Abstract
Animals on islands often exhibit dramatic differences in morphology and behaviour compared with mainland individuals, a phenomenon known as the ‘island syndrome’. These differences are thought to be adaptations to island environments, but the extent to which they have a genetic basis or instead represent plastic responses to environmental extremes is often unknown. Here, we revisit a classic case of island syndrome in deer mice (Peromyscus maniculatus) from British Columbia. We first show that Saturna Island mice and those from neighbouring islands are approximately 35% (approx. 5 g) heavier than mainland mice and diverged approximately 10 000 years ago. We then establish laboratory colonies and find that Saturna Island mice are heavier both because they are longer and have disproportionately more lean mass. These trait differences are maintained in second-generation captive-born mice raised in a common environment. In addition, island–mainland hybrids reveal a maternal genetic effect on body weight. Using behavioural testing in the laboratory, we also find that wild-caught island mice are less aggressive than mainland mice; however, laboratory-raised mice born to these founders do not differ in aggression. Together, our results reveal that these mice have different responses to the environmental conditions on islands—a heritable change in a morphological trait and a plastic response in a behavioural trait.
Keywords: aggression, gigantism, island syndrome, maternal genetic effect, Peromyscus, phenotypic plasticity
1. Introduction
Islands offer some of the most tractable examples of evolutionary adaptation and diversification [1]. Because of their geographical isolation, they form self-contained environments that facilitate the interpretation of evolutionary patterns and processes. For example, many island populations of animals share consistent differences in demography, body size, reproductive rate, anti-predator behaviour and territorial aggression—a phenomenon known as the ‘island syndrome’ [2–6]. The repeated appearance of similar traits in similar ecological conditions across taxa suggests that these traits evolved as adaptations during the establishment of island populations [7,8]. Thus, one implicit assumption is that these island traits have a genetic basis, resulting from directional selection and/or founder effects following island colonization [6,9,10]. However, island traits could also represent, in part or fully, plastic phenotypes that are expressed in novel environments, but the role of phenotypic plasticity in island evolution has received limited attention.
A key challenge in differentiating heritable and plastic components underlying island traits has been that most reports are based on experiments in the field, or for behavioural traits, wild-caught individuals subsequently tested in the laboratory (e.g. [11–14]). The detection of heritable differences in the wild, such as, through the quantitative-genetic analysis of trait variance [15], can be challenging. By contrast, a genetic component can be measured if wild-derived animals are born and raised in a common environment. By minimizing environmental variation, one can assess the extent to which morphological and behavioural traits have a heritable genetic basis.
Here, we revisit a classic case of island syndrome in deer mice (Peromyscus maniculatus). Field studies in the 1970s showed that ecological conditions differ for deer mice on Saturna Island off the coast of British Columbia—namely they have a higher population density, lower dispersal rate and smaller home range size than deer mice from a nearby mainland population [16]. In addition, wild-caught island deer mice had a larger body size [16,17] and displayed fewer aggressive and defensive interactions than mainland deer mice [12,13]. Here, we investigate the extent of and mechanisms driving the genetic and/or environmental components underlying morphological and behavioural island traits by measuring body size and aggressive behaviour both in wild-caught island and mainland deer mice as well as in their captive-born offspring.
2. Methods
(a). Body weight of museum specimens
We retrieved records from adult male museum specimens of P. maniculatus collected in Washington and British Columbia using the Arctos and Vertnet databases (see electronic supplementary material, S1). Our final sample comprised 504 mice (island, n = 328; mainland, n = 176), including 53 we collected.
(b). Fieldwork
We sampled deer mice (n = 120) in the Gulf Island National Park Reserve on Saturna Island and Pender Island (P. m. saturatus) and in the Malcolm Knapp Research Forest (MKRF) on the mainland (P. m. austerus and P. keeni) in October 2014. Of these, we collected 101 individuals and accessioned them in the Harvard Museum of Comparative Zoology (MCZ). We verified species identity using a fragment of the cytochrome b gene (following [18]) (electronic supplementary material, figure S1).
(c). Admixture analysis and divergence dating
We extracted genomic DNA from 65 wild-caught mice (Saturna Island, n = 28; Pender Island, n = 9; mainland, n = 28). We used a genotype-by-sequencing approach (ddRADseq [19]) to obtain genome-wide markers, filtered the dataset and then ran genetic principal component (PC) (based on n = 54 specimens after filtering), admixture (n = 54) and divergence time (n = 14) analyses (see electronic supplementary material, S1).
(d). Establishment of laboratory colonies and animal husbandry
After quarantine of the wild-caught mice at Charles River Laboratories, we established colonies at Harvard (Saturna Island, n = 9 females and 9 males; mainland, n = 8 females and 3 males; electronic supplementary material, S1). Subsequent experiments were conducted using either wild-caught mice or first-, second- and third-generation captive-born mice as noted.
(e). Weight measurements
Mice in the field were weighed using a Pesola spring scale to the nearest gram. Mice in the laboratory were weighed with a Jennings TB500 digital precision scale to the nearest 0.01 g. To weigh newborn litters before their first milk meal, we isolated and monitored pregnant females through remote cage surveillance, allowing us to intercept pups within 20–60 min of birth. We then checked for the presence of milk in the stomach.
(f). EchoMRI
Experiments to measure body composition were conducted in the Metabolic Core of Brigham and Women's Hospital Boston. Frozen mice were thawed to room temperature and measured individually in a calibrated EchoMRI 3-in-1 machine.
(g). X-ray measurements
To measure skeletal traits, we imaged mice on a digital X-ray system in the Digital Imaging Facility of the MCZ. We used ImageJ to measure 10 skeletal elements: length of the sacrum, skull, zygomatic bone, humerus, femur, ulna, tibia and metatarsal calcaneus, as well as the width of the skull and zygomatic bone.
