Abstract
Biological background data up to 11 weeks of age and tumorigenic susceptibility to xenotransplantation with HeLa cells were compared between severely immuno-deficient NOG and NSG mice. The body weight was lower in NOG mice than in NSG mice. Severe depletion of peripheral blood lymphocytes and lymphoid hypoplasia that are well-known characteristics of these mice were equally observed. No lymphoproliferative lesions developed in any mouse of either strain. The occurrence of ectopic exocrine gland and cyst was a common finding in the thymus of both strains. In addition, minimal spongiotic change was observed in the medulla oblongata and spinal cord in both strains, and its incidence in female NOG mice was a little higher than that in NSG mice. In the adrenal, subcapsular cell hyperplasia that is known as an age-related change in non-genetically modified mice developed earlier and its incidence was higher in NSG mice than in NOG mice. The development of female genital organs of NOG mice was slightly retarded in comparison with that of NSG mice. To evaluate tumorigenic susceptibility to xenotransplantation, female mice were implanted in the dorsal subcutis with 1×103 to 1×106 cells/head of HeLa cells, and were checked up to 16 weeks after implantation. As a result, there was no significant strain difference on tumor formation rate and tumor volume. In conclusion, the present study clearly demonstrated that NOG and NSG mice showed no distinct strain differences in either biological features or biological disadvantages.
Keywords: background data, comparison, NOG mice, NSG mice, tumorigenicity
Introduction
Recently, the NOG mouse (formal name: NOD.Cg-PrkdcscidIl2rgtm1Sug/ShiJic mouse) and the NSG mouse (formal name: NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mouse) have been established as severely immuno-deficient animals. Both strains of mice were generated by knockout of Il2rg responsible for development of NK cells on the NOD-scid mouse lacking functional T and B lymphocytes [4, 17]. Consequently, both mice lack functional T and B lymphocytes and have multifunctional defects in NK cell activity, macrophage function, complement activity and dendritic cell function, and the immunodeficiency mechanism is very similar.
The NOG mouse was developed by backcrossing the Il2rgnull mouse resulted from a truncation of the intracellular signaling domain onto NOD/ShiJic-Prkdcscid mouse [4]. In contrast, the NSG mouse was developed by backcrossing the Il2rgnull mouse resulted from a complete null mutation onto NOD/ShiLtSz-Prkdcscid mouse [17]. Thus, there is a difference in the Il2rg mutation between NOG and NSG mice. In NOG mice the Il2rg mutation produces a protein that is expressed and will bind cytokines but cannot signal, while the mutation in NSG mice is a complete null so that no Il2rg is expressed and cytokines cannot bind [16]. Moreover, it is suggested that the genetic backgrounds of these mice are not the same, because NOD/ShiJic mouse and NOD/ShiLtSz mouse were kept separate by different providers for a long time [12].
Up to the present, various strains of immuno-deficient mice have been developed for use in various fields including cancer research, evaluation of pharmacological efficacy, safety assessment of regenerative medicine, establishment of humanized models for human diseases and drug discovery. However, such disadvantages as short life span due to early occurrence of thymic lymphoma and a low level of engraftment due to remaining activity of NK cells have also been reported in NOD-scid and NOD-scid B2mnull mice [17]. In this connection, we clarified in our previous study on the biological background data of NOG mice that the survival rate was 95% at 52 weeks of age in both sexes, and that focal spongiotic change in the nervous system and tubular aggregates in the skeletal muscle were observed as noticeable histological changes. Moreover, we also clarified that although tumorous lesions such as malignant lymphoma, intestinal adenoma, hepatocellular adenoma, leukemia, cerebral lipomatous hamartoma, Harderian gland adenoma and uterine polyp developed in NOG mice, their incidences were very low except for that of uterine polyp [6].
The usefulness of NOG mice as a host for xenotransplantation has been reported by several researchers [2, 7, 9]. In this connection, Kanaji et al. [5] compared the susceptibility to xenotransplantation among NSG, BALB/c-nu, C.B-17 scid and NOD-scid mice, and they concluded that NSG mice might be the most suitable strain for testing tumorigenicity of lung cancer. However, there have been only a few reports of direct comparative studies on biological background data such as histopathology and tumorigenicity profile by xenotransplantation with Hela cells, a representative positive control human tumor cell line, between NOG and NSG mice.
Therefore, in the present study, we conducted two direct comparative experiments on biological features between NOG and NSG mice. The first was to evaluate the biological background data at 9 and 11 weeks of age, the termination age of 2- and 4-week toxicity studies, respectively. The second was to evaluate usefulness as a host for xenotransplantation by different numbers of HeLa cells. The present studies were performed according to the protocol of the regulatory study for tumorigenesis in regenerative medicine.
Materials and Methods
The present study was composed of two experiments. The first experiment was to compare the biological background data when the animals were housed untreated up to 11 weeks of age, and the second one was to compare the tumorigenesis by xenotransplantation with a human tumor cell line (HeLa cells, a human cervical cancer cell line).
These experiments were carried out after their approval at the Institutional Animal Care and Use Committee, Tsukuba Research Institute, BoZo Research Center Inc. They were conducted in accordance with the guidelines for the control and welfare of experimental animals specified by the test facility.
Experiment 1 (Comparison on the background data)
Animals: Twenty-two males and 22 females each of NOG and NSG mice, a specific pathogen free, were obtained at 5 weeks of age from the Central Institute for Experimental Animals (CIEA) (Kanagawa, Japan) and Charles River Laboratories Japan, Inc. (Kanagawa, Japan), respectively. After an approximately 2-week quarantine/acclimation, animals of each strain were divided into the 2-week group and the 4-week group, which consisted of 10 mice/sex and 12 mice/sex, respectively. All animals were housed in polycarbonate flat-bottomed cages (W160 × D370 × H130 mm, Tecniplast Japan Co., Ltd., Tokyo, Japan) installed in a positive pressure rack, individually for males and 3–5 animals per cage (W230 × D335 × H140 mm, Clea Japan Inc., Tokyo, Japan) for females in a clean animal room controlled to maintain the temperature at 23 ± 3°C, the relative humidity at 50 ± 20%, the air ventilation at 10 to 20 times per hour and illumination for 12 h a day, and were allowed free access to pelleted diet, CE-2 (irradiation sterilized, Clea Japan Inc.) and sterilized tap water.
