Abstract
Macroecologists seek to identify drivers of community turnover (β-diversity) through broad spatial scales. However, the influence of local habitat features in driving broad-scale β-diversity patterns remains largely untested, owing to the objective challenges of associating local-scale variables to continental-framed datasets. We examined the relative contribution of local- versus broad-scale drivers of continental β-diversity patterns, using a uniquely suited dataset of cave-dwelling spider communities across Europe (35–70° latitude). Generalized dissimilarity modelling showed that geographical distance, mean annual temperature and size of the karst area in which caves occurred drove most of β-diversity, with differential contributions of each factor according to the level of subterranean specialization. Highly specialized communities were mostly influenced by geographical distance, while less specialized communities were mostly driven by mean annual temperature. Conversely, local-scale habitat features turned out to be meaningless predictors of community change, which emphasizes the idea of caves as the human accessible fraction of the extended network of fissures that more properly represents the elective habitat of the subterranean fauna. To the extent that the effect of local features turned to be inconspicuous, caves emerge as experimental model systems in which to study broad biological patterns without the confounding effect of local habitat features.
Keywords: Araneae, cave, Europe, generalized dissimilarity model, latitudinal gradient, subterranean biodiversity
1. Background
Understanding why biological communities differ from one another is among the most basal research questions in ecology, yet answering this question represents a significant intellectual challenge [1,2]. For over a century, species richness (α-diversity) has been the most commonly used metric to quantify and explore biological diversity through the environmental space [3]. Yet, it is increasingly acknowledged that the extent of change in community composition along gradients (β-diversity) is a prominent and complementary feature to consider as well, possibly even more meaningful than α-diversity when dealing with macroecological patterns [4]. Substantial turnovers in the composition of communities along broad-scale ecological gradients have been observed in virtually all taxa [5]. Community changes following latitudinal clines or elevational extents (that is, essentially thermal seasonality gradients) [6–9], gradients of productivity [10], urbanization [11,12] or salinity [13,14], are all examples in which one or a few broad-scale environmental gradients well explained turnover in biological communities. Yet an equally important, but potentially inconspicuous and overlooked, facet of β-diversity analyses pertains the contribution of local-scale ecological factors in explaining patterns of biological diversity at a broader spatial scale [15]. Accounting for local features like microhabitat characteristics [16,17] and local land use [18] might provide complementary information to understand the ecological processes involved in filtering larger species pools to the subset of resident species that occurs within a given community. However, the objective challenge of associating local environmental and habitat features to continental and global biodiversity datasets has largely prevented macroecologists incorporating local-scale features in their modelling exercises.
Here, we use a uniquely suited continental dataset of cave-dwelling spider communities to examine the relative contribution of local- versus broad-scale predictors—i.e. the local geomorphological features of caves versus broad-scale environmental predictors commonly used in macroecological analyses—in driving community turnover through space. While subterranean habitats have largely been omitted in exploring β-diversity patterns across continental scales [19,20], cave communities provide the discrete boundaries and simplified biological assemblages which are often required for similar analyses [21]. The fact that cave systems are extensively replicated across the Earth [22] offers the unique opportunity of studying semi-closed habitats characterized by relatively homogeneous and recurrent structural characteristics distributed along broad-scale gradients of varying climatic conditions. A number of studies demonstrated the importance of local features in determining subterranean species richness at the level of a single cave or a few karst systems [23–27], suggesting that the signature of the environmental filtering posed by local habitat features could potentially be detected also at broader scales [28].
Gauging the relative contribution of local- and broad-scale drivers of macro-diversity patterns is challenging not only because of the general lack of suitable datasets for performing similar tasks but also because the organisms interacting within a typical community—even in a cave—are often spectacularly polyphyletic and functionally diverse [22]. To minimize noise, it is thus convenient to focus on specific model organisms deemed to be good representatives of the response of the biological communities owing to their clear and specific ecological role. Among other subterranean components, spiders (Arachnida: Araneae) are widespread and distinctive for their key role as predators in the subterranean trophic webs [29]. When accounting for the nearly 500 spider species inhabiting subterranean habitats in Europe, there are species with different levels of specialization and affinity to the subterranean environment, from obligate cave-dwellers (troglobionts) to species with only partial affinity to caves (troglophiles) [30]. This great diversity offers a wide analytical spectrum, insofar as differences in subterranean specialization and dispersal propensity might lead to diverse distribution patterns.
We assembled a dataset of 475 subterranean spider communities across Europe thanks to the effort of an international network of araneologists, biospeleologists and cavers. This dataset is unique in that it covers a large geographical extent on the one hand, and contains high-resolution local data on geomorphological and habitat features on the other. Analysing it by using generalized dissimilarity models (GDMs) [31], a novel modelling technique that accommodates for nonlinearity and non-stationarity in matrix regressions, we explored the following questions:
-
(i)
what is the contribution of local factors versus broad-scale environmental factors in determining community turnover among cave spider communities across Europe? and
-
(ii)
are the observed patterns influenced by the level of subterranean specialization of the different species (i.e. troglobiont versus troglophile spider assemblages)?
2. Methods
(a). Dataset assemblage
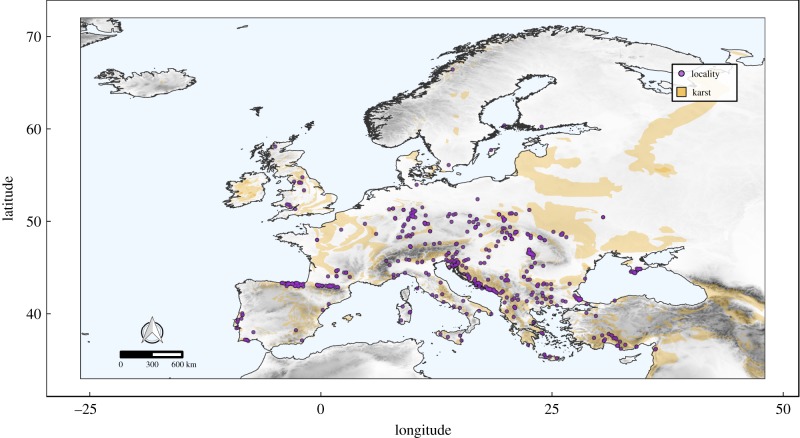
We compiled what we believe to be the first continental-scale geo-referenced dataset of subterranean spider communities across Europe [32]. The dataset comprises data from 475 caves from 27 European countries, and covers a latitudinal range from 35° to 70° (figure 1). In constructing the dataset, we deliberately choose caves for which we deemed the spider fauna to be exhaustively known and for which the morphological and environmental features were available, thus minimizing the number of missing data (NA) in the dataset. Although we acknowledge that different sampling bias exists when it comes to estimating the diversity of species within subterranean habitats [33], by selecting only well-studied caves, we assumed the sampling bias to be homogeneous within the caves included in the dataset.
