Abstract
Studies of environmental enrichment are progressing in the fields of nervous system, stress and exercise. Recently, housing in enriched environment have shown to influence to carcinogenesis and life span. However, the study for antitumor effect of environmental enrichment are difficult to reproduce due to the complexity of the experimental technique. Thus, a simpler experiment system is needed for antitumor study using environmental enrichment. In this research, we propose a minimum environmental enrichment, which is an experimental system by placing one mouse igloo which is normally used as a mouse shelter in the rearing environment. The experimental system of minimum environmental enrichment is not only easy to reproduce but also have enhanced activity to suppress the growth of transplanted tumor significantly. It was found that the activation of NK cells is involved also in the immune system related to tumor immunity of minimum environmental enrichment. Because minimum environmental enrichment is effective in activating antitumor immunity to transplanted tumor cells in mice, we believe this will be useful for promoting antitumor studies using environmental enrichment.
Keywords: antitumor immunity, environmental enrichment, mouse, mouse igloo
Introduction
It is thought that transformed cells arise continuously in animal’s body and that immune system usually eradicates these transformed cells [11]. This process that the immune system is constantly patrolling the body for foreign invaders and aberrant cells including tumor cells to destroy is commonly referred to as immune surveillance [6, 10, 17]. Immuno-deficient mice were more susceptible to spontaneous or chemical carcinogenesis and tumor transplantation [28], and previous study in patients with immune impairment caused by immunosuppressive treatment, as seen after kidney transplantation, reported increased incidence of a wide range of cancer types [5]; these results suggest that immune levels in animals are implicated in cancer susceptibility. Various methods, such as active immunotherapy and passive immunotherapy, have been developed as a method for increasing antitumor immune activity in vivo [26].
Environmental enrichment has been developed as a method for animal welfare. Since Hebb showed that rats raised as pets were better performing in problem solving test than rats raised in research cages [15], many studies were reported similar effects of environmental enrichment to rodents or other animals mainly in physical and mental activity [25]. Currently, environmental enrichment which are defined as that all animals should be provided with space of sufficient complexity to allow expression of a wide range of normal behavior and to control and choice over their environment to reduce stress-induced behavior is generally promoted as a way to improve animal welfare and is also legally required within the European Union by Directive 2010/63/EU [9]. Although it was reported since the 1990s that environmental enrichment also affects immunity, stress, excludability of tumor cells, and an increasing of life span, the effects were controversial [2,3,4, 18, 22, 27]. Since 2010, some studies demonstrated that environmental enrichment has effects to enhance reduction and eradication of tumors [7, 12, 14, 21, 36]. Antitumor study using environmental enrichment is expected to be important and beneficial to human health. However, non-reproducible result was also reported [34]. This non-reproducibility may be due to the fact that it is difficult to strictly re-test because the condition setting is complicated. Experiments of environmental enrichment include not only unavoidable differences such as laboratory facilities, mouse lots and expertise at handling animals, but also many parameters; breeding area, type or number of play equipment, number of breeding animals and difference of animal strains. The antitumor study using environmental enrichment so far places many and various equipment in wide breeding area [30, 32]. This seems to make the experiment complicated and difficult to reproduce. Therefore, in order to eliminate these parameters as much as possible and to provide an experiment that can be retested at any experimental facility, an experimental design which is a simpler and easier to reproduce is required for antitumor studies using environmental enrichment.
In this report, we propose a minimum environmental enrichment, which installs one shelter in a normal breeding cage. We observed the effect of minimum environmental enrichment on the elimination of transplanted tumor and found that minimum environmental enrichment has sufficient tumor suppressing effect. It was also revealed that activation of NK cells plays an important role in the mechanism. The experimental system of minimum environmental enrichment is not only easy to reproduce but also effective in activating antitumor immunity to transplanted tumor cells in mice. This report is useful in advancing research on environmental enrichment and antitumor studies in a future.