Clinical signs, body weight, food consumptions: Clinical signs including general condition and mortality were checked once every day, and body weights were measured once a week during the experimental period. Food consumption was calculated as an average value over three consecutive days.
Ophthalmology: All animals were subjected to ophthalmologic examinations before necropsy. The anterior portion and intermediate optic media were examined by a slit lamp (SL-15: Kowa Co., Ltd., Nagoya, Japan), and the fundus oculi after dilation of the pupil by mydriatic agent (Mydrin P: Santen Pharmaceutical Co., Ltd., Osaka, Japan) were examined by a binocular indirect ophthalmoscope (Omega 500: HEINE Optotechnik GmbH & Co. KG., Bayern, Germany).
Hematology: At each scheduled necropsy, all animals, without fasting, were subjected to laparotomy under isoflurane anesthesia and blood was collected via the vena cava inferior into blood collection tubes (MicrotainerTM, Nippon Becton Dickinson Co., Ltd., Tokyo, Japan) containing EDTA-2K using a syringe rinsed with heparin sodium. The parameters determined were red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocyte count, platelet count (PLT), white blood cell count (WBC) and differential leukocyte counts (ADVIA® 2120i Hematology System, Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA).
Blood chemistry: At the same time as hematological examination, plasma samples were obtained by centrifugation of the blood collected in tubes containing heparin lithium (Capiject Heparin Lithium: Terumo Co., Ltd., Tokyo, Japan), and were subjected to examine the following parameters; aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), alkaline phosphatase (ALP), total cholesterol (T-CHO), triglyceride (TG), phospholipid (PL), total bilirubin (T-BIL), glucose (GLU), blood urea nitrogen (BUN), creatinine (CRNN), sodium (Na), potassium (K), chloride (Cl), calcium (Ca), inorganic phosphorus (P), total protein (TP), albumin (ALB) using Clinical Laboratory System TBA-120FR (Toshiba Medical Systems Corp., Tokyo, Japan), and globulin, A/G ratio were calculated by the following formulas, globulin (g/dL) = TP − ALB, A/G = ALB / (TP − ALB) respectively.
Pathology: All animals were euthanized by exsanguination via the abdominal aorta and subjected to complete necropsy. The brain, spleen, heart, lungs (including bronchi), liver and gall bladder (as liver), kidneys and testes were weighed (absolute weight), and then organ weight per 100 g body weight (relative weight) was calculated based on the body weight at necropsy. In addition to these tissues, the following tissues were also dissected: medulla oblongata, spinal cord, sciatic nerves, eyeballs, optic nerves, Harderian glands, pituitary, thyroids, parathyroids, adrenals, thymus, submandibular lymph nodes, mesenteric lymph nodes, thoracic aorta, trachea, tongue, esophagus, stomach, duodenum, jejunum, ileum (including Peyer’s patches), cecum, colon, rectum, mandibular glands, sublingual glands, pancreas, urinary bladder, epididymides, prostate, seminal vesicles, ovaries, uterus, vagina, mammary glands (inguinal region, females only), sternum (including the bone marrow), femurs (including knee joint and bone marrow), femoral skeletal muscles, nasal cavity, skin (inguinal region) and any gross lesions. When the thymus, submandibular and mesenteric lymph nodes and Peyer’s patches were invisible, their anatomically addressed site tissues were removed. All tissues except for the eyeballs, optic nerves and testes were fixed in 10% phosphate-buffered formalin. The eyeballs and optic nerves were fixed in mixture of 3% glutaraldehyde and 2.5% formalin, and the testes were fixed with Bouin’s fluid. These tissues were trimmed, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E) and examined histopathologically.
Experiment 2 (Comparison of tumorigenesis by HeLa cell implantation)
Human cell lines: HeLa cells, a human cervical cancer cell line, were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank in the National Institute of Biomedical Innovation (Osaka, Japan). The cells were maintained in Eagle’s minimum essential medium (Thermo Fisher Scientific, Tokyo, Japan) supplemented with 0.1 mM non-essential amino acids (Thermo Fisher Scientific), 10% FBS (Fetal Bovine Serum, Thermo Fisher Scientific), 100 U/ml penicillin/100 µg/ml streptomycin (Thermo Fisher Scientific), incubated in a humidified incubator (5% CO2, 37°C).
MRC-5 cells, a human embryonic lung fibroblast, were obtained from JCRB and were maintained in Basal Medium Eagle (BME, Thermo Fisher Scientific) supplemented with 2 mM GlutaMAX-I (Thermo Fisher Scientific), 10% FBS (Fetal Bovine Serum, Thermo Fisher Scientific), 100 U/ml penicillin/100 µg/ml streptomycin (Thermo Fisher Scientific), incubated in a humidified incubator (5% CO2, 37°C). MRC-5 cells were used as negative control.
Animals and group composition: Forty females each of NOG and NSG mice were obtained at 6 weeks of age from CIEA and Charles River Laboratories, respectively. One NSG mouse was sacrificed in a moribund state during the quarantine/acclimation period. After 1-week of quarantine/acclimation, the animals were divided into 5 groups, consisting 8 animals each except for Group 5 of NSG mice (7 animals), and they were implanted with either HeLa cells or MRC-5 cells in the right dorsal subcutaneous region. The animals in Groups 1 to 4 were implanted with 1×103, 1×104, 1×105 and 1×106 cells/head of HeLa cells, respectively. Group 5 was a negative control group, which were implanted with 1×106 cells/head of MRC-5 cells.
