s post hoc test and were considered to be statistically significant at P<0.05.
Results
Effects of L-glutathione on GSH level in UVB-irradiated mice
Figure 1 showed the results for glutathione level in the various groups. The glutathione level of the UVB-irradiated group (0.376 ± 0.025 µmol/mg) decreased significantly as compared with that of the vehicle control group (0.554 ± 0.045 µmol/mg; P<0.05). Oral administration of L-glutathione 100 mg/kg increased the glutathione level (0.468 ± 0.029 µmol/mg) as compared with the level of UVB-irradiated group (0.376 ± 0.025 µmol/mg).
Fig. 1.
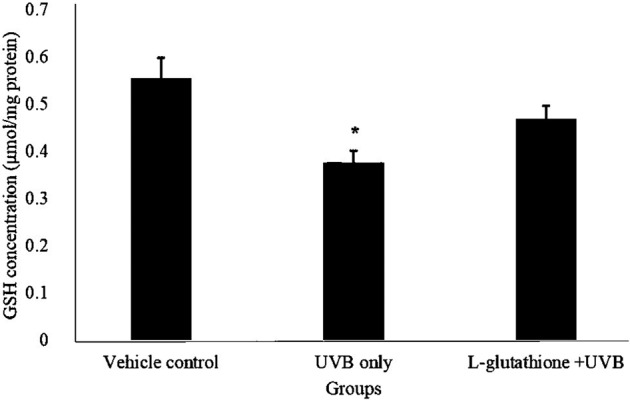
Determination of glutathione levels in ultraviolet B (UVB)-irradiated mice. The bar chart shows results for the concentration of glutathione in the different groups presented as the mean ± SEM; n=6. *Statistically significant difference in comparison with the vehicle control group (P<0.05).
Effects of L-glutathione on SOD activity in UVB-irradiated mice
Figure 2 showed the SOD activity level in various group. The SOD activity in UVB irradiated group (0.561 ± 0.034 U/min/mg) decreased significantly as compared to the vehicle control group (1.142 ± 0.156 U/min/mg). However, L-glutathione treatment group (1.153 ± 0.09 U/min/mg) increased significantly (P<0.05) as compared to UVB irradiated group (0.561 ± 0.034 U/min/mg).
Fig. 2.
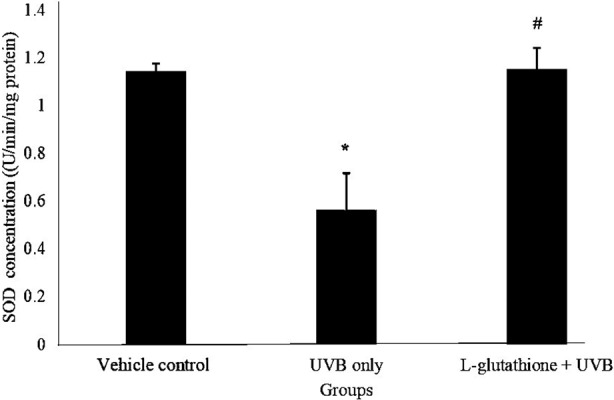
Determination of superoxide dismutase (SOD) activity in ultraviolet B (UVB)-irradiated mice. The barchart shows the results of concentration of glutathione in the different groups presented as by the mean ± SEM; n=6. *Statistically significant difference in comparison with the vehicle control group (P<0.05). #Statistically significant difference in comparison with the UVB-exposure group (P<0.05).
Effects of L-glutathione on lipid peroxidation activity in UVB irradiated mice
Figure 3 shows the levels of MDA, which indicated the lipid peroxidation activity, in various groups. The MDA level in the UVB-irradiated group increased (13.343 ± 1.35 nmol/g) significantly (P<0.05) as compared with that in the vehicle control group (2.67 ± 0.613 nmol/g). The MDA level in the L-glutathione treatment group (5.586 ± 0.746 nmol/g) was significantly reduced (P<0.05) as compared with that in the UVB-irradiated group (13.343 ± 1.35 nmol/g).
Fig. 3.
