Abstract
The senescence-accelerated mouse (SAM) strain has been established as an inbred strain with an accelerated aging phenotype. SAM prone-8 (SAMP8), one of the SAM strain, exhibits learning disability, immune deficiency, and circadian rhythm loss at a relatively young age. However, it has not been clarified whether aging affects the autonomic nervous activity in SAMP8. The aim of this study was to clarify the utility of SAMP8 in age-related studies of autonomic nervous function. Electrocardiogram (ECG), body temperature, and locomotor activity were recorded to evaluate bio-behavioral activities. Autonomic nervous activity was evaluated via power spectral analysis of heart rate variability from ECG recordings. SAMP8 significantly decreased both biological and autonomic nervous functions, and the animals exhibited circadian rhythm loss of locomotive activity at as early as 40 weeks of age compared with a control strain at the same age. We concluded that the SAMP8 strain can be used as an animal model for age-related studies of autonomic nervous function.
Keywords: aging, autonomic nervous function, circadian rhythm, heart rate variability, senescence-accelerated mouse prone-8
Introduction
In the last few decades, the advancement of life science technologies has led to an increase in the life span of humans in developed countries, resulting in a larger population of elderly people [18]. Age-related problems, such as cancer, cardiovascular diseases, and neurodegenerative diseases, are thereby growing problems in such countries and are strongly impacting health expectancy and quality of life (QOL). Generally, elderly people experience many biological dysfunctions, such as decrease in physical activity and immune function, learning disability, and disturbed life rhythm [2, 5]. Aging also alters the autonomic nervous activity, and changes in the autonomic nervous system functions can significantly impair the QOL of the elderly [10].
The autonomic nervous system is essential for the quick adaptation and modulation of visceral functions when external and/or internal environments change [10]. Like other biological functions, the autonomic nervous system follows a circadian rhythm, which is an important factor for adapting to environmental changes. The parasympathetic nervous activity is dominant during the resting phase, whereas the sympathetic nervous activity takes over during the active phase in most mammals [8, 9]. Heart rate variability (HRV) has been utilized as a noninvasive index of autonomic nervous activity. Among the numerous methods proposed for HRV assessment, power spectral analysis has been established to provide satisfactory information regarding the autonomic nervous functions in many animal species, including humans [12, 13]. Several studies on HRV have reported decreased parasympathetic nervous function with aging in humans [15, 19].
Mice demonstrate increased sympathetic nervous activity and decreased parasympathetic nervous activity with aging [6]. Experimental studies of senescence usually include many limitations and are by nature difficult to perform. In particular, long time periods are required to observe age-related changes. Generally, mammalian senescence is thought to start after sexual maturation and then progress gradually. In the case of rodents, such as rats and mice, senescence starts 12 months after sexual maturation when the rodent is aged <2 months [14]. Using appropriate models is essential for clarifying particular mechanisms of senescence for it to be translated in other animal species, including humans.
The senescence-accelerated mouse (SAM) strain was developed from AKR/J by Kyoto University and established as an inbred line displaying an accelerated aging phenotype. Nine SAM prone (SAMP) and three SAM resistant (SAMR) inbred strains have been established via selective breeding [23]. Each SAMP inbred line exhibits a different phenotype in terms of survival curves and grading scores [9]. The SAM prone 8 (SAMP8) strain exhibits learning disability, immune deficiency, and circadian rhythm loss in the adult stage (age, 4–12 months) [1, 17, 28]. The autonomic nervous system follows a circadian rhythm, and this could influence other bio-behavioral activities. In addition, oxidative stress increases with aging in SAMP8 males, and their mean life span is significantly shorter than that of SAMR1 males (381 vs 567 days, respectively) [25]. However, whether the autonomic nervous function of SAMP8 demonstrates adverse changes with aging or at a relatively young age remains to be clarified.
This study aimed to clarify whether changes in the autonomic nervous function as well as bio-behavioral activities can be detected at younger age in SAMP8 and whether SAMP8 can be used to study aging in the autonomic nervous system.
