Abstract
Osteoporosis is a common skeletal disorder resulting in elevated fracture risk. Improvement of osteogenic differentiation is thought to be the top priority in osteoporosis treatment projects. Significant characteristics of bone marrow mesenchymal stem cells (BMMSCs), especially attractive ability to differentiate into osteoblasts, have made them alternatives for osteoporosis treatment. However, therapeutic effect with BMMSCs remains to be improved. Here, osthole, a bioactive simple coumarin derivative extracted from many medicinal plants, was introduced to pre-stimulate BMMSCs and then applied in osteoporosis therapy. The results showed that osthole-treated-BMMSCs (OBMMSCs) brought a better outcome than BMMSCs alone in estrogen deficiency-induced osteoporosis model. And elevated autophagy level was suggested to be the underlying mechanism of the ability of osthole to promote osteoblast differentiation, which is indicated by the upregulation of protein and mRNA expression level of autophagy-associated genes, Beclin1 and LC3. We concluded from these experiments that OBMMSCs are more effective than BMMSCs in osteoporosis treatment maybe through upregulation level of autophagy level induced by osthole.
Keywords: autophagy, mesenchymal stem cells, osteoblast differentiation, osteoporosis, osthole.
Introduction
Osteoporosis (OP) is a physical status characterized by increased risk of fracture resulting from loss of bone mass and bone strength [1]. It’s predictable that incidence of OP will keep increasing among the elderly as life expectancy keeps going up, especially among women [32]. Nowadays, osteoanabolic drugs focusing on stimulating bone formation like parathyroid hormone [34] and antiresorptive reagents paying attention to inhibit bone resorption such as zoledronic acid [22], lasofoxifene [8], denosumab [7], and odanacatib [4] are the two major pharmacologic approaches to the therapy of OP. Increase in bone mineral density (BMD) and reduction in fractures are confirmed to happen after taking these medications. Although the symptoms and complications are the main targets of the drug [41] and long-term adverse effects are existed [18, 38]; so, a new treatment aiming at the imbalance of bone formation and resorption is expected to further advance the treatment of OP. Mesenchymal stem cells come into our sight for their advantages of multipotent differentiation potential [6] and easy access; therefore, they have been widely used for tissue repair [36]. Bone marrow mesenchymal stem cells (BMMSCs) derived from bone marrow stromal cells has drawn much attention for its ability to serve as a good therapy for skeletal disease [15, 29, 42]. It’s well believed that promoting osteoblasts differentiation and inhibiting adipocytes differentiation of BMMSCs can alleviate development of OP [3, 27]. Although BMMSCs have been much explored as a therapeutic agent for osteoporosis [15, 47], how to enhance the osteogenic ability of BMMSCs in vivo is still an issue waiting to be solved.
It’s well documented that autophagy plays an important role in bone metabolism [30]. Conditional knockout of autophagy-related gene Atg7 in osteoblasts results in decrease of trabecular bone mass, decline in numbers of osteoblasts, and reduction of bone formation [31], which figures out that autophagy is involved in maintaining skeletal homeostasis and the mineralization process [26]. Recent studies have also pointed out that autophagy dysfunction is related with OP [9] and autophagy can be a target of the treatment for glucocorticoid-induced osteoporosis treatment [11, 35]. In BMMSCs of estrogen deficiency-induced osteoporotic mice, autophagy level was reduced and ability of osteogenic differentiation was decreased, while rapamycin could rescue osteogenic differentiation of BMMSCs accompanying with elevation of autophagy level and attenuation of osteoporotic phenotype [33].
Osthole is a fundamental component with pharmacological activity extracted from Chinese Medicine cnidium monnieri [45]. Osthole has been reported to stimulate osteoblast differentiation and bone formation [17, 25, 39] which suggests that it may be an effective alternative of therapy for OP. In the present study, we investigated the therapeutic effect of BMMSCs pre-stimulated by osthole and demonstrated that osthole indeed promoted OP treatment by BMMSCs. It was futher elucidated that autophagy level has been elevated in BMMSCs after osthole stimulation. Through this research, we proposed that osthole may advance autophagy in BMMSCs and promote BMMSCs to differentiate into osteoblasts, which can further improve the treatment for OP.
Materials and Methods
Animals
C57BL/6J mice of eight weeks old were purchased from the animal center of Chongqing Medical University in Chongqing, China. Performances of all animal experiments were under the experimental animal guidelines of the National Institutes of Health and approved by the Animal Care and Use Committee of Chongqing Medical University. All mice were housed under specific pathogen-free conditions (22°C, 12-h light/12-h dark cycles, and 50%–55% humidity) with free access to food pellets and tap water.
BMMSCs isolation
The mouse femurs and tibias were harvested from 8-week-old mice, and then the bone marrow stromal cells were flushed out from the bone marrow cavity with a syringe to culture medium containing α-MEM (Invitrogen, Calsbad, CA, USA), 20% fetal bovine serum (FBS) (Hyclone, GE Healthcare Life Sciences, Logan, UT, USA), 10 U/ml penicillin (Invitrogen), 10 g/ml streptomycin (Introvigen) and 2 mM L-glutamine (Hyclone, GE Healthcare Life Sciences). The resultant medium was centrifuged at 1,000 revolutions per min (rpm) for 5 min and then the deposits were suspended with basal medium and cultured for 3 days. Next, the floating cells were removed from the suspension and the attached cells were cultured until they reached confluence. Afterwards, the limiting-dilution technique was used to obtain single-cell suspensions of primary cells, and passage 0 (P0) cells were cultured. BMMSCs were acquired after expansion. Cells at passage 3–5 (P3) were put to application in this study.
