Abstract
Tree cover differentiates forests from savannas and grasslands. In tropical floodplains, factors differentiating these systems are poorly known, even though floodplains cover 10% of the tropical landmass. Seasonal inundation potentially presents trees with both challenges (soil anoxia) and benefits (moisture and nutrient deposition), the relative importance of which may depend on ecological context, e.g. if floods alleviate water stress more in more arid ecosystems. Here, we use remotely sensed data across 13 large tropical and sub-tropical floodplain ecosystems on five continents to show that climatic water balance (i.e. precipitation—potential evapotranspiration) strongly increases floodplain tree cover in interaction with flooding, fire and topography. As predicted, flooding increases tree cover in more arid floodplains, but decreases tree cover in climatically wetter ones. As in uplands, frequent fire reduced tree cover, particularly in wet regions, but—in contrast with uplands—lower elevation and sandier soils decreased tree cover. Our results suggest that predicting the impacts of changing climate, land use and hydrology on floodplain ecosystems depends on considering climate-disturbance interactions. While outright wetland conversion proceeds globally, additional anthropogenic activities, including alteration of fire frequencies and dam construction, will also shift floodplain tree cover, especially in wet climates.
Keywords: wetland, flood pulse, disturbance regime, inundation, biome, vegetation
1. Introduction
Variation in tropical tree cover has been linked to rainfall [1–3], fire [2,4,5], soils [4,6], and, in places, herbivory [5,7,8]. These factors combine to differentiate high tree cover forests from low tree cover savannas and grasslands [2,3,5,9] with consequences for community composition [10] and ecosystem services [11]. Although broad, the existing literature has explicitly excluded or simply ignored tree cover determinants in seasonally flooded habitats [3,9], despite the fact that nearly 10% of the global terrestrial tropics is periodically inundated by floodwaters [12]. These habitats include highly biodiverse forests [13], large terrestrial carbon stocks [14], productive inland fisheries [15], and critical water sources for both people and wildlife [16], and tree cover shapes their local species composition [10], biogeochemical sinks [17] and hydrology [18]. Nonetheless, the relative importance of climate, soil composition, fire and hydrological regimes to structuring tropical floodplain vegetation is unknown.
As a starting point, the factors that determine tropical upland (non-flooded) vegetation structure also likely affect floodplain tree cover. In uplands, aridity [2,3,9] and fire frequency [2,5,6] limit tropical tree cover by reducing tree and sapling survival, growth, and competitive advantage against grasses. Higher soil sand content is associated with higher tree cover [3,6,19], likely because trees can grow deep roots to extract water from the deeper water tables in sandier regions [4,6,20]. By contrast, floodplain soils frequently have high water tables [21], potentially limiting niche separation and suggesting the relationship between soil composition and tree cover may be different from that in uplands.
A more obvious upland–floodplain contrast is that floodplain trees must deal with inundation. Indeed, the large literature on flood-induced mortality, plus plant physiological and morphological adaptations to inundation [22–24], suggest hydrological regimes might play a major role in determining floodplain vegetation structure. Flooding can present either a physiological challenge or an ecological opportunity for trees. On the one hand, flooding waterlogs soils, creating anoxic conditions that can kill roots, paradoxically starving plants of water needed for photosynthesis [22,25]. These physiological constraints can be alleviated by plastic or constitutive generation of hollow stems and other intercellular spaces called aerenchyma, that allow diffusion of oxygen from emergent tissues to submerged ones [22,25]. On the other hand, while anoxia is a barrier to survival for many tree species, floods can also benefit trees by recharging soil moisture [26] and depositing soil nutrients [27,28]. Tree cover in flooding ecosystems appears to reflect this tension: seasonally flooded tropical ecosystems exhibit a striking range of vegetation structure, from near-treeless grasslands (e.g. floodplains of northern Australia's Kakadu and Kimberley regions) to grassy systems with moderate tree cover (e.g. the Pantanal) to tropical forests with near-complete tree canopies (e.g. Amazonian várzea forests and igapó). We do not currently understand why these habitats vary in structure as much as they do, or specifically what the role of flooding is relative to other environmental factors in shaping these ecosystems.
The relative costs versus benefits of flooding for trees may depend on aspects of flood regimes themselves, including flood duration, frequency, depth and temporal predictability. For instance, in the Amazon—probably the best-studied tropical floodplain ecosystem—tree recruitment is slower in areas with long (greater than 150 days) compared to short (less than 70 days) hydroperiods [29], a pattern that is consistent across riparian plants [23]. Similarly, deeper floodwaters (relative to plant height) cause plant mortality [23]. In addition, where flooding (either its duration or seasonality) is more predictable, the evolution of flood adaptations such as aerenchyma and lenticels may be more likely [30,31], thereby easing tree survival in floodplains. Landscape position also can affect flood regime impacts; e.g. areas with raised and/or sloped topography tend to have water tables that drop more quickly following precipitation events, alleviating anoxic conditions in the rhizosphere [21]. Upland ecosystems do not have such elevational constraints on tree cover.
Finally, these tree cover determinants may interact. In arid, water-limited regions, floodwaters may alleviate water stress by maintaining or recharging soil moisture [26], whereas in high-rainfall regions where soils are already close to water-saturated, flooding may create anoxia. High-rainfall regions also burn relatively infrequently [32] and trees in these ecosystems tend to be poorly adapted to fire [33]; therefore, fires that do occur in these habitats may have especially strong effects on tree survival.
Here, we examine how climate, fire, soils and flood regimes affect tree cover in 13 seasonally flooded tropical and sub-tropical floodplains. We use globally consistent datasets including satellite-derived tree cover estimates [34] and remotely sensed monthly inundation maps [35] to compare among relatively well-studied (e.g. the Amazon) and poorly characterized (the majority of tropical floodplains; e.g. Sudan's Sudd) ecosystems. We expected (1) arid climates, (2) frequent fire and (3) frequent, long-duration, and unpredictable flooding to decrease tree cover, and that flood effects would depend on ecological context, especially climate.
2. Methods
(a). Study regions and data extraction
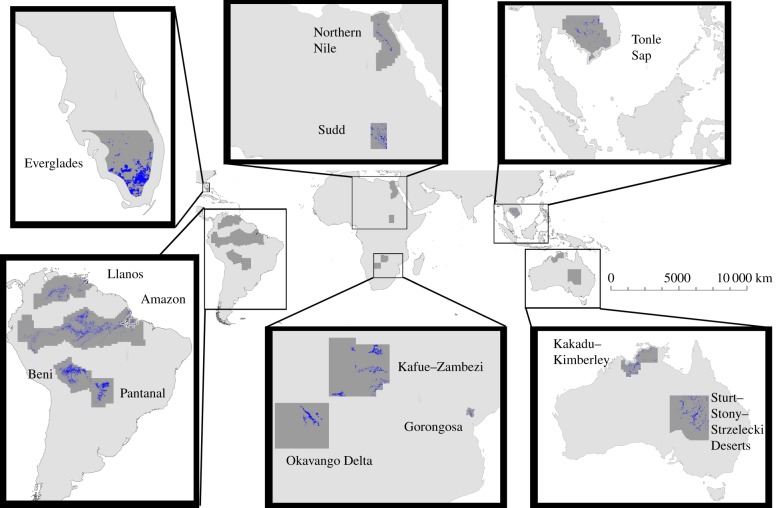
We studied 13 major tropical and sub-tropical floodplain ecosystems on five continents (figure 1). We selected the regions to capture hydrological and tree cover variation across the world's seasonal floodplains, but many also have significant biodiversity, carbon storage, water supply and tourism values. We hand-digitized the geographical extent of each ecosystem from a georeferenced map of global inundation probability (fig. 6 in ref. [35]), encompassing periodically flooded areas. In this step, we used wide boundaries because we later took data-driven subsets of each region.