(h). F1 hybrids
We reciprocally paired one female and one male litter mate each from five island and mainland parents. Of these 10 pairs, four island female × mainland male and three mainland female × island male pairs successfully reproduced. We weighed litters at birth and then a subset every 4 days until weaning.
(i). Cross-fostering
To test if parental care contributes to the differences in growth, we swapped island and mainland litters (i.e. island pups raised by mainland parents and vice versa) within 48 h of birth. We fostered the same number of pups from each litter. We then weighed the pups every 4 days until weaning (P23).
(j). Behavioural experiments
We measured territorial aggression using a resident–intruder paradigm. To induce territoriality, residents were first paired with a female. For one set of experiments, wild-caught residents were paired with a female for several months. For a second set of experiments, 9- to 13-week-old (non-breeding) residents were isolated with a female for one week. For a third set of experiments, 5- to 16-month-old (breeding) residents had been paired with a female for several months. Conspecific intruders were raised in social groups with at least two other mice, and were 9–13 weeks old. Resident and intruder had different parents and had not previously met. We tested each resident twice against different intruders on consecutive days. We conducted all behavioural experiments during the dark phase under red light (see electronic supplementary material, S1).
(k). Behavioural analysis
We annotated videos blind to population identity using the software Observer (Noldus). We scored aggressive (lunging, pindown, upright threats, boxing, wrestling and chasing), defensive (submission, defensive threat and defeat) and cohesive behaviours (following, mounting, grooming and huddling) [20,21] as start–stop events (duration) performed by both the resident and the intruder, and averaged behaviours across trials.
(l). Statistical analysis
We fitted linear models with lm {stats} or lmer {lme4} using R software [22]. p-values were calculated using lsmeans and contrast {lsmeans} or summary {lmerTest} (see electronic supplementary material, S1).
3. Results
(a). Island mice are heavier than mainland mice
Using data from museum specimens, we first compared weight records of adult deer mice in and around coastal British Columbia. As expected, island populations had a greater median weight than neighbouring mainland populations, with few exceptions (figure 1a). The overall median weight of island specimens was 21 g compared with 16 g in the mainland specimens (Kruskal–Wallis test, p < 0.0001), an increase of 31.25% (electronic supplementary material, figure S2).
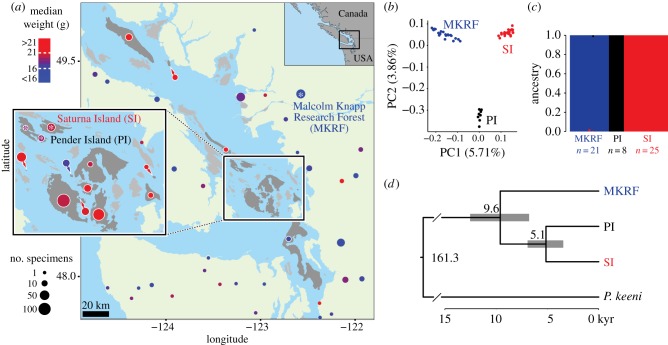
Figure 1.
Body weight of island and mainland populations in the study area. (a) Map of coastal British Columbia, Canada, showing the study area. The colour of collection points indicates the median weight of P. maniculatus populations, and circle area indicates sample size. Collection sites within 8 km of each other were combined into a single site. Only adult males were included. Sampled islands are shaded dark grey, non-sampled islands light grey and mainland areas (including Vancouver Island) light green. Live deer mice were collected from Saturna Island (SI) and the Malcolm Knapp Research Forest (MKRF) to found laboratory colonies (starred). (b) Genetic PCA of 54 deer mice from Saturna Island, Pender Island (PI) and the Malcolm Knapp Research Forest, based on 80 248 genome-wide variants. The percentage of variance explained by the first and second PC is provided. (c) Admixture analysis of the dataset from (b) with a cluster number of K = 3. (d) Bayesian divergence time estimates of island and mainland P. maniculatus populations and the outgroup P. keeni based on 7092 variants from 14 specimens. Grey bars represent 95% highest posterior density intervals. The scale bar is in thousands of years (kyr). (Online version in colour.)
We next collected wild mice from two focal populations, one island (Saturna Island, n = 21) and one on the mainland (MKRF, n = 23; figure 1a), following [12,13,16]. The median weight of adult Saturna Island mice was 20 g (±1 s.d. 16.8–23.2 g) compared with a median weight of 14.5 g (±1 s.d. 12.7–16.3 g) in mainland mice (Kruskal–Wallis test, p < 0.0001), an increase of 37.93% (electronic supplementary material, figure S2), largely recapitulating the general pattern of body weight observed in this region.
(b). Island and mainland mice are genetically distinct populations
To determine the genetic relatedness of our focal island and mainland populations, we conducted a genetic principal component analysis (PCA) using genome-wide SNPs. We found that these populations are genetically separable (figure 1b), and none of the individuals showed evidence of recent admixture, consistent with previous results based on electrophoresis of a single protein [17] (figure 1c). Next, we obtained time-calibrated divergence times of these populations, using P. keeni as an outgroup (figure 1d). We estimated island mice diverged from mainland mice approximately 9.6 (6.8–12.5, 95% highest posterior density interval) thousand years ago (ka), while Saturna Island mice diverged from Pender Island mice approximately 5.1 (3.5–6.9) ka. These data are consistent with a postglacial isolation of deer mice on islands in the Strait of Georgia [23] and little to no ongoing gene flow between island and mainland or among island populations.