BMMSCs pre-stimulated by osthole
It was reported before that confluent BMMSCs cultured with medium containing 10−5 M osthole for 3 days could acquire their most significant osteogenic capability as ALP activities were promoted and expression of osteogenic genes was increased [10]. So in our study, confluent cells were incubated with basal medium containing 10−5 M osthole for 3 days, then the resultant osthole-treated-BMMSCs (OBMMSCs) were analyzed and injected into mice as described below.
Flow-cytometric analysis
In vitro-expanded BMMSCs at early passages (P3) were examined by flow-cytometric analysis to characterize their immunophenotype, and mesenchymal and non-mesenchymal stem cell-associated surface markers were measured. 2 ml of 0.05% trypsin (Sigma-Aldrich, Darmstadt, Germany) was added to liberate adherent cells after being washed twice with PBS. The single-cell suspension was washed with PBS containing 3% FBS twice and re-suspended in PBS containing 3% FBS. With BMMSCs prepared, identification of the BMMSCs phenotype was performed. Antibodies combined with phcoerythrin (PE) or fluorescein isothiocyanate (FITC) against CD90, CD34, CD11b (BioLegend, San Diego, CA, USA), CD73-PE (eBioscience, San Diego, CA, USA), and Sca-1, CD14 [46] (Abcam, Cambridge, UK) were added into each tube loaded with nearly 5 × 105 BMMSCs/200 µl of PBS, and then the BMMSCs were incubated for 1 h at 4°C in the dark. BMMSCs which were incubated without any antibodies were used as negative control. Whereafter, BMMSCs were washed with 1 ml wash buffer twice and analyzed by a flow cytometer (Beckman Coulter, Fullerton, CA, USA). OBMMSCs had also been examined as described above.
Osteogenic differentiation
For osteogenic differentiation assay, 1 × 105/well BMMSCs (P3) reaching 80% confluence were incubated in 12-well plates with osteogenic medium containing 5 mM β-glycerophosphate (Sigma-Aldrich), 50 µg/ml ascorbic acid (Sigma-Aldrich) and 10 nM dexamethasone (Sigma-Aldrich). The osteogenic medium was refreshed every 3 days. And 21 days later, cells were stained with 2% Alizarin Red (Sigma-Aldrich). The same performance was done to OBMMSCs to confirm their osteogenic differentiation. The stain was eluted with 2% cetylpyridinium chloride (Sigma-Aldrich) for 30 min, and the absorbance was quantitatively measured at 520 nm.
Adipogenic differentiation
For adipogenic differentiation assay, 1 × 105/well BMMSCs (P3) reaching 100% confluence were incubated in 12-well plates with adipogenic medium, α-MEM added with 20% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM L-glutamine, 5 µg/ml insulin (Sigma-Aldrich), 50 µM indomethacin (Sigma-Aldrich), 1 × 10−6 M dexamethasone, and 0.5 µM 3-isobutyl-1-methylaxanthine (IBMX, Sigma Aldrich). The adipogenic medium was refreshed every 2–3 days. And 7 days later, 4% formaldehyde was applied to fix cells at room temperature (RT) for 30 min and then 0.5% oil red (Sigma-Aldrich) in methanol was added to stain cells for 20 min at RT. The same performance was done to OBMMSCs to confirm their adipogenic differentiation. The stain was eluted with methanol for 30 min, and the absorbance was quantitatively measured at 520 nm.
Cell proliferation assay
Cell proliferation of BMMSCs and OBMMSCs was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, which was carried out every day for the 7-day culture period. BMMSCs and OBMMSCs, which were incubated with basal medium containing with or without osthole for 3 days respectively, were plated at a density of 2 × 103 cells/well in 96-well plates, and incubated with 20 µl of 5 mg/ml MTT solution (Sigma-Aldrich, 5 mg/ml) was for 4 h. Afterwards, the medium was replaced by formazan salts dissolved in 150 µl of dimethylsulfoxide (DMSO, Sigma-Aldrich), and the absorbance was quantitatively measured at 490 nm.
Western blot
Lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) was employed to lyse harvested cells, and the concentration of total protein isolated from cells was assessed with BCA protein assay reagent (Bio-Rad, Hercules, CA, USA). In SDS-polyacrylamide gels, 30 micrograms of each sample was loaded and then after separation, proteins were transferred to immunoblot PVD membranes (Millipore, Billerica, MA, USA). The membranes were blocked with blocking buffer containing 5% non-fat milk and 2 mg/ml BSA in PBST for 1 h and then were incubated overnight with the primary antibodies against Beclin1 (1:1,000; Abcam), LC3 (1:1,000; Abcam) and β-actin (1:2,000; Abcam). The membranes were incubated with secondary antibody (Boster, Pleasanton, NJ, USA) in blocking solution for 2 h at RT after washing in PBST for 10 min. The membranes were washed again with PBST and then be visualized by an enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ, USA) and the results were quantified by ImageJ.
RNA isolation and real-time polymerase chain reaction
Total RNA was extracted with Trizol (Sigma-Aldrich) reagent according to manufacture’s protocol. Then, 1,000 ng mRNA was reverse-transcribed to cDNA using a PrimeScript RT reagent kit (Takara Bio Inc., Kusatsu, Japan). SYBR Premix Ex Taq II kit (Takara) and a CFX96TM Real-time RT-PCR System (Bio-Rad) were used to perform the real-time RT-PCR analysis respectively. The primer sequences for real-time RT-PCR are listed below:
Beclin1 (F:CAGTACCAGCGGGAGTATAGTGA; R:TGTGGAAGGTGGCATTGAAGA),
LC3 (F:CCTGTCCTGGATAAGACCAAGTT; R:CTCCTGTTCATAGATGTCAGCGAT),
β-actin (F:CTGGCACCACACCTTCTACA; R:GGTACGACCAGAGGCATACA).