Figure 1.
Map of cave localities in Europe included in the dataset. Shades of grey represent elevation. Brown areas indicate karst areas. (Online version in colour.)
To capture the diversity of subterranean habitats across Europe, the selection of the sites was driven by the necessity to maximize the ranges of environmental gradients therein. First, in order to account for the wide variety of habitats inhabited by subterranean spiders [29], we considered as individual sites different types of caves: limestone, volcanic, talus and salt caves, but also artificial sites such as mines, blockhouses, and cellars. The general term ‘cave’ is used hereafter. Furthermore, we selected cave openings in different types of habitats and substrates, at different elevations (0–2000 m above sea level), covering a wide range of linear planimetric development (3.5–70 000 m), prevalent drops (from –877 to +815 m) and main entrance sizes (0.1–45 000 m2). Spatially, we selected caves so to cover the study area as homogeneously as possible. Yet, the need for choosing only well-studied caves and the often clumped distribution of caves within karst areas [34,35] prevented us obtaining a fully homogeneous distribution of sites (figure 1).
(b). Spider composition and environmental gradients
We associated high-resolution data to each cave, namely spider community composition along with information on local geomorphological and environmental features. Spider community composition was represented as incidence data—presence/absence of both described species and species under description. To evaluate if drivers of β-diversity varied depending on the subterranean specialization of different species, we classified each species as either ‘troglophile' or ‘troglobiont'. In subterranean biology, the term ‘troglophile' is used to refer to species that are able to complete their life cycles both in the subterranean and the surface environments, often forming populations in both habitats. Conversely, the term ‘troglobiont' refers to species that are obligate subterranean dwellers [36]. We use the partitioning of European spiders into these two classes found in the checklist of subterranean spiders [30], species not included in the checklist were classified using the same criteria. When lacking information on the distribution, habitat preference and autoecology [36] the classification of a species into these two categories was based on morphological traits associated with the subterranean life—depigmentation, leg elongation and eye regression. Morphological traits were derived from species descriptions, taking advantage of the fact that taxonomic literature on spiders is fully digitalized and freely available online [37]. Accidental species, i.e. surface species not showing any morphological adaptation or association with the subterranean habitats, were not included in the dataset.
We used as local-scale predictors all the geomorphological features of the different caves, namely the elevation, number of entrances, the main entrance size (a numerical estimation of the dimension of the main entrance in m2), cave development (total planimetric development of the cave in m), prevalent drop (total positive minus total negative drop in m), as well as additional categorical features (type of cave, geological substrate, the presence of a subterranean river, entrance habitat, touristic use). We extracted broad-scale predictors for each locality from environmental rasters at a resolution of 2.5 min. Climatic data were derived from WORLDCLIM2 [38]: mean annual temperature, annual range of temperature, cumulative precipitations and solar radiation. The reliability of these surface variables as surrogates for subterranean conditions has been extensively discussed elsewhere [20,39,40]. To consider the possible effects related to the biogeographical history, we further included the distance from the last glacial maximum (LGM) glacier as an additional broad-scale predictor. We constructed this raster by buffering the shapefile of LGM glaciers with distance rings of 5 km [41]. Furthermore, a shapefile of carbonate extent for the study area was obtained from the World Map of Carbonate Rock Outcrops (v.3.0). We rasterized the shapefile and calculated the area of each karst patch (karst area; figure 1). We assigned to each raster pixel the area value of the corresponding karst patch (value of 0 for non-karst pixel). A full description of all variables is given in the electronic supplementary material, appendix S1.
(c). Statistical analyses
We used a Wilcoxon rank-sum test with continuity correction to compare median values of richness of troglophile and troglobiont spider communities. To compare β-diversity, we made pairwise comparisons of the 475 cave communities and computed a Sørensen dissimilarity index. We used GDMs to compare patterns of β-diversity between communities of troglophile and troglobiont spiders and evaluate the relative contribution of local- versus broad-scale environmental gradients in explaining these patterns. GDM represents a nonlinear extension of a traditional distance approach of matrix regression. It permits us to analyse patterns in the compositional dissimilarity among sites and to quantify how much sites differ in their environmental conditions (environmental distance) and how isolated they are from one another (geographical distance). In contrast with standard linear matrix regressions, a GDM accommodates for the variation in the rate of compositional turnover (non-stationarity) at different positions along a given gradient, and nonlinear relations between compositional dissimilarity and both environmental and geographical distances between sites [31].
We performed matrix regressions in R (v.3.5.1) with the functions available in the ‘gdm' package [42]. We used as input data site-by-environment and site-by-species matrices for troglophile and troglobiont spiders, which is available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qz612jm8z [43]. Prior to model fitting, we performed data exploration in order to detect outlying observations in the dataset and to evaluate collinearity among predictors. We graphically explored the presence of outliers using Cleveland's dot plots. We calculated pairwise Pearson's correlations to detect collinearity among predictors using a standard |r| > 0.70 threshold to cull variables [44]. We also used boxplots to graphically assess collinearity between continuous and categorical variables.