Figure 1.
Study regions. Blue shading (dark grey in print) in each region depicts the areal extent of natural vegetation floodplain pixels included in the analysis. Dark grey areas show the larger extent from which final study pixels were subset. (Online version in colour.)
We then identified areas within each ecosystem that were (a) temporarily but not permanently flooded and (b) not deforested, urban, or agricultural areas to leave only natural vegetation from locations flooded at least once in 1993–2007. Specifically, we first removed permanent water defined as locations inundated for greater than or equal to 95% of observations in either of two global, monthly inundation geo-databases, the Global Surface Water dataset [36] and GIEMS-D3 [35]. The Global Surface Water dataset was produced from the entire 1984 to 2015 Landsat archive (30 m resolution optical imagery); GIEMS-D3 is a monthly topographic downscaling (3 arc-second or approx. 90 m resolution) of inundation detections by a suite of visible, near-infrared, and microwave sensors covering January 1993–December 2007 [37,38]. GIEMS-D3 allows mapping of inundation beneath vegetation canopies, not detectable by the Global Surface Water product [35,36,39]. We also removed areas that were never inundated in the GIEMS-D3 dataset, and locations within 25 km of a coastline because GIEMS-D3 data are contaminated by ocean signals (C. Prigent 2018, personal communication [35]).
To identify urban areas, we used the GlobCover v. 2.2 land cover classification for December 2004–June 2006 and removed from our study regions all pixels classified as ‘Artificial surfaces and associated areas (urban areas greater than 50%).’ Deforested areas identified using the ‘lossyear’ layer from the Landsat-based Global Forest Change dataset [34] were also removed study regions.
We used two datasets to define and remove agricultural areas. First, we removed all areas covered by the 1 km crop mask from the Global Food Security Support Analysis Data to exclude croplands [40,41]. Then, we additionally removed all areas with greater than or equal to 20 tropical livestock units per km2 as defined by Jahnke & Jahnke [42] and Jahnke et al. [43] using modelled cattle, pig, goat and sheep densities from the Livestock Geo-Wiki Project [44].
In each region, we extracted per cent tree cover and a set of 11 covariates hypothesized to affect vegetation structure for 3 arc-second pixels. Per cent tree cover was derived from a Landsat-based product produced by Hansen et al. [45] for the year 2010.
We included three predictors—climatic water balance, soil sand content and fire frequency (number of times burned)—that are well-known determinants of tree cover in upland tropical ecosystems [3,4]. Annual climatic water balance (hereafter ‘water balance’) was computed as the mean annual precipitation minus annual potential evapotranspiration, both derived from the WorldClim dataset [46]. Soil sand content at 5 cm depth was extracted from the SoilGrids dataset [47] at 250 m resolution, and the number of times burned between May 2000 (the start of the dataset) and December 2010 was computed from NASA's MODIS MCD64A1 burned area product [48].
Hydrological regime descriptors were computed from GIEMS-D3 [35]. We calculated metrics for (i) flood frequency (number of calendar years with flooding in at least one month), (ii) flood duration (mean number of months flooded annually), (iii) variance of flood duration; and (iv) variability of flood seasonality. The last metric captures the across-years frequency with which a pixel is in its most common state—flooded or not—for each calendar month, and was calculated for each pixel as follows:
where x is the number of years in which the focal pixel was flooded for a given month and 15 is the number of years in the full GIEMS-D3 dataset. For example, a pixel that was flooded in January, February and March in 5 of 15 years, and in April in 3 of 15 years, but never between May and December would take the value:
Larger values indicate greater within-month variability among years in the status of inundation (flooded or dry) and possible values for variability of flood seasonality in our 15-year dataset range from 0 (complete year-to-year consistency in monthly flood status) to 7 (all months are flooded in exactly 7 or 8 of 15 years).
We calculated the metric variation in flood seasonality in place of the more common Colwell's predictability [49] because our metric was computationally more efficient by several orders of magnitude. We tested the validity of our more efficient metric for the three smallest regions in this study, Gorongosa, the Everglades and the Okavango, finding that our metric was correlated to Colwell predictability at r = −0.992, −0.985 and −0.972.
Because the GIEMS-D3 data are limited in their ability to resolve floods with small spatial extent (less than 60 km2; [35,37]) and cannot capture centennial-to-millennial periodicities or short-term (e.g. 1 day) inundation, we included four topographic variables as proxies for these flood regime characteristics. First, because higher elevation areas may escape flood impacts, we computed the relative elevation for each pixel as metres above the mean within-region elevation of floodplain pixels from the 3 arc-s SRTM digital elevation model [50]. Second, we included topographic slope, computed as a percentage and also from the SRTM dataset, because higher-slope areas may drain faster and have lower water tables, helping to alleviate anoxia [21]. Third and fourth, we computed the distance from the centre of each GIEMS-D3 grid cell to the nearest river and the nearest large river. Any river course may facilitate riparian/gallery forest tree cover by increasing soil moisture, but larger rivers are more likely to flood extensive areas. We used the 15 arc-s HydroSHEDS river network [51] and defined large river cells in the data as those draining an upstream region with area greater than or equal to 4000 15 arc-s grid cells (approx. 850 km2).
(b). Statistical modelling
We began analyses with random forest regressions to find the relative importance of predictors for determining floodplain tree cover. Random forest regression is a decision-tree-based method that finds split-points in predictor values at which the response can be partitioned into increasingly homogeneous groups [52]. Random forest modelling is highly robust to non-normal data, interactions, and to collinear predictors [53–55]. In addition, and of most interest here, random forest regression allows quantification of predictors' relative importance. Specifically, variable importance is assessed by permuting predictor values from a subset of the data held out of the model training set, then comparing the fit of model-predicted tree cover to actual tree cover values using the randomly permuted versus original predictor values. We completed this procedure for each predictor and computed the average increase in mean squared error of predicted versus actual tree cover across regression trees [53,56]. Larger values indicate a greater variable importance, because they show that permuting a given predictor's values reduces fit to a greater degree.
