Abstract
The Center for Animal Resources and Development (CARD), Kumamoto University was established in 1998. We provide advanced research support services for the mouse-based biomedical research community via an official and a premium mouse bank system. To efficiently manage these mouse banks, we have actively developed and modified basic mouse reproductive techniques. We shall introduce these techniques in this paper.
Keywords: genetically engineered mice, mouse bank, reproductive techniques, the Center for Animal Resources and Development (CARD), training course
Introduction
In 1998, the Center for Animal Resources and Development (CARD) Mouse Bank was established to share genetically engineered mice by supporting their production, preservation and supply [5, 6]. Mouse banks all over the world collaborate on a global scale and share archived strains of genetically engineered mice via the International Mouse Strain Resource (http://www.findmice.org/). We have hereto archived more than 2,500 strains of genetically engineered mice in the CARD Mouse Embryo and Sperm Bank System. Our system allows researchers to deposit their mice free of charge. Meanwhile, information concerning the archived mice is published on our CARD R-BASE website (http://cardb.cc.kumamoto-u.ac.jp/transgenic/). Using the mouse bank system, we have supplied mice to both domestic and foreign institutes (717 strains and 223 strains respectively).
In addition to the aforementioned CARD Mouse Bank System, CARD has offered another type of mouse bank service since 2005. This is called the CARD Premium Mouse Bank System. Our premium mouse bank system enables the efficient preservation and production of genetically engineered mice for an additional charge. Using the system, researchers are able to produce mice based on an experimental design, or recover a mouse line that is affected by infertility or subfertility during natural mating.
To effectively manage these mouse banks, we have developed and modified basic reproductive techniques in mice. We shall describe these techniques briefly in this paper.
1. In Vitro Fertilization
We generally produce embryos from mouse strains using in vitro fertilization. In vitro fertilization (IVF) is a useful technique for producing many embryos for the storage or production of genetically engineered mice via embryo cryopreservation or embryo transfer. Since the establishment of our center, we have carried out IVF on more than 5,000 occasions, using 2,400 different strains of genetically engineered mice. During this time, we have improved our IVF technique to stabilize and enhance the fertilization rate [21]. Our IVF method now incorporates sperm preincubation medium [30] and a modified fertilization medium [26]. Furthermore, we demonstrated that the coadministration of inhibin antiserum (IAS) and equine chorionic gonadotropin (eCG) during superovulation increased the number of ovulated oocytes in four-week old C57BL/6J female mice [23]. Using this technique, we are able to obtain fifty pups derived from a single oocyte donor. Now, we are applying the technique to many other strains so as to enable the efficient production of embryos and pups of genetically engineered mice [22].
2. Cold Storage of Cauda Epididymides
Storing cauda epididymides at refrigerated temperatures is extremely useful for the transportation of genetically engineered mice between research facilities. There are various benefits to transporting cauda epididymides in this manner, including user-friendliness, low shipment costs, a reduced risk of spreading infectious disease and no risk of losing animals due to death or escape. The transported epididymides are immediately available for IVF. We have continuously improved our transport system for cauda epididymides [25, 32, 33] and have established a robust system for shipping genetically engineered mice using the CARD Cold Transport Kit (Kyudo Co., Ltd., Saga, Japan). Using this system, all samples from other institutes arrive at our center within 72 h of shipment, and a large number of embryos are obtained via IVF using sperm collected from the samples. We have hereto overseen the transportation of cauda epididymides taken from genetically engineered mice on 425 occasions.
3. Sperm Cryopreservation
Sperm cryopreservation offers many benefits, such as quick and simple operations, low storage cost and efficient embryo production via IVF. Now, sperm cryopreservation is the preferred method for the efficient preservation of genetically engineered mice [7,8,9,10, 13, 18,19,20, 24, 26, 28, 30, 31]. In our center, we have frequently performed IVF using cryopreserved sperm prepared using various methods. We also developed a novel IVF method for legacy stock of cryopreserved sperm [3]. In addition, we have conducted the cryopreservation of sperm derived from the cauda epididymides of genetically engineered mice after shipment under refrigerated temperatures, and subsequently carried out IVF using the sperm [24]. In total, we possess more than 4,400 frozen sperm samples of genetically engineered mice.
4. Embryo/Oocyte Vitrification
Embryo cryopreservation is useful for the efficient storage of genetically engineered mice. In addition, if we freeze unfertilized oocytes in advance, we can use these oocytes along with sperm collected from genetically engineered male mice to carry out IVF whenever needed. The embryos/oocytes can be cryopreserved via a simple vitrification method using a cryoprotectant which we developed (DAP213: 2 M dimethyl sulfoxide, 1 M acetoamide and 3 M propanediol) [2, 4, 7, 9, 11, 14,15,16,17]. We have hereto archived over 1,200,000 cryopreserved 2-cell embryos derived from 2,500 strains of genetically engineered mice.