We fitted individual GDMs for troglophile and troglobiont β-diversity matrices with default parameters of three I-splines per predictor and knot values of 0 (minimum), 50 (median) and 100 quantiles (maximum). Models were weighted by species richness—for troglophiles, we also filtered caves with less than four species to avoid sampling artefacts. We quantified variable importance and significance using Monte Carlo matrix permutation [31,45]. We retained in the final GDMs only predictors that explained model variance. We plotted the I-splines of significant predictors to assess how magnitudes and rates of species turnover varied along and between gradients and how these patterns differed between troglophiles and troglobionts. We estimated confidence intervals around the fitted I-splines using bootstrapping. Finally, to visualize multi-dimensional biological patterns of β-diversity in the environmental space, we used a principal component analysis (PCA) to reduce dimensionality among predictors and assigned the first three PCA components to an red, green, blue colour palette.
3. Results
(a). European spider assemblages by numbers
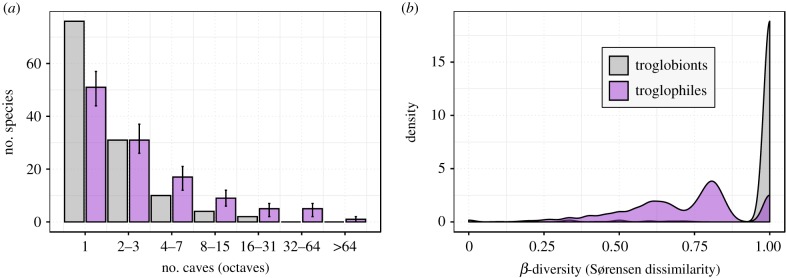
The site-by-species dataset consisted of 475 caves and 331 unique species, of which 132 were troglobionts and 199 were troglophiles. This diversity accounts for nearly 70% of the subterranean spider species reported to occur in Europe, i.e. 486 species [30]. The overall number of species per cave community ranged from 0 to 15 (mean ± s.d. = 4.44 ± 2.25). The number of troglophile species ranged from 0 to 11 (mean ± s.d. = 3.84 ± 2.10), whereas troglobiont species were numerically lower (range = 0–5; mean ± s.d. = 0.60 ± 0.99) (figure 2a). The median number of species per cave was significantly higher in troglophile rather than troglobiont spider communities (Wilcoxon rank-sum test, W = 14927, p < 0.01).
Figure 2.
(a) Abundance classes (octaves) of the numbers of troglophile and troglobiont spider species in caves included in the analysis. Error bars indicate 95% confidence limits for troglophile species (n = 199) when resampled to the number of troglobiont species (n = 132). (b) Density of β-diversity values for troglophile and troglobiont spider communities. (Online version in colour.)
β-diversity between caves was generally higher for troglobionts than troglophiles (figure 2b). The β-diversity values for troglophiles were mostly concentrated between 0.6 and 0.8. Lower values were mostly owing to the presence of a few widespread troglophile species, namely Metellina merianae (Scopoli) (Tetragnathidae), present in 50% of the considered caves (n = 238), Meta menardi (Latreille) (Tetragnathidae) (30%; n = 147), Tegenaria silvestris L. Koch (Agelenidae) (19%; n = 92) and Porrhomma convexum (Westring) (Linyphiidae) (18%; n = 86). Values of β-diversity for troglobionts approached 1 in most cases (figure 2b). On average, each troglophile species appeared in nine caves (mean ± s.d. = 9.16 ± 27.08; range = 1–238). Conversely, troglobiont species rarely occurred in more than two caves (mean ± s.d. = 2.15 ± 2.70; range = 1–21).
(b). Drivers of β-diversity
As a result of data exploration, we log-transformed the cave development and main entrance size variables to homogenize their distribution and account for a few outliers. We found mean annual temperature to be collinear with elevation (|r| = 0.7) and solar radiation (|r| = 0.8), hence we culled the latter predictors. Mean annual temperature was also correlated with entrance habitat—caves in forested areas generally displayed lower temperatures than caves opening in shrubs, grass and rocky habitats—and hence we also excluded the latter categorical predictor. As a large proportion of caves in the dataset were formed in limestone rocks (n = 411), we found the levels of the categorical variable type of cave and geological substrate to be unbalanced and the variables to be correlated with karst area. Thus, we dropped these predictors. We also excluded the presence of a subterranean river and the touristic use of cave variables owing to a significant unbalance between the distribution of the observations at the two levels of these factors. Yet, a preliminary exploration with a χ2 test revealed no difference in spider richness relative to these factors (presence of subterranean river: , p = 0.91; touristic use of cave: , p = 0.50). The list of local and regional predictors used in the GDMs and their significance is presented in table 1, whereas the full list of predictors is reported in the electronic supplementary material, appendix S1.
Table 1.
Relative importance and significance of non-collinear predictor variables for β-diversity of subterranean spider communities across Europe, as determined by permutating 50 times the generalized dissimilarity models (*p < 0.05). (Em dashes indicate predictors which explained no model variance.)