Measurement of tumor volume: The length and width of the tumor developed from the transplanted cells was measured with a caliper once a week for up to 16 weeks after the implantation with HeLa cells or MRC-5 cells. Tumor volume was calculated using the following formula.
Tumor volume (mm3) = Length (mm) × Width (mm)2 × 0.5
Pathology: At 13 weeks after implantation, one NOG mouse from Group 1 was euthanized under isoflurane gas anesthesia, due to rapid enlargement of a tumor which developed in the pubic region. At 16 weeks after implantation, all survivors were euthanized by exsanguination via the abdominal aorta and subjected to complete necropsy. The implanted sites and all tumors in any sites detected at necropsy were resected and stored in 10% phosphate-buffered formalin, and routinely processed for histology slides. Histopathologic examination was conducted on hematoxylin and eosin stained slides. In addition, the tumor tissues were stained immunohistochemically with HLA (anti-HLA class I-A, B, C, mouse monoclonal antibody, Hokudo Co., Ltd., Hokkaido, Japan), HNA (anti-nuclei, clone 3E1.3, monoclonal antibody, Merck Millipore, Darmstadt, Germany) and human specific Ki-67 (monoclonal mouse antibody anti-human Ki-67, Dako, Glostrup, Denmark) to confirm whether or not these tissues were derived from the implanted cells.
Statistical Analysis: For numerical data including body weight, food consumption, hematology, blood chemistry and organ weights in experiment 1, and tumor volume in experiment 2, the significance of differences between NOG and NSG mice was tested after calculating Mean ± SD or Mean ± SE in each mouse. Homogeneity of variance in each group was analyzed by F test [18] (level of significance: 5%, one-tailed), and the homogeneous data were analyzed by Student’s t-test [18] (level of significance: 5% and 1%, two-tailed) while the heterogeneous data were analyzed by Aspin-Welch’s t-test [18] (level of significance: 5% and 1%, two-tailed) for the mean difference between NOG and NSG mice. Tumor formation rates were analyzed by Fisher’s exact test (level of significance: 5%, two-tailed).
Results
Experiment 1
Mortality and clinical signs: All animals survived until the end of the experimental period. As for clinical signs, except for alopecia in the parietal region that was observed in 5 female NSG mice from 7 to 9 weeks of age, no abnormal clinical signs were found in any other mice through the experimental period.
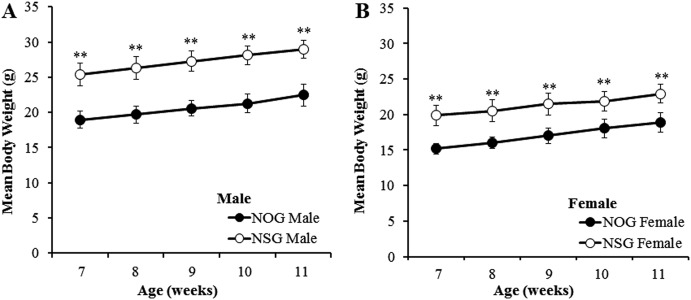
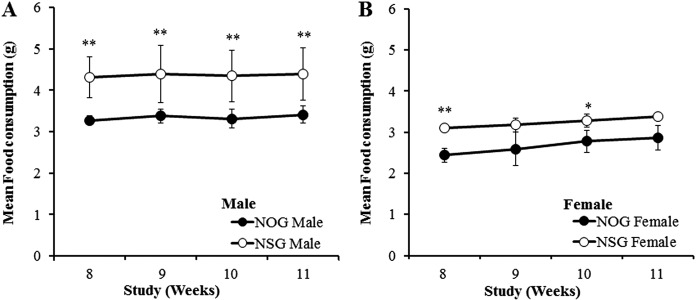
Body weights and food consumption: The body weight of NOG mice was significantly lighter than that of NSG mice through the experimental period (Figs. 1A and B), while the body weight gain was almost similar between the two mouse strains. The mean food consumption was significantly lower in NOG mice than in NSG mice through the experimental period (Figs. 2A and B).
Fig. 1.
Mean body weights in males (A) and females (B) of NOG and NSG mice. **: Significantly different from NOG mice at P<0.01 by Student’s t-test.
Fig. 2.
Mean food consumption in males (A) and females (B) of NOG and NSG mice. * and **: Significantly different from NOG mice at P<0.05 and P<0.01 by Student’s t-test, respectively.
Ophthalmology, hematology and blood chemistry: Ophthlmologically, focal opacity in the lens and persistence of tunica vasculosa lentis in the vitreous body were occasionally observed in both NOG and NSG mice, and their incidences showed no strain differences (Table 1).
Table 1. Ophthalmological abnormalities in NOG and NSG mice.
| Region | Findings | Sex Age (weeks) |
Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 11 | 9 | 11 | |||||||
| Straina No. of mice |
NOG | NSG | NOG | NSG | NOG | NSG | NOG | NSG | ||
| 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |||
| Lens | Focal opacity, nucleus | 0 | 2 | 1 | 2 | 0 | 1 | 0 | 1 | |
| Focal opacity, posterior capsule | 0 | 0 | 1 | 2 | 0 | 1 | 1 | 3 | ||
| Vitreous body | Persistent tunica vasculosa lentis | 2 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | |
Among the hematological parameters examined, the value of RBC, HCT, MCHC, MCV, MCH, PLT, lymphocytes and eosinophils showed a statistically significant difference between NOG and NSG mice, however, these differences were generally small (Table 2).