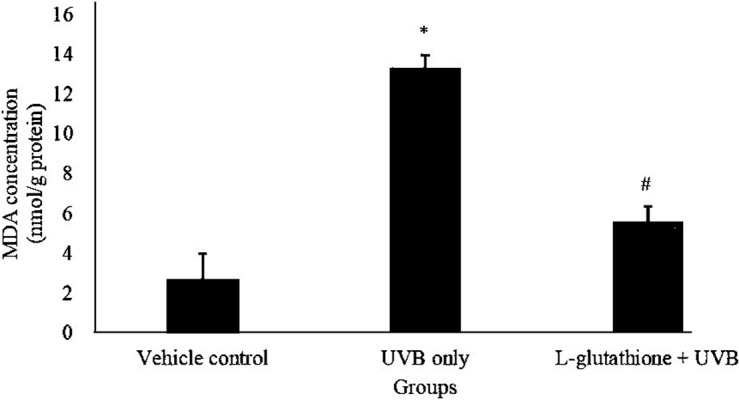
Determination of lipid peroxidation activity in ultraviolet B (UVB)-irradiated mice. The bar chart shows the results for the concentration of malondialdehyde (MDA) in the different groups presented as the mean ± SEM; n=6. *Statistically significant difference in comparison with the vehicle control group (P<0.05). #Statistically significant difference in comparison with the UVB-exposure group (P<0.05).
Effects of L-glutathione on melanin content in UVB-irradiated mice
Figure 4 shows the levels of melanin content in the various groups. The melanin content in the UVB-irradiated group (1.546 ± 0.097 µg/mg of skin) was significantly increased (P<0.05) as compared with that in the vehicle control group (1.188 ± 0.091 µg/mg of skin). However, oral administration of L-glutathione (1.122 ± 0.056 µg/mg of skin) reduced the melanin content significantly (P<0.05) as compared with the level in the UVB irradiated group (1.546 ± 0.097 µg/mg of skin). Meanwhile, the melanin content in the vehicle control group (1.188 ± 0.091 µg/mg of skin) showed no significant difference as compared with that in the L-glutathione treatment group (1.122 ± 0.056 µg/mg of skin).
Fig. 4.
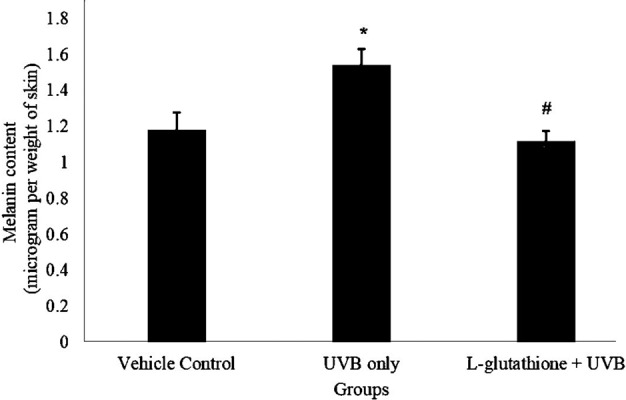
Determination of melanin content in ultraviolet B (UVB)-irradiated mice. The bar chart shows the results for the melanin level in the different groups presented as the mean ± SEM; n=6. *Statistically significant difference in comparison with vehicle control group (P<0.05). #Statistically significant difference in comparison with UVB-exposure group (P<0.05).
Effects of L-glutathione on tyrosinase activity in UVB-irradiated mice
Figure 5 shows the levels of dopa oxidase, which indicated the tyrosinase activity, in the various groups. The dopa oxidase level in the UVB-irradiated group (10.907 ± 2.059 units/min) was significantly increased as compared with that in the vehicle control group (1.533 ± 0.349 units/min). Meanwhile, the L-glutathione treatment group (3.933 ± 0.858 units/min) showed significantly reduced dopa oxidase activity as compared with the UVB-irradiated group (10.907 ± 2.059 units/min).
Fig. 5.