| variable | scale | troglophiles | troglobionts |
|---|---|---|---|
| geographical distance (°) | broad | 13.03* | 43.37* |
| cumulative precipitation (mm) | broad | 0.42 | 1.11 |
| mean annual temperature (°C) | broad | 48.96* | 11.38* |
| annual range of temperature (°C) | broad | — | — |
| distance from the LGM glacier (km) | broad | — | — |
| karst area (km2) | broad | 16.74* | 5.36* |
| no. of entrances | local | — | — |
| main entrance size (m) | local | 0.73 | — |
| cave development (m) | local | — | 0.44 |
| prevalent drop (m) | local | 0.02 | 2.25 |
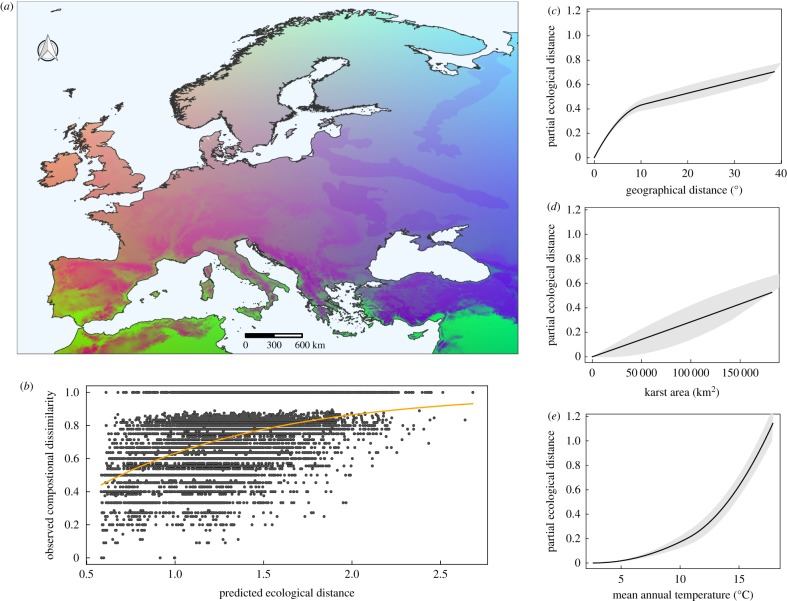
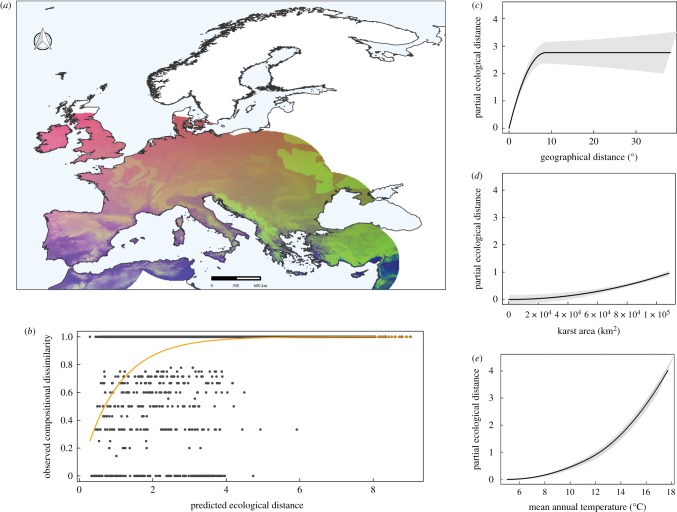
In both troglophiles and troglobionts, patterns of species turnover varied by environmental gradients and geographical distance. Yet, the percentage of variance explained by the models differed considerably between the two groups (18% for troglophiles and 43% for troglobionts). Spatially, community turnover was greater in southern than northern Europe for both troglophiles (figure 3a) and troglobionts (figure 4a). Troglobiont spider assemblages in the Dinaric karst (Balkan Peninsula) and Turkey emerged as the most unique. The assemblages from the Alps and the Iberian Peninsula were, in general, more similar. Communities at northern latitudes were the most homogeneous. In the case of troglobionts, northern communities virtually consisted of a single species, Porrhomma rosenhaueri (L. Koch) (Linyphiidae). For troglophiles, mean annual temperature (figure 3c) was the most important gradient for determining community turnover, followed by karst area (figure 3d) and geographical distance (figure 3e). The rate and magnitude of turnover along the gradients were exponential for mean annual temperature and linear for karst area. Turnover also increased nonlinearly with geographical distance, without reaching an asymptote. The contribution of additional drivers, both local and broad, was negligible. Predictors identified as significant by the GDM for troglobionts were, in order of importance: geographical distance (figure 4c), mean annual temperature, (figure 4e) and karst area (figure 4d). The model excluded other local and regional predictors (table 1). The rates and magnitude of turnover along the geographical distance gradient were nonlinearly asymptotic, with rates of turnover steeply increasing up to 8° when they reach a plateau of full community dissimilarity (figure 4e). The rates and magnitude of turnover of mean annual temperature and karst area were exponential. In the latter case, the contribution of additional drivers was also negligible.
Figure 3.
Results of generalized dissimilarity model for troglophile spider communities across Europe. (a) Predicted spatial variation in troglophile spider community composition. Colours represent gradients in species composition derived from transformed environmental predictors, whereby areas with similar colours are expected to contain more similar communities. (b) Relationship between observed compositional dissimilarity in troglophile spider community between each cave pair and the linear predictor of the regression equation from generalized dissimilarity model. (c–e) Fitted I-splines (partial regression fits) for variables significantly associated with β-diversity of troglophile spiders. The maximum height reached by each curve indicates the total amount of compositional turnover explained by that variable (holding all other variables constant), whereas the shape of each spline indicates how the rate of compositional turnover varies along the environmental gradient. (Online version in colour.)
Figure 4.
Results of generalized dissimilarity model for troglobiont spider communities across Europe. (a) Predicted spatial variation in communities of troglobiont spiders. Colours represent gradients in species composition derived from transformed environmental predictors, whereby areas with similar colours are expected to contain more similar communities. Colour gradient was constrained within a radius of 500 km from all cave localities with troglobionts to avoid extending predictions to areas lacking data on troglobionts. (b) Relationship between observed compositional dissimilarity in troglobiont spider community between each cave pair and the linear predictor of the regression equation from generalized dissimilarity model. (c–e) Fitted I-splines (partial regression fits) for variables significantly associated with β-diversity of troglobiont spiders. The maximum height reached by each curve indicates the total amount of compositional turnover explained by that variable (holding all other variables constant), whereas the shape of each spline indicates how the rate of compositional turnover varies along the environmental gradient. (Online version in colour.)
4. Discussion
Spider species richness (α-diversity) in European caves was generally low, with the majority of species being distributed in one or a very few caves (figure 2a). Number of troglobionts per cave was consistently lower than number of troglophiles, an expected pattern that both reflects the limited dispersal ability of troglobiont spiders [29] and the reduced availability of trophic resources in the deep and inner areas of caves where they usually reside [21,22]. Yet, it must be kept in mind that α-diversity values are expected to be higher—and number of caves per several species to be lower—than those reported here, as recent molecular studies revealed that cryptic diversity in subterranean lineages is often high [46–48]. Interestingly, it was demonstrated that this is not a critical shortcoming in subterranean macroecological studies, as cryptic species diversity should be homogeneously distributed along environmental gradients [48].