ELISA for serological markers
To detect biochemical markers of bone turnover, blood samples were acquired from the aorta ventralis upon euthanasia. Serum was isolated and stored at −80°C. Serum levels of procollagen 1 N-terminal peptide (P1NP), bone alkaline phosphatase (ALP), and tartrate-resistant acid phosphatase (TRAP) were measured using the relevant enzymelinked immunoassay (ELISA) kits (IDS, Frankfurt, Germany). Serum levels of calcium were measured using a Plasma Emission Spectrometer (iCAP 6000; Thermo Fisher Scientific, Waltham, MA, USA).
Induction of OP and study design
Thirty female C57BL/6J mice (8-weeks-old) were randomly divided into two groups and underwent either sham surgery or bilateral ovariectomy (OVX) under general anesthesia by the dorsal approach. Before injection, 1 × 106 BMMSCs or 1.0 × 106 OBMMSCs were suspended in 200 ml PBS. Mice in the OVX group were injected intravenously with 1.0 × 106 mouse BMMSCs, 1.0 × 106 OBMMSCs, or equivalent PBS alone at 8 weeks after surgery. Mice were sacrificed one month later, and all femurs were isolated intactly and scanned by micro CT (Siemens Inveon Micro CT, Munich, Germany).
Micro-computed tomography analysis
Femurs were scanned with the Inveon micro-CT (Siemens Inveon Micro CT). Setting of X-ray source were 80 kV and 500 µA microfocus. Interested region was selected manually in the marrow cavity. Bone morphometric parameters, including bone mineral density (BMD, mg/cc), bone volume relative to total volume (BV/TV, %), trabecular number (Tb. N, mm−1), trabecular thickness (Tb. Th, mm) and trabecular spacing (Tb. Sp, mm) were assessed.
Statistical analysis
Data are exhibited as mean ± SD. Comparisons were performed by Student’s t-test or one-way ANOVA. All analyses were performed using the SPSS statistical package (version 16.0, SPSS Inc., Chicago, IL, USA) and GraphPad Prism software (version 7.05). P<0.05 was considered statistically significant.
Results
Characterization of BMMSCs and OBMMSCs
To characterize BMMSCs and BMMSCs pre-stimulated with osthole for 3 days as documented [10], specific cell surface markers were tested. Results demonstrated that the obtained BMMSCs and osthole-treated-BMMSCs (OBMMSCs) were positive for CD73, CD90, and Sca-1 but negative for the CD45, CD34, and CD11b (Fig. 1A). Besides, BMMSCs and OBMMSCs still maintained their abilities to differentiate into osteoblasts and adipocytes (Figs. 1B and C). Quantitative analysisshowed that OBMMSCs decreased adipogenic differentiation (Fig. 1D) and increased osteogenic differentiation (Fig. 1E), compared with BMMSCs. In addition, OBMMSCs proliferation activity was higher than that of BMMSCs after 7 days of incubation, though the proliferation rate was similar in the first 3 days (Fig. 1F).
Fig. 1.
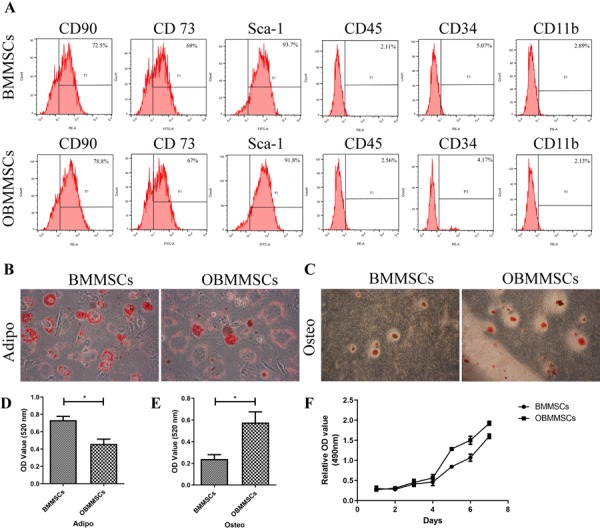
Characterization of bone marrow mesenchymal stem cells (BMMSCs) and osthole-treated-BMMSCs (OBMMSCs). (A) The analysis of surface antigens of BMMSCs and OBMMSCs by flow cytometry. Adipogenic (B, D) and osteogenic (C, E) differentiation of BMMSCs and OBMMSCs were determined by Oil Red O staining and Alizarin Red S staining respectively. (F) Proliferation activity of BMMSCs and OBMMSCs. Data were analyzed by Student’s t-test. Results are presented as means ± SD, *P<0.05.
Together these data revealed that OBMMSCs satisfied BMMSCs criteria and OBMMSCs exhibited a higher potential for osteogenic differentiation and cell proliferation rate than BMMSCs.
Therapeutic outcome of osteoporosis with BMMSCs and OBMMSCs
To test effects of OBMMSCs on therapy of osteoporosis, estrogen deficiency induced osteoporosis mice model was constructed by ovariectomy (OVX), and sham surgery was simultaneously performed in another group of mice as control. All mice were assessed by micro CT (Fig. 2A), which showed that trabecular bone value/total value (BV/TV) (Fig. 2C), bone mineral density (BMD) (Fig. 2D), trabecular thickness (Tb. Th) (Fig. 2E) and trabecular number (Tb. N) (Fig. 2F) were decreased, while trabecular spacing (Tb. Sp) (Fig. 2B) was significantly increased in the OVX model compared with the sham group.
