Abstract
High-flow nasal oxygen therapy warms and humidifies gases, allows better clearance of secretions, along with providing added benefits like preventing dehydration of airway surface, while decreasing atelectasis and thereby, offering comfort to the patient. While its effect on critically ill patients is still in its pioneering phase, there is lack of substantial evidence on the use of high-flow nasal cannula in cardiac patients with type I respiratory failure. We found it worthwhile to share our experience of its use in elderly and postpartum patients with moderate-to-severe pulmonary hypertension, with associated comorbidities and type I respiratory failure, with do-not-intubate or defer intubation status. In patients with pulmonary hypertension (PHT) and respiratory failure, endotracheal intubation followed by initiation of mechanical ventilation may have detrimental hemodynamic effects. Increase in lung volumes and decrease in functional residual capacity lead to increase in pulmonary hypertension and right ventricle afterload. If a patient has right heart failure, lung hyperinflation can fatally reduce cardiac output. High-flow nasal oxygen therapy may be of an advantage in these scenarios.
How to cite this article: Gupta B, Kerai S, Kakkar K, Gupta L. Role of High-flow Nasal Oxygen Therapy in Cases with Pulmonary Hypertension in an Intensive Care Unit Setting. Indian J Crit Care Med 2019;23(10):458–461.
Keywords: High-flow nasal cannula, High-flow nasal oxygen therapy, Pulmonary hypertension
INTRODUCTION
High-flow nasal oxygen (HFNO) therapy is increasingly being used as part of ward, operation theater and critical care setting in management of the patients with respiratory failure. Respiratory failure is distressing for the patients and its treatment with the help of invasive ventilation is associated with increased risk of ventilator-associated pneumonia and other complications, and on the other hand, if managed with noninvasive modes using tightly fitting masks causes discomfort to the patients, and is associated with facial skin problems. High-flow nasal oxygen therapy provides warm humidified gases at flows up to 60 liters/minute and allows delivery of gases between 33°C and 43°C, and 95–100% humidity.
CASE DESCRIPTION
We report the first case of a 75-year-old female, a known case of rheumatic heart disease with severe tricuspid regurgitation, moderate PHT and atrial fibrillation. Her echocardiogram was suggestive of right ventricular hypertrophy and dilatation, severe tricuspid regurgitation, moderate systolic dysfunction with moderate PHT. She was diagnosed with the condition 1 year ago and put on digoxin, warfarin, and sildenafil subsequently. She underwent an emergency laparotomy for perforation peritonitis, after which she landed up in ICU with features suggestive of right heart failure (raised jugular venous pressure, bilateral fine crepitations with low-systemic blood pressure (MAP <65 mm Hg), loud P2, pansystolic murmur, parasternal heave, lower limb edema and atrial fibrillation. She was put initially on CPAP device, but her oxygen saturation values were persistently in range of 75–78%. We decided not to intubate our patient, owing to her age, comorbidities, possibility of long-term ventilation compounded by relative's refusal for invasive ventilation. It was then decided to manage her with high-flow nasal oxygen therapy initially starting at 60 liters/minute and FiO2 of 60%, gradually tapered in accordance to her serial arterial blood gas values and vital parameters. Her oxygen saturation improved to 94% and she had improvement in her symptoms clinically as well. Simultaneously, she was also put on infusion of inj. dobutamine to manage her blood pressure, and inj. amiodarone infusion for management of her atrial fibrillation. Her clinical course of 7 days in the ICU was uneventful and she was referred to cardiothoracic vascular surgeons for definitive repair of her valvular lesion and treatment of pulmonary hypertension.
Second case was of a 69-year-old female, diagnosed with severe primary pulmonary hypertension with chronic kidney disease, associated with lower limb deep venous thrombosis, multi-organ dysfunction owing to sepsis, severe anemia, pulmonary tuberculosis, and was admitted with complaints of tachypnea, dyspnea with progressive breathlessness. Her echocardiogram was suggestive of severe pulmonary hypertension with right ventricular hypertrophy and dilatation and 50% ejection fraction. Her ABGs were suggestive of type I respiratory failure and she was put on high-flow nasal cannula (HFNC). She responded well to HFNC with dramatic improvement in PaO2. Simultaneously, she was put on broad spectrum antibiotics, antitubercular therapy, warfarin, anti-thrombotic therapy, erythropoietin, hematinic, diuretics and other conservative therapy. Although her clinical stay lasted for 1 month in ICU, she was discharged with stable vitals and improved clinical condition.
Our third case was of a 29-year-old female, a known case of primary pulmonary hypertension, who landed up in acute respiratory and right heart failure, after delivery of her live baby via lower segment cesarean section. Her respiratory rate was 35–40/minute, with fine basal crepts, oxygen saturation in range of 82–84% and features suggestive of right heart failure (raised jugular venous pressure, pedal edema and hypotension). She was put on high-flow nasal cannula (HFNC) initially with the flow rate of 50 liters/minute and FiO2 of 0.5, along with diuretics, broad spectrum antibiotics, sildenafil, etc. She responded well to the treatment and her oxygen requirement decreased gradually, and was able to maintain on a low-flow nasal cannula over a period of 96 hours.