Materials and Methods
Animals and environmental enrichment
Specific-pathogen-free female B6C3F1 mice at six weeks of age were purchased from an animal breeding facility (Japan CLEA, Tokyo, Japan). After 2 weeks acclimation, mice of 8 weeks of age were divided into two groups, standard environment (SE) and enriched environment (EE), and used for experiments. Four mice were housed in a normal cage. In the case of mice housed under minimum enriched environment (mEE), a mouse igloo (BioServ, Flemington, NJ, USA) was placed in the same cage (Fig. 1A) from 8 weeks of age until the end of experiment. The mouse igloos were replaced every week with a clean (washed) unit. All animals were housed in rooms maintained at a temperature of 23 ± 2°C with 50 ± 10% humidity and a 12-h light/dark cycle and were allowed ad libitum access to commercially prepared and gamma-sterilized feed pellets (FR-2; Funabashi Farm Co., Chiba, Japan) and chlorinated drinking water. In both SE and EE, 4 mice were housed together in a plastic cage (TM-TPX-10, 218W × 320D × 133H mm, floor area of 481 cm2, Tokiwa Kagaku Kikai Co., Tokyo, Japan) with wood shavings as bedding.
Fig. 1.
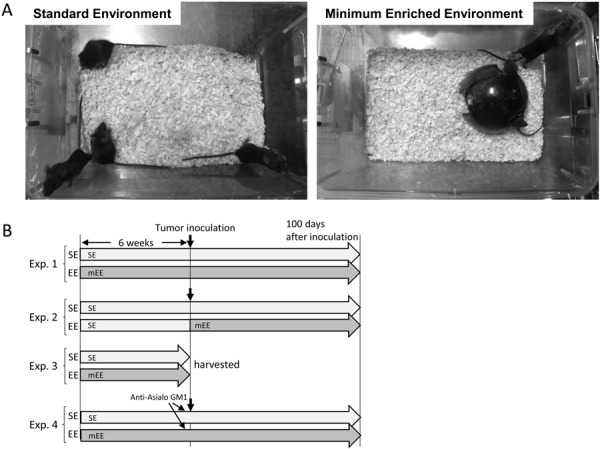
Experimental design. (A) Four mice were housed in a cage of standard environment and enriched environment. (B) Schematic diagram of the experimental protocol.
Four experiments were designed as followed (Fig. 1B).
Experiment 1: Mice of SE (n=24) or EE (n=24) were housed in each condition for 6 weeks prior to tumor cell transplantation with tumor cells as described in below. The period of EE treatment was determined to have sufficient effect previously. The EE housing was continued up to 100 days after tumor cell transplantation.
Experiment 2: Both SE (n=12) and EE (n=12) mice were housed in SE for 6 weeks. Then, tumor cells were transplanted into all mice as described in below. After that, only EE mice were housed in mEE. The EE housing was continued up to 100 days after tumor cell transplantation.
Experiment 3: Mice of SE (n=8) or EE (n=8) were housed in each condition for 6 weeks. Then blood and spleen cells were collected for analysis for flow cytometry and cytotoxic activity.
Experiment 4: Mice of SE (n=24) or EE (n=23) were housed in each condition for 6 weeks. Before 3 days of tumor cell transplantation, for NK cell depletion, mice of SE+Asialo (n=12) and EE+Asialo (n=11) were given an intraperitoneal administration of 0.01 ml of anti-asialo GM1 (10 mg/ml, Fujifilm Wako Pure Chemical Corp., Tokyo, Japan) for three consecutive days. The other mice of SE+PBS (n=12) and EE+PBS (n=12) were given an intraperitoneal administration of 0.01 ml of PBS. Then, tumor cells were transplanted into all mice as described in below. The EE housing was continued up to 100 days after tumor cell transplantation.
The experiments were conducted according to the legal regulations in the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Guidelines for Animal Experiments of the Institute for Environmental Sciences.
Tumor cell transplantation
The tumor cell line OV3121, derived from an ovarian granulosa cell tumor from female B6C3F1 mice after irradiation with high dose-rate gamma rays [37], was obtained from Health Science Research Resources Bank (Osaka, Japan). OV3121 cells cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal calf serum were trypsinized and suspended in normal saline to obtain 5 × 106 cells/ml cell suspensions. The cell suspensions (0.1 ml including 5 × 105 cells) were subcutaneously transplanted onto the back of the mice. Tumor formation and development were examined twice a week. The number of tumor-bearing mice, in which a palpable tumor was examined during the observation period of 100 days after tumor cell transplantation, was counted to assess tumor formation. The cell number inoculated to mice was determined to make tumor formation in approximately 80% of the mice.