Table 2. Hematological data in NOG and NSG mice.
| Age (weeks) |
Strain (No. of mice) |
RBC (×104/µl) |
HCT (%) |
MCV (fl) |
MCH (pg) |
MCHC (g/dl) |
PLT (×104/µl) |
WBC (×102/µl) |
LYMP (×102/µl) |
EOS (×102/µl) |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 9 | NOG (10) | Mean | 849 | 45.1 | 53.1 | 15.6 | 29.4 | 139.3 | 14.5 | 1.8 | 0.5 |
| SD | 20 | 1.0 | 0.9 | 0.2 | 0.4 | 8.9 | 4.0 | 0.4 | 0.3 | |||
| NSG (10) | Mean | 841 | 44.5 | 52.9 | 15.7 | 29.7 | 131.0 | 11.6 | 1.4 | 0.3** | ||
| SD | 25 | 1.3 | 0.8 | 0.2 | 0.3 | 12.0 | 1.9 | 0.5 | 0.1 | |||
| 11 | NOG (12) | Mean | 859 | 44.2 | 51.5 | 15.4 | 29.9 | 139.8 | 12.9 | 1.8 | 0.5 | |
| SD | 16 | 0.9 | 0.5 | 0.2 | 0.3 | 12.9 | 2.5 | 0.4 | 0.2 | |||
| NSG (12) | Mean | 842 | 44.0 | 52.3* | 15.7* | 30.0 | 128.2* | 14.0 | 1.7 | 0.3* | ||
| SD | 33 | 1.4 | 1.0 | 0.2 | 0.5 | 8.2 | 3.6 | 0.5 | 0.2 | |||
| Female | 9 | NOG (5) | Mean | 867 | 44.7 | 51.6 | 15.6 | 30.2 | 117.2 | 13.5 | 1.8 | 0.6 |
| SD | 11 | 0.4 | 0.6 | 0.3 | 0.5 | 18.4 | 3.8 | 0.3 | 0.1 | |||
| NSG (5) | Mean | 840** | 43.7** | 52.0 | 15.8 | 30.3 | 113.1 | 11.8 | 1.1** | 0.3** | ||
| SD | 7 | 0.5 | 0.6 | 0.2 | 0.4 | 8.5 | 1.6 | 0.2 | 0.1 | |||
| 11 | NOG (6) | Mean | 862 | 44.9 | 52.1 | 15.8 | 30.2 | 120.4 | 15.2 | 1.8 | 0.8 | |
| SD | 19 | 1.5 | 0.8 | 0.2 | 0.3 | 20.0 | 11.0 | 0.6 | 0.6 | |||
| NSG (6) | Mean | 833 | 43.0* | 51.6 | 15.8 | 30.7* | 107.9 | 11.0 | 1.3 | 0.2 | ||
| SD | 28 | 1.2 | 0.8 | 0.3 | 0.3 | 14.6 | 3.4 | 0.3 | 0.1 | |||
RBC: red blood cell, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, PLT: platelet, WBC: white blood cell, LYMP: lymphocyte, EOS: eosinophil. * and **: Significantly different from NOG mice at P<0.05 and P<0.01by Student’s t-test, respectively.
Among the blood chemical parameters, the values of ALT, ALP, T-BIL and CRNN showed a statistically significant difference between NOG and NSG mice, these differences were generally small (Table 3).
Table 3. Blood chemical data in NOG and NSG mice.
| Age (weeks) |
Strain (No. of mice) |
ALT (IU/l) |
ALP (IU/l) |
T-BIL (mg/dl) |
CRNN (mg/dl) |
||
|---|---|---|---|---|---|---|---|
| Male | 9 | NOG (10) | Mean | 36 | 352 | 0.1 | 0.08 |
| SD | 6 | 61 | 0.0 | 0.02 | |||
| NSG (10) | Mean | 32 | 291* | 0.2 | 0.08 | ||
| SD | 6 | 36 | 0.0 | 0.03 | |||
| 11 | NOG (12) | Mean | 40 | 260 | 0.2 | 0.12 | |
| SD | 14 | 38 | 0.0 | 0.03 | |||
| NSG (12) | Mean | 30 | 224* | 0.2 | 0.09* | ||
| SD | 12 | 35 | 0.0 | 0.03 | |||
| Female | 9 | NOG (5) | Mean | 30 | 443 | 0.1 | 0.08 |
| SD | 6 | 25 | 0.0 | 0.00 | |||
| NSG (5) | Mean | 33 | 356** | 0.2** | 0.07 | ||
| SD | 4 | 25 | 0.0 | 0.01 | |||
| 11 | NOG (6) | Mean | 47 | 396 | 0.1 | 0.08 | |
| SD | 9 | 34 | 0.0 | 0.02 | |||
| NSG (6) | Mean | 31** | 331** | 0.2 | 0.08 | ||
| SD | 5 | 25 | 0.1 | 0.02 | |||
ALT: alanine aminotransferase, ALP: alkaline phosphatase, T-BIL: total bilirubin, CRNN: creatinine. * and **: Significantly different from NOG mice at P<0.05 and P<0.01 by Student’s t-test, respectively.
Pathology: The absolute organ weights of the brain, heart, lung, liver, kidney and testis were statistically significantly lighter in NOG mice than in NSG mice. The relative organ weights of the brain, heart, lung and liver were statistically significantly smaller in NSG mice than in NOG mice (Table 4).
Table 4. Absolute and relative organ weights in NOG and NSG mice.