(c). Differences in body weight appear heritable and are driven by both differences in body length and proportional lean mass
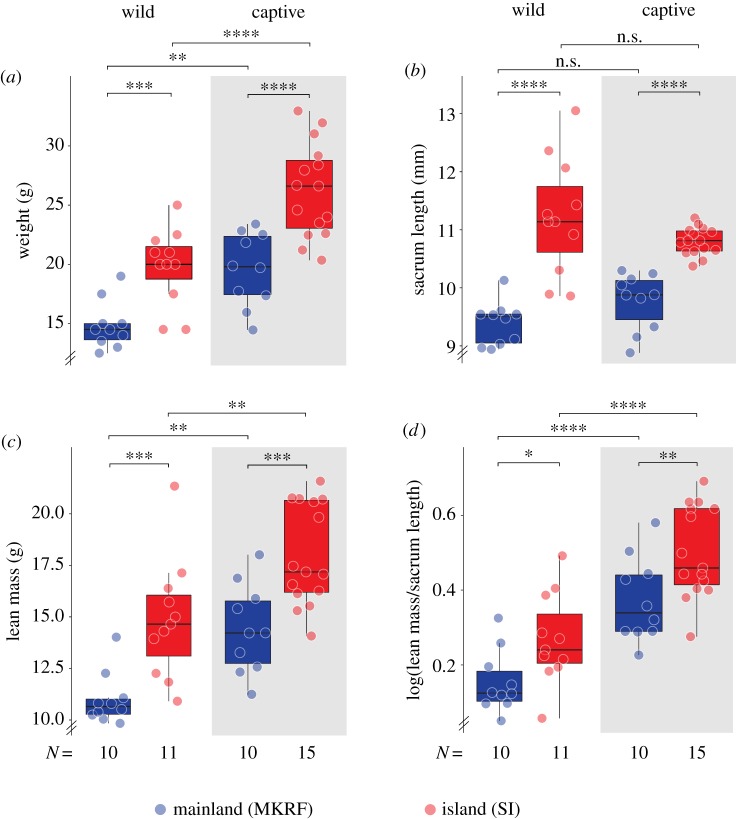
To test whether differences in weight between island and mainland mice observed in the field were retained across generations in a common environment, we established laboratory colonies and then compared wild-caught and captive-born mice. We used captive-born mice that were themselves born to captive-born parents (as two generations in captivity should minimize environmental, maternal and other non-heritable variation), and focused our analyses on males, the sex with the larger difference in weight (figure 2a; electronic supplementary material, figure S3). We found that the magnitude of difference in the median weight between island and mainland mice observed in the field was retained in the laboratory: island mice are 37.93% bigger in the field and 34.02% bigger in the laboratory (figure 2a). However, the overall weight of all mice was higher in the laboratory: captive-born mice were on average 34.99% heavier than wild-caught mice (figure 2a), probably due to an enriched and ad libitum diet.
Figure 2.
The genetic basis of body size traits in island mice. (a) Body weight, (b) sacrum length, (c) lean mass and (d) size-corrected lean mass in male wild- and captive-born island and mainland mice. Box plots indicate median, interquartile range (IQR) and at most ± 1.5 IQR from hinges. See Methods for statistical details. n.s., not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (Online version in colour.)
We next tested whether island mice were heavier because they are larger (i.e. longer) and/or because they are heavier relative to their body length. We found that the median sacrum length, a proxy for body length, was approximately 14.39% larger in island than mainland mice for both field and laboratory individuals, but did not differ between field and laboratory populations (figure 2b), suggesting that environmental conditions had little effect on body length. This pattern was largely recapitulated in other skeletal traits (electronic supplementary material, figure S4).
We next quantified body composition and found that island mice had more lean mass (figure 2c), but not fat mass (electronic supplementary material, figure S5), than mainland mice. Moreover, this increase in lean mass was not simply due to differences in body length (figure 2d). Collectively, these data show that island mice are heavier both because they are larger (i.e. longer) and because they have disproportionately more lean mass than mainland mice. In addition, because these trait differences are maintained both in a common environment and across generations, they probably have a strong genetic basis.
(d). Island mice are born heavier with growth pulses at birth and weaning
To determine when these weight differences emerge, we weighed litters across postnatal development. We noted that island mice have slightly smaller litter sizes than mainland mice (electronic supplementary material, figure S6; Kruskal–Wallis test, p = 0.034); as litter size is often inversely correlated with birth weight, we included litter size as an explanatory variable in subsequent analyses. We first found that island litters are heavier than mainland litters at birth (figure 3; linear fixed effects model, t = −6.259, p ≤ 0.0001). This difference is not simply due to island mice ingesting more milk because island litters were already heavier before their first milk meal (electronic supplementary material, figure S7; repeated-measures linear mixed-effects model; t = 6.901, p = 6.96 × 10−5 (pre- versus post-feeding); t = 3.602, p = 0.004 (strain)). Furthermore, growth at birth in island mice is greater than in mainland mice (electronic supplementary material, figure S8A; linear fixed effects model, t = −2.852, p = 0.033), but this significant difference disappears by postnatal day 4 (P4), and only reappears around weaning at P23 (electronic supplementary material, figure S8A; linear fixed effects model, t = −2.748, p = 0.039). Together, these results suggest that growth pulses around birth, and with the onset of sexual maturity at weaning, underlie the main differences in weight between island and mainland mice.
Figure 3.
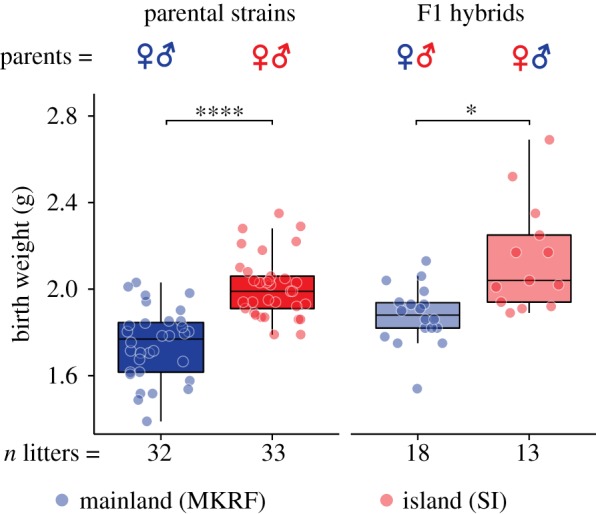
A maternal effect contributes to birth weight in island mice. Birth weight in island and mainland litters (left panel) and F1 hybrids by maternal strain (right panel). Points represent the mean weight of litter. See Methods for statistical details. *p < 0.05, ****p < 0.0001. (Online version in colour.)