5. Transport of Mouse Embryos at Refrigerated Temperatures
The transport of mouse embryos at refrigerated temperatures is an effective alternative method of transporting genetically engineered mice to the transport of live animals or cryopreserved samples. Benefits of the cold-transport of mouse embryos include low shipping costs and easy sample handling. We have developed a method for the short-term storage of 2-cell embryos at refrigerated temperatures, thus enabling the acquisition of many pups derived therefrom [1, 27, 29].
6. Embryo Transfer
Embryo transfer is a useful technique for the efficient production of genetically engineered mice. In addition, the obtained pups are free from various pathogens derived from oocytes or sperm donors. In our laboratory, we transfer 2-cell embryos through the wall of the oviduct of pseudopregnant recipients [12]. This procedure is much simpler to conduct than the conventional embryo transfer procedure. We have hereto conducted embryo transfer using over 2,300 different strains of genetically engineered mice. We transferred over 400,000 embryos and obtained an average birth rate of 40% (around 160,000 pups).
7. Cesarean Section
A cesarean section is performed when a recipient mouse has not given birth by day 19 after 2-cell embryo transfer (Day 0 is the day on which the 2-cell embryos were transferred to recipients). Delivery dysfunction is frequently observed in pregnant females with few fetuses. We have hereto performed cesarean sections on around 900 recipients which had not given birth on their expected date of delivery. In almost all cases, all pups were successfully recovered after the cesarean section.
Future Prospects
We continue to efficiently manage the CARD Mouse Bank using the reproductive techniques explained above (Fig. 1) (http://card.medic.kumamoto-u.ac.jp/card/japanese/manual/index.html). In addition, we have provided 3–4 training courses on these techniques per year for 20 years, in order to spread the use of these techniques throughout institutes worldwide (http://card.medic.kumamoto-u.ac.jp/card/japanese/mousebank/contribution/trainingcourse.html).
Fig. 1.
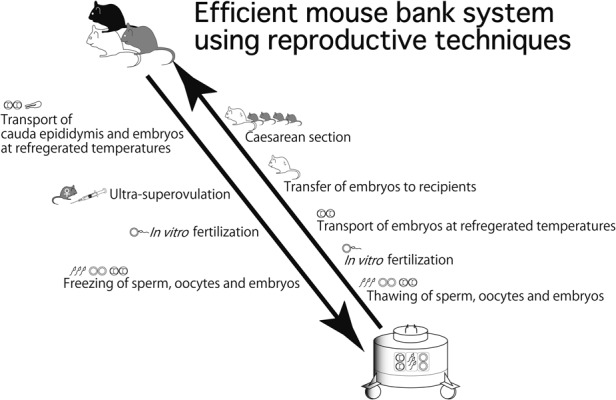
An efficient mouse bank system at The Center for Animal Resources and Development (CARD) using reproductive techniques.
Looking to the future, we would like to develop new reproductive techniques in order to further expand our mouse bank and to provide various genetically engineered mouse strains promptly to research communities all over the world. Finally, I would like to impart a few words of advice to the next generation of researchers. Remember that Rome was not built a day. Do not rush your work. Instead, always maintain a sense of purpose, then make efforts and never give up until you accomplish your purpose.
Acknowledgments
We would like to thank our staff members Yuko Nakamuta, Tomoko Kondo, Yukie Haruguchi, Kiyoko Yamashita, Eri Ishida, Yumi Takeshita, Kanoko Iki, Kayo Tou, Sanae Onishi, Yoshiko Nakagawa, Wataru Sakamoto, Mari Iwamoto, Fumi Takahashi, Kaori Sakaguchi, Naoko Nakamura, Satomi Kawabe and Shuuji Tsuchiyama for their constant helpful assistance and discussion.