We built one ‘global’ model pooling data from all regions in our analysis to find the importance of predictors for determining tree cover across our 13 globally distributed floodplain ecosystems and built a separate model for each ecosystem to examine the same within regions. In total, all 13 ecosystems included 41 164 320 three arc-s pixels. Individual regions ranged in size from 27 618 (Gorongosa) to 14 442 434 (Amazon) pixels (electronic supplementary material, table S1) and encompassed a wide range of tree cover and predictor values (electronic supplementary material, figures S1 and S2).
Despite its strengths for identifying important predictors and dealing with collinearities, non-normality and higher-order interactions, it can be difficult to interpret the directional effects of weaker and non-monotonic predictors from random forest regression. Often the direction of effects are visualized using partial dependence plots [54], but interpretation of these plots is subjective. We, therefore, used linear models—again one global and one for each region—to determine the direction, strength and significance of predictors' effects on tree cover.
We centred and standardized the predictor variables by subtracting the mean from each value and then dividing by the predictor's standard deviation. This rescales the variables (means = 0, s.d. = 1) and expresses all parameter estimates as the rate of change in tree cover per standard-deviation change in the predictor, facilitating comparison of slopes among parameters. Where predictors were collinear at greater than 0.70 [57] we removed the less important predictor(s)— as determined by variable importance scores in the random forest regression—from the linear models.
In addition to main effects of all variables, we included the interactive effects of water balance with fire frequency, slope, elevation, flood frequency and duration, distance to the nearest river and nearest large river, soil sand content and slope. We included these interactions because our a priori hypotheses included the expectation that climatic context might alter the effects of fire and flooding.
We validated linear regression models in light of the assumptions of linear regression by inspecting diagnostic plots of the model residuals. We used histograms of residuals to verify their normality, plots of the residuals against fitted tree cover to check for homogeneity of variance, and plots of standardized residuals versus leverage to check for data points with disproportionate influence on the regression results [58]. We found no indication that these assumptions were violated.
All modelling was completed in software R [59]. Where we report means, errors are given as ± 1 s.d. Further methodological and modelling details are presented in the online electronic supplementary material.
3. Results
R2 ranged from 0.16 to 0.81 in the random forest regressions and 0.13 to 0.52 in the linear models, excepting the two driest regions, the SSS Deserts and Northern Nile (electronic supplementary material, table S2). In the case of the SSS Deserts and Northern Nile, linear models explained very little variation in the near constantly low tree cover and so are not considered further.
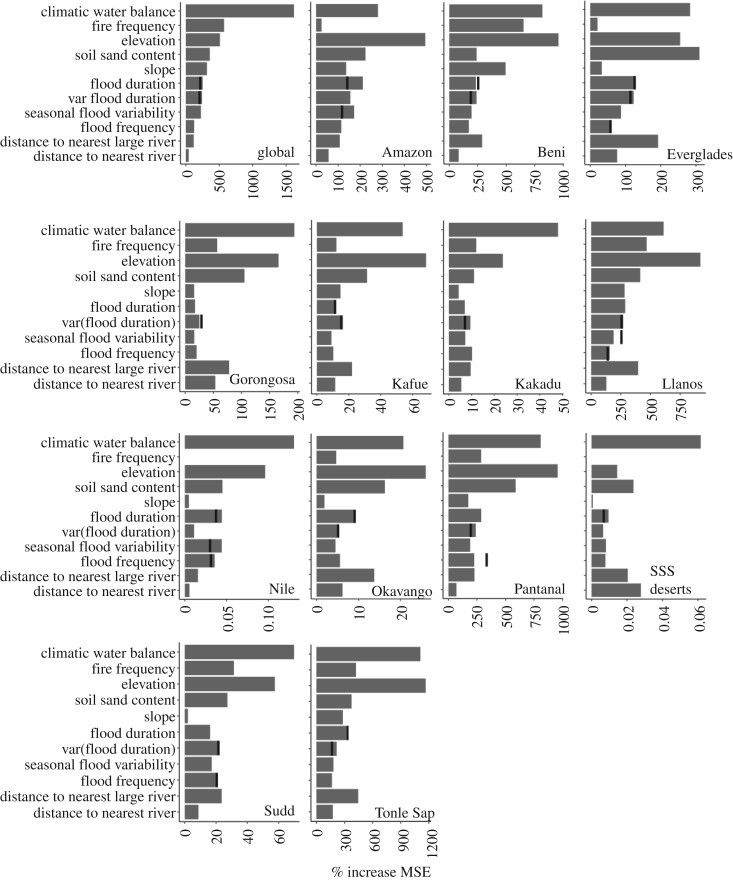
Random forest regression models identified water balance (average rank among predictors within regional models = 1.62 ± 0.50) and elevation (1.77 ± 1.2) as consistently the most important predictors of floodplain tree cover (figure 2). Water balance had a positive effect on floodplain tree cover both at the global scale (table 1 and figure 3) and within most regions (electronic supplementary material, table S3 and figures S3–S13). However, in four of the six climatically wettest regions (Llanos, Tonle Sap, Beni and the Pantanal) tree cover declined with increasing water balance.
Figure 2.
Variable importance plots illustrating the relative influence of predictors on floodplain tree cover across 13 ecosystems (‘global’ model) and within each ecosystem. Importance is measured as the average increase in mean squared error of predicted versus actual tree cover when using permuted predictor values. Black vertical bars indicate variable importance for hydrological variables retained in the sensitivity analysis (see electronic supplementary material, text) after removal of less important and highly collinear (r ≥ 0.70) hydrological predictors.
Table 1.
Parameter estimates and their standard errors from the global linear model for the effect of centered and standardized predictors on floodplain tree cover. CWB, climatic water balance; % sand, soil percent sand. Main effects are ordered from greatest to least variable importance in the global random forest model. All parameters were significant (p < 0.05), except slope and the CWB:slope interaction (marginally significant) in Gorongosa.