(e). A maternal effect contributes to birth weight and early growth in island mice
We next asked if these differences in weight are influenced by a maternal genetic effect. We found that F1 litters born to island mothers are on average 14.11% heavier at birth than those born to mainland mothers (figure 3; linear fixed effects model, t = −2.386, p = 0.0241), even when we controlled for family effects (wild cluster bootstrapped generalized linear model, 95% CI = 0.0022, 0.1749, p = 0.029). Furthermore, growth differences were greatest at birth but not different on days 8–20 or at weaning (electronic supplementary material, figure S8B). Finally, in F1 adults in which sex could be determined, we found a larger difference between offspring with island versus mainland mothers in male hybrids compared to female hybrids (electronic supplementary material, figure S8C,D). These results suggest that the growth difference in island versus mainland mice around birth, but not at the onset of sexual maturity at weaning, is likely to be governed by a maternal effect.
(f). Parental care does not affect early growth differences
We found little evidence that cross-fostering affected growth in either island or mainland mice (electronic supplementary material, figure S9A,B). For example, mainland litters raised by island parents grew the same (days 0–20) or slower (day 23) compared with non-fostered mainland litters (electronic supplementary material, figure S9A). These data show that the increases in growth in island litters and F1 hybrids born to island mothers are not due to increased parental care by island parents.
(g). Wild-caught island mice are less aggressive than wild-caught mainland mice
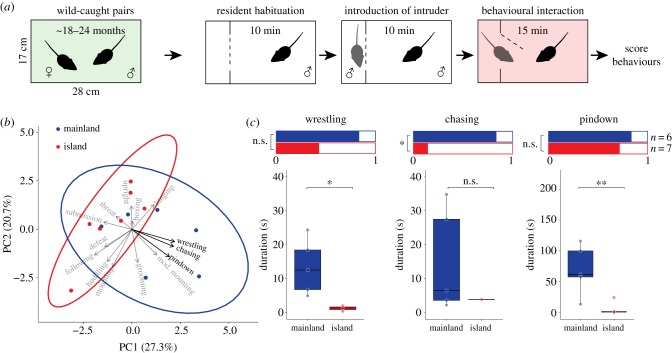
We next compared levels of territorial aggression in wild-caught males from island and mainland populations (figure 4a). We performed a PCA to reduce the dimensionality of the behavioural dataset (figure 4b). The first two PCs explained a cumulative proportion of 47.92% of the variance; 95% confidence intervals for the two strains only partially overlapped, and 67% of mainland mice were outside the confidence interval for island mice. The main behavioural contributors to the first PC were wrestling (12.94%), chasing (12.65%) and pindown duration (11.42%).
Figure 4.
Territorial aggression in wild-caught island and mainland mice. (a) Schematic of the behavioural assay. (b) PCA of the duration of wild-caught resident behaviours. Ellipses represent 95% confidence intervals. Arrow directions indicate how behaviours contribute to the two PCs, and arrow lengths are proportional to the strength of contribution. Behaviours highlighted in bold were selected for additional analysis. (c) Aggressive behaviour in wild-caught founder mice. Bar graphs (top) show the proportions of mice engaging in wrestling, chasing and pindown behaviours. Boxplots (bottom) show the duration of each behaviour for those individuals that engaged in that behaviour. Sample sizes are provided. See Methods for statistical details. n.s., not significant; *p < 0.05, **p < 0.01. (Online version in colour.)
We next tested for differences in these three behaviours between wild-caught island and mainland males. We used a two-stage hurdle model approach to separately test for differences in the proportion of mice displaying a behaviour as well as the number and duration of bouts once a behaviour was initiated. We found no significant difference in the proportion of wild-caught island and mainland mice showing wrestling behaviour (83.3 versus 42.86%, logistic regression model, z = −1.421, p = 0.155), but mainland mice that wrestled did so significantly longer than island mice (figure 4c; electronic supplementary material, figure S10A; linear fixed effects model, t = −2.512, p = 0.0458). In addition, significantly more mainland mice chased than island mice (83.3 versus 14.29%, logistic regression model, z = −2.211, p = 0.027), but we found no difference in duration once mice chased (figure 4c; electronic supplementary material, figure S10A; linear fixed effects model, t = −0.666, p = 0.5418). Similar to wrestling behaviour, we found no significant difference in the proportion of mice exhibiting pindown behaviour (83.3 versus 71.43%, logistic regression model, z = −0.503, p = 0.615), but mainland mice that exhibited pindown behaviour did so significantly longer than island mice (figure 4c; electronic supplementary material, figure S10A; linear fixed effects model, t = −3.460, p = 0.0086). These differences in aggression could not be explained by differences in the time that mice had spent in captivity, the time since a litter was last sired, or the weight difference between resident and intruder (electronic supplementary material, figure S11). Together, these results suggest that wild-caught island mice show reduced territorial aggression compared with wild-caught mainland mice, consistent with previous observations [12,13].