References
- 1.Horikoshi Y., Takeo T., Nakagata N.2016. N-acetyl cysteine prolonged the developmental ability of mouse two-cell embryos against oxidative stress at refrigerated temperatures. Cryobiology 72: 198–204. doi: 10.1016/j.cryobiol.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 2.Nakagata N., Sztein J., Takeo T.2019. The CARD method for simple vitrification of mouse oocytes: advantages and applications. Methods Mol. Biol. 1874: 229–242. doi: 10.1007/978-1-4939-8831-0_13 [DOI] [PubMed] [Google Scholar]
- 3.Nakagata N., Takeo T., Fukumoto K., Haruguchi Y., Kondo T., Takeshita Y., Nakamuta Y., Umeno T., Tsuchiyama S.2014. Rescue in vitro fertilization method for legacy stock of frozen mouse sperm. J. Reprod. Dev. 60: 168–171. doi: 10.1262/jrd.2013-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakagata N., Takeo T., Fukumoto K., Kondo T., Haruguchi Y., Takeshita Y., Nakamuta Y., Matsunaga H., Tsuchiyama S., Ishizuka Y., Araki K.2013. Applications of cryopreserved unfertilized mouse oocytes for in vitro fertilization. Cryobiology 67: 188–192. doi: 10.1016/j.cryobiol.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 5.Nakagata N., Yamamura K.2009. Current activities of CARD as an international core center for mouse resources. Exp. Anim. 58: 343–350. doi: 10.1538/expanim.58.343 [DOI] [PubMed] [Google Scholar]
- 6.Nakagata N.2007. [Mouse bank]. Nippon Yakurigaku Zasshi 129: 343–348(in Japanese). doi: 10.1254/fpj.129.343 [DOI] [PubMed] [Google Scholar]
- 7.Nakagata N.1996. Use of cryopreservation techniques of embryos and spermatozoa for production of transgenic (Tg) mice and for maintenance of Tg mouse lines. Lab. Anim. Sci. 46: 236–238. [PubMed] [Google Scholar]
- 8.Nakagata N., Ueda S., Yamanouchi K., Okamoto M., Matsuda Y., Tsuchiya K., Nishimura M., Oda S., Koyasu K., Azuma S., Toyoda Y.1995. Cryopreservation of wild mouse spermatozoa. Theriogenology 43: 635–643. doi: 10.1016/0093-691X(94)00069-7 [DOI] [PubMed] [Google Scholar]
- 9.Nakagata N.1993. Production of normal young following transfer of mouse embryos obtained by in vitro fertilization between cryopreserved gametes. J. Reprod. Fertil. 99: 77–80. doi: 10.1530/jrf.0.0990077 [DOI] [PubMed] [Google Scholar]
- 10.Nakagata N., Takeshima T.1993. Cryopreservation of mouse spermatozoa from inbred and F1 hybrid strains. Jikken Dobutsu 42: 317–320. [DOI] [PubMed] [Google Scholar]
- 11.Nakagata N.1993. Survival of mouse morulae and blastocysts derived from in vitro fertilization after ultra rapid freezing. Jikken Dobutsu 42: 229–231. [PubMed] [Google Scholar]
- 12.Nakagata N.1992. [Embryo transfer through the wall of the fallopian tube in mice]. Jikken Dobutsu 41: 387–388(in Japanese). [DOI] [PubMed] [Google Scholar]
- 13.Nakagata N., Takeshima T.1992. High fertilizing ability of mouse spermatozoa diluted slowly after cryopreservation. Theriogenology 37: 1283–1291. doi: 10.1016/0093-691X(92)90183-R [DOI] [Google Scholar]
- 14.Nakagata N.1990. Cryopreservation of unfertilized mouse oocytes from inbred strains by ultrarapid freezing. Jikken Dobutsu 39: 303–305. [DOI] [PubMed] [Google Scholar]
- 15.Nakagata N.1989. High survival rate of unfertilized mouse oocytes after vitrification. J. Reprod. Fertil. 87: 479–483. doi: 10.1530/jrf.0.0870479 [DOI] [PubMed] [Google Scholar]
- 16.Nakao K., Nakagata N., Katsuki M.1997. Simple and efficient vitrification procedure for cryopreservation of mouse embryos. Exp. Anim. 46: 231–234. doi: 10.1538/expanim.46.231 [DOI] [PubMed] [Google Scholar]
- 17.Nakao K., Nakagata N., Katsuki M.1998. Production of chimeric mice from cryopreserved blastocysts. Exp. Anim. 47: 167–171. doi: 10.1538/expanim.47.167 [DOI] [PubMed] [Google Scholar]
- 18.Sztein J.M., Takeo T., Nakagata N.2018. History of cryobiology, with special emphasis in evolution of mouse sperm cryopreservation. Cryobiology 82: 57–63. doi: 10.1016/j.cryobiol.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 19.Takeo T., Sztein J., Nakagata N.2019. The CARD Method for Mouse Sperm Cryopreservation and In Vitro Fertilization Using Frozen-Thawed Sperm. Methods Mol. Biol. 1874: 243–256. doi: 10.1007/978-1-4939-8831-0_14 [DOI] [PubMed] [Google Scholar]