| Age (weeks) |
Strain (No. of mice) |
FBW | Brain† | Heart | Lung | Liver | Kidney-RL | Testis-RL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | mg | mg/100g | mg | mg/100g | mg | mg/100g | mg | mg/100g | mg | mg/100g | mg | mg/100g | ||||
| Male | 9 | NOG (10) | Mean | 21.1 | 460 | 2,187 | 104 | 493 | 122 | 581 | 1,140 | 5,400 | 357 | 1,696 | 131 | 624 |
| SD | 1.2 | 17 | 165 | 9 | 36 | 11 | 49 | 90 | 210 | 18 | 101 | 18 | 90 | |||
| NSG (10) | Mean | 26.9 | 483** | 1,797** | 130** | 482 | 153** | 568 | 1,300** | 4,820** | 456** | 1,691 | 183** | 679 | ||
| SD | 1.4 | 13 | 74 | 11 | 33 | 9 | 25 | 60 | 110 | 31 | 53 | 8 | 29 | |||
| 11 | NOG (12) | Mean | 22.5 | 460 | 2,057 | 103 | 460 | 120 | 538 | 1,090 | 4,860 | 365 | 1,629 | 146 | 654 | |
| SD | 1.5 | 9 | 137 | 8 | 30 | 10 | 51 | 120 | 280 | 18 | 65 | 17 | 85 | |||
| NSG (12) | Mean | 29.0 | 487** | 1,681** | 132** | 456 | 157** | 541 | 1,390** | 4,800 | 487** | 1,677 | 178** | 616 | ||
| SD | 1.3 | 17 | 86 | 10 | 26 | 16 | 49 | 100 | 270 | 48 | 124 | 20 | 78 | |||
| Female | 9 | NOG (10) | Mean | 16.6 | 454 | 2,739 | 82 | 496 | 114 | 685 | 770 | 4,650 | 211 | 1,272 | ||
| SD | 1.0 | 13 | 119 | 6 | 18 | 11 | 39 | 80 | 250 | 16 | 67 | |||||
| NSG (10) | Mean | 21.6 | 474** | 2,202** | 99** | 461** | 138** | 640* | 910** | 4,200** | 272** | 1,261 | ||||
| SD | 1.9 | 17 | 131 | 7 | 25 | 18 | 54 | 120 | 270 | 26 | 45 | |||||
| 11 | NOG (5) | Mean | 18.9 | 471 | 2,501 | 90 | 477 | 118 | 623 | 840 | 4,450 | 231 | 1,223 | |||
| SD | 1.4 | 27 | 131 | 9 | 40 | 13 | 38 | 110 | 390 | 21 | 40 | |||||
| NSG (5) | Mean | 22.9 | 496* | 2,171** | 103** | 450 | 141** | 616 | 940* | 4,120* | 280** | 1,222 | ||||
| SD | 1.3 | 22 | 116 | 7 | 25 | 10 | 37 | 100 | 290 | 21 | 53 | |||||
FBW: final body weight. †: Values in left and right columns show absolute and relative organ weight, respectively. * and **: Significantly different from NOG mice at P<0.05 and P<0.01 by Student's t-test, respectively.
Macroscopically, the thymus, submandibular and mesenteric lymph nodes and Peyer’s patches were extremely small or undetectable in both NOD and NSG mice. However, there were no significant differences in their frequency and severity between the two mouse strains. Furthermore, the following gross lesions were observed in a few NOG and/or NSG mice: white foci in the spleen and liver that were microscopically confirmed respectively as osseous metaplasia and focal necrosis, jejunal diverticulum, and small-sized testes and epididymides (Table 5).
Table 5. Macroscopic findings in NOG and NSG mice.
| Tissue | Observation | Sex Age (weeks) |
Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 11 | 9 | 11 | |||||||
| Strain No. of mice |
NOG | NSG | NOG | NSG | NOG | NSG | NOG | NSG | ||
| 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |||
| Thymus | Rudimentary | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |
| Spleen | Focus, white | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Lymph node, submandibular | Rudimentary | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |
| Lymph node, mesenteric | Rudimentary | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |
| Peyer’s patch | Unobservable | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |
| Intestine, jejunum | Diverticulum | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Liver | Focus, white | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Testis | Small | 1 | 0 | 0 | 1 | – | – | – | – | |
| Epididymis | Small | 1 | 0 | 0 | 0 | – | – | – | – | |
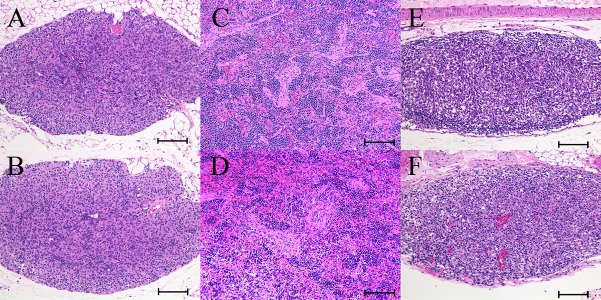
Microscopically, hypoplasia of the lymphoid tissues was generally detected in NOG and NSG mice without any strain differences in its severity and incidence (Table 6). This change was characterized by poor- or under-development of the cortex and medulla in the thymus (Figs. 3A and 3B), the marginal zone of lymphofollicles and periarteriolar lymphoid sheath (PALS) in the spleen (Figs. 3C and D), and the cortex and medulla in the submandibular and mesenteric lymph nodes (Figs. 3E and F), with lymphoid cell hypocellularity. The lymphoid tissues which normally distribute through the intestinal, bronchopulmonary and nasopharyngeal mucosa could not be detected in any mice of either strain. Moreover, ectopic exocrine gland and cyst in the thymus were frequently and equally observed in mice of both strains (Table 6).
Table 6. Microscopic findings in NOG and NSG mice.