| intercept | climatic water balance | fire | elev. | sand | slope | flood dur. | var. of flood duration | distance to large river | distance to river | CWB: fire | CWB: elevation | CWB: sand | CWB: slope | CWB: flood duration | CWB: large river | CWB: river | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 47.89 | 18.57 | −18.18 | 2.26 | −4.08 | 5.33 | −2.1 | 1.78 | −0.98 | 0.93 | −12.13 | 0.71 | 0.13 | 0.45 | −2.07 | −0.84 | −0.21 |
| SE | 0.006 | 0.008 | 0.007 | 0.006 | 0.006 | 0.005 | 0.005 | 0.006 | 0.005 | 0.005 | 0.007 | 0.005 | 0.004 | 0.005 | 0.005 | 0.005 | 0.005 |
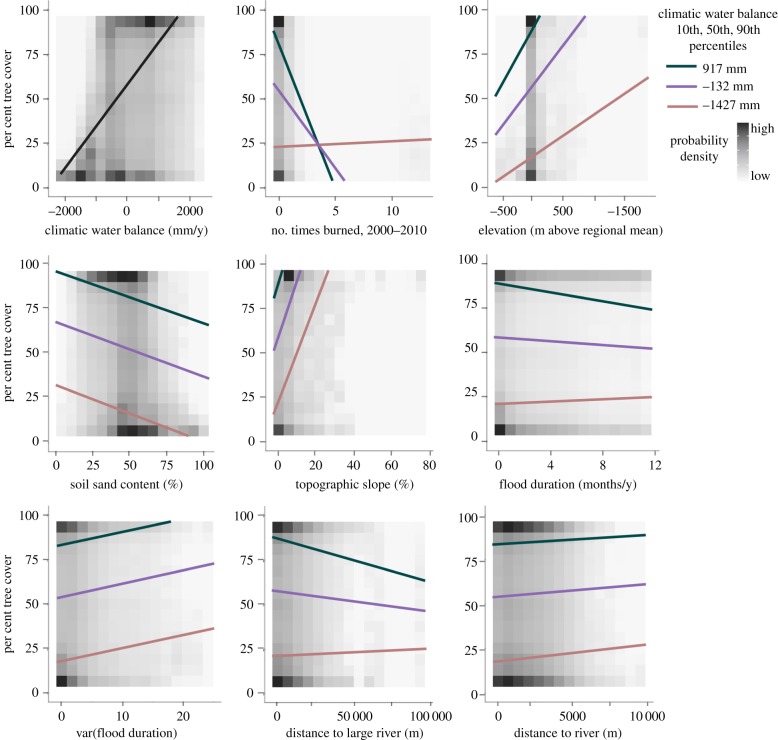
Figure 3.
Conditional regression plots for linear models of floodplain tree cover across 13 floodplain ecosystems (‘global’ linear model). Model-predicted tree cover is plotted for x-variable values at the 10th, 50th and 90th percentile of climatic water balance (red, purple and green lines online; top, middle, and bottom lines at left of each panel in hard-copy). Note the often-weaker effects (shallower slopes) in drier ecosystems. Predictors not plotted in each panel are held constant at their medians. Ninety-five per cent confidence intervals are all thinner than the regression lines. Plot backgrounds show the relative density of tree cover observations and plots are ordered by global variable importance.
Water balance affected tree cover not only through strong direct effects but also by altering several other predictors' effects on tree cover. For instance, floodplain tree cover generally increased strongly at higher elevations, but the effect was especially robust in wetter regions, as indicated by the positive water balance–elevation interaction in the global linear model and in several within-region models (electronic supplementary material, table S3, figure 3). Only in one region (Kakadu–Kimberly) did tree cover increase at lower elevation.
The effects of flood duration (and, in the two models where flood duration was removed due to collinearity, flood frequency) were not as strong as those of elevation or the direct effects of water balance, but did vary with regional water balance. Tree cover declined with increased flooding in five of the six climatically wettest ecosystems (from the Pantanal (−552 mm y−1) to the Amazon (713 mm y−1)) but increased in the Kakadu–Kimberley (−1119 mm y−1), Sudd (−1189 mm y−1) and Okavango (−1417 mm y−1) regions (electronic supplementary material, table S3). In addition, the water balance–flood duration interaction in the global linear model and most regional models indicated that flood duration had a stronger negative effect on tree cover in climatically wetter areas (figure 3 and table 1; electronic supplementary material, table S3).
Fire frequency had strong negative effects on floodplain tree cover, again, especially in regions with high water balance. Tree cover in the global model and in all seven of the climatically wettest regions decreased with increasing fire frequency (table 1; electronic supplementary material, table S3; figures 3, S3–S13). By contrast, fire effects on tree cover were weakly negative or near-absent in the drier Kafue–Zambezi, Kakadu–Kimberley, Sudd and Okavango regions. Furthermore, negative water balance–fire frequency interactions in the global model and in six of 11 regions indicated fire had a stronger negative (or less positive) effect in wetter locations (table 1; electronic supplementary material, table S3 and figure 3).
Floodplain tree cover was positively related to slope, except in the Sudd where there was a weak but statistically negative effect, and in Gorongosa where there was no effect of slope (table 1; electronic supplementary material, table S3 and figures S3–S13).
At the global scale, floodplain tree cover declined with increasing soil sand content (figure 2) and there was only a small (though statistically significant) positive interaction of water balance with soil sand content, indicating that sandier soil was somewhat less of a hindrance to tree cover in wetter climates. Within individual regions, soil sand content was often ranked as an important predictor (figure 2), but the direction of its effect was idiosyncratic (electronic supplementary material, table S3 and figures S3–S13), sometimes increasing (Tonle Sap, Everglades, Gorongosa, Kafue–Zambezi and Sudd) and sometimes decreasing (Amazon, Llanos, Beni, Pantanal, Kakadu–Kimberly, Okavango) tree cover.
Neither metric of flood predictability had a reliably strong influence on tree cover. Variance of flood duration and variability of flood seasonality were ranked 7th and 8th, respectively, in the global random forest model's variable importance scores and variance of flood duration was only weakly positively associated with tree cover in the associated linear model. The directional influence of variance in flood duration differed among regions, and this predictor was never ranked better than fifth most important, meaning there was little support for the idea that more seasonally predictable flood regimes would increase tree cover; indeed, the strongest responses were positive associations of Llanos and Sudd tree cover with variance of flood duration (electronic supplementary material, table S3 and figures S9 and S12).
Finally, distance to the nearest large river was negatively related to tree cover globally and in all ecosystems except the Everglades (table 1; electronic supplementary material, table S3 and figures S3–S13). Although weak (10th-ranked variable importance globally) relative to the effects of water balance, elevation, fire, soil composition and flood duration, the effect of distance to the nearest large river was moderate in the Beni, Gorongosa and Llanos. Distance to the nearest river of any size was consistently a weak predictor (11th-ranked globally), positively associated with floodplain tree cover globally, but negatively related to seven regions' tree cover.
4. Discussion
Here, we found that more arid climates and more frequent fire both had generally strong, negative effects on tree cover in tropical floodplains. Moreover, floodplain tree cover decreased with increasing soil sand content, and tree cover was much greater at higher elevations, likely a result of long-term limitation of tree cover by flooding. The climatic and fire regime results are consistent with those from non-flooding systems [1–3,5]; however, the effect of soil sand content is opposite to that in uplands [3,6,19], and there is no consistent effect of elevation on tropical tree cover outside floodplains, save for the near-binary effect of montane tree lines.
Increasing climatic water availability increased tree cover, but this effect saturated in climatically wetter regions (table 1; electronic supplementary material table S3; and figure S3–S13), as it does in upland savannas [3]. However, in contrast to the effects in uplands, we even observed a reversal of this effect—declining tree cover with increasing rainfall—in some of the climatically wettest floodplain regions (Beni, Tonle Sap and Pantanal). Trees in these wettest regions are likely released from persistent water limitation and may experience nutrient leaching [60] or decreased light availability due to cloudiness [61], with potentially reduced productivity as a consequence.