Cytotoxic activity of NK cells
Mice were euthanized with CO2 and peripheral blood were collected aseptically. Erythrocytes were lysed using BD PharmLys (BD Biosciences, San Jose, CA, USA) and lymphocytes were collected. Yac-1 cells were obtained from DS Pharma Biomedical Co., Ltd., (Osaka, Japan). Yac-1 cells were labeled with Bromodeoxyuridine as described in Cellular DNA Fragmentation ELISA (Merck KGaA, Darmstadt, Germany). After the labeled cells were co-cultured with the lymphocytes for 4 h, the supernatants were collected. The cytotoxic activities were analysed according to the procedure for measuring cell-mediated cytotoxicity of Cellular DNA Fragmentation ELISA.
Flow cytometric analysis
The lymphocytes were stained with 7-Aminoactinomycin D (7-AAD) and antibodies of fluorescein isothiocyanate (FITC)-conjugated anti-mouse cluster of differentiation (CD) 335 (BD Biosciences). The proportions of NK cells in blood cells were calculated as 7-AAD negative and FITC positive cells using FACSCalibureTM (BD Biosciences).
Statistical analysis
Differences in the tumor cell elimination activity between mice housed in enriched environment and standard environment were examined by the log-rank test. The body weight, cytotoxic activity and cell proportion were examined using the t-test. P<0.05 was considered statistically significant.
Results and Discussion
Animals housed in enriched environment
For mEE condition, a mouse igloo has enough space to be used at the same time for 4 mice of normal size. In this report, we confirmed that 4 B6C3F1 female mice of normal size could use one mouse igloo together. It may be preferable to install 2 mouse igloos at the same time for larger mice or 5 mice. Some mice did not use the mouse igloo by burying or flipping over it, in that case the cage of the mice was not used for experiments. Furthermore, in the case of a male mouse, because of a possibility that fighting frequently occurs by installing a mouse igloo [16], it is necessary to observe mice carefully after installing mouse igloo(s).
In the experiment 1, the body weights of the mice after 6 weeks housing in mEE (n=24) were 26.5 ± 1.56 g, which was significantly (P<0.01) heavier than the body weight of 25.5 ± 1.06 g of mice housed in SE (n=24). Though the significant difference of body weight continues over 500 days after the installation of mEE, the difference is resolved because mice of SE and EE become obese after that (data not shown). Some previous study using comparably simple enrichment reported that housing in EE had no influence to body weight of BALBc/OlaHsd, C57BL/6JOlaHsd and A/JOlaHsd female mice [33], C57BL/6JOlaHsd female mice [20], and C57BL/6JOlaHsd and BALB/cOlaHsd female and male mice [35]. But the other study using comparably simple enrichment reported that housing in EE induced increased body weight of BALB/c male mice [13]. Although it is not clear why such body weight differences occur, mouse strain, sex, age and methods of enrichment may cause such differences.
Tumor elimination activity of mice housed in enriched environment (Experiment 1 and 2)
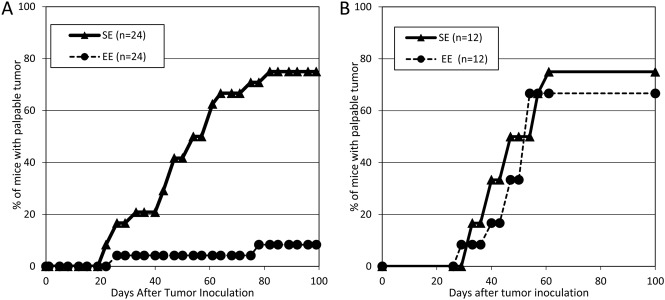
To assess tumor formation, 5 × 105 cells of OV3121 were transplanted into the mice at 14 weeks of age, housed under SE or mEE. Compared with SE group, the tumor elimination activity of mice housed in mEE was enhanced significantly (Experiment 1, Fig. 2A). As mentioned above, mice housed in mEE are heavier than SE mice. Because there was no correlation between the body weight and tumor elimination activity, it seems that the difference of body weight didn’t affect the tumor elimination activity. All mice were dissected at the end of experiment. Mice without tumor formation were appeared to be healthy without tumor metastasis or organ abnormalities. When the enriched treatment started after tumor cell transplantation, the tumor elimination activity was not significantly different between SE and EE group (Experiment 2, Fig. 2B). It indicates that mEE has a marked effect only when it was started before tumor cell transplantation but not after the transplantation. It suggests that the effect of EE may need time to affect the antitumor effect, for such as NK cell distribution.