| Tissue | Observation | Sex Age (weeks) |
Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 11 | 9 | 11 | |||||||
| Strain No. of mice |
NOG | NSG | NOG | NSG | NOG | NSG | NOG | NSG | ||
| 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |||
| Thymus | Number examined | 10 | 8 | 11 | 10 | 7 | 8 | 11 | 11 | |
| Hypocellularity, lymphoid, cortex / medulla | 10 | 8 | 11 | 10 | 7 | 8 | 11 | 11 | ||
| Hypoplasia, cortex / medulla | 10 | 8 | 11 | 10 | 7 | 8 | 11 | 11 | ||
| Debris, apoptotic | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | ||
| Ectopic thyroid | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | ||
| Ectopic exocrine gland | 3 | 2 | 4 | 2 | 1 | 0 | 3 | 1 | ||
| Ectopic parathyroid | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Cyst | 6 | 6 | 10 | 8 | 5 | 4 | 6 | 8 | ||
| Spleen | Hypocellularity, lymphoid, white pulp | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |
| Hypoplasia, follicle / marginal zone / PALSa) | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | ||
| Metaplasia, osseous | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| Hematopoiesis, extramedullary | 10 | 10 | 12 | 12 | 10 | 12 | 12 | 12 | ||
| Lymph node, submandibular | Number examined | 10 | 8 | 10 | 12 | 9 | 8 | 10 | 11 | |
| Hypocellularity, lymphoid | 10 | 8 | 10 | 12 | 9 | 8 | 10 | 11 | ||
| Hypoplasia, cortex / medulla | 10 | 8 | 10 | 12 | 9 | 8 | 10 | 11 | ||
| Debris, apoptotic | 2 | 1 | 3 | 4 | 2 | 4 | 4 | 6 | ||
| Lymph node, mesenteric | Number examined | 10 | 8 | 8 | 9 | 9 | 9 | 12 | 12 | |
| Hypocellularity, lymphoid | 10 | 8 | 8 | 9 | 9 | 9 | 12 | 12 | ||
| Hypoplasia, cortex / medulla | 10 | 8 | 8 | 9 | 9 | 9 | 12 | 12 | ||
| Debris, apoptotic | 7 | 2 | 6 | 9 | 7 | 9 | 11 | 11 | ||
| Other lymphoid tissuesb) | Hypoplasia, lymphoid tissue | 10 | 10 | 12 | 12 | 10 | 10 | 12 | 12 | |
| Medulla oblongata/ Spinal cord | Spongiotic change | 6 | 5 | 6 | 5 | 6 | 1 | 9 | 4 | |
| Adrenal | Hyperplasia, subcapsular cell | 0 | 2 | 0 | 6 | 0 | 7 | 2 | 12 | |
| Liver | Necrosis, focal | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Testis | Infarction | 1 | 0 | 0 | 0 | – | – | – | – | |
| Bone, sternal | Degeneration, chondromucinous | 10 | 9 | 12 | 12 | 10 | 9 | 12 | 12 | |
| Genimal organ, female | Immature | – | – | – | – | 4 | 1 | 2 | 0 | |
a)Periarterial lymphatic sheath. b)Mucosa associated lymphatic tissues including intestinal, bronchopulmonary and nasopharyngeal mucosa.
Fig. 3.
Representative histology images of the lymphoid tissues. Image A, C and E were from male NOG mice and image B, D and F were from male NSG mice, 11 weeks of age. (A) and (B) Thymus. Severe lymphoid depletion with disappearance of the cortico-medullary junction. Bar=100 µm. (C) and (D) Spleen. Severe lymphoid depletion with loss of normal architectures of the white pulp and marginal zone. Bar=100 µm. (E) and (F) Mesenteric lymph node. Severe lymphoid depletion with loss of normal cortical and medullary architectures. Bar=100 µm. H&E stain. There was no histological difference in each tissue between NOG and NSG mice.
Minimal spongiotic change in the medulla oblongata and spinal cord was noted in both strains, and its incidence in female NOG mice was a little higher than that in female NSG mice (Table 6). Subcapsular cell hyperplasia in the adrenal developed earlier and its incidence was higher in NSG mice than in NOG mice. In the sternum, chondromucinous degeneration, showing similar severity, was noted in most mice of both strains. Moreover, immaturity of the female genital organs was found showing a little higher incidence in NOG mice than in NSG mice.
Although other changes than the above-mentioned were also observed in various organs, they were all detected only in a small number of NOG and/or NSG mice (Table 6).
Experiment 2
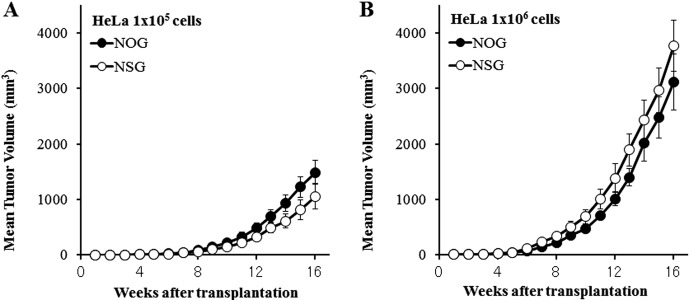
At 16 weeks after inoculation, the tumor formation rate was 100% (8/8) in both NOG and NSG mice inoculated with 1×105 and 1×106 cells/head, with no statistically significant strain differences (Table 7). In addition, the tumor growth curve was similar between NOG and NSG mice, when inoculated with 1×105 and 1×106 cells/head (Figs. 4A and B). Moreover, the tumor volume showed no statistically significant differences between NOG and NSG mice at each point of measurement, although it was slightly bigger in NOG mice than in NSG mice when inoculated with 1×105 cells/head while it was slightly smaller in NOG mice than in NSG mice when inoculated with 1×106 cells/head.
Table 7. Tumor formation rate of HeLa cells in NOG and NSG mice.
| Group | Inoculated cell | Cell dose (cells/head) |
Number of mice with tumors (% formation)* | |
|---|---|---|---|---|
| NOG | NSG | |||
| 1 | HeLa | 1 × 103 | 0/8 (0%) | 0/8 (0%) |
| 2 | HeLa | 1 × 104 | 0/8 (0%) | 1/8 (13%) |
| 3 | HeLa | 1 × 105 | 8/8 (100%) | 8/8 (100%) |
| 4 | HeLa | 1 × 106 | 8/8 (100%) | 8/8 (100%) |
| 5 | MRC-5 | 1 × 106 | 0/8 (0%) | 0/7 (0%) |
*: Tumor formation rate was evaluated 16 weeks after inoculation by 1×103, 104, 105 and 106 HeLa cells, and 1×106 MRC-5 cells.
Fig. 4.
Mean growth curve of engrafted subcutaneous HeLa tumors in NOG and NSG mice at 1×105(A) and 1×106(B) cells/head.