Materials and Methods
Male SAMP8/TaSlc and SAMR1/TaSlc were purchased from Japan SLC (Hamamatsu, Japan). SAMP8 (17–20 weeks old, n=5; 37–40 weeks old, n=3) and SAMR1 (17–20 weeks old, n=4; 37–40 weeks old, n=3) were used for the experiments. The mice were individually housed under a 12:12-h light:dark cycle (light on at 08:00) in a temperature-controlled conventional room (23–24°C). Standard mouse chow (MF; Oriental Yeast, Tokyo, Japan) and water were supplied adlibitum. All animal experiments were performed in accordance with the Ethical Guidelines of the Institute under the protocols approved by the Animal Experimental Expert Committee of the University of Tokyo.
The mice were anesthetized with pentobarbital sodium (40 mg/kg; i.p.) or isoflurane (2.0%; inhalation), and telemetric transmitters (TA10ETA-F20; Data science international, St. Paul, MN, USA) were implanted in their neck for continuous electrocardiogram (ECG) recording with an ECG processor (SBP2000, Softron, Tokyo, Japan) at 17 (younger group) or 37 weeks of age (older group). Heart rate (HR), body temperature (BT), and locomotive activity (LA) data were recorded every 5 min using the Dataquest A.R.T. 4.1 acquisition system (Data Science International, St. Paul, MN, USA). The actual experimental recordings were started 3 weeks after the implantation.
An off-line power spectral analysis of HRV was performed using an ECG analyzing software (SRV2W; Softron, Tokyo, Japan) from R-R intervals of recorded ECG data, as described previously [16]. Briefly, one dataset for fast Fourier transform was constructed by 512 points resampled at 70 msec. A hamming window, which subsequently underwent fast Fourier transform to obtain the power spectrum of the fluctuation, was applied to each dataset. The frequency range in classification was selected based on previous studies, classified with low frequency (LF) if between 0.1 and 1.0 Hz and high frequency (HF) if between 1.0 and 5.0 Hz [12, 13]. HF power indicates the parasympathetic nervous activity, and LF power is affected by both the sympathetic and parasympathetic nervous activities. The ratio of LF to HF (LF/HF) indicates the balance of autonomic nervous functions [26]. Data were summarized for every 24 h as well as in each dark phase (20:00–08:00) and light phase (08:00–20:00), corresponding to the active and resting phases in mice, respectively.
The circadian rhythmicity of each parameter was evaluated with the Lomb-Scargle periodogram using the free stat software (R.3.5.0). This method is a useful, all-round-method that handles all types of data collection and solves problems of missing data [20]. These data were compared by chi-square analysis. Data were presented as mean ± SEM. The Mann-Whitney U-test was used to compare 20-and 40-week-old mice of the same strain. P<0.05 was considered significant.
Results
The circadian rhythm and bio-behavioral activities
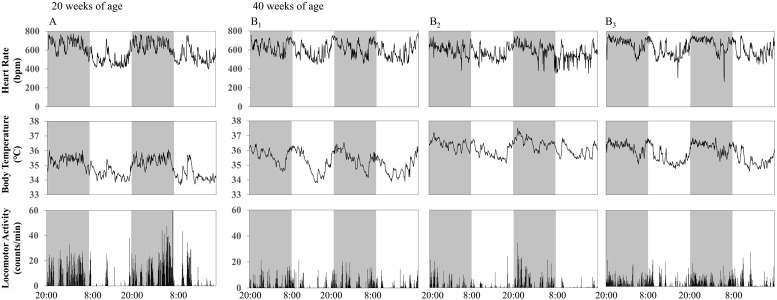
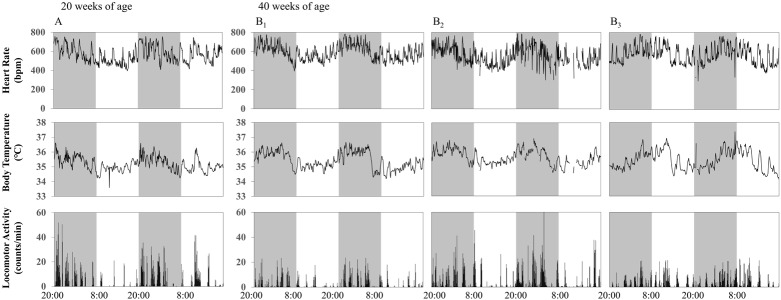
Individual data of HR, BT, and LA for 2 days in the one 20- and three 40-week-old SAMP8 animals are presented in Fig. 1. HR, BT, and LA were higher in the dark phase in the 20-week-old SAMP8, indicating circadian (24-h) rhythm, which was confirmed using the Lomb-Scargle periodogram (Table 1). However, in the 40-week-old SAMP8, the circadian rhythm seemed to be blunted, and most parameters showed a strong 12-h rhythm (Figs. 1B1 and 1B2), which was never seen in the 20-week-old SAMP8 (Table 1). In the SAMR1 controls, the 24-h rhythm in HR, BT, and LA was strongly observed even at 40 weeks of age (Fig. 2, Table 1). In addition, the 12-h rhythm in HR and BT was observed at 20 weeks of age, but the rhythm was lost at 40 weeks of age (Fig. 2, Table 1).