Fig. 2.
Comparison of transplanted tumor elimination activity. (A) Experiment 1: After mice housed in SE (triangle, n=24) or mEE (circle, n=24) for 6 weeks, tumor cells were transplanted. Mice with palpable tumors were counted to assess transplanted tumor formation. (B) Experiment 2: Tumor cells were transplanted into mice of 14 weeks of age, then the mice were housed in SE (triangle, n=12) and mEE (circle, n=12). Mice with palpable tumors were counted to assess transplanted tumor formation.
NK cells of mice housed in enriched environment (Experiment 3 and 4)
It is known that innate immunity such as NK cells have an important role in the early elimination of tumors [19] and NK cell activity was enhanced by housing in mEE [2, 3, 23, 31]. So, we compared NK cell activity of lymphocytes from mice housed in mEE and SE. The NK cell activities were enhanced after mEE housing (Experiment 3, Fig. 3A). Because NK cells were known to have essential roles on tumor immunity [19, 29], it is possible that NK cells induced by mEE contribute to enhancing tumor elimination. Moreover, NK cell proportion in lymphocytes of mouse housed in mEE were not affected by mEE (Experiment 3, Fig. 3B). Recently, Meng et al. showed that EE housing induce NK cell maturation without affecting the proportion of total NK cells in peripheral blood [23]. Also in mice housed in mEE, mature NK cells may enhance the tumor elimination activity. To confirm the involvement of NK cells to enhance the tumor elimination activity, NK cells were depleted by the administration of anti-asialo GM1 to mice (Experiment 4, Fig. 3C). The tumor elimination activity was activated significantly when mice housed in mEE (EE+PBS vs. SE+PBS). On the other hand, administration of anti-Asialo GM1 canceled the effect of mEE (EE+PBS vs. EE+Asialo and SE+PBS vs. SE+Asialo). This result, again, suggested that NK cells induced by mEE contribute to enhancing tumor elimination.
Fig. 3.
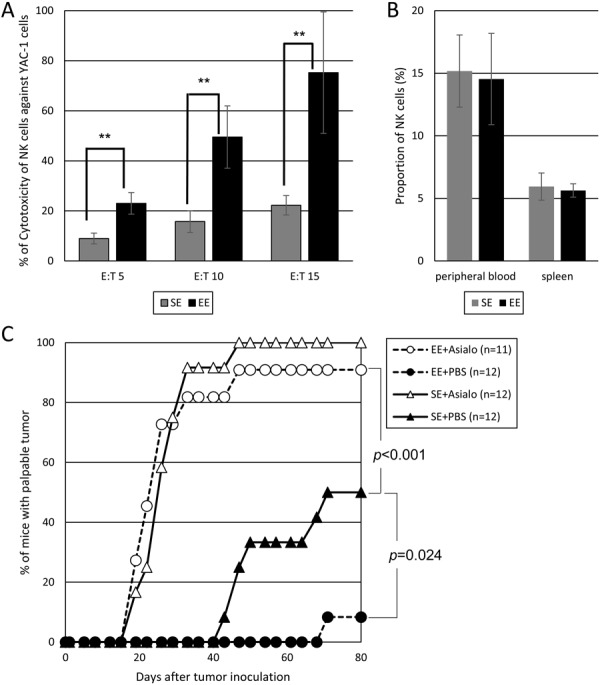
(A) Experiment 3: Comparison of cytotoxic activity of NK cells against Yac-1 cells. Bromodeoxyuridine-labeled Yac-1 cells were incubated in triplicate with lymphocytes derived from peripheral blood of mice housed in SE or mEE. Results are expressed as mean ± SD percent of specific lysis at some effector: target (E:T) ratios. **, P<0.01. (B) Experiment 3: Comparison of proportion of NK cells in peripheral blood and spleen of mice housed in SE or mEE. (C) Experiment 4: Comparison of transplanted tumor elimination activity of mice with or without anti-Asialo GM1 administration.