Water balance also created context-dependence in the effects of fire, topography and flooding on tree cover. We found that frequent fire was more strongly associated with lower tree cover in wetter climates, mirroring upland ecosystems where fire also has the biggest effects on woody plant abundance at higher-rainfall sites [2]. Trees tend to be poorly adapted to fire in moist tropical forests [33] where fire is relatively rare [48], meaning trees are more likely to die when fires do occur there. In contrast, many trees in more arid climates survive regular, low-intensity fires [19,33], accounting for the relative lack of an effect of fire in climatically drier floodplains (figure 3). In addition, wetter climates encourage higher fuel loads and fire intensity in savannas [62]. Indeed, fire frequency had stronger negative effects on tree cover in the Pantanal and Gorongosa, floodplain savannas with relatively wet climates compared to the Okavango and Sudd, which are relatively dry floodplain savannas and where fire frequency had little impact on tree cover (electronic supplementary material, figures S6, S10–S12). Fire-vegetation feedbacks are well known from upland savannas [5], meaning that not only do fires create ecosystems more dominated by herbaceous plants but also that more herbaceous ecosystems tend to have higher fuel loads, promoting fire frequency and intensity. The same feedbacks are likely in floodplains.
The effects of topography and flooding also depended on climate. Flooding increased tree cover in the driest systems, perhaps by supplementing rainfall [63], but acted mainly to limit tree cover in climatically wetter regions, likely via the effects of anoxic soils and physical disturbance on tree growth and survival [24]. However, the effects of our flood regime descriptors on floodplain tree cover were surprisingly weak—given that even short-term inundation can cause soil anoxia and decrease the growth and survival of many woody plants—compared to the effects of climatic water balance, elevation and fire [23,29].
While our flood regime descriptors were relatively weak tree cover determinants in our study, our topographic variables provided an additional proxy for flood regimes, especially over longer timescales than captured by the 15-year GIEMS-D3 dataset. Indeed, areas with higher elevations and steeper slopes tended to have higher tree cover, both globally and within regions. That these topographic effects on tree cover are a proxy for flooding effects is further supported by the fact that only in the Kakadu–Kimberley region—that with the lowest flood duration (0.39 ± 1.06 months per year; electronic supplementary material, figure S1) and frequency (2.77 ± 3.65 out of 15 years)—were elevation and slope effects weak. Field studies from the Okavango and Everglades similarly show that topography influences flood susceptibility: islands rising only slightly above the surrounding floodplain floor are home to relatively dense aggregations of trees [64,65]. Water tables beneath sloped and high-elevation terrain tend to drop more quickly following rainfall and inundation, likely reducing soil anoxia and its negative effects on trees [21]. Moreover, topographic effects were stronger in climatically wetter regions, as for flood frequency and duration, and suggesting that the negative effects of flooding on trees (likely via soil anoxia) are exacerbated where trees are less limited by water. The importance of elevation versus flood frequency, duration, and predictability may indicate that it is the longer-term hydrological regimes, or the ages of floodplain ecosystems, and not short-term hydrological dynamics, which have shaped floodplain vegetation structure [24]. We found little support for the longstanding theory (derived in part from research in Amazonian flooded forests) which suggests that more predictable flood regimes might increase tree cover because they are more conducive to the evolution of flood-adaptive traits [27,30,66]. Still, variability of flood regimes over longer timescales than the annual-to-decadal scale captured by GIEMS-D3 inundation data may influence functional trait evolution (e.g. [67]). The relatively long-term stability of hydrological regimes over geological time in some floodplains may have resulted in the regionally widespread prevalence of effective flood-adaptive traits [24,68]. For example, the heavily treed Amazon floodplains have flooded for millions of years compared to only several thousand years of flooding in other climatically wet regions like the Pantanal and Tonle Sap [24,66,69], resulting in variation in flood responses that are biogeographic rather than local in scale.
Tree responses to flooding include various morphological traits that aid gas diffusion to the roots from tissues both beneath and above the water line (e.g. aerenchyma, adventitious roots, hypertrophied lenticels), glycolysis and other anaerobic respiration pathways (especially for short-term anoxic events), and annual dormancy during the flood season [22,25,68]. At present, we have no comprehensive understanding of the geographic or taxonomic distribution of these traits, and future work in flooding ecosystems should consider the distribution and effectiveness of these biotic factors relative to flood regimes, phylogeny, biogeographic history and other environmental characteristics. Despite the spatially extensive and species-rich nature of some floodplain tree communities [24], targeted sampling across local and regional flood frequency, duration, and predictability gradients for comparison of flood-adaptive traits may allow better understanding of the within and between-species factors that allow tree persistence in seasonally flooded ecosystems and apparently somewhat irrespective of flood regimes.
Unfortunately, we were not able to directly consider the effects of flood depth, which local field studies show influences vegetation structure and composition [23], on tree cover in flooding systems because flood depth is not yet measurable or modelled at high spatio-temporal resolution at the global scale (GIEMS-D3 relies on satellite observations, which are extensive but not yet applied to modelling water depth globally). However, it is possible that our analysis captured the statistical effects of flood depth, since it is often highly correlated with flood duration [26]. We also did not consider the temporal sequence of flooded versus non-flooded periods, although there is some evidence that alternation between flooding and drought can limit tree diversity and seedling recruitment in floodplains [70].
Higher soil sand content was associated with lower tree cover at the global scale, although this effect was idiosyncratic within individual floodplain ecosystems. This contrasts with its effects in upland savannas, where sandier soils tend to increase tree cover by promoting water infiltration and therefore root niche separation of trees and grasses [3,6,19,20]. Infiltration depth may be less important in floodplains because wetland water tables are generally shallower [21], precluding the possibility of root depth differentiation between grasses and trees. Rather, our result that sandier soil decreased tree cover suggests that negative effects of high sand content on plants due to reduced nutrient availability [3,71] may predominate in floodplains. Further, whereas previous studies of upland ecosystems have focused on savannas and grasslands [3,6,19], we also included forested floodplains, which are less likely to be water-limited and which have little-if-any grass, again reducing the expected influence of any mechanism reliant on competition for soil water.
Proximity to rivers was also a relatively minor determinant; somewhat higher floodplain tree cover closer to large rivers (table 1; electronic supplementary material, table S3 and figures S3–S13; except in the Everglades) may indicate that trees benefit from year-round access to water in riparian zones [21].
Taken together, our results reveal that a suite of environmental factors affect tree cover in flooding regions. Climatic water balance (largely determined by rainfall and temperature) is fundamental and differentiates between systems in dry versus wet contexts. Tree cover in dry floodplains tended to increase slightly from flooding, which suggests that soil moisture subsidies from floods probably help to alleviate water limitation/stress in those systems. By contrast, tree cover in wetter floodplains tended to decline from increased flooding, suggesting that the alleviation of water stress increases the relative importance of anoxic stress. So too, fire frequency: the effects of fire were much stronger in wet than in dry systems, consistent with previous findings in the non-flooding tropics.