Fig. 1.
Individual data of 48-h recordings of heart rate (HR), body temperature (BT), and locomotor activity (LA) in one 20-week-old (A) and three 40-week-old senescence accelerated mouse-prone 8 (SAMP8) animals (B). (A) 24-h rhythm was observed in all parameters. (B1) 24-h rhythm was observed in BT and 12-h rhythm was observed in all parameters. (B2) 24-h rhythm was observed in HR and BT, and 12-h rhythm was observed in all parameters. (B3) 24-h rhythm was observed in all parameters and 12-h rhythm was observed in BT.
Table 1. The results of Lomb-Scargle periodogram in bio-behavioral function.
| Periods | Measure | SAM prone 8 | SAM resistant 1 | ||
|---|---|---|---|---|---|
| 20-week-old | 40-week-old | 20-week-old | 40-week-old | ||
| 24 h | HR | 100% (5/5) | 67% (2/3) | 100% (4/4) | 100% (3/3) |
| BT | 100% (5/5) | 100% (3/3) | 100% (4/4) | 100% (3/3) | |
| LA | 100% (5/5) | 33% (1/3)* | 100% (4/4) | 100% (3/3) | |
| 12 h | HR | 0% (0/5) | 67% (2/3)* | 100% (4/4) | 0% (0/3)* |
| BT | 0% (0/5) | 100% (3/3)* | 75% (3/4) | 0% (0/3)* | |
| LA | 0% (0/5) | 67% (2/3)* | 25% (1/4) | 0% (0/3) | |
The number of parentheses indicate the number of animals showing 24 h or 12 h rhythm. HR: heart rate, BT: body temperature, LA: locomotor activity. *P<0.05 by chi-square test between 20-week-old and 40-week-old.
Fig. 2.
Individual data of 48-h recordings of heart rate (HR), body temperature (BT), and locomotor activity (LA) in one 20-week-old (A) and three 40-week-old SAMR1 animals (B). (A) 24-h rhythm was observed in HR and BT. (B) 24-h rhythm was observed in all parameters.
There was no significant difference in bio-behavioral parameters between SAMP8 and SAMR1 at 20 weeks of age (Supplementary Table 1). Age-related changes in bio-behavioral activities in SAMP8 are summarized in Fig. 3. HR did not change with aging between 20 and 40 weeks (Fig. 3A), however, BT significantly increased at 40 weeks of age, particularly in the light phase (Fig. 3B). LA in SAMP8 significantly decreased at 40 weeks of age in the dark phase but not in the light phase (Fig. 3C). In contrast, bio-behavioral activities were similar between SAMR1 of 20 and 40 weeks of age (Figs. 3D–F).
Fig. 3.