The pathway by which EE treatment activates NK cells is not yet clear. Cao et al. [7] showed that hypothalamic brain-derived neurotrophic factor contributed to NK activity in EE housing mice and suggested that the enhanced NK activity after EE treatment could be partly explained by increased sympathetic activity similar to that shown for “mirthful laughter” [24]. Garofalo et al. [12] showed that cerebral level of IL-15 in EE mice induced NK cell accumulation to the tumor site. Song et al. [31] explained that enhancing NK cell antitumor immunity of EE mice resulted from sympathetic nerve-dependent regulation of NK-cell-activating receptor, NKG2, and the chemokine receptor, CCR5, in NK cells. Further research is necessary to explain how mEE increased NK activity in our study.
In the present study, we used female B6C3F1 mice and a tumor cell line derived from an ovarian granulosa cell tumor from the same strain mouse. It is necessary to certain the effect of mEE in antitumor immunity to transplanted tumor cells in other mouse strains as well other tumor cell lines. Depending on the type of cancer, it is quite possible that the effects of mEE may be differ (and sometimes opposite), hence selection of the appropriate mEE would be important. Furthermore, differences of sex and age could also affect the effectiveness of mEE.
In conclusion, an experiment design of environmental enrichment using mEE is simple and easy to reproduce even in many animal experimental facilities. Furthermore, mEE is effective in activating antitumor immunity to transplanted tumor cells in mice. Some reports using simple enrichment items resembling mEE showed physiological and behavioral effects of enriched environment [1, 8, 13, 16, 20, 33, 35]. However, because they didn’t investigate immunological parameters including antitumor immunity, this is the first report to show that mEE is effective in activating antitumor immunity in mice. We propose that mEE is an experimental system suitable for antitumor studies using environmental enrichment.
Acknowledgments
We thank Mr. M. Yoneya (IES) for providing technical assistance with breeding mice. This study was performed under a contract with the Aomori Prefectural Government, Japan.
References
- 1.André V., Gau C., Scheideler A., Aguilar-Pimentel J.A., Amarie O.V., Becker L., Garrett L., Hans W., Hölter S.M., Janik D., Moreth K., Neff F., Östereicher M., Racz I., Rathkolb B., Rozman J., Bekeredjian R., Graw J., Klingenspor M., Klopstock T., Ollert M., Schmidt-Weber C., Wolf E., Wurst W., Gailus-Durner V., Brielmeier M., Fuchs H., Hrabé de Angelis M.2018. Laboratory mouse housing conditions can be improved using common environmental enrichment without compromising data. PLoS Biol. 16: e2005019. doi: 10.1371/journal.pbio.2005019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arranz L., De Castro N.M., Baeza I., Maté I., Viveros M.P., De la Fuente M.2010. Environmental enrichment improves age-related immune system impairment: long-term exposure since adulthood increases life span in mice. Rejuvenation Res. 13: 415–428. doi: 10.1089/rej.2009.0989 [DOI] [PubMed] [Google Scholar]
- 3.Benaroya-Milshtein N., Hollander N., Apter A., Kukulansky T., Raz N., Wilf A., Yaniv I., Pick C.G.2004. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur. J. Neurosci. 20: 1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x [DOI] [PubMed] [Google Scholar]
- 4.Benaroya-Milshtein N., Apter A., Yaniv I., Kukulansky T., Raz N., Haberman Y., Halpert H., Pick C.G., Hollander N.2007. Environmental enrichment augments the efficacy of idiotype vaccination for B-cell lymphoma. J. Immunother. 30: 517–522. doi: 10.1097/CJI.0b013e31804efc5e [DOI] [PubMed] [Google Scholar]
- 5.Birkeland S.A., Storm H.H., Lamm L.U., Barlow L., Blohmé I., Forsberg B., Eklund B., Fjeldborg O., Friedberg M., Frödin L., Glattre E., Halvorsen S., Holm N.V., Jakobsen A., Jorgensen H.E., Ladefoged J., Lindholm T., Lundgren G., Pukkala E.1995. Cancer risk after renal transplantation in the Nordic countries, 1964-1986. Int. J. Cancer 60: 183–189. doi: 10.1002/ijc.2910600209 [DOI] [PubMed] [Google Scholar]
- 6.Burkholder B., Huang R.Y., Burgess R., Luo S., Jones V.S., Zhang W., Lv Z.Q., Gao C.Y., Wang B.L., Zhang Y.M., Huang R.P.2014. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 1845: 182–201. [DOI] [PubMed] [Google Scholar]
- 7.Cao L., Liu X., Lin E.J., Wang C., Choi E.Y., Riban V., Lin B., During M.J.2010. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 142: 52–64. doi: 10.1016/j.cell.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coke-Murphy C., Buendia M.A., Saborido T.P., Stanwood G.D.2014. Simple shelter-style environmental enrichment alters behavior in mice. Transl. Neurosci. 5: 185–196. doi: 10.2478/s13380-014-0228-4 [DOI] [Google Scholar]
- 9.Directive 2010/63/EU of the european parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes.