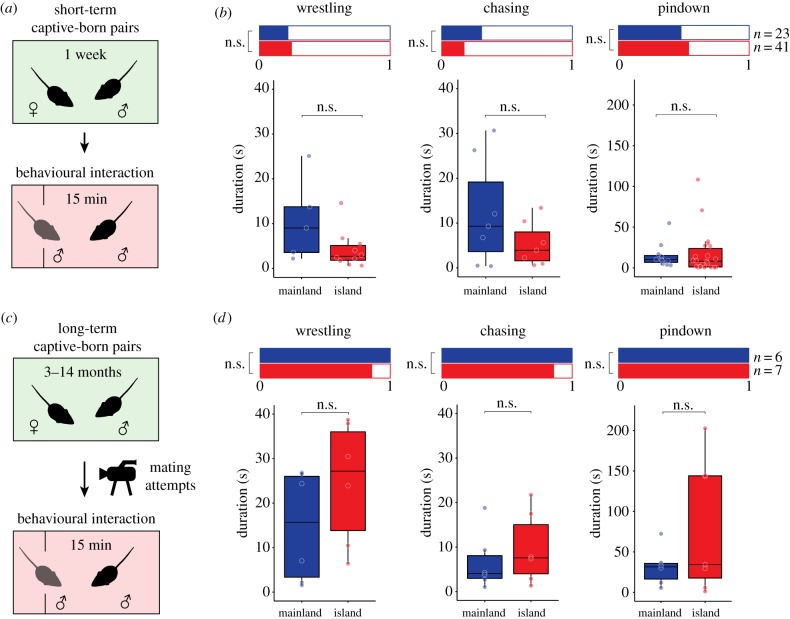
(h). Captive-born mice do not differ in aggression levels, regardless of reproductive experience
We next tested if these behavioural differences observed in wild mice were retained in a controlled laboratory environment over generations. We initially paired residents with a virgin female for one week prior to behavioural testing (figure 5a), a well-established procedure to induce territoriality and aggression in laboratory mice [24]. However, both captive-born island and mainland mice displayed low levels of aggression (figure 5b; electronic supplementary material, figure S10B). We found no significant difference in the proportion of island versus mainland mice that displayed wrestling (21.73 versus 24.39%, logistic regression model, z = 0.240, p = 0.81), chasing (30.43 versus 17.07%, z = −1.227, p = 0.22) or pindown (47.83 versus 53.66%, z = 0.448, p = 0.654), nor did we find a significant difference in the duration of wrestling (linear fixed effects model, t = −1.938, p = 0.0746), chasing (t = −1.432, p = 0.1777) or pindown (t = 0.268, p = 0.7905) once mice engaged in these behaviours.
Figure 5.
Territorial aggression in captive-born island and mainland mice. (a) Schematic of the resident–intruder behavioural assay using captive-born males paired with a female for one week. (b) Aggressive behaviours in short-term pairings. Bar graphs (top) show proportions of mice engaging in each behaviour. Boxplots (bottom) show the duration of each behaviour for those individuals that engaged in that behaviour. See Methods for statistical details. (c) Schematic of the behavioural assay using captive-born males paired with a female for 3–14 months, and then testing males approximately 1 h after the onset of mating attempts and again 1 day later. (d) Aggressive behaviours in long-term pairings. Sample sizes are provided. n.s., not significant. (Online version in colour.)
While one week of pairing with a female typically induces sexual activity and territoriality in male laboratory mice, it may not be sufficient for deer mice. Indeed, we found that the majority of males sired litters approximately three weeks after pairing (electronic supplementary material, figure S12A), suggesting that few mice had become sexually active in the one week before testing. We thus identified mice among this subset that had not sired a litter by the time of first testing, and retested them after they had sired and been co-housed with a litter until weaning. As expected, residents wrestled significantly more after they had reproduced (electronic supplementary material, figure S12B; repeated-measures linear mixed-effects model; t = 2.578, p = 0.0327 (trial); t = 0.337, p = 0.7462 (strain)), suggesting that reproductive experience is crucial to induce aggression in these populations.
As aggression in male deer mice peaks at the time of and just following copulation with a receptive female [25–27], we next aimed to test for island–mainland differences in aggression at this time (figure 5c). We identified pregnant females and continuously video-monitored their behaviour in their home cage. We found that male mice will begin to attempt copulation, as evidenced by vigorous pursuit [28], about 12 h after the birth of a litter for about 1–2 h (electronic supplementary material, figure S13). Therefore, we tested male mice in our assay approximately 1 hour after the onset of copulation and again 1 day later.
As expected, aggression levels were high in reproductively active mice. Nearly all residents of both populations exhibited wrestling, chasing and pindown behaviours (figure 5d; electronic supplementary material, figure S10C). Surprisingly, however, there was no significant difference in the proportion of island and mainland mice exhibiting wrestling (100 versus 85.71%, logistic regression model, z = −0.003, p = 0.998), chasing (100 versus 85.71%, z = −0.003, p = 0.998) or pindown behaviour (100 versus 100%, z = 0, p = 1), nor was there a difference in the duration of wrestling (linear fixed effects model, t = 1.309, p = 0.2199), chasing (t = 0.725, p = 0.4851) or pindowns (t = 1.409, p = 0.186). Thus, different levels of reproductive experience are unlikely to drive the behavioural differences between wild-caught island and mainland mice. Consistent with this, direct comparison of data from mice that were exposed to comparable reproductive experience and experimental conditions in captivity (i.e. data from captive-born mice in electronic supplementary material, figure S12B and from wild-caught mice in figure 4) shows that wild-caught island mice are less aggressive than wild-caught mainland mice, but that both island and mainland captive-born mice match the aggressiveness of wild-caught mainland mice (electronic supplementary material, figure S14), implying that the aggression differences observed in wild-caught mice are driven, at least in large part, by environmental effects.
4. Discussion
Here, we investigated the genetic basis of morphological and behavioural traits associated with the island syndrome in deer mice. Previous work in the 1970s reported that wild-caught Saturna Island deer mice had higher body weight [16,17] and reduced aggression levels compared with mainland mice [12,13]. As an initial step, we confirmed these differences in our populations of wild-caught deer mice. First, body weight is indeed higher on Saturna Island (as well as most other islands in the region) than mainland mice, which we show is driven by both the increased body length and disproportionately larger lean mass. Second, we found striking differences in aggression: wild-caught island mice showed reduced aggression in several behaviours compared to mainland mice. Thus, wild Saturna Island mice conform to the predicted island syndrome phenotype. However, because measurements were taken only using wild-caught individuals, it was unclear if these differences had a genetic basis or instead were plastic responses to environmental extremes [12,13].