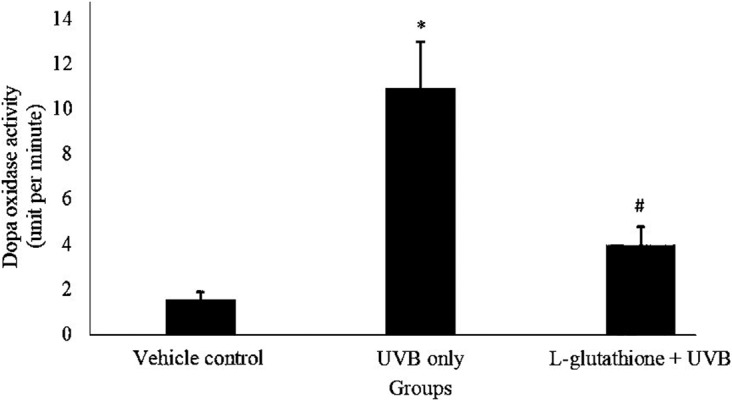
Determination of tyrosinase activity in ultraviolet B (UVB)-irradiated mice. The bar chart shows the results of dopa oxidase activity level in different groups presented as the mean ± SEM; n=6. *Statistically significant difference in comparison with vehicle control group (P<0.05). #Statistically significant difference in comparison with UVB-exposure group (P<0.05).
Effects of L-glutathione on histopathological changes in UVB-irradiated mice
Figure 6 shows the observed histopathological changes in the various groups. UVB irradiated group showed an obvious deposition of melanin in the basal layer of the epidermis as compared with the vehicle control group. Meanwhile, the L-glutathione treatment group showed very minimal deposition of melanin in the basal layer as compared with the UVB irradiated group. Figure 7 shows the melanin pigmentation indexes of the various groups. The UVB irradiated group (index: 15.082 ± 1.001) showed a significant increase in pigmentation index as compared with the vehicle control group (index: 3.621 ± 0.442). Meanwhile, the L-glutathione group (4.766 ± 0.337) showed a significantly decreased the pigmentation index compared with UVB-irradiated group (index: 15.082 ± 1.001).
Fig. 6.
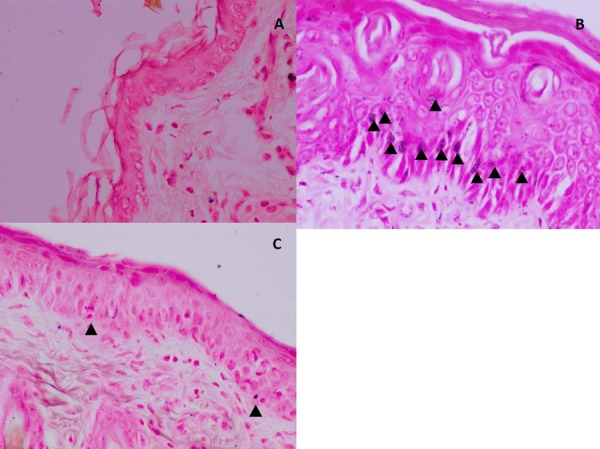
Histological observation of melanin pigmentation (arrowheads) in the basal layer of the epidermis of mice skin. (A) Vehicle control group; (B) ultraviolet B (UVB)-irradiated group. (C) L-glutathione treatment group. Fontana Masson staining. Images are shown at 40× magnification. Scale bar: 100 µm.
Fig. 7.
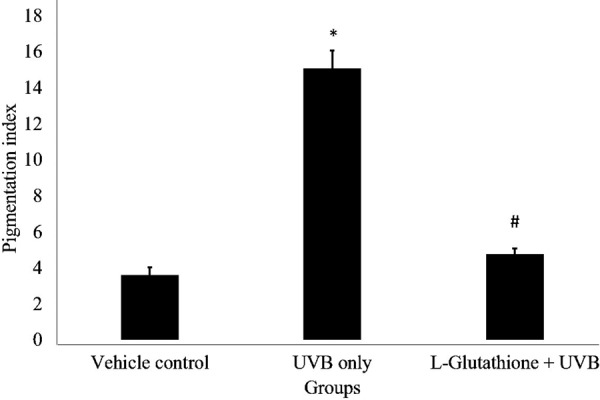
Determination of melanin pigmentation index in histology of various groups. The bar chart shows the results for the pigmentation index in the different groups presented as the mean ± SEM; n=6. *Statistically significant difference in comparison with vehicle control group (P<0.05). #Statistically significant difference in comparison with ultraviolet B (UVB)-exposure group (P<0.05).