These findings have implications for the responses of tree cover to ongoing global changes in climate, fire regimes, land use and hydrology. Our models predict that declines in precipitation and/or increasing evapotranspiration due to climate change and climate–landcover feedbacks [72] will reduce floodplain tree cover in all but the wettest regions, where rainfall does not at present limit tree cover, consistent with effects of aridification in upland ecosystems [2,3]. Meanwhile, savanna fire frequencies are decreasing [73], which will encourage woody encroachment—a globally distributed threat to grassland biodiversity [10,74]—in all but the most arid grassy floodplains. By contrast, fires are rare but increasing in frequency in wet tropical forests [32,73], which may promote savannafication in some floodplain forests (table 1; electronic supplementary material, figures S3, S4, S13, ref. [81]) via extensive tree mortality and slow post-fire recovery [75]. Our results also suggest that the ongoing proliferation of dams [76] and the continuous inundation their impoundments create could decrease local tree cover by up to 15 percentage points in the climatically wettest regions (figure 3, flood duration). Finally, while we focused on floodplains that retain their natural vegetation, the extensive and continuing loss of wetlands [36]—and the myriad services they provide—due to human encroachment and land development underscores the importance of understanding and protecting the structure and function of remaining wetlands.
Supplementary Material
Acknowledgements
We thank C. Prigent for assistance with GIEMS-D3, Staver laboratory members for project feedback, and T. Bouncé for long-term inspiration.
Data accessibility
All data are available from the Dryad Digital Repository, along with example R scripts for running random forest models: https://doi.org/10.5061/dryad.pj77fc4 [77].
Authors' contributions
J.H.D. conceived of and designed the study and analysed the data. F.A. provided GIEMS-D3 data. A.C.S. contributed to the analytical design. All three authors wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
J.H.D. was supported by a Donnelley Postdoctoral Fellowship from the Yale Institute of Biospheric Studies.
References
- 1.Lehmann CE, et al. 2014. Savanna vegetation–fire–climate relationships differ among continents. Science 343, 548–552. ( 10.1126/science.1247355) [DOI] [PubMed] [Google Scholar]
- 2.Staver AC, Archibald S, Levin S. 2011. Tree cover in sub-Saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92, 1063–1072. ( 10.1890/10-1684.1) [DOI] [PubMed] [Google Scholar]
- 3.Sankaran M, et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849. ( 10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 4.Staver AC, Botha J, Hedin L. 2017. Soils and fire jointly determine vegetation structure in an African savanna. New Phytol. 216, 1151–1160. ( 10.1111/nph.14738) [DOI] [PubMed] [Google Scholar]
- 5.Van Langevelde F, et al. 2003. Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84, 337–350. ( 10.1890/0012-9658(2003)084[0337:EOFAHO]2.0.CO;2) [DOI] [Google Scholar]
- 6.Sankaran M, Ratnam J, Hanan N. 2008. Woody cover in African savannas: the role of resources, fire and herbivory. Global Ecol. Biogeogr. 17, 236–245. ( 10.1111/j.1466-8238.2007.00360.x) [DOI] [Google Scholar]
- 7.Daskin JH, Stalmans M, Pringle RM. 2016. Ecological legacies of civil war: 35-year increase in savanna tree cover following wholesale large-mammal declines. J. Ecol. 104, 79–89. ( 10.1111/1365-2745.12483) [DOI] [Google Scholar]
- 8.Asner GP, Levick SR. 2012. Landscape-scale effects of herbivores on treefall in African savannas. Ecol. Lett. 15, 1211–1217. ( 10.1111/j.1461-0248.2012.01842.x) [DOI] [PubMed] [Google Scholar]
- 9.Staver AC, Archibald S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232. ( 10.1126/science.1210465) [DOI] [PubMed] [Google Scholar]
- 10.Parr CL, Gray EF, Bond WJ. 2012. Cascading biodiversity and functional consequences of a global change-induced biome switch. Divers. Distributions 18, 493–503. ( 10.1111/j.1472-4642.2012.00882.x) [DOI] [Google Scholar]
- 11.de Groot R, et al. 2012. Global estimates of the value of ecosystems and their services in monetary units. Ecosyst. Serv. 1, 50–61. ( 10.1016/j.ecoser.2012.07.005) [DOI] [Google Scholar]
- 12.Fluet-Chouinard E, Lehner B, Rebelo L-M, Papa F, Hamilton SK. 2015. Development of a global inundation map at high spatial resolution from topographic downscaling of coarse-scale remote sensing data. Remote Sens. Environ. 158, 348–361. ( 10.1016/j.rse.2014.10.015) [DOI] [Google Scholar]
- 13.Junk WJ, Piedade MTF, Wittmann F, Schöngart J, Parolin P (eds). 2010. Amazonian floodplain forests: ecophysiology, biodiversity and sustainable management, 2011 edn Dordrecht, Netherlands: Springer. [Google Scholar]
- 14.Dargie GC, Lewis SL, Lawson IT, Mitchard ET, Page SE, Bocko YE, Ifo SA. 2017. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 542, 86 ( 10.1038/nature21048) [DOI] [PubMed] [Google Scholar]
- 15.Sabo JL, Ruhi A, Holtgrieve GW, Elliott V, Arias ME, Ngor PB, Räsänen TA, Nam S. 2017. Designing river flows to improve food security futures in the Lower Mekong Basin. Science 358, 1–11. [DOI] [PubMed] [Google Scholar]
- 16.Tockner K, Stanford JA. 2002. Riverine flood plains: present state and future trends. Environ. Conserv. 29, 308–330. ( 10.1017/S037689290200022X) [DOI] [Google Scholar]
- 17.Coetsee C, Bond WJ, February EC. 2010. Frequent fire affects soil nitrogen and carbon in an African savanna by changing woody cover. Oecologia 162, 1027–1034. ( 10.1007/s00442-009-1490-y) [DOI] [PubMed] [Google Scholar]
- 18.Joffre R, Rambal S. 1993. How tree cover influences the water balance of Mediterranean rangelands. Ecology 74, 570–582. ( 10.2307/1939317) [DOI] [Google Scholar]
- 19.Case MF, Staver AC. 2016. Fire prevents woody encroachment only at higher-than-historical frequencies in a South African savanna. J. Appl. Ecol. 54, 955–962. ( 10.1111/1365-2664.12805) [DOI] [Google Scholar]
- 20.Scholes RJ, Archer SR. 1997. Tree–grass interactions in savannas. Annu. Rev. Ecol. Syst. 28, 517–544. ( 10.1146/annurev.ecolsys.28.1.517) [DOI] [Google Scholar]