Fig. 2.
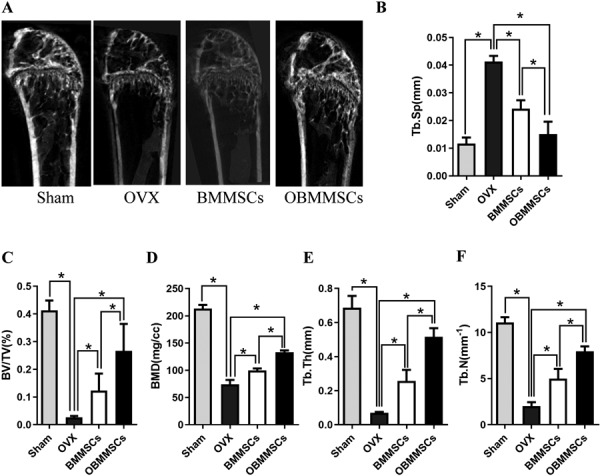
Therapeutic outcome of osteoporosis with bone marrow mesenchymal stem cells (BMMSCs) and osthole-treated-BMMSCs (OBMMSCs). (A) Micro-CT analysis of trabecular bone mass in the femora of mice in each group one month later after injection. Quantitative analysis was performed including (B) trabecular spacing (Tb. Sp), (C) trabecular bone volume (BV/TV), (D) bone mineral density (BMD), (E) trabecular thickness (Tb. Th), and trabecular number (Tb. N). Data were analyzed by one-way ANOVA. Results are presented as means ± SD, *P<0.05.
With the OVX model well established, we intravenously injected OBMMSCs or BMMSCs into the OVX mice 8 weeks after surgical operation as previously described. The femur bone mass was analyzed by micro CT one month later. Newly formed bone tissues were observed, and it was markedly more in OBMMSC-treated mice than in BMMSCs-treated mice (Fig. 2A). In addition, the distal femoral trabecular BV/TV, BMD, Tb. Th, Tb. N of OBMMSCs-treated mice also showed remarkably higher than those of BMMSCs-treated mice (Figs. 2C–F), whereas, Tb. Sp was lower in OBMMSCs-treated mice than that in BMMSCs-treated mice (Fig. 2B).
Results mentioned above consistently indicate that OBMMSCs are more effective in the prevention of osteoporosis than BMMSCs.
Osteoblastic and osteoclastic activity of BMMSCs and OBMMSCs
Knowing OBMMSCs can attenuate development of osteoporosis, we were interested in the problem that how OBMMSCs affect bone formation and resorption. So serological assessment was carried out in sham group, OVX group (PBS injected), OBMMSCs group, and BMMSCs group one month after injection. ELISA of osteogenic markers, P1NP and ALP, was performed in the OVX and sham group first. Serum levels of P1NP and ALP in the OVX group were considerably lower than those in the sham group (Figs. 3A and B). In addition, serum level of calcium in the OVX group was also decreased than that in the sham group (Fig. 3D). Whereafter, ELISA of osteoclastic marker, TRAP, was also conducted. Serum level of TRAP in the OVX group was significantly higher than in the sham group as expected (Fig. 3C).
Fig. 3.
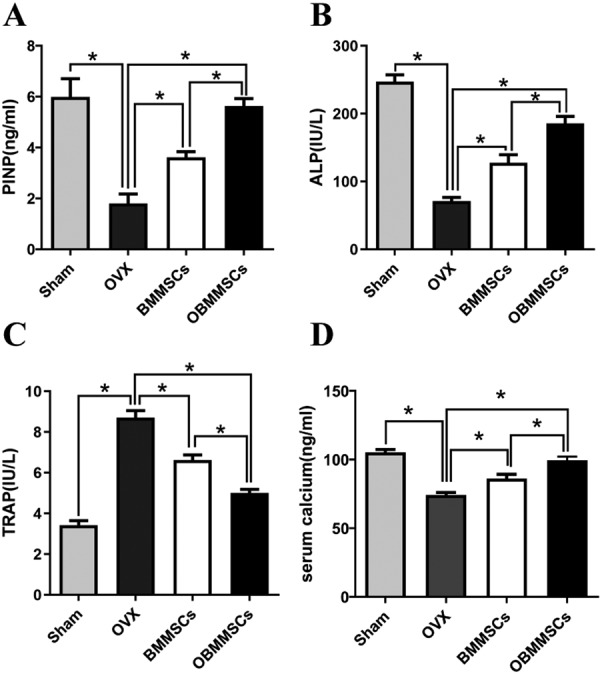
Osteoblastic and osteoclastic activity of bone marrow mesenchymal stem cells (BMMSCs) and osthole-treated-BMMSCs (OBMMSCs). Serum procollagen 1 N-terminal peptide (P1NP) (A), bone alkaline phosphatase (ALP) (B), and tartrate-resistant acid phosphatase (TRAP) (C), calcium (D) concentrations in mice from each group. Data were analyzed by one-way ANOVA. Results are presented as means ± SD, *P<0.05.
Serum levels of P1NP and ALP were increased in OBMMSCs and BMMSCs group when compared with those in the OVX group (Fig. 3A). Importantly, serum levels of P1NP and ALP in the OBMMSCs group were dramatically higher than those in the BMMSCs group. Serum level of calcium was also elevated after OBMMSCs and BMMSCs treatments, and OBMMSCs improved calcium level more than BMMSCs. On the contrary, serum level of TRAP was reduced in the OBMMSCs and the BMMSCs group, and its level in the OBMMSCs group was much lower than in the BMMSCs group (Fig. 3C).