We observed that spider communities progressively became more homogeneous from the south to the north for both troglophiles (figure 3a) and troglobionts (figure 4a), a typical pattern in the Northern Hemisphere [9,49]. The same environmental gradients explained β-diversity variations for both categories of taxa: geographical distance, temperature and availability of karst. Nevertheless, the relative importance of these three drivers differed substantially depending on the level of subterranean specialization. Also, model fit was significantly better in the case of troglobionts, whereas approximately 80% of the model deviance for troglophiles remained unexplained. This latter group comprises a great variety of species, highly diverse in terms of Linnean distance, but also in morphological and life-history traits [29,30,36]. An ongoing collection of subterranean spider traits will possibly allow the subdivision of troglophiles into more coherent functional subgroups, hence increasing the explanatory power of the models.
Rather than environmental distance, geographical distance emerged as the most important factor explaining β-diversity patterns in troglobiont spider communities. Across our study area, two randomly sampled caves are predicted to be fully dissimilar in terms of community composition if they are at a distance greater than 8° (figure 4c). This result is consistent with the high rate of endemism generally observed in subterranean obligate species [50] and parallels similar predictions obtained for groundwater crustaceans in Europe [20]. Although significant, the geographical effect was less strong in the case of troglophile communities, consistently with their broad distribution patterns and higher dispersal propensity. It seems likely that the steep increase in β-diversity with geographical distance reflects dispersal limitations. In the case of communities at northern latitudes (greater than 48–50° N), this pattern might also reflect Pleistocene local extirpation of faunas, and the subsequent post-glacial dispersal limitation [51]. However, at this analytical scale, the influence of the distance from LGM glaciers in our models was negligible—a variable often found to be highly significant to explain the distribution of European subterranean arachnids at smaller scales [41,52].
The most important environmental gradient explaining dissimilarity in troglophile communities was mean annual temperature. This variable was recovered as significant and important also for troglobionts, although to a lesser degree. Mean annual temperature at the surface is deemed to be an ideal proxy-variable for the largely constant thermal conditions of subterranean habitats [40,53]. The variable was also collinear with elevation, meaning that caves at different altitudes tend to be more dissimilar from one another. Insofar as the climatic distance between two caves explains the dissimilarity between their communities, it is possible to infer that the specialization to habitats with contrasted temperatures may have contributed to promote isolation [54]. It is also worth noting that mean annual temperature strongly correlates with surface productivity; in turn, a high surface productivity is deemed to correlate with high organic input to subterranean habitats, thus potentially exerting an influence on diversity patterns [39,55,56]. Interestingly, a few studies on groundwater fauna [20,48] recovered thermal seasonality as the most important factor explaining species range size, whereas temperature range was not significant in this study.
Finally, amount of karst was an additional important predictor for both troglophiles and troglobionts. Karst area is a good proxy for subterranean habitat availability and connectivity [34,39], so this result was somewhat expected. Yet, it is worth noting that 88% of records in the database were obtained from limestone caves. Thus, our analysis might underestimate the availability of suitable habitats to subterranean spiders in non-karst substrates, such as talus caves, shallow subterranean habitats [57] or artificial subterranean habitats opening in other substrates, all poorly represented in the dataset.
Interestingly, there was virtually no contribution of local cave features in explaining β-diversity patterns. We realize that this result might seem counterintuitive, because different geomorphological features have been documented to directly or indirectly correlate with subterranean diversity. For example, it is documented how a cave with a large entrance or a vertical cave with a high drop often accumulates more external trophic resources than a horizontal cave with a very narrow entrance [58] hence probably supporting a dissimilar and possibly more diverse community. Similarly, one might expect a cave with a greater planimetric development to support a more diverse community than a smaller cave. There are different explanations for this pattern. First, it is possible that local features exercise their primary effect on the species abundance, rather than on the simple presence/absence of species. Second, the effect of local variables may be evident exclusively locally; when analysing diversity patterns at regional to continental scales, such effects may be masked by the stronger influence of large-scale gradients. Third, it is worth noting that caves are only part of an extended network of fissures which more properly represents the elective habitat of the subterranean fauna [59]. The fact that we recovered a major effect of the karst area (that is, a proxy for the extent of habitat availability across the landscape), rather than that of the planimetric development of the cave (that is, an anthropocentric view of the habitat available to the subterranean species), suggests that this explanation might be reasonable. In a way, the lack of local effects is a possible clue reflecting our inability to truly capture the local conditions governing subterranean habitats. Because the effect of local features turned out to be inconspicuous, caves emerge as ideal experimental model systems in which to study broad biological patterns without the confounding effect of local features.
5. Conclusion
Even though caves represent island-like habitats well-suited for α- and β-diversity studies [20,25,33,60–63], it is only recently that researchers began to consistently explore broad-scale patterns of subterranean diversity [21,64]. We demonstrated a limited influence of local-scale cave features in determining the continental pattern of β-diversity in subterranean spider communities in Europe. On the other hand, we proved how geographical distance, in synergy with the environmental gradients of habitat and temperature, explained most of the community turnover. This pattern is consistent with the dispersal limitations that are typically observed in subterranean obligate species. Overall, this analysis was possible thanks to the collaboration of 30 researchers, providing their expertise and their own field-collected data. Such a collaborative attitude is a crucial premise to tackle macroecological issues, where the quality and the amount of data is an essential condition that is rarely met. This point is particularly important in cave-based science, as the harsh conditions of the working environment delays the acquisition of the much-need data for exploring global diversity patterns [21,64]. Accordingly, we reaffirm the need to pursue collaborative databasing and data sharing [65].
Supplementary Material
Acknowledgements
One of the experts who provided data for this study, Boyan Petrov (1973–2018), sadly disappeared while climbing the Shishapangma (8027 m above sea level) in the Himalaya. This work is dedicated to his memory. We are grateful to Ingi Agnarsson and an anonymous referee for constructive comments.
Data accessibility
Response and environmental predictors, as well as the R code to generate the analyses, are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qz612jm8z [43]. The full dataset of subterranean spider communities across Europe are available in an associated data paper [32] and updated as long as new data becomes available.