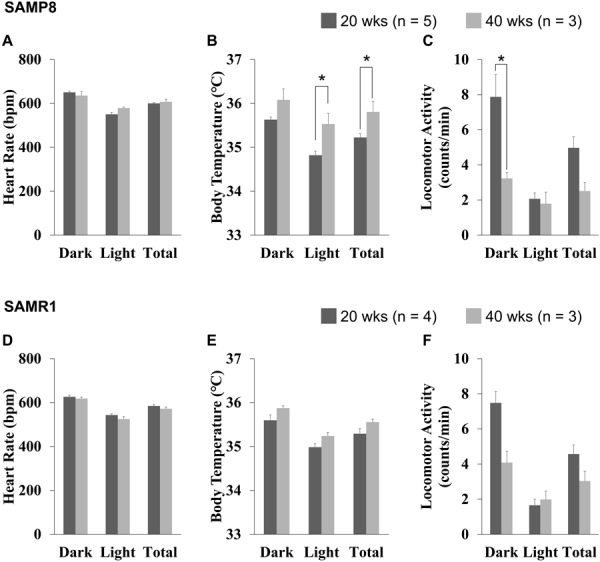
Group data of bio-behavioral activities in senescence accelerated mouse-prone 8 (SAMP8) (A–C) and SAMR1 (D–F). Heart rate (HR) (A), body temperature (BT) (B), and locomotor activity (LA) (C) of SAMP8 and HR (D), BT (E), and LA (F) in SAMR1 in the dark and light phase as well as during 24 h from 20- and 40-week-old SAMP8 animals. Data were averaged from 12- or 24-h consecutive recordings. *P<0.05 by the Mann-Whitney U-test between 20 week-old and 40-week-old.
Autonomic nervous function
There was no significant difference parameters of HRV between SAMP8 and SAMR1 at 20 weeks of age (Supplementary Table 2). Parameters of HRV in SAMP8 are shown in Fig. 4. LF and HF powers were greater in the light phase than in the dark phase regardless of age. Particularly in the light phase, both LF and HF powers were significantly smaller in the SAMP8 at 40 weeks of age than at 20 weeks of age (Figs. 4A and B). LF/HF did not show any photoperiod-related differences or age-related changes (Fig. 4C). These results in SAMR1 are summarized in Figs. 4D–F. SAMR1 did not show significant age-related changes in autonomic nervous functions.
Fig. 4.
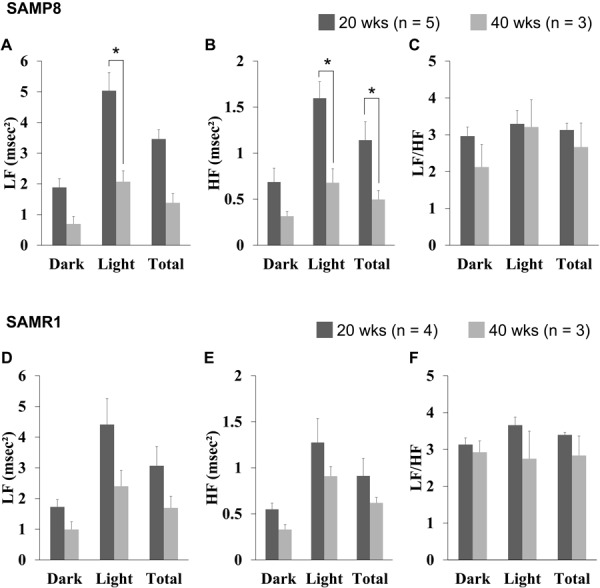
Group data of heart rate variability in the dark and light phase as well as during 24 h from 20- and 40-week-old senescence accelerated mouse-prone 8 (SAMP8) (A–C) and SAMR1 (D–F) animals. Data were averaged from 12- or 24-h consecutively analyzed values. LF: low frequency (A, D), HF: high frequency (B, E), and LF/HF: LF to HF ratio (C, F). *P<0.05 by the Mann-Whitney U-test between 20 week-old and 40-week-old.