We concluded from those data that osthole had a great impact on osteoblastic and osteoclastic activities.
Osthole promotes autophagy in BMMSCs
Based on previous reports that osteogenic differentiation of BMMSCs in OVX mice can be rescued through elevating autophagy level [33], we were wondering whether there was relationship between autophagy and osthole-prestimulation. So we examined expression levels of autophagy-associated genes, LC3 and Beclin1. First of all, both protein and mRNA expressions of LC3 and Beclin1 of BMMSCs isolated from the OVX mice were significantly reduced compared with those of the Sham mice, indicating that autophagy level declined in the OVX mice (Figs. 4A, B and E). Further study testified that osthole treatment resulted in upregulation of LC3 and Beclin1 protein and mRNA production in BMMSCs (Figs. 4C, D and F).
Fig. 4.
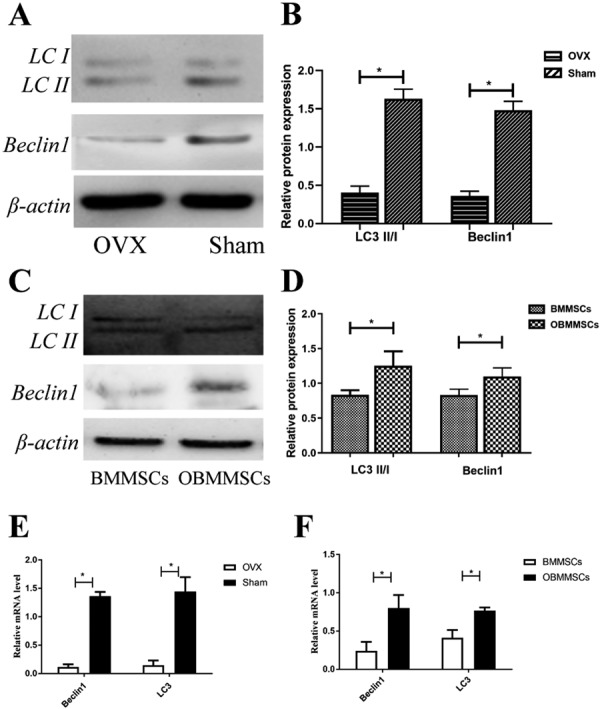
Autophagy level in bone marrow mesenchymal stem cells (BMMSCs) and osthole-treated-BMMSCs (OBMMSCs). (A, B) Western blot was performed to examine expression of Beclin1 and LC3 in the ovariectomy (OVX) and Sham group at protein level. (E) Real-time PCR was performed to detect the mRNA expression of Beclin1 and LC3. (C, D, F) Expression of Beclin1 and LC3 in BMMSCs and OBMMSCs at protein and mRNA level was also observed. Data were analyzed by Student’s t-test. Results are presented as means ± SD, *P<0.05.
From the above data, we suggest that osthole promotes autophagy in BMMSCs, leading to a better treatment effect for OP.
Discussion
Osteoporosis is a common metabolic skeletal disease which makes the trabecular bone be prone to fracture and leads to high incidence of fractures, mortality and morbidity, which seriously influences the quality of aging individuals [41, 44]. It’s a worldwide concern waiting for more safe and effective therapeutic methods. BMMSCs are an option embracing cell supply and gene therapy. Gene modified stem cell transplantation has been studied to enhance the osteogenic differentiation ability of BMMSCs and offers broad prospects for osteoporosis treatment [2, 16, 20, 22]. However, growth factors added to culture medium cannot display its complete function for its short half-lives. As a consequence, its effects on promoting differentiation and proliferation couldn’t come up to our expectations. And in recent several decades, many Chinese herbal medicines attract much interest for their regulation in stem cell differentiation and function in tissue regeneration [43]. It has been well established that application of the Chinese Medicine (CM) facilitates osteoblast differentiation which benefits in therapy for bone fracture. These facts point out that CM can be important alternatives of growth factors [21].
Osthole is a coumarin derivative possessing efficiacy of anti-inflammatory, anti-apoptosis, anti-antitumor, anti-bacterial, anti-allergic, anti-osteoporotic [5, 24, 25, 28, 52]. It also plays protective roles in cardiovascular system, reproductive system and skeletal diseases [25, 48]. It’s approved that osthole exhibits estrogen-like effects, promoting osteoblast proliferation and differentiation [17]. Osthole has been demonstrated to have relationship with several signaling pathways involved in osteoblast differentiation. BMP-2/p38 signaling pathway has been proved to regulate the early differentiation stage while ERK1/2 plays a role in late differentiation stage of osteoblast differentiation mediated by osthole [17, 25]. Wnt/β-catenin-BMP pathway has also been suggested to take part in promotion of osteoblast differentiation resulted from osthole treatment [39]. And importantly, osthole promotes osteoblast differentiation in a strictly dose-dependent manner, as Hu et al. [14] reported that osthole suppressed osteogenic differentiation of MSCs with concentrations 6.25, 12.5, and 25 µM. In contrast, 10−5 M osthole is widely reported to enhance osteoblast differentiation [10, 17, 25, 49]. Additionally, the tissue where MSCs derived from can also influence the role of osthole in osteoblast differentiation [10, 14]. So in our study, we combined BMMSCs and 10−5 M osthole to treat OP and the results indicate that BMMSCs pre-stimulated by osthole can produce a better amelioration of development of OP.