Authors' contribution
S.M. and M.I. planned the line of enquiry. M.I. coordinated the network of experts. S.M. and P.C. analysed the data. All other authors are listed alphabetically. A.M. and L.K. provided data from Slovakia. C.D., M.N. and S.Ć. provided data from Albania, Bulgaria, Greece and Serbia. C.K. provided data from Austria. T.B. and S.Z. provided data from Germany. D.A., L.D. and G.B. provided data from Hungary. C.E.P., C.R. and J.F. provided data from Spain. F.G. provided data from Slovenia. K.B.K. and M.E. provided data from Turkey. M.K. and M.P. provided data from North Macedonia and Montenegro. M.I. and S.M. provided data from Italy. M.P. provided data from Croatia and Bosnia and Herzegovina. O.T.M. provided data from Romania. P.C. provided data from Finland and Portugal. R.R. provided data from Poland. R.S.V. provided data from Ukraine. V.R. provided data from the Czech Republic.
Competing interests
We declare we have no competing interests.
Funding
S.M. is supported by Bando per l'Internazionalizzazione della Ricerca—Anno 2018 (Compagnia di San Paolo). M.I. and S.M. are supported by the project ‘The Dark Side of Climate Change', funded by the University of Turin and the Compagnia di San Paolo (grant award: CSTO162355). M.P. is supported by the MSCA Individual Fellowships ‘HiddenLife' project (grant agreement: 749867). O.T.M. is supported by a grant of the Romanian Ministry of Research and Innovation, CNCS - UEFISCDI, project no. PN-III-P4-ID-PCCF-2016–0016, within PNCDI III. S.Ć. is supported by the Serbian Ministry of Education, Science and Technological Development (grant no. 173038). A.M. and L.K. were supported by the Slovak Scientific Grant Agency, Project VEGA 1/0346/18, and the Agency for Research and Development, Project APVV–17–0477.
References
- 1.Gaston KJ. 2000. Global patterns in biodiversity. Nature 405, 220 ( 10.1038/35012228) [DOI] [PubMed] [Google Scholar]
- 2.Legendre P, De Cáceres M.. 2013. Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol. Lett. 16, 951–963. ( 10.1111/ele.12141) [DOI] [PubMed] [Google Scholar]
- 3.Gotelli NJ, Colwell RK. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391. ( 10.1046/j.1461-0248.2001.00230.x) [DOI] [Google Scholar]
- 4.Mori AS, Isbell F, Seidl R. 2018. β-diversity, community assembly, and ecosystem functioning. Trends Ecol. Evol. 33, 549–564. ( 10.1016/j.tree.2018.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricklefs RE. 2004. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15. ( 10.1046/j.1461-0248.2003.00554.x) [DOI] [Google Scholar]
- 6.Chan W-P, Chen I-C, Colwell RK, Liu W-C, Huang C, Shen S-F. 2016. Seasonal and daily climate variation have opposite effects on species elevational range size. Science 351, 1437–1439. ( 10.1126/science.aab4119) [DOI] [PubMed] [Google Scholar]
- 7.Kraft NJB, et al. 2011. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 333, 1755–1758. ( 10.1126/science.1208584) [DOI] [PubMed] [Google Scholar]
- 8.Polato NR, et al. 2018. Narrow thermal tolerance and low dispersal drive higher speciation in tropical mountains. Proc. Natl Acad. Sci. USA 115, 12 471–12 476. ( 10.1073/pnas.1809326115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian H, Ricklefs RE. 2007. A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecol. Lett. 10, 737–744. ( 10.1111/j.1461-0248.2007.01066.x) [DOI] [PubMed] [Google Scholar]
- 10.Chase JM. 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328, 1388–1391. ( 10.1126/science.1187820) [DOI] [PubMed] [Google Scholar]
- 11.Ferenc M, Sedláček O, Fuchs R, Dinetti M, Fraissinet M, Storch D. 2014. Are cities different? Patterns of species richness and beta diversity of urban bird communities and regional species assemblages in Europe. Glob. Ecol. Biogeogr. 23, 479–489. ( 10.1111/geb.12130) [DOI] [Google Scholar]
- 12.Kelly RP, O'Donnell JL, Lowell NC, Shelton AO, Samhouri JF, Hennessey SM, Feist BE, Williams GD. 2016. Genetic signatures of ecological diversity along an urbanization gradient. PeerJ 4, e2444 ( 10.7717/peerj.2444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herlemann DPR, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF. 2011. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5, 1571 ( 10.1038/ismej.2011.41) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martiny JBH, Eisen JA, Penn K, Allison SD, Horner-Devine MC. 2011. Drivers of bacterial β-diversity depend on spatial scale. Proc. Natl Acad. Sci. USA 108, 7850–7854. ( 10.1073/pnas.1016308108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huston MA. 1999. Local processes and regional patterns: appropriate scales for understanding variation in the diversity of plants and animals. Oikos 86, 393–401. ( 10.2307/3546645) [DOI] [Google Scholar]
- 16.Angermeier PL, Winston MR. 1998. Local vs. regional influences on local diversity in stream fish communities of Virginia. Ecology 79, 911–927. ( 10.1890/0012-9658(1998)079[0911:LVRIOL]2.0.CO) [DOI] [Google Scholar]
- 17.Hewitt JE, Thrush SF, Halliday J, Duffy C. 2005. The importance of small-scale habitat structure for maintaining beta diversity. Ecology 86, 1619–1626. ( 10.1890/04-1099) [DOI] [Google Scholar]
- 18.Ferger SW, Peters MK, Appelhans T, Detsch F, Hemp A, Nauss T, Otte I, Böhning-Gaese K, Schleuning M. 2017. Synergistic effects of climate and land use on avian beta-diversity. Divers. Distrib. 23, 1246–1255. ( 10.1111/ddi.12615) [DOI] [Google Scholar]