Discussion
We aimed to evaluate the age-related changes in autonomic nervous functions using the model animal SAMP8, which allows for studying aging at relatively younger ages. In addition, we aimed to validate, if possible, the utility of this inbred line for future senescence studies in terms of autonomic nervous function. Our data demonstrate the blunted circadian rhythm of the bio-behavioral activities in elderly SAMP8 animals at 40 weeks of age, suggesting that senescence in bio-behavioral activities had started earlier than normally expected [2]. In addition, HRV was also changed in SAMP8 at 40 weeks of age, indicating that senescence was accelerated at a relatively young age and that this animal model could be used for aging studies related particularly to autonomic nervous function. On the other hand, SAMR1 showed clear circadian (24-h) rhythm of the bio-behavioral activities even at 40 weeks of age, while some of SAMR1 showed 12-h rhythm in bio-behavioral activities at 20 weeks of age but not at 40 weeks of age. The 12-h rhythm (i.e., circadian “harmonics”) can be ‘overwhelmed’ by the 24-h rhythm [11], and therefore 40-week-old SAMR1 may have strong circadian rhythm and/or weaken 12-h rhythm in bio-behavioral activities, which should be clarified in future studies. In addition, although further studies, what causes 12-h rhythm found in this study and whether 12-h rhythm is influenced by aging ,will be required, it seems possible that 20-week old SAMP8 had relatively strong 24-h rhythm and/or weaken 12-h rhythm like 40-week old SAMR1 had. On the contrary, some SAMP8 showed significant 12-h rhythm in bio-behavioral activities at 40 weeks of age, which may be due to the disturbance as well as the weakened of 24-h rhythms with aging. It should be noted that losing 24-h rhythm under the controlled light:dark condition can be crucial, probably suggesting accelerated senescence in bio-behavioral activities.
As previously reported, SAMP8 strains exhibit a disrupted circadian rhythm in behavior at 4 months of age in constant dark conditions [29]. Yanai et al. [29] also reported that in SAMP8 at 4 months (approximately 16 weeks) of age, the circadian rhythm could be reset by light, indicating that the resetting mechanism had been preserved. In this study, obvious circadian rhythm was observed in SAMP8 even at 20 weeks of age, maybe because the resetting mechanism was stimulated every 24 h via 12-h light:dark cycle to activate/inactivate autonomic nervous function. Therefore, SAMP8 at 20 weeks of age can represent the normal phenotype in studies of autonomic nervous function.
Age-related changes in bio-behavioral activities were also observed in this study. Interestingly, LA decreased in the dark phase in SAMP8 at 40 weeks of age; however, BT was not changed, suggesting that decreased LA did not affect BT. Alternatively, LA was not influenced by aging in the light phase but BT was significantly increased. This may result from neurogenic changes in the thermoregulatory system involving the hypothalamus [7]. HR did not show any age-related changes, indicating that changes in LA or BT did not influence the HR or the rhythmic outputs of autonomic nervous activity.
Basso et al. [2] reported that in rodents, LA and BT decreased with aging and that a moderate drop of BT could be beneficial for longevity. In the present study, age-related decrease in LA was also observed in SAMP8, suggesting that LA decrease is specific for aging. On the contrary, age-related increase in BT was observed in SAMP8, which was not consistent with elder BALB/c mice or humans [3]. However, the increase in BT may correspond to an accelerated senescence, or increase in cell death resulting in accelerating cell cycle for the repair, since p38 MAPK that promotes cell death and cell cycle induces thermogenesis [4].
Although HR was similar in both SAMP8 at 20 and 40 weeks of age, autonomic nervous function decreased at 40 weeks of age. Both LF and HF powers particularly decreased in the light phase, indicating a decrease in parasympathetic nervous function. In addition, LF to HF ratio did not change but LF power decreased with aging, suggesting sympathetic nervous function also decreased. These results are similar to those from earlier studies about mice [6] as well as human showing decreases in HF and LF with aging [21]. These changes might result from the disturbance of the circadian rhythm observed in this study. Thus, sensitive biomarker of aging could be HRV but not HR in SAMP8 and possibly in human, too.