- 21.Fan Y, Miguez-Macho G, Jobbágy EG, Jackson RB, Otero-Casal C. 2017. Hydrologic regulation of plant rooting depth. Proc. Natl Acad. Sci. USA 114, 10 572–10 577. ( 10.1073/pnas.1712381114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson MB, Colmer TD. 2005. Response and adaptation by plants to flooding stress. Ann. Botany 96, 501–505. ( 10.1093/aob/mci205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garssen AG, Baattrup-Pedersen A, Voesenek LACJ, Verhoeven JTA, Soons MB. 2015. Riparian plant community responses to increased flooding: a meta-analysis. Glob. Change Biol. 21, 2881–2890. ( 10.1111/gcb.12921) [DOI] [PubMed] [Google Scholar]
- 24.Parolin P, Wittmann F. 2010. Struggle in the flood: tree responses to flooding stress in four tropical floodplain systems. AoB Plants 2010, 1–18. ( 10.1093/aobpla/plq003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlowski TT. 1997. Responses of woody plants to flooding and salinity. Tree Physiol. 17, 490 ( 10.1093/treephys/17.7.490) [DOI] [Google Scholar]
- 26.Arias ME, Cochrane TA, Norton D, Killeen TJ, Khon P. 2013. The flood pulse as the underlying driver of vegetation in the largest wetland and fishery of the Mekong Basin. Ambio 42, 864–876. ( 10.1007/s13280-013-0424-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junk WJ, Bayley PB, Sparks RE. 1989. The flood pulse concept in river–floodplain systems. Can. J. Fish. Aquat. Sci. 106, 110–127. [Google Scholar]
- 28.Kvist LP, Nebel G. 2001. A review of Peruvian flood plain forests: ecosystems, inhabitants and resource use. Forest Ecol. Manage. 150, 3–26. ( 10.1016/S0378-1127(00)00679-4) [DOI] [Google Scholar]
- 29.Lucas CM, Sheikh P, Gagnon PR, Mcgrath DG. 2016. How livestock and flooding mediate the ecological integrity of working forests in Amazon River floodplains. Ecol. Appl. 26, 190–202. ( 10.1890/14-2182) [DOI] [PubMed] [Google Scholar]
- 30.Poff NL. 1992. Why disturbances can be predictable: a perspective on the definition of disturbance in streams. J. North American Benthological Soc. 11, 86–92. ( 10.2307/1467885) [DOI] [Google Scholar]
- 31.Lytle DA, Poff NL. 2004. Adaptation to natural flow regimes. Trends Ecol. Evol. 19, 94–100. ( 10.1016/j.tree.2003.10.002) [DOI] [PubMed] [Google Scholar]
- 32.van der Werf GR, Randerson JT, Giglio L, Gobron N, Dolman AJ. 2008. Climate controls on the variability of fires in the tropics and subtropics. Global Biogeochem. Cycles 22, 1–13. ( 10.1029/2007GB003122) [DOI] [Google Scholar]
- 33.Pellegrini AF, Anderegg WR, Paine CE, Hoffmann WA, Kartzinel T, Rabin SS, Sheil D, Franco AC, Pacala SW. 2017. Convergence of bark investment according to fire and climate structures ecosystem vulnerability to future change. Ecol. Lett. 20, 307–316. ( 10.1111/ele.12725) [DOI] [PubMed] [Google Scholar]
- 34.Hansen MC, et al. 2013. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. ( 10.1126/science.1244693) [DOI] [PubMed] [Google Scholar]
- 35.Aires F, Miolane L, Prigent C, Pham B, Fluet-Chouinard E, Lehner B, Papa F. 2017. A global dynamic long-term inundation extent dataset at high spatial resolution derived through downscaling of satellite observations. J. Hydrometeor. 18, 1305–1325. ( 10.1175/JHM-D-16-0155.1) [DOI] [Google Scholar]
- 36.Pekel J-F, Cottam A, Gorelick N, Belward AS. 2016. High-resolution mapping of global surface water and its long-term changes. Nature 540, 418–422. ( 10.1038/nature20584) [DOI] [PubMed] [Google Scholar]
- 37.Prigent C, Papa F, Aires F, Rossow WB, Matthews E. 2007. Global inundation dynamics inferred from multiple satellite observations, 1993–2000. J. Geophys. Res. Atmos. 112, 1–13. ( 10.1029/2006JD007847) [DOI] [Google Scholar]
- 38.Prigent C, Papa F, Aires F, Jimenez C, Rossow WB, Matthews E. 2012. Changes in land surface water dynamics since the 1990s and relation to population pressure. Geophys. Res. Lett. 39, L08403 ( 10.1029/2012GL051276) [DOI] [Google Scholar]
- 39.Pham-Duc B, Prigent C, Aires F, Papa F. 2017. Comparisons of global terrestrial surface water datasets over 15 years. J. Hydrometeor. 18, 993–1007. ( 10.1175/JHM-D-16-0206.1) [DOI] [Google Scholar]
- 40.Thenkabail PS, et al. 2012. Assessing future risks to agricultural productivity, water resources and food security: how can remote sensing help? Photogramm. Eng. Remote Sens. 78, 773–782. [Google Scholar]
- 41.Teluguntla PG, et al. 2016. Global Cropland Area Database (GCAD) derived from remote sensing in support of food security in the twenty-first century: current achievements and future possibilities. In Remote sensing handbook: volume 2 - land resources monitoring, modeling, and mapping with remote sensing (ed. Thenkabail PS.), pp. 1–45. Boca Raton, FL: Taylor & Francis. [Google Scholar]
- 42.Jahnke HE, Jahnke HE. 1982. Livestock production systems and livestock development in tropical Africa. Kiel, Germany: Kieler Wissenschaftsverlag Vauk. [Google Scholar]
- 43.Jahnke HE, Tacher G, Kiel P, Rojat D. 1988. Livestock production tsetse affected areas of Africa. In Livestock production in tropical Africa, with special reference to the tsetse-affected zone, pp. 3–21. Nairobi, Kenya: International Livestock Centre for Africa and the International Laboratory for Research on Animal Diseases. [Google Scholar]
- 44.Robinson TP, et al. 2014. Mapping the global distribution of livestock. PLoS ONE 9, e96084 ( 10.1371/journal.pone.0096084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansen MC, et al. 2013. Data from: High-resolution global maps of 21st-century forest cover change. Science 342, 850–853. See https://www.glad.umd.edu/dataset/global-2010-tree-cover-30-m. [DOI] [PubMed] [Google Scholar]
- 46.Fick SE, Hijmans RJ. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315. ( 10.1002/joc.5086) [DOI] [Google Scholar]
- 47.Hengl T, et al. 2016. SoilGrids250 m: global gridded soil information based on machine learning. PLoS ONE 9, e105992 ( 10.1371/journal.pone.0105992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giglio L, Boschetti L, Roy D, Hoffman AA, Humber M. 2016. Collection 6 MODIS burned area product user's guide version 1.0, 26. See https://modis-land.gsfc.nasa.gov/pdf/MODIS_C6_BA_User_Guide_1.0.pdf.