Autophagy is a cellular degradation tool for large molecules and also involves in the removal of dysfunctional organelles to maintain cellular metabolism [13]. It’s reported that upregulation of autophagy can extend the lifespan of aged mice and restore the self-renewal capacity of stem cells [12, 37]. Autophagy level is reduced in aged BMMSCs according to mainstream view [23, 51], and it’s reported that autophagy activation can repair degenerative properties of aged BMMSCs [23]. We take an observation on the correlation of autophagy and osthole-mediated osteoblast differentiation. Autophagy level in the OVX mice was lower than that in the Sham mice, while the level in OBMMSCs is higher than that in BMMSCs. We speculate that OBMMSCs have a better therapeutic effect than BMMSCs due to the elevated autophagy level induced by osthole. In vitro study proposed that upregulated autophagy could protect osteoblast cells from tumor necrosis-α-induced [50] and oxidative stress-mediated apoptosis [19]. Induction of osteoblast autophagic activity by β-ecdysterone inactivating mTOR is reported to be the mechanism for treating glucocorticoid-induced osteoporosis [40]. So in our study, the exact molecular targets for osthole to induce autophagy activity and the mechanisms for osthole-induced autophagy to promote osteoblast differnentiation of BMMSCs still needs and is worthy of further investigations. Autophagy level of BMMSCs from OVX mice, which are either treated by BMMSCs stimulated or not stimulated by osthole, is needed to be explored to strengthen our conclusion. In addition, experiments on proliferation and apoptosis of osteoblasts could give further understandings of the mechanisms on the improvement of OP by osthole treatment.
In summary, the therapy combining BMMSCs and osthole remarkably ameliorated the condition of osteoporosis due to low estrogen level. And autophagy is associated with promotion of osteoblast differentiation and osthole application.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31571508, 31371473 to D.Y, 81800979 to Y.Y and 81700958 to L.C).
Reference
- 1.Ahmed S.F., Elmantaser M.2009. Secondary osteoporosis. Endocr. Dev. 16: 170–190. doi: 10.1159/000223695 [DOI] [PubMed] [Google Scholar]
- 2.Baltzer A.W., Whalen J.D., Wooley P., Latterman C., Truchan L.M., Robbins P.D., Evans C.H.2001. Gene therapy for osteoporosis: evaluation in a murine ovariectomy model. Gene Ther. 8: 1770–1776. doi: 10.1038/sj.gt.3301594 [DOI] [PubMed] [Google Scholar]
- 3.Beresford J.N., Bennett J.H., Devlin C., Leboy P.S., Owen M.E.1992. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 102: 341–351. [DOI] [PubMed] [Google Scholar]
- 4.Bone H.G., McClung M.R., Roux C., Recker R.R., Eisman J.A., Verbruggen N., Hustad C.M., DaSilva C., Santora A.C., Ince B.A.2010. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J. Bone Miner. Res. 25: 937–947. [DOI] [PubMed] [Google Scholar]
- 5.Cai J., Yu B., Xu G., Wu J.1991. [Studies on the quality of fructus Cnidii--comparison of antibacterial action]. Zhongguo Zhongyao Zazhi 16: 451–453, 510.(in Chinese) [PubMed] [Google Scholar]
- 6.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., Péault B.2008. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313. doi: 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., Delmas P., Zoog H.B., Austin M., Wang A., Kutilek S., Adami S., Zanchetta J., Libanati C., Siddhanti S., Christiansen C.; FREEDOM Trial.2009. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361: 756–765. doi: 10.1056/NEJMoa0809493 [DOI] [PubMed] [Google Scholar]
- 8.Cummings S.R., Ensrud K., Delmas P.D., LaCroix A.Z., Vukicevic S., Reid D.M., Goldstein S., Sriram U., Lee A., Thompson J., Armstrong R.A., Thompson D.D., Powles T., Zanchetta J., Kendler D., Neven P., Eastell R.;PEARL Study Investigators.2010. Lasofoxifene in postmenopausal women with osteoporosis. N. Engl. J. Med. 362: 686–696. doi: 10.1056/NEJMoa0808692 [DOI] [PubMed] [Google Scholar]
- 9.Florencio-Silva R., Sasso G.R., Simões M.J., Simões R.S., Baracat M.C., Sasso-Cerri E., Cerri P.S.2017. Osteoporosis and autophagy: What is the relationship? Rev Assoc Med Bras 1992 63: 173–179. doi: 10.1590/1806-9282.63.02.173 [DOI] [PubMed] [Google Scholar]
- 10.Gao L.N., An Y., Lei M., Li B., Yang H., Lu H., Chen F.M., Jin Y.2013. The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials 34: 9937–9951. doi: 10.1016/j.biomaterials.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 11.Han Y., Zhang L., Xing Y., Zhang L., Chen X., Tang P., Chen Z.2018. Autophagy relieves the function inhibition and apoptosis‑promoting effects on osteoblast induced by glucocorticoid. Int. J. Mol. Med. 41: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., Pahor M., Javors M.A., Fernandez E., Miller R.A.2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He C., Klionsky D.J.2009. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43: 67–93. doi: 10.1146/annurev-genet-102808-114910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H., Chen M., Dai G., Du G., Wang X., He J., Zhao Y., Han D., Cao Y., Zheng Y., Ding D.2016. An Inhibitory Role of Osthole in Rat MSCs Osteogenic Differentiation and Proliferation via Wnt/β-Catenin and Erk1/2-MAPK Pathways. Cell. Physiol. Biochem. 38: 2375–2388. doi: 10.1159/000445590 [DOI] [PubMed] [Google Scholar]