- 19.Malard F, Boutin C, Camacho AI, Ferreira D, Michel G, Sket B, Stoch F. 2009. Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe. Freshw. Biol. 54, 756–776. ( 10.1111/j.1365-2427.2009.02180.x) [DOI] [Google Scholar]
- 20.Zagmajster M, Eme D, Fišer C, Galassi D, Marmonier P, Stoch F, Cornu J-F, Malard F. 2014. Geographic variation in range size and beta diversity of groundwater crustaceans: insights from habitats with low thermal seasonality. Glob. Ecol. Biogeogr. 23, 1135–1145. ( 10.1111/geb.12200) [DOI] [Google Scholar]
- 21.Mammola S. 2019. Finding answers in the dark: caves as models in ecology fifty years after Poulson and White. Ecography 42, 1331–1351. ( 10.1111/ecog.03905) [DOI] [Google Scholar]
- 22.Culver DC, Pipan T. 2019. The biology of caves and other subterranean habitats, 2nd edn. Oxford, UK: Oxford University Press. [Google Scholar]
- 23.Hutchison NL, Lance RF, Pekins CE, Noble ME, Leberg PL. 2016. Influence of geomorphology and surface features on the genetic structure of an important trogloxene, the secret cave cricket (Ceuthophilus secretus). Conserv. Genet. 17, 969–983. ( 10.1007/s10592-016-0836-3) [DOI] [Google Scholar]
- 24.Jaffe R, et al. 2018. Conserving relics from ancient underground worlds: assessing the influence of cave and landscape features on obligate iron cave dwellers from the eastern Amazon. PeerJ 6, e4531 ( 10.7717/peerj.4531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiménez-Valverde A, Sendra A, Garay P, Reboleira ASPS. 2017. Energy and speleogenesis: key determinants of terrestrial species richness in caves. Ecol. Evol. 7, 10 207–10 215. ( 10.1002/ece3.3558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lunghi E, Manenti R, Ficetola GF. 2017. Cave features, seasonality and subterranean distribution of non-obligate cave dwellers. PeerJ 5, e3169 ( 10.7717/peerj.3169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellegrini TG, Sales LP, Aguiar P, Ferreira RL. 2016. Linking spatial scale dependence of land-use descriptors and invertebrate cave community composition. Subterr. Biol. 18, 17–38. ( 10.3897/subtbiol.18.8335) [DOI] [Google Scholar]
- 28.Mammola S, Aharon S, Seifan M, Lubin Y, Gavish-Regev E. 2019. Exploring the interplay between local and regional drivers of distribution of a subterranean organism. Diversity 11, 1–19. ( 10.3390/d11080119) [DOI] [Google Scholar]
- 29.Mammola S, Isaia M. 2017. Spiders in caves. Proc. R. Soc. B 284, 20170193 ( 10.1098/rspb.2017.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammola S, Cardoso P, Ribera C, Pavlek M, Isaia M. 2018. A synthesis on cave-dwelling spiders in Europe. J. Zool. Syst. Evol. Res. 56, 301–316. ( 10.1111/jzs.12201) [DOI] [Google Scholar]
- 31.Ferrier S, Manion G, Elith J, Richardson K. 2007. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 13, 252–264. ( 10.1111/j.1472-4642.2007.00341.x) [DOI] [Google Scholar]
- 32.Mammola S, et al. 2019. Continental data on cave-dwelling spider communities across Europe (Arachnida: Araneae) Biodivers. Data J. 7, e38492 ( 10.3897/BDJ.7.e38492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zagmajster M, Culver DC, Christman MC, Sket B. 2010. Evaluating the sampling bias in pattern of subterranean species richness: combining approaches. Biodivers. Conserv. 19, 3035–3048. ( 10.1007/s10531-010-9873-2) [DOI] [Google Scholar]
- 34.Christman MC, Culver DC. 2001. The relationship between cave biodiversity and available habitat. J. Biogeogr. 28, 367–380. ( 10.1046/j.1365-2699.2001.00549.x) [DOI] [Google Scholar]
- 35.Niemiller ML, Zigler KS. 2013. Patterns of cave biodiversity and endemism in the Appalachians and Interior Plateau of Tennessee, USA. PLoS ONE 8, e64177 ( 10.1371/journal.pone.0064177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trajano E, de Carvalho MR.. 2017. Towards a biologically meaningful classification of subterranean organisms: a critical analysis of the Schiner-Racovitza system from a historical perspective, difficulties of its application and implications for conservation. Subterr. Biol. 22, 1–26. ( 10.3897/subtbiol.22.9759) [DOI] [Google Scholar]
- 37.Nentwig W, Gloor D, Kropf C. 2015. Spider taxonomists catch data on web. Nature 528, 479 ( 10.1038/528479a) [DOI] [PubMed] [Google Scholar]
- 38.Fick SE, Hijmans RJ. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. ( 10.1002/joc.5086) [DOI] [Google Scholar]
- 39.Christman MC, Doctor DH, Niemiller ML, Weary DJ, Young JA, Zigler KS, Culver DC. 2016. Predicting the occurrence of cave-inhabiting fauna based on features of the Earth surface environment. PLoS ONE 11, e0160408 ( 10.1371/journal.pone.0160408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammola S, Leroy B. 2018. Applying species distribution models to caves and other subterranean habitats. Ecography 41, 1194–1208. ( 10.1111/ecog.03464) [DOI] [Google Scholar]
- 41.Mammola S, Schönhofer AL, Isaia M. 2019. Tracking the ice: subterranean harvestmen distribution matches ancient glacier margins. J. Zool. Syst. Evol. Res. 57, 548–554. ( 10.1111/jzs.12264) [DOI] [Google Scholar]
- 42.Manion G, Lisk M, Ferrier S, Nieto-Lugilde D, Mokany K, Fitzpatrick MC.. 2018. gdm: generalized dissimilarity modeling. See https://cran.r-project.org/package=gdm.