DISCUSSION
High-flow nasal oxygen therapy consists of an air/oxygen blender, an active humidifier, a nasal cannula and a heated circuit. Traditional oxygen delivery devices, e.g. low-flow nasal cannula, nonreservoir bag masks can only deliver oxygen flow at the rate of 6–15 liters/minute which is insufficient in most of patients with type I respiratory failure; however, HFNC is an efficient noninvasive device which can deliver 100% humidified and heated oxygen at flow rate as high as 60 liters/minute.
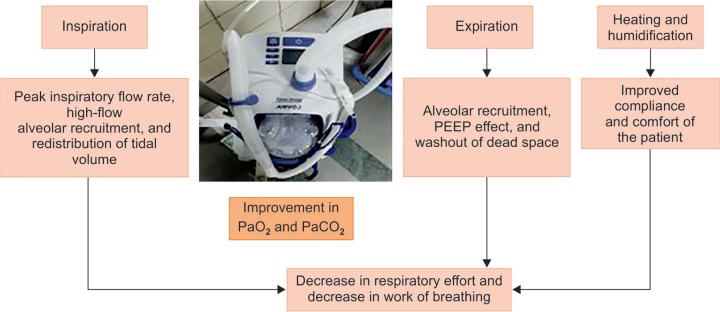
HFNC is known to have many desirable properties: constant fraction of oxygen, humidified gases, reduction of dead anatomical space, along with reduction in nasopharyngeal resistance and increasing alveolar recruitment in lungs (Fig. 1).
Fig. 1.
High-flow nasal cannula
There are lots of well-known beneficial effects of HFNC over conventional oxygen therapy as the gas is effectively warmed to 37°C with humidification of gases, thereby maintaining the mucociliary function and is more convenient for patients. Undoubtedly, Noninvasive ventilation in the form of Bipap/CPAP has been established as an effective therapy, but the mask remains claustrophobic to a large number of patients and the tight seal maintenance is cumbersome and unpleasing to the patients for long-term maintenance which is not an issue with HFNC. High flow of gases also helps wash out carbon dioxide in anatomical dead space as well. The characteristic fact is that the difference between the inspiratory flow of patients and delivered flow is small and FiO2 remains constant relatively, unlike the conventional technique.
All of our three patients had marked improvement in oxygen saturations, decrease in respiratory rate, improvement in PaO2 levels in serial arterial blood gases and compliance with the device (Table 1). All three patients had perceived level of comfort with the device, they could eat properly and have a sound sleep with the device, and we could defer the need of intubation after achieving adequate oxygenation levels. All three patients had different etiologies for pulmonary hypertension — the first case had PHT secondary to valvular heart disease, in the second case it was due to pulmonary tuberculosis, whereas the third one had primary PHT. The first case and third case developed right ventricular failure following noncardiac surgery in postoperative period, which is the most common cause of death in this patient population.1 PHT is viewed as a predictor of adverse postoperative outcome especially for those with concurrent cardiovascular disease, undergoing emergency operations and with higher pulmonary artery systolic failure.2 In patients with PHT and RV failure, initial approach of care should focus on management of any reversible cause of acute RV dysfunction and formulation of strategy to improve RV function. The latter may be achieved by modifying RV preload, contractility and RV afterload. Hypoxemia in these patients contribute to increase in RV afterload and hence, lead to deterioration of function. Oxygen supplementation has been shown to decrease PA pressure and cardiac functions, regardless of etiology of PHT.3
Table 1.