By establishing wild-derived colonies in the laboratory, we minimized environmental variation and found that body size differences persisted in captive-born mice, suggesting these differences are largely heritable. Although examples of density-dependent plasticity in body weight are known from natural populations (e.g. [29]), our results are consistent with studies that have demonstrated the genetic basis of gigantism and skeletal evolution in island rodents [30–32]. In addition, our data on island–mainland hybrid growth showed that the larger body weight in island mice was also partly driven by a maternal genetic effect. The maternal effect was not recapitulated by cross-fostering, suggesting that it cannot be explained simply by postnatal nursing differences. A study in island and mainland deer mice from California showed that 4-day-old mainland embryos that were transferred into island foster mothers had a significantly increased neonatal weight [31], suggesting that increased maternal uterine investment also could contribute to the large size of Saturna Island deer mice. However, we cannot exclude the possibility of maternal genomic imprinting (e.g. [33]) without similar experiments. Regardless, it is clear that both offspring and maternal genotypes driving larger body size have evolved in island deer mice.
Unlike body size differences, however, our laboratory-based behavioural experiments demonstrated that aggression levels in captive-born island and mainland deer mice remained indistinguishable across a spectrum of low to high overall aggression intensity. These results suggest that additional environmental factors are necessary to induce the aggression differences observed in wild-caught mice. One important factor may be population density [5,6,34]. Consistent with our findings in P. maniculatus, increased body size and reduced aggression have been documented in other island rodents with high population density (e.g. Microtus breweri [35,36] and Myodes glareolus skomerensis [37]). Population density in island rodents is typically increased, probably because of the absence of emigration, fewer competing species and thus more available resources, and fewer predators on islands. The rate of social interactions, and thereby the cost of territorial defence, increases with density. In addition, reproductive output is often adjusted to population density. Rodents in high-density island populations could thus face pressure to reduce direct conflict in favour of indirect competition (territorial defence hypothesis) and to reallocate resources to produce fewer, but more competitive offspring (reallocation hypothesis).
Adaptive evolution is the result of selection on heritable variation, and both the strength of selection and the degree of heritability can influence trait evolution, raising the question of why, in this case, differences in body weight, but not aggressive behaviour, appear genetically encoded. One possibility is that behaviour in general is less heritable than morphology. However, data on the heritability of behaviour and morphology do not fully support this hypothesis [38,39]. Heritability of behaviours varies substantially: for example, a comparative study of captive and wild insular macropodid marsupials suggested that the loss of some anti-predator behaviours is heritable, while the loss of other behaviours is phenotypically plastic [40]. Moreover, selection on certain behaviours, like aggression, may be labile over time (e.g. if selection was density-dependent and density changed periodically). Thus, if selection on aggression was weak and/or heritability low, 10 000 years (or approx. 25 000 generations) simply may not be enough time for a behavioural difference to evolve between island and mainland populations. By contrast, plasticity in aggression could facilitate the evolution of heritable changes in body size by enabling the selective environment, that is, high population density, in which the maternal and offspring genotypes driving larger offspring body size became advantageous, consistent with growing evidence that behavioural plasticity often precedes and promotes the adaptive evolution of other traits, especially morphology [41–51].
In summary, while the island syndrome has been reported in a wide range of organisms, few studies have tested if these traits are genetically encoded. More research is needed into the drivers, sequence and mechanisms of trait evolution during the colonization of islands and subsequent establishment of populations to fully understand one of the most iconic and widespread patterns of repeated evolution in nature.
Supplementary Material
Supplementary Material
Acknowledgements
We thank N. Bedford, J. Chupasko, E. Hager, J. Kenagy and K. Turner for help with fieldwork and logistics. We also thank H. Wellington for taking skeletal trait measurements; A. Shultz for advice on the population-genetic analysis; M. Omura for help accessioning specimens; A. Nelson for advice on the behavioural assay design; and S. Worthington and S. Goshev for statistical support. K. Pritchett-Corning helped set up breeding colonies from wild-caught mice.
Ethics
All experiments were approved by the Harvard University Faculty of Arts and Sciences Institutional Animal Care and Use Committee (protocol no. 14-08-211). Fieldwork was conducted with permits from the Gulf Island National Park Reserve (GINP-2014-17276), the Pender Island Parks and Recreation Commission, the Malcolm Knapp Research Forest at the University of British Columbia and the Ministry of Forests, Lands and Natural Resource Operations (NASU14-154228, NASU14-154230).
Data accessibility
Raw sequence data (fastq files) and mtDNA sequences were submitted to the Sequence Read Archive (PRJNA508981, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA508981/) and GenBank (MK265122–MK265240, https://www.ncbi.nlm.nih.gov/genbank/), respectively. Morphological and behavioural data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vx0k6djmk [52]. Museum specimens were accessioned into the Harvard Museum of Comparative Zoology Mammal Department (69713–69813).