- 43.Mammola S, et al. 2019. Data from: Local- versus broad-scale environmental drivers of continental β-diversity patterns in subterranean spider communities across Europe Dryad Digital Repository. ( 10.5061/dryad.qz612jm8z) [DOI] [PMC free article] [PubMed]
- 44.Zuur AF, Ieno EN, Elphick CS. 2009. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. ( 10.1111/j.2041-210X.2009.00001.x) [DOI] [Google Scholar]
- 45.Fitzpatrick MC, Sanders NJ, Ferrier S, Longino JT, Weiser MD, Dunn R. 2011. Forecasting the future of biodiversity: a test of single- and multi-species models for ants in North America. Ecography 34, 836–847. ( 10.1111/j.1600-0587.2011.06653.x) [DOI] [Google Scholar]
- 46.Esposito LA, Bloom T, Caicedo-Quiroga L, Alicea-Serrano AM, Sánchez-Ruíz JA, May-Collado LJ, Binford GJ, Agnarsson I. 2015. Islands within islands: diversification of tailless whip spiders (Amblypygi, Phrynus) in Caribbean caves. Mol. Phylogenet. Evol. 93, 107–117. ( 10.1016/j.ympev.2015.07.005) [DOI] [PubMed] [Google Scholar]
- 47.Hedin M. 2015. High-stakes species delimitation in eyeless cave spiders (Cicurina, Dictynidae, Araneae) from central Texas. Mol. Ecol. 24, 346–361. ( 10.1111/mec.13036) [DOI] [PubMed] [Google Scholar]
- 48.Eme D, et al. 2018. Do cryptic species matter in macroecology? Sequencing European groundwater crustaceans yields smaller ranges but does not challenge biodiversity determinants. Ecography 41, 424–436. ( 10.1111/ecog.02683) [DOI] [Google Scholar]
- 49.Qian H, Badgley C, Fox DL. 2009. The latitudinal gradient of beta diversity in relation to climate and topography for mammals in North America. Glob. Ecol. Biogeogr. 18, 111–122. ( 10.1111/j.1466-8238.2008.00415.x) [DOI] [Google Scholar]
- 50.Gibert J, Deharveng L. 2002. Subterranean ecosystems: a truncated functional biodiversity. Bioscience 52, 473–481. ( 10.1641/0006-3568(2002)052[0473:SEATFB]2.0.CO;2) [DOI] [Google Scholar]
- 51.Růžička V, Šmilauer P, Mlejnek R. 2013. Colonization of subterranean habitats by spiders in Central Europe . Int. J. Speleol. 42: 133–140. ( 10.5038/1827-806X.42.2.5) [DOI] [Google Scholar]
- 52.Mammola S, Goodacre SL, Isaia M. 2018. Climate change may drive cave spiders to extinction. Ecography 41, 233–243. ( 10.1111/ecog.02902) [DOI] [Google Scholar]
- 53.Sánchez-Fernández D, et al. 2018. The deep subterranean environment as a potential model system in ecological, biogeographical and evolutionary research. Subterr. Biol. 25, 1–7. ( 10.3897/subtbiol.25.23530) [DOI] [Google Scholar]
- 54.Mammola S, Piano E, Malard F, Vernon P, Isaia M. 2019. Extending Janzen's hypothesis to temperate regions: a test using subterranean ecosystems. Funct. Ecol. 33, 1638–1650. ( 10.1111/1365-2435.13382) [DOI] [Google Scholar]
- 55.Bregović P, Zagmajster M. 2016. Understanding hotspots within a global hotspot: identifying the drivers of regional species richness patterns in terrestrial subterranean habitats. Insect Conserv. Divers. 9, 268–281. ( 10.1111/icad.12164) [DOI] [Google Scholar]
- 56.Culver DC, Deharveng L, Bedos A, Lewis JJ, Madden M, Reddell JR, Sket B, Trontelj P, White D. 2006. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 29, 120–128. ( 10.1111/j.2005.0906-7590.04435.x) [DOI] [Google Scholar]
- 57.Culver DC, Pipan T. 2014. Shallow subterranean habitats: ecology, evolution, and convervation. Oxford, UK: Oxford University Press. [Google Scholar]
- 58.Silva MS, Martins PR, Ferreira RL. 2011. Trophic dynamics in a Neotropical limestone cave. Subterr. Biol. 9, 127–138. ( 10.3897/subtbiol.9.2515) [DOI] [Google Scholar]
- 59.Giachino PM, Vailati D. 2010. The subterranean environment. Hypogean life, concepts and collecting techniques. Verona, Italy: WBA Handbooks. [Google Scholar]
- 60.Cardoso P. 2012. Diversity and community assembly patterns of epigean vs. troglobiont spiders in the Iberian Peninsula. Int. J. Speleol. 41, 83–94. ( 10.5038/1827-806X.41.1.9) [DOI] [Google Scholar]
- 61.Fiera C, Habel JC, Ulrich W. 2018. Neutral colonisations drive high beta-diversity in cavernicole springtails (Collembola). PLoS ONE 13, e0189638 ( 10.1371/journal.pone.0189638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fattorini S, Borges PAV, Fiasca B, Galassi DMP. 2016. Trapped in the web of water: groundwater-fed springs are island-like ecosystems for the meiofauna. Ecol. Evol. 6, 8389–8401. ( 10.1002/ece3.2535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pipan T, Culver DC, Papi F, Kozel P. 2018. Partitioning diversity in subterranean invertebrates: the epikarst fauna of Slovenia. PLoS ONE 13, e0195991 ( 10.1371/journal.pone.0195991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Culver DC, Trontelj P, Zagmajster M, Pipan T. 2013. Paving the way for standardized and comparable subterranean biodiversity studies. Subterr. Biol. 10, 1–43. ( 10.3897/subtbiol.10.4759) [DOI] [Google Scholar]
- 65.Costello MJ, Horton T, Kroh A. 2018. Sustainable biodiversity databasing: international, collaborative, dynamic, centralised. Trends Ecol. Evol. 33, 803–805. ( 10.1016/j.tree.2018.08.006) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mammola S, et al. 2019. Data from: Local- versus broad-scale environmental drivers of continental β-diversity patterns in subterranean spider communities across Europe Dryad Digital Repository. ( 10.5061/dryad.qz612jm8z) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Response and environmental predictors, as well as the R code to generate the analyses, are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.qz612jm8z [43]. The full dataset of subterranean spider communities across Europe are available in an associated data paper [32] and updated as long as new data becomes available.