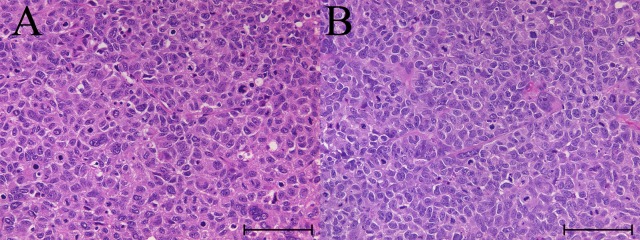
Histologically, the engrafted tumor was composed of large epithelium-like cells with nuclear pleomorphism and surrounded by fibrous tissues and infiltration of inflammatory cells excluding lymphocytes. Such histologic features of the engrafted tumor were essentially similar between NOG and NSG mice (Figs. 5A and B).
Fig. 5.
Representative histology images of the engrafted tumor. Image A and B were from NOG and NSG mice, respectively. Both tumors from NOG and NSG mice were composed of large epithelium-like cells with nuclear pleomorphism and increased mitotic figures. Bar=100 µm. H&E stain.
The tumor observed in the pubic region of a NOG mouse in Group 1 was diagnosed as a spontaneous osteosarcoma, and it was immunohistochemically negative for both anti-HLA antibody and anti-human specific Ki-67 antibody. Therefore, the tumor was judged not to be derived from inoculated HeLa cells.
Discussion
To provide fundamental biological data of NOG and NSG mice, two experiments were conducted. The first was to collect biological background data up to 11 weeks of age, and the second was to assess their susceptibility to tumorigenesis by xenotransplantation using HeLa cells.
Neither death nor spontaneous tumors were noted in both strains during the experimental periods until 11 weeks of age. The body and organ weights were significantly lighter in NOG mice than in NSG mice. The food consumption was correspondingly lower in NOG mice than in NSG mice. With regard to the body weight difference, it is considered to reflect the genetic predisposition, whereas exogenous factors such as breeding environment difference between the vendors could not be totally excluded. Moreover, in the clinical observation, alopecia in the parietal region was observed transiently from 7 to 9 weeks of age only in a small number of female NSG mice, though its cause and meaning were obscure.
The most typical findings in NOG and NSG mice were extremely small numbers of peripheral blood lymphocytes and severe systemic hypoplasia of the lymphoid tissues, and the severities of such changes were almost similar between the two mouse strains. Hypoplastic lymphoid tissues were characterized by severe lymphocyte depletion with poorly developed tissue architecture. The above-mentioned hematological and histological changes in NOG and NSG mice were consistent with those in previous reports [6, 8, 17]. On the other hand, some hematological parameters showed statistically significant, but minimal, differences between NOG and NSG mice. Such differences were considered not to be biologically meaningful. Taken together with the similarity in gene mutation [4, 17], the severity of the immuno-deficient state was judged to be similar between NOG and NSG mice.
As for the histological changes other than those in the lymphoid system, subcapsular cell hyperplasia in the adrenal was more frequently observed in NSG mice than in NOG mice. This finding is known as an age-related change and more commonly found in females than in males [11]. Our previous study to collect biological background data of NOG mice up to 52 weeks of age revealed that subcapsular hyperplasia in the adrenal increased with age, although the occurrence at 7 weeks of age was low (0.5%) in both sexes [6]. Although the real meaning of this histological change is obscure, it is thought to be less meaningful because it is supposed to have less influence on adrenal function.
NOG and NSG mice have the genetic background of NOD-scid mice which develop thymic lymphoma. Therefore, the occurrence of thymic lymphoma was the matter of main concern in NOG and NSG strains. However, the incidence of thymic lymphoma has been reported to be very low in NOG mice [19], and its occurrence has not been reported in NSG mice [15]. In the present study, neither thymic lymphoma nor lymphoid hyperplasia was observed in mice of either strain.
Spongiotic changes having minimal severity appeared focally or multifocally in the medulla oblongata and spinal cord, and the incidence was slightly higher in NOG mice than in NSG mice. In addition, our previous study revealed that such change appeared at 7 weeks of age without neurological signs, and disappeared until 52 weeks of age [6]. Further investigation of this change may be required, as its pathogenesis is still obscure.
The incidence of immaturity of female genital organs was slightly higher in NOG mice than in NSG mice at 9 weeks of age. This seems to reflect minor differences in the developmental phase between the two strains of mice. Moreover, ectopic exocrine gland and cyst in the thymus were found in NOG and NSG mice without strain differences, and the incidence of the latter was high. It seems to be reasonable to consider that the former is congenital in nature and the latter is secondary to thymic hypoplasia. Therefore, these changes seem not to reduce the usefulness of NOG and NSG mice as a host of xenotransplantation.
There are many references in the literature that NOG or NSG mice were the most suitable mouse strain for human cell engraftment and human hematopoietic stem cell transplantation [1,2,3, 5, 9, 14, 17]. However, as mentioned in the introduction, there have been no publications of direct comparison between NOG and NSG mice except for the report by McDermott et al. [10]. They reported that as compared with NOG mice, NSG mice generated a higher graft in human cord blood engraftment in the bone marrow. In the present study, there was no significant difference between NOG and NSG mice in HeLa cell engraftment. Xenograft efficiency is well known to differ by type of tumor, site of implantation and tumor aggressiveness [13]. Therefore, the difference in the results between our present study and McDermott et al. might be due to the differences in the engrafted cell type and/or site. Both NOG and NSG mice are known to be superior in xenograft efficiency compared with other immune-deficient mouse strains [5, 7, 9, 10]. To confirm which strain of NOG and NSG mice is more useful to evaluate xenograft efficiency will require more direct comparative studies between NOG and NSG mice using various human tumor cell lines.
In conclusion, this study revealed that there were no biologically meaningful differences between NOG and NSG mice in clinical and histological background data up to 11 weeks of age and in tumorigenesis by HeLa cell implantation.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgments
The authors gratefully acknowledge Dr. Kunio Doi, Professor Emeritus of the University of Tokyo, for critical review of the manuscript, Mr. Pete Aughton, D.A.B.T., Dip.R.C.Path., ITR Laboratories Canada Inc., for proofreading and the Central Institute for Experimental Animals and Charles River Laboratories Japan for providing NOG and NSG mice respectively. We also thank Atsushi Wakita, Kazuhisa Yamada, Kenji Ohtani, Eiichi Kobayashi, Kazuko Tsurumoto, Maki Noguchi, Minatsu Haruki and Satomi Hirochika for technical assistance.