- 15.Ichioka N., Inaba M., Kushida T., Esumi T., Takahara K., Inaba K., Ogawa R., Iida H., Ikehara S.2002. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells. Stem Cells 20: 542–551. doi: 10.1634/stemcells.20-6-542 [DOI] [PubMed] [Google Scholar]
- 16.Kumar S., Mahendra G., Nagy T.R., Ponnazhagan S.2004. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum. Gene Ther. 15: 1197–1206. doi: 10.1089/hum.2004.15.1197 [DOI] [PubMed] [Google Scholar]
- 17.Kuo P.L., Hsu Y.L., Chang C.H., Chang J.K.2005. Osthole-mediated cell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J. Pharmacol. Exp. Ther. 314: 1290–1299. doi: 10.1124/jpet.105.085092 [DOI] [PubMed] [Google Scholar]
- 18.Lewiecki E.M.2010. Treatment of osteoporosis with denosumab. Maturitas 66: 182–186. doi: 10.1016/j.maturitas.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 19.Li D.Y., Yu J.C., Xiao L., Miao W., Ji K., Wang S.C., Geng Y.X.2017. Autophagy attenuates the oxidative stress-induced apoptosis of Mc3T3-E1 osteoblasts. Eur. Rev. Med. Pharmacol. Sci. 21: 5548–5556. [DOI] [PubMed] [Google Scholar]
- 20.Lien C.Y., Chih-Yuan Ho K., Lee O.K., Blunn G.W., Su Y.2009. Restoration of bone mass and strength in glucocorticoid-treated mice by systemic transplantation of CXCR4 and cbfa-1 co-expressing mesenchymal stem cells. J. Bone Miner. Res. 24: 837–848. doi: 10.1359/jbmr.081257 [DOI] [PubMed] [Google Scholar]
- 21.Lin P.C., Chang L.F., Liu P.Y., Lin S.Z., Wu W.C., Chen W.S., Tsai C.H., Chiou T.W., Harn H.J.2011. Botanical drugs and stem cells. Cell Transplant. 20: 71–83. doi: 10.3727/096368910X532747 [DOI] [PubMed] [Google Scholar]
- 22.Lyles K.W., Colón-Emeric C.S., Magaziner J.S., Adachi J.D., Pieper C.F., Mautalen C., Hyldstrup L., Recknor C., Nordsletten L., Moore K.A., Lavecchia C., Zhang J., Mesenbrink P., Hodgson P.K., Abrams K., Orloff J.J., Horowitz Z., Eriksen E.F., Boonen S., HORIZON Recurrent Fracture Trial2007. Zoledronic acid and clinical fractures and mortality after hip fracture. N. Engl. J. Med. 357: 1799–1809. doi: 10.1056/NEJMoa074941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y., Qi M., An Y., Zhang L., Yang R., Doro D.H., Liu W., Jin Y.2018. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell 17: e12709. doi: 10.1111/acel.12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda H., Tomohiro N., Ido Y., Kubo M.2002. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 25: 809–812. doi: 10.1248/bpb.25.809 [DOI] [PubMed] [Google Scholar]
- 25.Ming L.G., Zhou J., Cheng G.Z., Ma H.P., Chen K.M.2011. Osthol, a coumarin isolated from common cnidium fruit, enhances the differentiation and maturation of osteoblasts in vitro. Pharmacology 88: 33–43. doi: 10.1159/000328776 [DOI] [PubMed] [Google Scholar]
- 26.Nollet M., Santucci-Darmanin S., Breuil V., Al-Sahlanee R., Cros C., Topi M., Momier D., Samson M., Pagnotta S., Cailleteau L., Battaglia S., Farlay D., Dacquin R., Barois N., Jurdic P., Boivin G., Heymann D., Lafont F., Lu S.S., Dempster D.W., Carle G.F., Pierrefite-Carle V.2014. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 10: 1965–1977. doi: 10.4161/auto.36182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuttall M.E., Gimble J.M.2000. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 27: 177–184. doi: 10.1016/S8756-3282(00)00317-3 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto T., Masuda Y., Kawasaki T.2001. Prevention of concanavalin A-induced mice hepatitis by molsidomine. Int. J. Mol. Med. 7: 307–309. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto Y., Tateishi H., Kinoshita K., Tsuchiya S., Hibi H., Ueda M.2011. An experimental study of bone healing around the titanium screw implants in ovariectomized rats: enhancement of bone healing by bone marrow stromal cells transplantation. Implant Dent. 20: 236–245. doi: 10.1097/ID.0b013e3182199543 [DOI] [PubMed] [Google Scholar]
- 30.Onal M., Piemontese M., Xiong J., Wang Y., Han L., Ye S., Komatsu M., Selig M., Weinstein R.S., Zhao H., Jilka R.L., Almeida M., Manolagas S.C., O’Brien C.A.2013. Suppression of autophagy in osteocytes mimics skeletal aging. J. Biol. Chem. 288: 17432–17440. doi: 10.1074/jbc.M112.444190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piemontese M., Onal M., Xiong J., Han L., Thostenson J.D., Almeida M., O’Brien C.A.2016. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci. Rep. 6: 24262. doi: 10.1038/srep24262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietschmann P., Rauner M., Sipos W., Kerschan-Schindl K.2009. Osteoporosis: an age-related and gender-specific disease--a mini-review. Gerontology 55: 3–12. doi: 10.1159/000166209 [DOI] [PubMed] [Google Scholar]