Discussion
Solar radiation is one of the important external factors in skin pathogenesis that can lead to many complications. Exposure to UVB irradiation can generate ROS which can increase oxidative stress and results in an imbalance between ROS and endogenous antioxidant defense system [20]. It also activates melanocytes to produce melanin, which accelerates the melanogenesis biosynthesis pathway [2]. The use of antioxidant supplements that absorb UV rays and protect skin against UVB irradiation is of great interest in dermatology and cosmeceutical fields. In the present study, we investigated the inhibitory effects of L-glutathione on oxidative stress and melanogenesis activity in mice.
Exposure of skin to UVB irradiation leads to depletion of the antioxidant defense system, including SOD and GSH, which are used up to counter the surge in ROS production [23]. In this study, we discovered that both SOD and GSH decreased significantly after exposure of mouse skin to UVB irradiation, suggesting that the levels of antioxidants depleted. L-glutathione treatment increased the SOD level significantly. However, no significant difference in reduced glutathione level was observed, which suggested that L-glutathione quenched the ROS by increasing the enzymatic antioxidant (SOD) level.
Oxidative stress is one of the detrimental outcomes of UVB-induced skin photodamage [12]. MDA is the end product of lipid peroxidation and can also serve as an important indicator of the presence of ROS [34]. Our results showed that the lipid peroxidation activity was higher in the UVB-irradiated group, suggesting that oxidative degradation of lipids occurred in the mice. Interestingly, L-glutathione protected the UVB-irradiated mice by decreasing the lipid peroxidation activity. This finding is similar a finding in another study [33] that showed that quercetin significantly reduced the lipid peroxidation induced by UVB. In addition, caffeic acid, a dietary antioxidant supplement- also result reduction of lipid peroxidation in UVB-induced mice skin [3].
Melanin plays a vital role in protecting the skin from photodamage. However, overexposure to UV radiation could lead to hyperpigmentation, melasma, and melanoma [6]. In our study, the melanin content increased in UVB-irradiated mouse skin suggesting that UVB activated the melanogenesis process and resulted in accumulation of melanin in skin. Treatment with L-glutathione had decreased the melanin content in the UVB-irradiated mice skin, suggesting that L-glutathione was able to inhibit or downregulate the melanin biosynthesis pathway. To understand the mechanism behind the significant decrease in melanin content caused by L-glutathione, we investigated tyrosinase which is the key enzyme involved in melanogenesis activity [2]. L-glutathione decreased the tyrosinase enzyme level in UVB-irradiated mice and this showed that the mechanism of the photoprotecting action of L-glutathione could be the direct inactivation of tyrosinase by binding with the copper-containing active site [29]. Histopathological observation also showed that UVB-irradiated mouse skin has more visible melanin pigmentation, meanwhile mice treated with L-glutathione showed less melanin pigmentation. The findings are also supported by another similar study conducted on the effects of Diospyros lotus leaf extract against UVB-induced skin damage in BALB/c mice.
In short, we demonstrated that oral administration of L-glutathione prior to UVB irradiation could influence the cutaneous response. L-glutathione was able to improve the antioxidant status level, decreased lipid peroxidation activity, and could inhibit melanogenesis activity.
Conclusion
Oral administration of L-glutathione was able to inhibit melanogenesis activity by decreasing the melanin content and tyrosinase activity. L-glutathione also reduced the oxidative stress by increasing antioxidant levels and preventing lipid peroxidation activity. Hence, it could protect the skin from photodamage and has potential as a natural alternative agent for photoprotection.
Acknowledgments
This research work was funded by Universiti Kebangsaan Malaysia (Grant number: GUP-2016-081 & DIP-2018-034). We would also like to thank the Faculty of Health Sciences, Universiti Kebangsaan Malaysia.