Authors' contributions
F.B. and H.E.H. conceived the study, F.B. carried out the experiments and analysed the data supervised by H.E.H., and F.B. and H.E.H. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
Fieldwork was supported by a Putnam Expedition Grant of the Harvard Museum of Comparative Zoology. F.B. was supported by an HHMI International Student Research Fellowship, a Joan Brockman Williamson Graduate Research Fellowship and a Herchel Smith Graduate Fellowship. H.E.H. is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830–836. ( 10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 2.Foster JB. 1964. Evolution of mammals on islands. Nature 202, 234–235. ( 10.1038/202234a0) [DOI] [Google Scholar]
- 3.Van Valen LM. 1973. Pattern and the balance of nature. Evol. Theory 1, 31-49. [Google Scholar]
- 4.Lomolino MV. 1985. Body size of mammals on islands: the island rule reexamined. Am. Nat. 125, 310–316. ( 10.1086/284343) [DOI] [Google Scholar]
- 5.Stamps JA, Buechner M. 1985. The territorial defense hypothesis and the ecology of insular vertebrates. Q. Rev. Biol. 60, 155–181. ( 10.2307/2828392) [DOI] [PubMed] [Google Scholar]
- 6.Adler GH, Levins R. 1994. The island syndrome in rodent populations. Q. Rev. Biol. 69, 473-490. ( 10.2307/3036435) [DOI] [PubMed] [Google Scholar]
- 7.Clegg SM, Owens IPF. 2002. The ‘island rule’ in birds: medium body size and its ecological explanation. Proc. R. Soc. Lond. B 269, 1359–1365. ( 10.1098/rspb.2002.2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novosolov M, Raia P, Meiri S. 2013. The island syndrome in lizards. Glob. Ecol. Biogeogr. 22, 184–191. ( 10.1111/j.1466-8238.2012.00791.x) [DOI] [Google Scholar]
- 9.Mayr E. 1954. Change of genetic environment and evolution. In Evolution as a process (eds Huxley J, Hardy AC, Ford EB), pp. 157–180. London, UK: Allen and Unwin. [Google Scholar]
- 10.Berry RJ. 1996. Small mammal differentiation on islands. Phil. Trans. R. Soc. Lond. B 351, 753–764. ( 10.1098/rstb.1996.0070) [DOI] [PubMed] [Google Scholar]
- 11.Gray SJ, Hurst JL. 1998. Competitive behaviour in an island population of house mice, Mus domesticus. Anim. Behav. 56, 1291–1299. ( 10.1006/anbe.1998.0890) [DOI] [PubMed] [Google Scholar]
- 12.Halpin Z. 1981. Adult–young interactions in island and mainland populations of the deermouse Peromyscus maniculatus. Oecologia 51, 419–425. ( 10.1007/BF00540916) [DOI] [PubMed] [Google Scholar]
- 13.Halpin ZT, Sullivan TP. 1978. Social interactions in island and mainland populations of the deer mouse, Peromyscus maniculatus. J. Mammal. 59, 395-401. ( 10.2307/1379924) [DOI] [Google Scholar]
- 14.Orrock JL. 2010. When the ghost of predation has passed: do rodents from islands with and without fox predators exhibit aversion to fox cues? Ethology 116, 338–345. ( 10.1111/j.1439-0310.2010.01740.x) [DOI] [Google Scholar]
- 15.Schlichting CD, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective, p. 387 Sunderland, MA: Sinauer Associates. [Google Scholar]
- 16.Sullivan TP. 1977. Demography and dispersal in island and mainland populations of the deer mouse, Peromyscus maniculatus. Ecology 58, 964–978. ( 10.2307/1936918) [DOI] [Google Scholar]
- 17.Redfield JA. 1976. Distribution, abundance, size, and genetic variation of Peromyscus maniculatus on the Gulf Islands of British Columbia. Can. J. Zool. 54, 463–474. ( 10.1139/z76-053) [DOI] [Google Scholar]
- 18.Zheng X, Arbogast BS, Kenagy GJ. 2003. Historical demography and genetic structure of sister species: deermice (Peromyscus) in the North American temperate rain forest. Mol. Ecol. 12, 711–724. ( 10.1046/j.1365-294X.2003.01770.x) [DOI] [PubMed] [Google Scholar]
- 19.Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE. 2012. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7, e37135 ( 10.1371/journal.pone.0037135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenberg JF. 1962. Studies on the behavior of Peromyscus maniculatus gambelii and Peromyscus californicus parasiticus. Behaviour 19, 177–207. ( 10.2307/4533012) [DOI] [Google Scholar]
- 21.Eisenberg JF. 1963. The intraspecific social behavior of some cricetine rodents of the genus Peromyscus. Am. Midl. Nat. 69, 240–246. ( 10.2307/2422858) [DOI] [Google Scholar]
- 22.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 23.Mathews WH, Fyles JG, Nasmith HW. 1970. Postglacial crustal movements in southwestern British Columbia and adjacent Washington state. Can. J. Earth Sci. 7, 690–702. ( 10.1139/e70-068) [DOI] [Google Scholar]
- 24.Blanchard DC, Blanchard RJ. 1988. Ethoexperimental approaches to the biology of emotion. Annu. Rev. Psychol. 39, 43–68. ( 10.1146/annurev.ps.39.020188.000355) [DOI] [PubMed] [Google Scholar]
- 25.de Catanzaro D. 1981. Facilitation of intermale aggression in mice through exposure to receptive females. J. Comp. Physiol. Psychol. 95, 638–645. ( 10.1037/h0077791) [DOI] [Google Scholar]
- 26.Dewsbury DA. 1981. Social dominance, copulatory behavior, and differential reproduction in deer mice (Peromyscus maniculatus). J. Comp. Physiol. Psychol. 95, 880–895. ( 10.1037/h0077842) [DOI] [Google Scholar]
- 27.Dewsbury DA. 1984. Aggression, copulation, and differential reproduction of deer mice (Peromyscus maniculatus) in a semi-natural enclosure. Behaviour 91, 1–23. ( 10.2307/4534377) [DOI] [Google Scholar]
- 28.Dewsbury DA. 1979. Copulatory behavior of deer mice (Peromyscus maniculatus). I. Normative data, subspecific differences, and effects of cross-fostering. J. Comp. Physiol. Psychol. 93, 151–160. ( 10.1037/h0077573) [DOI] [Google Scholar]