- 49.Colwell RK. 1974. Predictability, constancy, and contingency of periodic phenomena. Ecology 55, 1148–1153. ( 10.2307/1940366) [DOI] [Google Scholar]
- 50.USGS. 2013. NASA shuttle radar topography mission global 3 arc second v.003. See https://lpdaac.usgs.gov/products/srtmgl3sv003/.
- 51.Lehner B, Verdin K, Jarvis A. 2006. HydroSHEDS technical documentation, version 1.0, pp. 1–27. Washington, DC: World Wildlife Fund US. [Google Scholar]
- 52.Breiman L. 2001. Random forests. Mach. Learn. 45, 5–32. ( 10.1023/A:1010933404324) [DOI] [Google Scholar]
- 53.Prasad AM, Iverson LR, Liaw A. 2006. Newer classification and regression tree techniques: bagging and random forests for ecological prediction. Ecosystems 9, 181–199. ( 10.1007/s10021-005-0054-1) [DOI] [Google Scholar]
- 54.Cutler DR, Edwards TC, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ. 2007. Random forests for classification in ecology. Ecology 88, 2783–2792. ( 10.1890/07-0539.1) [DOI] [PubMed] [Google Scholar]
- 55.Vincenzi S, Zucchetta M, Franzoi P, Pellizzato M, Pranovi F, De Leo GA, Torricelli P. 2011. Application of a random forest algorithm to predict spatial distribution of the potential yield of Ruditapes philippinarum in the Venice lagoon, Italy. Ecol. Modell. 222, 1471–1478. ( 10.1016/j.ecolmodel.2011.02.007) [DOI] [Google Scholar]
- 56.Strobl C, Boulesteix A-L, Kneib T, Augustin T, Zeileis A. 2008. Conditional variable importance for random forests. BMC Bioinf. 9, 307 ( 10.1186/1471-2105-9-307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dormann CF, et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27–46. ( 10.1111/j.1600-0587.2012.07348.x) [DOI] [Google Scholar]
- 58.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, 2009 edn New York, NY: Springer. [Google Scholar]
- 59.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 60.Schuur EA, Matson PA. 2001. Net primary productivity and nutrient cycling across a mesic to wet precipitation gradient in Hawaiian montane forest. Oecologia 128, 431–442. ( 10.1007/s004420100671) [DOI] [PubMed] [Google Scholar]
- 61.Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ. 2003. Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc. Natl Acad. Sci. USA 100, 572–576. ( 10.1073/pnas.0133045100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Govender N, Trollope WSW, Wilgen BWV. 2006. The effect of fire season, fire frequency, rainfall and management on fire intensity in savanna vegetation in South Africa. J. Appl. Ecol. 43, 748–758. ( 10.1111/j.1365-2664.2006.01184.x) [DOI] [Google Scholar]
- 63.Hughes FMR. 1990. The influence of flooding regimes on forest distribution and composition in the Tana River floodplain, Kenya. J. Appl. Ecol. 27, 475–491. ( 10.2307/2404295) [DOI] [Google Scholar]
- 64.Ellery WN, Ellery K, McCarthy TS. 1993. Plant distribution in islands of the Okavango Delta, Botswana: determinants and feedback interactions. African J. Ecol. 31, 118–134. ( 10.1111/j.1365-2028.1993.tb00526.x) [DOI] [Google Scholar]
- 65.van der Valk A, Sklar FH. 2012. What we know and should know about tree islands. In Tree islands of the everglades (eds Sklar FH, van der Valk A). Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 66.Junk WJ, Wantzen KM. 2004. The flood pulse concept: new aspects, approaches, and applications. An update. In Proceedings of the second international symposium on the management of large rivers for fisheries (eds Welcomme RL, Petr T). Bangkok: Food and Agriculture Organization and Mekong River Commission, FAO Regional Office for Asia and the Pacific. [Google Scholar]
- 67.Gunderson LH. 1994. Vegetation of the Everglades: determinants of community composition. In Everglades: the ecosystem and its restoration (eds Davis SM, Ogden JC, Park WA), pp. 323–340. Boca Raton, FL: St Lucie Press. [Google Scholar]
- 68.Parolin P, Oliveira AC, Piedade MTF, Wittmann F, Junk WJ. 2002. Pioneer trees in Amazonian floodplains: three key species form monospecific stands in different habitats. Folia Geobotanica 37, 225–238. ( 10.1007/BF02804233) [DOI] [Google Scholar]
- 69.Junk WJ, Brown M, Campbell IC, Finlayson M, Gopal B, Ramberg L, Warner BG. 2006. The comparative biodiversity of seven globally important wetlands: a synthesis. Aquat. Sci. 68, 400–414. ( 10.1007/s00027-006-0856-z) [DOI] [Google Scholar]
- 70.Lopez OR, Kursar TA. 2007. Interannual variation in rainfall, drought stress and seedling mortality may mediate monodominance in tropical flooded forests. Oecologia 154, 35–43. ( 10.1007/s00442-007-0821-0) [DOI] [PubMed] [Google Scholar]
- 71.Schlesinger WH. 1997. Biogeochemistry: an analysis of global change, 2nd edn Cambridge, MA: Academic Press Inc. [Google Scholar]
- 72.Malhi Y, Roberts JT, Betts RA, Killeen TJ, Li W, Nobre CA. 2008. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172. ( 10.1126/science.1146961) [DOI] [PubMed] [Google Scholar]
- 73.Andela N, et al. 2017. A human-driven decline in global burned area. Science 356, 1356–1362. ( 10.1126/science.aal4108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stevens N, Lehmann CER, Murphy BP, Durigan G. 2017. Savanna woody encroachment is widespread across three continents. Glob. Change Biol. 23, 235–244. ( 10.1111/gcb.13409) [DOI] [PubMed] [Google Scholar]
- 75.Flores BM, Holmgren M, Xu C, van Nes EH, Jakovac CC, Mesquita RCG, Scheffer M. 2017. Floodplains as an Achilles' heel of Amazonian forest resilience. Proc. Natl Acad. Sci. USA 114, 4442–4446. ( 10.1073/pnas.1617988114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zarfl C, Lumsdon AE, Berlekamp J, Tydecks L, Tockner K. 2015. A global boom in hydropower dam construction. Aquat. Sci. 77, 161–170. ( 10.1007/s00027-014-0377-0) [DOI] [Google Scholar]
- 77.Daskin JH, Aires F, Staver AC. 2019. Data from: Determinants of tree cover in tropical floodplains Dryad Digital Repository. ( 10.5061/dryad.pj77fc4) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Daskin JH, Aires F, Staver AC. 2019. Data from: Determinants of tree cover in tropical floodplains Dryad Digital Repository. ( 10.5061/dryad.pj77fc4) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available from the Dryad Digital Repository, along with example R scripts for running random forest models: https://doi.org/10.5061/dryad.pj77fc4 [77].