References
- 1.Allen J., Bradley R.D.2011. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 17: 827–833. doi: 10.1089/acm.2010.0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldea I., Mocan T., Cosgarea R.2009. The role of ultraviolet radiation and tyrosine stimulated melanogenesis in the induction of oxidative stress alterations in fair skin melanocytes. Exp. Oncol. 31: 200–208. [PubMed] [Google Scholar]
- 3.Balupillai A., Ramasamy K., Prasad R.2015. Caffeic acid prevents lipid peroxidation and antioxidant stress in chronic UVB exposed mice skin. Life Sci. Arch. 1: 277–286. [Google Scholar]
- 4.Beyer W.F., Jr, Fridovich I.1987. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 161: 559–566. doi: 10.1016/0003-2697(87)90489-1 [DOI] [PubMed] [Google Scholar]
- 5.Billings P.C., Sanzari J.K., Kennedy A.R., Cengel K.A., Seykora J.T.2015. Comparative analysis of colorimetric staining in skin using open-source software. Exp. Dermatol. 24: 157–159. doi: 10.1111/exd.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao H.C., Najjaa H., Villareal M.O., Ksouri R., Han J., Neffati M., Isoda H.2013. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 22: 131–136. doi: 10.1111/exd.12089 [DOI] [PubMed] [Google Scholar]
- 7.Costin G.E., Hearing V.J.2007. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 21: 976–994. doi: 10.1096/fj.06-6649rev [DOI] [PubMed] [Google Scholar]
- 8.D’Orazio J., Jarrett S., Amaro-Ortiz A., Scott T.2013. UV radiation and the skin. Int. J. Mol. Sci. 14: 12222–12248. doi: 10.3390/ijms140612222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson D.A., Forman H.J.2002. Glutathione in defense and signaling: lessons from a small thiol. Ann. N. Y. Acad. Sci. 973: 488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x [DOI] [PubMed] [Google Scholar]
- 10.Ellman G.L.1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82: 70–77. doi: 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 11.Furukawa T., Meydani S.N., Blumberg J.B.1987. Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mech. Ageing Dev. 38: 107–117. doi: 10.1016/0047-6374(87)90071-6 [DOI] [PubMed] [Google Scholar]
- 12.Halliday G.M.2005. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 571: 107–120. doi: 10.1016/j.mrfmmm.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 13.Kim E., Hwang K., Lee J., Han S.Y., Kim E.M., Park J., Cho J.Y.2018. Skin protective effect of epigallocatechin gallate. Int. J. Mol. Sci. 19: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K.S., Kim J.A., Eom S.Y., Lee S.H., Min K.R., Kim Y.2006. Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression. Pigment Cell Res. 19: 90–98. doi: 10.1111/j.1600-0749.2005.00281.x [DOI] [PubMed] [Google Scholar]
- 15.Kim S.J., Han D., Ahn B.H., Rhee J.S.1997. Effect of glutathione, catechin, and epicatechin on the survival of Drosophila melanogaster under paraquat treatment. Biosci. Biotechnol. Biochem. 61: 225–229. doi: 10.1271/bbb.61.225 [DOI] [PubMed] [Google Scholar]
- 16.Matsui Y., Sugiyama K., Kamei M., Takahashi T., Suzuki T., Katagata Y., Ito T.2010. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 58: 11112–11118. doi: 10.1021/jf102650d [DOI] [PubMed] [Google Scholar]
- 17.Meyskens F.L., Jr, Chau H.V., Tohidian N., Buckmeier J.1997. Luminol-enhanced chemiluminescent response of human melanocytes and melanoma cells to hydrogen peroxide stress. Pigment Cell Res. 10: 184–189. doi: 10.1111/j.1600-0749.1997.tb00482.x [DOI] [PubMed] [Google Scholar]
- 18.Nagapan T.S., Ghazali A.R., Basri D.F., Lim W.N.2018a Photoprotective Effect Of Stilbenes And Its Derivatives against Ultraviolet Radiation-Induced Skin Disorders. Biomed. Pharmacol. J. 11: 1199–1208. doi: 10.13005/bpj/1481 [DOI] [Google Scholar]