- 29.Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340, 1215–1217. ( 10.1126/science.1235765) [DOI] [PubMed] [Google Scholar]
- 30.Gray MM, Parmenter MD, Hogan CA, Ford I, Cuthbert RJ, Ryan PG, Broman KW, Payseur BA. 2015. Genetics of rapid and extreme size evolution in island mice. Genetics 201, 213–228. ( 10.1534/genetics.115.177790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth VL, Klein MS. 1986. Maternal effects on body size of large insular Peromyscus maniculatus: evidence from embryo transfer experiments. J. Mammal. 67, 37–45. ( 10.2307/1380999) [DOI] [Google Scholar]
- 32.Parmenter MD, Gray MM, Hogan CA, Ford IN, Broman KW, Vinyard CJ, Payseur BA. 2016. Genetics of skeletal evolution in unusually large mice from Gough Island. Genetics 204, 1559–1572. ( 10.1534/genetics.116.193805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheverud JM, Hager R, Roseman C, Fawcett G, Wang B, Wolf JB. 2008. Genomic imprinting effects on adult body composition in mice. Proc. Natl Acad. Sci. USA 105, 4253–4258. ( 10.1073/pnas.0706562105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gliwicz J. 1980. Island populations of rodents: their organization and functioning. Biol. Rev. 55, 109-138. ( 10.1111/j.1469-185X.1980.tb00690.x) [DOI] [Google Scholar]
- 35.Tamarin RH. 1978. Dispersal, population regulation, and K-selection in field mice. Am. Nat. 112, 545–555. ( 10.2307/2460122) [DOI] [Google Scholar]
- 36.Reich LM, Wood KM, Rothstein BE, Tamarin RH. 1982. Aggressive behaviour of male Microtus breweri and its demographic implications. Anim. Behav. 30, 117–122. ( 10.1016/S0003-3472(82)80245-5) [DOI] [Google Scholar]
- 37.Johnson RP. 1975. Scent marking with urine in two races of the bank vole (Clethrionomys glareolus). Behaviour 55, 81–93. ( 10.2307/4533697) [DOI] [PubMed] [Google Scholar]
- 38.Stirling DG, Réale D, Roff DA. 2002. Selection, structure and the heritability of behaviour. J. Evol. Biol. 15, 277–289. ( 10.1046/j.1420-9101.2002.00389.x) [DOI] [Google Scholar]
- 39.Postma E. 2014. Four decades of estimating heritabilities in wild vertebrate populations: improved methods, more data, better estimates? In Quantitative genetics in the wild (eds Charmantier A, Garant D, Kruuk LEB), pp. 16–33. Oxford, UK: Oxford University Press. [Google Scholar]
- 40.Blumstein DT, Daniel JC. 2005. The loss of anti-predator behaviour following isolation on islands. Proc. R. Soc. B 272, 1663–1668. ( 10.1098/rspb.2005.3147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levis NA, Pfennig DW. 2016. Evaluating ‘plasticity-first’ evolution in nature: key criteria and empirical approaches. Trends Ecol. Evol. 31, 563–574. ( 10.1016/j.tree.2016.03.012) [DOI] [PubMed] [Google Scholar]
- 42.Price TD, Qvarnström A, Irwin DE. 2003. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. Lond. B 270, 1433–1440. ( 10.1098/rspb.2003.2372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West-Eberhard MJ. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549. ( 10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wcislo WT. 1989. Behavioral environments and evolutionary change. Annu. Rev. Ecol. Syst. 20, 137–169. ( 10.1146/annurev.es.20.110189.001033) [DOI] [Google Scholar]
- 45.Foster SA. 2013. Evolution of behavioural phenotypes: influences of ancestry and expression. Anim. Behav. 85, 1061–1075. ( 10.1016/j.anbehav.2013.02.008) [DOI] [Google Scholar]
- 46.Aubret F, Shine R. 2009. Genetic assimilation and the postcolonization erosion of phenotypic plasticity in island tiger snakes. Curr. Biol. 19, 1932–1936. ( 10.1016/j.cub.2009.09.061) [DOI] [PubMed] [Google Scholar]
- 47.Corl A, Bi K, Luke C, Challa AS, Stern AJ, Sinervo B, Nielsen R. 2018. The genetic basis of adaptation following plastic changes in coloration in a novel environment. Curr. Biol. 28, 2970–2977. ( 10.1016/j.cub.2018.06.075) [DOI] [PubMed] [Google Scholar]
- 48.Losos JB, Creer DA, Glossip D, Goellner R, Hampton A, Roberts G, Haskell N, Taylor P, Ettling J. 2000. Evolutionary implications of phenotypic plasticity in the hindlimb of the lizard Anolis sagrei. Evolution 54, 301–305. ( 10.1111/j.0014-3820.2000.tb00032.x) [DOI] [PubMed] [Google Scholar]
- 49.Losos JB, Schoener TW, Spiller DA. 2004. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432, 505 ( 10.1038/nature03039) [DOI] [PubMed] [Google Scholar]
- 50.Price TD, Yeh PJ, Harr B. 2008. Phenotypic plasticity and the evolution of a socially selected trait following colonization of a novel environment. Am. Nat. 172, S49–S62. ( 10.1086/588257) [DOI] [PubMed] [Google Scholar]
- 51.Yeh PJ, Price TD. 2004. Adaptive phenotypic plasticity and the successful colonization of a novel environment. Am. Nat. 164, 531–542. ( 10.1086/423825) [DOI] [PubMed] [Google Scholar]
- 52.Baier F, Hoekstra HE. 2019. Data from: The genetics of morphological and behavioural island traits in deer mice Dryad Digital Repository. ( 10.5061/dryad.vx0k6djmk) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Baier F, Hoekstra HE. 2019. Data from: The genetics of morphological and behavioural island traits in deer mice Dryad Digital Repository. ( 10.5061/dryad.vx0k6djmk) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw sequence data (fastq files) and mtDNA sequences were submitted to the Sequence Read Archive (PRJNA508981, https://www.ncbi.nlm.nih.gov/bioproject/PRJNA508981/) and GenBank (MK265122–MK265240, https://www.ncbi.nlm.nih.gov/genbank/), respectively. Morphological and behavioural data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vx0k6djmk [52]. Museum specimens were accessioned into the Harvard Museum of Comparative Zoology Mammal Department (69713–69813).