- 10.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D.2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3: 991–998. doi: 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich P.1909. Über den jetzigen stand der karzinomforschung. Ned. Tijdschr. Geneeskd. 5: 273–290. [Google Scholar]
- 12.Garofalo S., D’Alessandro G., Chece G., Brau F., Maggi L., Rosa A., Porzia A., Mainiero F., Esposito V., Lauro C., Benigni G., Bernardini G., Santoni A., Limatola C.2015. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat. Commun. 6: 6623. doi: 10.1038/ncomms7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurfein B.T., Stamm A.W., Bacchetti P., Dallman M.F., Nadkarni N.A., Milush J.M., Touma C., Palme R., Di Borgo C.P., Fromentin G., Lown-Hecht R., Konsman J.P., Acree M., Premenko-Lanier M., Darcel N., Hecht F.M., Nixon D.F.2012. The calm mouse: an animal model of stress reduction. Mol. Med. 18: 606–617. doi: 10.2119/molmed.2012.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gurfein B.T., Davidenko O., Premenko-Lanier M., Milush J.M., Acree M., Dallman M.F., Touma C., Palme R., York V.A., Fromentin G., Darcel N., Nixon D.F., Hecht F.M.2014. Environmental enrichment alters splenic immune cell composition and enhances secondary influenza vaccine responses in mice. Mol. Med. 20: 179–190. doi: 10.2119/molmed.2013.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hebb D.O.1947. The effects of early experience on problem solving at maturity. Am. Psychol. 2: 306–307. [Google Scholar]
- 16.Howerton C.L., Garner J.P., Mench J.A.2008. Effects of a running wheel-igloo enrichment on aggression, hierarchy linearity and stereotypy in group-housed male CD-1 (ICR) mice. Appl. Anim. Behav. Sci. 115: 90–103. doi: 10.1016/j.applanim.2008.05.004 [DOI] [Google Scholar]
- 17.Kim R., Emi M., Tanabe K.2007. Cancer immunoediting from immune surveillance to immune escape. Immunology 121: 1–14. doi: 10.1111/j.1365-2567.2007.02587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kingston S.G., Hoffman-Goetz L.1996. Effect of environmental enrichment and housing density on immune system reactivity to acute exercise stress. Physiol. Behav. 60: 145–150. doi: 10.1016/0031-9384(95)02241-4 [DOI] [PubMed] [Google Scholar]
- 19.Koebel C.M., Vermi W., Swann J.B., Zerafa N., Rodig S.J., Old L.J., Smyth M.J., Schreiber R.D.2007. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450: 903–907. doi: 10.1038/nature06309 [DOI] [PubMed] [Google Scholar]
- 20.Kulesskaya N., Rauvala H., Voikar V.2011. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS One 6: e24755. doi: 10.1371/journal.pone.0024755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G., Gan Y., Fan Y., Wu Y., Lin H., Song Y., Cai X., Yu X., Pan W., Yao M., Gu J., Tu H.2015. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci. Rep. 5: 7856. doi: 10.1038/srep07856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marashi V., Barnekow A., Ossendorf E., Sachser N.2003. Effects of different forms of environmental enrichment on behavioral, endocrinological, and immunological parameters in male mice. Horm. Behav. 43: 281–292. doi: 10.1016/S0018-506X(03)00002-3 [DOI] [PubMed] [Google Scholar]