References
- 1.Brayton C.2017. In defense of parity, females, and pathology in research. Vet. Pathol. 54: 731–733. doi: 10.1177/0300985817717772 [DOI] [PubMed] [Google Scholar]
- 2.Chijiwa T., Kawai K., Noguchi A., Sato H., Hayashi A., Cho H., Shiozawa M., Kishida T., Morinaga S., Yokose T., Katayama M., Takenaka N., Suemizu H., Yamada R., Nakamura Y., Ohtsu T., Takano Y., Imai K., Miyagi Y., Nakamura M.2015. Establishment of patient-derived cancer xenografts in immunodeficient NOG mice. Int. J. Oncol. 47: 61–70. doi: 10.3892/ijo.2015.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L.D., Harada M.2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chain(null) mice. Blood 106: 1565–1573. doi: 10.1182/blood-2005-02-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., Heike T., Nakahata T.2002. NOD/SCID/γ(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100: 3175–3182. doi: 10.1182/blood-2001-12-0207 [DOI] [PubMed] [Google Scholar]
- 5.Kanaji N., Tadokoro A., Susaki K., Yokokura S., Ohmichi K., Haba R., Watanabe N., Bandoh S., Ishii T., Dobashi H., Matsunaga T.2014. Higher susceptibility of NOD/LtSz-scid Il2rg (-/-) NSG mice to xenotransplanted lung cancer cell lines. Cancer Manag. Res. 6: 431–436. doi: 10.2147/CMAR.S71185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasahara K., Fukunaga Y., Igura S., Andoh R., Saito T., Suzuki I., Kanemitsu H., Suzuki D., Goto K., Nakamura D., Mochizuki M., Yasuda M., Inoue R., Tamura K., Nagatani M.2017. Background data on NOD/Shi-scid IL-2Rγnull mice (NOG mice). J. Toxicol. Sci. 42: 689–705. doi: 10.2131/jts.42.689 [DOI] [PubMed] [Google Scholar]
- 7.Kusakawa S., Machida K., Yasuda S., Takada N., Kuroda T., Sawada R., Okura H., Tsutsumi H., Kawamata S., Sato Y.2015. Characterization of in vivo tumorigenicity tests using severe immunodeficient NOD/Shi-scid IL2Rγnull mice for detection of tumorigenic cellular impurities in human cell-processed therapeutic products. Regen Ther 1: 30–37. doi: 10.1016/j.reth.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knibbe-Hollinger J.S., Fields N.R., Chaudoin T.R., Epstein A.A., Makarov E., Akhter S.P., Gorantla S., Bonasera S.J., Gendelman H.E., Poluektova L.Y.2015. Influence of age, irradiation and humanization on NSG mouse phenotypes. Biol. Open 4: 1243–1252. doi: 10.1242/bio.013201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida K., Suemizu H., Kawai K., Ishikawa T., Sawada R., Ohnishi Y., Tsuchiya T.2009. Higher susceptibility of NOG mice to xenotransplanted tumors. J. Toxicol. Sci. 34: 123–127. doi: 10.2131/jts.34.123 [DOI] [PubMed] [Google Scholar]
- 10.McDermott S.P., Eppert K., Lechman E.R., Doedens M., Dick J.E.2010. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 116: 193–200. doi: 10.1182/blood-2010-02-271841 [DOI] [PubMed] [Google Scholar]
- 11.Nyska A., Maronpot R.R.1999. Adrenal gland. pp 509–536. In: Pathology of the mouse (Maronpot, R.R., Boorman, G.A., and Gaul, B.W. eds.), Cache River Press, Vienna. [Google Scholar]
- 12.Okada S.2017. Application of highly immunodeficient mice for biomedical research. Cytometry Res 27: 25–31. [Google Scholar]
- 13.Okada S., Vaeteewoottacharn K., Kariya R.2018. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine. Chem. Pharm. Bull. (Tokyo) 66: 225–230. doi: 10.1248/cpb.c17-00789 [DOI] [PubMed] [Google Scholar]
- 14.Puchalapalli M., Zeng X., Mu L., Anderson A., Hix Glickman L., Zhang M., Sayyad M.R., Mosticone Wangensteen S., Clevenger C.V., Koblinski J.E.2016. NSG mice provide a better spontaneous model of breast cancer metastasis than athymic (nude) mice. PLoS One 11: e0163521. doi: 10.1371/journal.pone.0163521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santagostino S.F., Arbona R.J.R., Nashat M.A., White J.R., Monette S.2017. Pathology of aging in NOD scid gamma female mice. Vet. Pathol. 54: 855–869. doi: 10.1177/0300985817698210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shultz L.D., Goodwin N., Ishikawa F., Hosur V., Lyons B.L., Greiner D.L.2014. Human cancer growth and therapy in immunodeficient mice. Cold Spring Harb. Protoc. 2014: 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S., Kotb M., Gillies S.D., King M., Mangada J., Greiner D.L., Handgretinger R.2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R γ null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174: 6477–6489. doi: 10.4049/jimmunol.174.10.6477 [DOI] [PubMed] [Google Scholar]
- 18.Snedecor G.W., Cochran W.G.1989. Statistical methods. 8th ed. Ames: Iowa State University Press. [Google Scholar]
- 19.Yasuda M., Ogura T., Goto T., Yagoto M., Kamai Y., Shimomura C., Hayashimoto N., Kiyokawa Y., Shinohara H., Takahashi R., Kawai K.2017. Incidence of spontaneous lymphomas in non-experimental NOD/Shi-scid, IL-2Rγnull (NOG) mice. Exp. Anim. 66: 425–435. doi: 10.1538/expanim.17-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]