- 33.Qi M., Zhang L., Ma Y., Shuai Y., Li L., Luo K., Liu W., Jin Y.2017. Autophagy Maintains the Function of Bone Marrow Mesenchymal Stem Cells to Prevent Estrogen Deficiency-Induced Osteoporosis. Theranostics 7: 4498–4516. doi: 10.7150/thno.17949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin M.R., Bilezikian J.P.2005. Parathyroid hormone as an anabolic skeletal therapy. Drugs 65: 2481–2498. doi: 10.2165/00003495-200565170-00005 [DOI] [PubMed] [Google Scholar]
- 35.Shen G., Ren H., Shang Q., Qiu T., Yu X., Zhang Z., Huang J., Zhao W., Zhang Y., Liang D., Jiang X.2018. Autophagy as a target for glucocorticoid-induced osteoporosis therapy. Cell. Mol. Life Sci. 75: 2683–2693. doi: 10.1007/s00018-018-2776-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y., Hu G., Su J., Li W., Chen Q., Shou P., Xu C., Chen X., Huang Y., Zhu Z., Huang X., Han X., Xie N., Ren G.2010. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 20: 510–518. doi: 10.1038/cr.2010.44 [DOI] [PubMed] [Google Scholar]
- 37.Simonsen A., Cumming R.C., Brech A., Isakson P., Schubert D.R., Finley K.D.2008. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4: 176–184. doi: 10.4161/auto.5269 [DOI] [PubMed] [Google Scholar]
- 38.Spanou A., Lyritis G.P., Chronopoulos E., Tournis S.2015. Management of bisphosphonate-related osteonecrosis of the jaw: a literature review. Oral Dis. 21: 927–936. doi: 10.1111/odi.12333 [DOI] [PubMed] [Google Scholar]
- 39.Tang D.Z., Hou W., Zhou Q., Zhang M., Holz J., Sheu T.J., Li T.F., Cheng S.D., Shi Q., Harris S.E., Chen D., Wang Y.J.2010. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J. Bone Miner. Res. 25: 1234–1245. doi: 10.1002/jbmr.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y.H., Yue Z.S., Li G.S., Zeng L.R., Xin D.W., Hu Z.Q., Xu C.D.2018. Effect of β‑ecdysterone on glucocorticoid‑induced apoptosis and autophagy in osteoblasts. Mol. Med. Rep. 17: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C., Meng H., Wang X., Zhao C., Peng J., Wang Y.2016. Differentiation of Bone Marrow Mesenchymal Stem Cells in Osteoblasts and Adipocytes and its Role in Treatment of Osteoporosis. Med. Sci. Monit. 22: 226–233. doi: 10.12659/MSM.897044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z., Goh J., Das De S., Ge Z., Ouyang H., Chong J.S., Low S.L., Lee E.H.2006. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 12: 1753–1761. doi: 10.1089/ten.2006.12.1753 [DOI] [PubMed] [Google Scholar]
- 43.Wong H.L., Siu W.S., Shum W.T., Gao S., Leung P.C., Ko C.H.2012. Application of Chinese herbal medicines to revitalize adult stem cells for tissue regeneration. Chin. J. Integr. Med. 18: 903–908. doi: 10.1007/s11655-012-1293-3 [DOI] [PubMed] [Google Scholar]
- 44.Xia W.B., He S.L., Xu L., Liu A.M., Jiang Y., Li M., Wang O., Xing X.P., Sun Y., Cummings S.R.2012. Rapidly increasing rates of hip fracture in Beijing, China. J. Bone Miner. Res. 27: 125–129. doi: 10.1002/jbmr.519 [DOI] [PubMed] [Google Scholar]
- 45.You L., Feng S., An R., Wang X.2009. Osthole: a promising lead compound for drug discovery from a traditional Chinese medicine (TCM). Nat. Prod. Commun. 4: 297–302. [PubMed] [Google Scholar]
- 46.Yu Y., Zhao T., Yang D.2017. Cotransfer of regulatory T cells improve the therapeutic effectiveness of mesenchymal stem cells in treating a colitis mouse model. Exp. Anim. 66: 167–176. doi: 10.1538/expanim.16-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Recker R., Lee W.N., Xiao G.G.2010. Proteomics in bone research. Expert Rev. Proteomics 7: 103–111. doi: 10.1586/epr.09.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Jiang G., Yao F., He Y., Liang G., Zhang Y., Hu B., Wu Y., Li Y., Liu H.2012. Growth inhibition and apoptosis induced by osthole, a natural coumarin, in hepatocellular carcinoma. PLoS One 7: e37865. doi: 10.1371/journal.pone.0037865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Qin L., He W., Van Puyvelde L., Maes D., Adams A., Zheng H., De Kimpe N.2007. Coumarins from Cnidium monnieri and their antiosteoporotic activity. Planta Med. 73: 13–19. doi: 10.1055/s-2006-951724 [DOI] [PubMed] [Google Scholar]
- 50.Zheng L., Wang W., Ni J., Mao X., Song D., Liu T., Wei J., Zhou H.2017. Role of autophagy in tumor necrosis factor-α-induced apoptosis of osteoblast cells. J. Investig. Med. 65: 1014–1020. doi: 10.1136/jim-2017-000426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y., Hu C.J., Zhuo R.H., Lei Y.S., Han N.N., He L.2014. Inhibition of autophagy alleviates the senescent state of rat mesenchymal stem cells during long-term culture. Mol. Med. Rep. 10: 3003–3008. doi: 10.3892/mmr.2014.2624 [DOI] [PubMed] [Google Scholar]
- 52.Zimecki M., Artym J., Cisowski W., Mazol I., Włodarczyk M., Gleńsk M.2009. Immunomodulatory and anti-inflammatory activity of selected osthole derivatives. Z. Natforsch. C J. Biosci. 64: 361–368. [DOI] [PubMed] [Google Scholar]