- 23.Meng Z., Liu T., Song Y., Wang Q., Xu D., Jiang J., Li M., Qiao J., Luo X., Gu J., Tu H., Gan Y.2019. Exposure to an enriched environment promotes the terminal maturation and proliferation of natural killer cells in mice. Brain Behav. Immun. 77: 150–160. doi: 10.1016/j.bbi.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 24.Nagatomi R.2006. The implication of alterations in leukocyte subset counts on immune function. Exerc. Immunol. Rev. 12: 54–71. [PubMed] [Google Scholar]
- 25.Olsson I.A., Dahlborn K.2002. Improving housing conditions for laboratory mice: a review of “environmental enrichment”. Lab. Anim. 36: 243–270. doi: 10.1258/002367702320162379 [DOI] [PubMed] [Google Scholar]
- 26.Peto J.2001. Cancer epidemiology in the last century and the next decade. Nature 411: 390–395. doi: 10.1038/35077256 [DOI] [PubMed] [Google Scholar]
- 27.Schapiro S.J.2002. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacol. Biochem. Behav. 73: 271–278. doi: 10.1016/S0091-3057(02)00779-7 [DOI] [PubMed] [Google Scholar]
- 28.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J., Schreiber R.D.2001. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410: 1107–1111. doi: 10.1038/35074122 [DOI] [PubMed] [Google Scholar]
- 29.Sharma P., Kumar P., Sharma R.2017. Natural Killer Cells − Their role in tumour immunosurveillance. J. Clin. Diagn. Res. 11: BE01–BE05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slater A.M., Cao L.2015. A protocol for housing mice in an enriched environment. J. Vis. Exp. 100: e52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y., Gan Y., Wang Q., Meng Z., Li G., Shen Y., Wu Y., Li P., Yao M., Gu J., Tu H.2017. Enriching the housing environment for mice enhances their NK cell antitumor immunity via sympathetic nerve-dependent regulation of NKG2D and CCR5. Cancer Res. 77: 1611–1622. doi: 10.1158/0008-5472.CAN-16-2143 [DOI] [PubMed] [Google Scholar]
- 32.Sztainberg Y., Chen A.2010. An environmental enrichment model for mice. Nat. Protoc. 5: 1535–1539. doi: 10.1038/nprot.2010.114 [DOI] [PubMed] [Google Scholar]
- 33.Tsai P.P., Pachowsky U., Stelzer H.D., Hackbarth H.2002. Impact of environmental enrichment in mice. 1: effect of housing conditions on body weight, organ weights and haematology in different strains. Lab. Anim. 36: 411–419. doi: 10.1258/002367702320389071 [DOI] [PubMed] [Google Scholar]
- 34.Westwood J.A., Darcy P.K., Kershaw M.H.2013. Environmental enrichment does not impact on tumor growth in mice. F1000 Res. 2: 140. doi: 10.12688/f1000research.2-140.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirz A., Mandillo S., D’Amato F.R., Giuliani A., Riviello M.C.2015. Response, use and habituation to a mouse house in C57BL/6J and BALB/c mice. Exp. Anim. 64: 281–293. doi: 10.1538/expanim.14-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y., Gan Y., Yuan H., Wang Q., Fan Y., Li G., Zhang J., Yao M., Gu J., Tu H.2016. Enriched environment housing enhances the sensitivity of mouse pancreatic cancer to chemotherapeutic agents. Biochem. Biophys. Res. Commun. 473: 593–599. doi: 10.1016/j.bbrc.2016.03.128 [DOI] [PubMed] [Google Scholar]
- 37.Yanagihara K., Nii M., Tsumuraya M., Numoto M., Seito T., Seyama T.1995. A radiation-induced murine ovarian granulosa cell tumor line: introduction of v-ras gene potentiates a high metastatic ability. Jpn. J. Cancer Res. 86: 347–356. doi: 10.1111/j.1349-7006.1995.tb03063.x [DOI] [PMC free article] [PubMed] [Google Scholar]