Patient's stay in ICU and their vital parameters with HFNC setting
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age/sex | 75 years/female | 69 years/female | 29 years/female |
| Diagnosis | RHD, severe TR, moderate pulmonary hypertension, atrial fibrillation, hypertension and diabetes | Chronic kidney disease, hypertensive, diabetic, sepsis, MODS, mod-severe pulmonary hypertension, pulmonary tuberculosis | Postpartum day one, via LSCS, primary pulmonary hypertension |
| Day 1 of admission Vitals PR BP (mm Hg) RR SPO2 ABG pH PO2 PCO2 HFNC setting |
130 158/100 40 78–80% 7.31 43 40 60 L/min, 0.6 |
140 130/98 44 82–84% 7.21 48 39 50 L/min, 0.6 |
128 108/76 39 82–84% 7.28 56 38 45 L/min, 0.5 |
| Day 3 of admission Vitals PR BP RR SPO2 ABG pH PO2 PCO2 HFNC setting |
102 129/82 28 93% 7.4 60 34 45 L/min, 0.6 |
90 130/80 30 98% 7.3 72 38 35 L/min, 0.5 |
88 120/85 28 99% 7.44 80 38 30 L/min, 0.4 |
| Day 7 of admission Vitals PR BP RR SPO2 ABG pH PO2 PCO2 HFNC setting |
90 120/86 24 95% 7.44 69 38 35 L/min, 0.5 |
88 127/88 24 98% 7.38 78 34 30 L/min, 0.4 |
Discharged from ICU |
In critically ill patients receiving mechanical ventilation (MV), functional residual capacity (FRC) is reduced. Application of PEEP increases FRC and tidal ventilation above closing capacity, improves lung compliance and corrects ventilation/perfusion abnormalities. In patients with decompensated heart failure, PEEP has a broad spectrum of hemodynamic consequences which can alternatively be favorable or unfavorable, depending on the clinical scenario in which it is being used.4 MV and PEEP reduce venous return and RV preload. At the same time, ventilator-induced rise in transpulmonary pressure increases pulmonary vascular resistance and RV afterload. Therefore, application of PEEP can produce unfavorable hemodynamic effects when managing patient with PHT and RV failure.5,6
HFNC is increasingly being used for patients with right and left heart failures.7 Apart from improving oxygenation and respiratory mechanics, HFNC could potentially improve hemodynamics in these patients. Roca et al. showed that HFNC in heart failure patients cause a decrease in inspiratory collapse of inferior vena cava. As the right atrium is surrogate for right ventricular preload, and right atrial pressure is usually estimated from inferior vena collapsibility, they concluded that the decrease in inspiratory collapse indicated a reduced RV preload.8
Therefore, every attempt should be made to avoid endotracheal intubation in patients with RV failure. In cases with worsening hypoxemia, noninvasive ventilation has been first line of intervention to avoid ET intubation.9 However, the NIV with a tight fitting face mask has various limitations. As a result, long duration of NIV is not feasible for all patients.10
HFNO provides humidity; high FiO2, improves patient compliance, decreases heat and moisture loss from the airway, reduces anatomical dead space, provides PEEP, and improves oxygenation. There is increasing evidence for its use in acute respiratory failure and in avoiding impending tracheal intubation. Its role in pulmonary hypertension is important as invasive ventilation is associated with unfavorable hemodynamic and cardiac effects. In our experience, HFNO has the potential to significantly impact the management of cardiac patients presenting with respiratory failure by improving patient morale, avoiding ill effects of invasive ventilation, with the added advantage that the patient can eat, drink and communicate with HNFC in situ.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 2.Lai HC, Lai HC, Wang KY, Lee WL, Ting CT, Liu TJ. Severe pulmonary hypertension complicates postoperative outcome of non-cardiac surgery. Br J Anaesth. 2007;99:184–190. doi: 10.1093/bja/aem126. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med. 2011;184:1114–1124. doi: 10.1164/rccm.201104-0662CI. [DOI] [PubMed] [Google Scholar]
- 4.Wiensen J, Ornstein M, Tonelli AR, Menon V, Ashton RW. State of evidence: mechanical ventilation with PEEP in patients with cardiogenic shock. Heart. 2103;99(24):1812–1817. doi: 10.1136/heartjnl-2013-303642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Disselkamp M, Adkins D, Pandey S, Coz Yataco AO. Physiologic approach to mechanical ventilation in right ventricular failure. Ann Am Thorac Soc. 2018;15(3):383–389. doi: 10.1513/AnnalsATS.201707-533CC. [DOI] [PubMed] [Google Scholar]
- 6.Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J. 2003;21:720–727. doi: 10.1183/09031936.03.00120102. [DOI] [PubMed] [Google Scholar]
- 7.Chua MT, Kuan WS. The use of high flow nasal cannula in acute decompensated heart failure: ready for prime time yet? J Emerg Crit Care Med. 2017;1:22. [Google Scholar]
- 8.Roca O, Pérez-Terán P, Masclans JR, Galve E, Evangelista A, Rello J. Patients with New York Heart Association class III heart failure may benefit with high flow nasal cannula supportive therapy: high flow nasal cannula in heart failure. J Crit Care. 2013;28:741–746. doi: 10.1016/j.jcrc.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Jardin F, Vieillard-Baron A. Right ventricular function and positive pressure ventilation in clinical practice: from hemodynamic subsets to respiratory settings. Intensive Care Med. 2003;29:1426–1434. doi: 10.1007/s00134-003-1873-1. [DOI] [PubMed] [Google Scholar]
- 10.Koutsogiannidis CP, Ampatzidou FC, Ananiadou OG, Karaiskos TE, Drossos GE. Noninvasive ventilation for post-pneumonectomy severe hypoxemia. Respir Care. 2012;57:1514–1516. doi: 10.4187/respcare.01493. [DOI] [PubMed] [Google Scholar]