- 19.Nagapan T.S., Ghazali A.R., Basri D.F., Lim W.N.2018b Oral administration of resveratrol ameliorates epidermal hyperplasia in ultraviolet B irradiated BALB/c mice. J. Appl. Pharm. Sci. 8: 47–52. doi: 10.7324/JAPS.2018.81007 [DOI] [Google Scholar]
- 20.Oberley T.D.2002. Oxidative damage and cancer. Am. J. Pathol. 160: 403–408. doi: 10.1016/S0002-9440(10)64857-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.M., Cho J.K., Mok J.Y., Jeon I.H., Kim H.S., Kang H.J., Jang S. Il. 2012. Protective effect of astragalin and quercetin on ultraviolet (UV)-irradiated damage in HaCaT cells and Balb/c mice. J. Korean Soc. Appl. Biol. Chem. 55: 443–446. doi: 10.1007/s13765-012-2072-y [DOI] [Google Scholar]
- 22.Pérez-Sánchez A., Barrajón-Catalán E., Herranz-López M., Castillo J., Micol V.2016. Lemon balm extract (Melissa officinalis, L.) promotes melanogenesis and prevents UVB-induced oxidative stress and DNA damage in a skin cell model. J. Dermatol. Sci. 84: 169–177. doi: 10.1016/j.jdermsci.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 23.Petrova A., Davids L.M., Rautenbach F., Marnewick J.L.2011. Photoprotection by honeybush extracts, hesperidin and mangiferin against UVB-induced skin damage in SKH-1 mice. J. Photochem. Photobiol. B 103: 126–139. doi: 10.1016/j.jphotobiol.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 24.Schwartz J.L., Shklar G.1996. Glutathione inhibits experimental oral carcinogenesis, p53 expression, and angiogenesis. Nutr. Cancer 26: 229–236. doi: 10.1080/01635589609514479 [DOI] [PubMed] [Google Scholar]
- 25.Solano F.2014. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014: 1–28. doi: 10.1155/2014/498276 [DOI] [Google Scholar]
- 26.Sonthalia S., Daulatabad D., Sarkar R.2016. Glutathione as a skin whitening agent: Facts, myths, evidence and controversies. Indian J. Dermatol. Venereol. Leprol. 82: 262–272. doi: 10.4103/0378-6323.179088 [DOI] [PubMed] [Google Scholar]
- 27.Stocks J., Dormandy T.L.1971. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Haematol. 20: 95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x [DOI] [PubMed] [Google Scholar]
- 28.Talwar H.S., Griffiths C.E.M., Fisher G.J., Russman A., Krach K., Benrazavi S., Voorhees J.J.1993. Differential regulation of tyrosinase activity in skin of white and black individuals in vivo by topical retinoic acid. J. Invest. Dermatol. 100: 800–805. doi: 10.1111/1523-1747.ep12476615 [DOI] [PubMed] [Google Scholar]
- 29.Villarama C.D., Maibach H.I.2005. Glutathione as a depigmenting agent: an overview. Int. J. Cosmet. Sci. 27: 147–153. doi: 10.1111/j.1467-2494.2005.00235.x [DOI] [PubMed] [Google Scholar]
- 30.Winder A.J., Harris H.1991. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur. J. Biochem. 198: 317–326. doi: 10.1111/j.1432-1033.1991.tb16018.x [DOI] [PubMed] [Google Scholar]
- 31.Yabuki Y., Fukunaga K.2013. Oral administration of glutathione improves memory deficits following transient brain ischemia by reducing brain oxidative stress. Neuroscience 250: 394–407. doi: 10.1016/j.neuroscience.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 32.Yamakoshi J., Otsuka F., Sano A., Tokutake S., Saito M., Kikuchi M., Kubota Y.2003. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 16: 629–638. doi: 10.1046/j.1600-0749.2003.00093.x [DOI] [PubMed] [Google Scholar]
- 33.Yin Y., Li W., Son Y.O., Sun L., Lu J., Kim D., Wang X., Yao H., Wang L., Pratheeshkumar P., Hitron A.J., Luo J., Gao N., Shi X., Zhang Z.2013. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharmacol. 269: 89–99. doi: 10.1016/j.taap.2013.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S., Lu B., Han X., Xu L., Qi Y., Yin L., Xu Y., Zhao Y., Liu K., Peng J.2013. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 55: 60–69. doi: 10.1016/j.fct.2012.12.041 [DOI] [PubMed] [Google Scholar]