In present study, SAMR1 was used as a control strain, because SAMR strains has been known as strains expressing “normal aging” [24]. In fact, the median survival of SAMR1 strains is 18.9 months [23], which is similar to conventional strains, such as ICR (about 17 months)[22] and BALB/c (about 17 months)[27]. In addition, SAMR1 showed similar HRV to those of >6 months old ICR mice [12]. Thus, SAMR1 could be an adequate control strain in terms of autonomic nervous function and aging.
In conclusion, 40-week-old SAMP8 exhibited a decrease in LA and parasympathetic nervous function and a dominant sympathetic nervous function the at rest phase, suggesting that SAMP8 is an appropriate model for age-related studies of autonomic nervous function.
Supplementary Material
Reference
- 1.Akiguchi I., Pallàs M., Budka H., Akiyama H., Ueno M., Han J., Yagi H., Nishikawa T., Chiba Y., Sugiyama H., Takahashi R., Unno K., Higuchi K., Hosokawa M.2017. SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda’s legacy and future directions. Neuropathology 37: 293–305. doi: 10.1111/neup.12373 [DOI] [PubMed] [Google Scholar]
- 2.Basso A., Del Bello G., Piacenza F., Giacconi R., Costarelli L., Malavolta M.2016. Circadian rhythms of body temperature and locomotor activity in aging BALB/c mice: early and late life span predictors. Biogerontology 17: 703–714. doi: 10.1007/s10522-016-9635-y [DOI] [PubMed] [Google Scholar]
- 3.Blatteis C.M.2012. Age-dependent changes in temperature regulation - a mini review. Gerontology 58: 289–295. doi: 10.1159/000333148 [DOI] [PubMed] [Google Scholar]
- 4.Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessì-Fulgheri P., Zhang C., Takahashi N., Sarzani R., Collins S.2012. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122: 1022–1036. doi: 10.1172/JCI59701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castelo-Branco C., Soveral I.2014. The immune system and aging: a review. Gynecol. Endocrinol. 30: 16–22. doi: 10.3109/09513590.2013.852531 [DOI] [PubMed] [Google Scholar]
- 6.Freeling J.L., Li Y.2015. Age-related attenuation of parasympathetic control of the heart in mice. Int. J. Physiol. Pathophysiol. Pharmacol. 7: 126–135. [PMC free article] [PubMed] [Google Scholar]
- 7.Greaney J.L., Kenney W.L., Alexander L.M.2016. Sympathetic regulation during thermal stress in human aging and disease. Auton. Neurosci. 196: 81–90. doi: 10.1016/j.autneu.2015.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto M., Kuwahara M., Tsubone H., Sugano S.1999. Diurnal variation of autonomic nervous activity in the rat: investigation by power spectral analysis of heart rate variability. J. Electrocardiol. 32: 167–171. doi: 10.1016/S0022-0736(99)90095-X [DOI] [PubMed] [Google Scholar]
- 9.Hosokawa M., Kasai R., Higuchi K., Takeshita S., Shimizu K., Hamamoto H., Honma A., Irino M., Toda K., Matsumura A., et al. 1984. Grading score system: a method for evaluation of the degree of senescence in senescence accelerated mouse (SAM). Mech. Ageing Dev. 26: 91–102. doi: 10.1016/0047-6374(84)90168-4 [DOI] [PubMed] [Google Scholar]
- 10.Hotta H., Uchida S.2010. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatr. Gerontol. Int. 10:(Suppl 1): S127–S136. doi: 10.1111/j.1447-0594.2010.00592.x [DOI] [PubMed] [Google Scholar]
- 11.Hughes M.E., Hong H.K., Chong J.L., Indacochea A.A., Lee S.S., Han M., Takahashi J.S., Hogenesch J.B.2012. Brain-specific rescue of Clock reveals system-driven transcriptional rhythms in peripheral tissue. PLoS Genet. 8: e1002835. doi: 10.1371/journal.pgen.1002835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishii K., Kuwahara M., Tsubone H., Sugano S.1996. The telemetric monitoring of heart rate, locomotor activity, and body temperature in mice and voles (Microtus arvalis) during ambient temperature changes. Lab. Anim. 30: 7–12. doi: 10.1258/002367796780744992 [DOI] [PubMed] [Google Scholar]