- 20.Takeo T., Nakagata N.2018. Mouse sperm cryopreservation using cryoprotectant containing l-glutamine. Cold Spring Harb. Protoc. 2018: pdb.prot094516. doi: 10.1101/pdb.prot094516 [DOI] [PubMed] [Google Scholar]
- 21.Takeo T., Nakagata N.2018. In Vitro Fertilization in Mice. Cold Spring Harb. Protoc. 2018: pdb.prot094524. doi: 10.1101/pdb.prot094524 [DOI] [PubMed] [Google Scholar]
- 22.Takeo T., Nakagata N.2016. Immunotherapy using inhibin antiserum enhanced the efficacy of equine chorionic gonadotropin on superovulation in major inbred and outbred mice strains. Theriogenology 86: 1341–1346. doi: 10.1016/j.theriogenology.2016.04.076 [DOI] [PubMed] [Google Scholar]
- 23.Takeo T., Nakagata N.2015. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 10: e0128330. doi: 10.1371/journal.pone.0128330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeo T., Fukumoto K., Kondo T., Haruguchi Y., Takeshita Y., Nakamuta Y., Tsuchiyama S., Yoshimoto H., Shimizu N., Li M.W., Kinchen K., Vallelunga J., Lloyd K.C., Nakagata N.2014. Investigations of motility and fertilization potential in thawed cryopreserved mouse sperm from cold-stored epididymides. Cryobiology 68: 12–17. doi: 10.1016/j.cryobiol.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeo T., Tsutsumi A., Omaru T., Fukumoto K., Haruguchi Y., Kondo T., Nakamuta Y., Takeshita Y., Matsunaga H., Tsuchiyama S., Sakoh K., Nakao S., Yoshimoto H., Shimizu N., Nakagata N.2012. Establishment of a transport system for mouse epididymal sperm at refrigerated temperatures. Cryobiology 65: 163–168. doi: 10.1016/j.cryobiol.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 26.Takeo T., Nakagata N.2011. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol. Reprod. 85: 1066–1072. doi: 10.1095/biolreprod.111.092536 [DOI] [PubMed] [Google Scholar]
- 27.Takeo T., Kondo T., Haruguchi Y., Fukumoto K., Nakagawa Y., Takeshita Y., Nakamuta Y., Tsuchiyama S., Shimizu N., Hasegawa T., Goto M., Miyachi H., Anzai M., Fujikawa R., Nomaru K., Kaneko T., Itagaki Y., Nakagata N.2010. Short-term storage and transport at cold temperatures of 2-cell mouse embryos produced by cryopreserved sperm. J. Am. Assoc. Lab. Anim. Sci. 49: 415–419. [PMC free article] [PubMed] [Google Scholar]
- 28.Takeo T., Nakagata N.2010. Combination medium of cryoprotective agents containing L-glutamine and methyl-beta-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab. Anim. 44: 132–137. doi: 10.1258/la.2009.009074 [DOI] [PubMed] [Google Scholar]
- 29.Takeo T., Kaneko T., Haruguchi Y., Fukumoto K., Machida H., Koga M., Nakagawa Y., Takeshita Y., Matsuguma T., Tsuchiyama S., Shimizu N., Hasegawa T., Goto M., Miyachi H., Anzai M., Nakatsukasa E., Nomaru K., Nakagata N.2009. Birth of mice from vitrified/warmed 2-cell embryos transported at a cold temperature. Cryobiology 58: 196–202. doi: 10.1016/j.cryobiol.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 30.Takeo T., Hoshii T., Kondo Y., Toyodome H., Arima H., Yamamura K., Irie T., Nakagata N.2008. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol. Reprod. 78: 546–551. doi: 10.1095/biolreprod.107.065359 [DOI] [PubMed] [Google Scholar]
- 31.Takeshima T., Nakagata N., Ogawa S.1991. [Cryopreservation of mouse spermatozoa]. Jikken Dobutsu 40: 493–497(in Japanese). [DOI] [PubMed] [Google Scholar]
- 32.Yoshimoto H., Takeo T., Nakagata N.2017. Dimethyl sulfoxide and quercetin prolong the survival, motility, and fertility of cold-stored mouse sperm for 10 days. Biol. Reprod. 97: 883–891. doi: 10.1093/biolre/iox144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshimoto H., Takeo T., Irie T., Nakagata N.2017. Fertility of cold-stored mouse sperm is recovered by promoting acrosome reaction and hyperactivation after cholesterol efflux by methyl-beta-cyclodextrin. Biol. Reprod. 96: 446–455. doi: 10.1095/biolreprod.116.142901 [DOI] [PubMed] [Google Scholar]