- 13.Ishii K., Kuwahara M., Tsubone H., Sugano S.1996. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab. Anim. 30: 359–364. doi: 10.1258/002367796780739880 [DOI] [PubMed] [Google Scholar]
- 14.Kitani K.1988. Neurohumoral control of liver functions during aging. Gerontology 34: 55–63. doi: 10.1159/000212931 [DOI] [PubMed] [Google Scholar]
- 15.Korkushko O.V., Shatilo V.B., Plachinda YuI, Shatilo T.V.1991. Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. J. Auton. Nerv. Syst. 32: 191–198. doi: 10.1016/0165-1838(91)90113-H [DOI] [PubMed] [Google Scholar]
- 16.Kuwahara M., Yayou K., Ishii K., Hashimoto S., Tsubone H., Sugano S.1994. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J. Electrocardiol. 27: 333–337. doi: 10.1016/S0022-0736(05)80272-9 [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto M.1997. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp. Gerontol. 32: 139–148. doi: 10.1016/S0531-5565(96)00061-7 [DOI] [PubMed] [Google Scholar]
- 18.Oeppen J., Vaupel J.W.2002. Demography. Broken limits to life expectancy. Science 296: 1029–1031. doi: 10.1126/science.1069675 [DOI] [PubMed] [Google Scholar]
- 19.Poller U., Nedelka G., Radke J., Pönicke K., Brodde O.E.1997. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. J. Am. Coll. Cardiol. 29: 187–193. doi: 10.1016/S0735-1097(96)00437-8 [DOI] [PubMed] [Google Scholar]
- 20.Refinetti R.1993. Laboratory instrumentation and computing: comparison of six methods for the determination of the period of circadian rhythms. Physiol. Behav. 54: 869–875. doi: 10.1016/0031-9384(93)90294-P [DOI] [PubMed] [Google Scholar]
- 21.Shannon D.C., Carley D.W., Benson H.1987. Aging of modulation of heart rate. Am. J. Physiol. 253: H874–H877. [DOI] [PubMed] [Google Scholar]
- 22.Storer J.B.1966. Longevity and gross pathology at death in 22 inbred mouse strains. J. Gerontol. 21: 404–409. doi: 10.1093/geronj/21.3.404 [DOI] [PubMed] [Google Scholar]
- 23.Takeda T., Hosokawa M., Higuchi K.1997. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp. Gerontol. 32: 105–109. doi: 10.1016/S0531-5565(96)00036-8 [DOI] [PubMed] [Google Scholar]
- 24.Takeda T., Hosokawa M., Higuchi K.2013. Senescence-accelerated mouse (SAM). pp. 3–14. In: The senescence-accelerated mouse (SAM) achievements and future directions (Takeda, T., Akiguchi, I., Higuchi, K., Hosokawa, M., Hosokawa, T. and Nomura, Y. eds.), Elsevier, Amsterdam. [Google Scholar]
- 25.Taniguchi S., Hanafusa M., Tsubone H., Takimoto H., Yamanaka D., Kuwahara M., Ito K.2016. Age-dependency of the serum oxidative level in the senescence-accelerated mouse prone 8. J. Vet. Med. Sci. 78: 1369–1371. doi: 10.1292/jvms.16-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- 27.Tazume S., Umehara K., Matsuzawa H., Aikawa H., Hashimoto K., Sasaki S.1991. Effects of germfree status and food restriction on longevity and growth of mice. Jikken Dobutsu 40: 517–522. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita Y., Chiba Y., Xia C., Hirayoshi K., Satoh M., Saitoh Y., Shimada A., Nakamura E., Hosokawa M.2005. Different adaptive traits to cold exposure in young senescence-accelerated mice. Biogerontology 6: 133–139. doi: 10.1007/s10522-005-3499-x [DOI] [PubMed] [Google Scholar]
- 29.Yanai S., Endo S.2016. Early onset of behavioral alterations in senescence-accelerated mouse prone 8 (SAMP8). Behav. Brain Res. 308: 187–195. doi: 10.1016/j.bbr.2016.04.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.